Published online Jan 28, 2016. doi: 10.3748/wjg.v22.i4.1617
Peer-review started: May 6, 2015
First decision: August 26, 2015
Revised: September 12, 2015
Accepted: November 13, 2015
Article in press: November 13, 2015
Published online: January 28, 2016
Processing time: 266 Days and 18.9 Hours
Living donor liver transplantation (LDLT) has been widely used to treat end-stage liver disease with improvement in surgical technology and the application of new immunosuppressants. Vascular complications after liver transplantation remain a major threat to the survival of recipients. LDLT recipients are more likely to develop vascular complications because of their complex vascular reconstruction and the slender vessels. Early diagnosis and treatment are critical for the survival of graft and recipients. As a non-invasive, cost-effective and non-radioactive method with bedside availability, conventional gray-scale and Doppler ultrasonography play important roles in identifying vascular complications in the early postoperative period and during the follow-up. Recently, with the detailed vascular tracing and perfusion visualization, contrast-enhanced ultrasound (CEUS) has significantly improved the diagnosis of postoperative vascular complications. This review focuses on the role of conventional gray-scale ultrasound, Doppler ultrasound and CEUS for early diagnosis of vascular complications after adult LDLT.
Core tip: Vascular complications are among the most severe complications after living donor liver transplantation (LDLT), which may lead to graft loss and death of the recipients. Conventional gray-scale and Doppler ultrasound, contrast-enhanced ultrasound (CEUS) play important roles in identifying vascular complications in the early postoperative period and during follow-up. This review focuses on the current applications of conventional ultrasound and CEUS in the diagnosis of vascular complications in the early period after adult LDLT, including the diagnostic efficacy, controversial diagnostic criteria and current issues requiring further investigations.
- Citation: Ma L, Lu Q, Luo Y. Vascular complications after adult living donor liver transplantation: Evaluation with ultrasonography. World J Gastroenterol 2016; 22(4): 1617-1626
- URL: https://www.wjgnet.com/1007-9327/full/v22/i4/1617.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i4.1617
Because of the severe shortage of donor livers, living donor liver transplantation (LDLT) has undergone rapid development with advances in surgical technology and application of new immunosuppressive drugs since first introduced in 1969 by Smith[1-4]. Vascular complications after liver transplantation remain a major threat to the survival of recipients, especially in the early postoperative period[5-8]. Compared with cadaver liver transplantation, LDLT recipients have a higher risk for postoperative vascular complications because of their complex vascular reconstruction and slender vessels[5-6]. The vascular complications after LDLT mainly involved the hepatic artery, portal vein, hepatic vein and other outflow tracts. Hepatic artery thrombosis (HAT) and portal vein thrombosis (PVT) are the most severe complications which may lead to graft dysfunction and liver failure. The consequences of severe hepatic artery stenosis (HAS) and portal vein stenosis (PVS) are similar to thrombosis. Although the obstruction of the hepatic vein and other outflow tracts is relatively rare, it may induce congestions in the drained area, which may lead to small-for-size syndrome and graft loss. Therefore, early detection and timely treatment of vascular complications are critical for the survival of the graft and recipients[6-13].
Imaging techniques play a decisive role in the diagnosis of vascular complications. Although angiography is the traditional gold standard, it is an invasive procedure. Computed tomography (CT) causes radiation effects and magnetic resonance imaging (MRI) is more costly. Moreover, these techniques are not available at the bedside of severely ill patients in the intensive care unit (ICU). As a non-invasive, cost-effective and non-radioactive modality with bedside availability, ultrasonography serves as a first-line imaging technique to identify vascular complications in the early postoperative period and long-term follow-up[14-17]. Gray-scale ultrasound can be used to reveal the structure of blood vessels, parenchymal changes in the liver graft and perihepatic conditions. Doppler ultrasound can evaluate the hemodynamics including patency, direction, velocity and spectrum of the blood flow[17]. Contrast-enhanced ultrasound (CEUS) can be used to assess microcirculation of the liver graft and facilitates visualization of blood vessels, providing real-time angiographic-like images with a high diagnostic efficiency[18]. Furthermore, CEUS causes rare adverse reactions and can be applied in the recipients with renal insufficiency, because the gas within micro-bubbles is metabolized by respiration[19,20]. In this article, we review the current applications of conventional ultrasound and CEUS in the diagnosis of vascular complications in the early period after adult LDLT.
Hepatic artery complications are among the most severe complications after LDLT[6-10]. These complications include HAT, HAS, hepatic artery pseudoaneurysm (HAP) and splenic arterial steal syndrome (SASS).
HAT is the most severe hepatic artery complication with an incidence of 3%-5%, and a fatality rate of 20%-60%[6,8-10,21]. Risk factors for early HAT (occurring within 30 d after liver transplantation) include ABO blood type incompatibility, increased cold ischemic time of the donor liver, acute rejection and surgical factors such as hepatic artery spasm, intimal injury, perianastomotic hematoma compression, artery distortion, small artery caliber and artery anastomosis inversion. In contrast, late HAT (occurring more than 30 d after liver transplantation) is associated with chronic rejection and sepsis[22-24]. Typical HAT is manifested as severe hepatalgia, fever, ascites, sudden increase in serum transaminases, reduced bile flow, changed bile properties, prolonged prothrombin time and sepsis, whereas the symptoms of late HAT are often atypical due to the formation of collateral circulation. HAT can rapidly lead to biliary complications, graft necrosis and even patient death; therefore, early diagnosis and prompt treatment are critical[9-10].
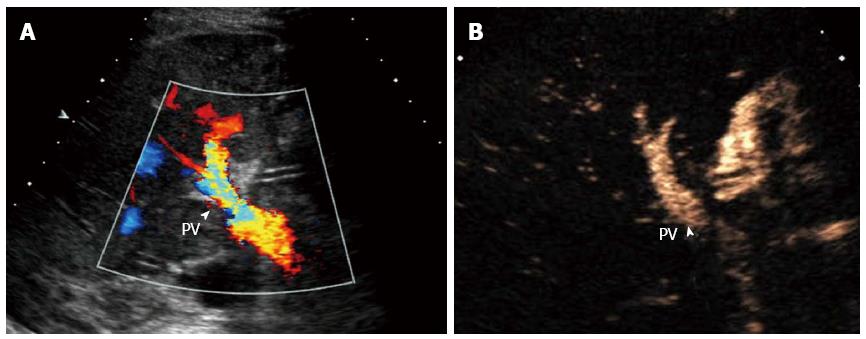
The normal hepatic artery is slender (2-5 mm in diameter) with a rapid systolic upstroke and continuous diastolic flow on ultrasound. The resistive index (RI) should be in the range from 0.5 to 0.8 and the systolic acceleration time (SAT) should be less than 80 ms. High resistance (RI > 0.8) may occur within 72 h postoperatively, and return to normal values afterwards[25]. It is difficult to observe hepatic artery directly by gray-scale ultrasound. The Doppler ultrasound diagnostic criteria for HAT include the disappearance of arterial blood flow at the hilus hepatis (Figure 1A) and inside the liver on color Doppler flow imaging. When HAT is complicated with collateralization, abnormal intrahepatic blood flow with a tardus-parvus spectrum (RI < 0.5 and SAT > 80 ms) can be detected. The secondary changes mainly include the biliary complications, hepatic infarction and abscess[26-30]. The sensitivity of Doppler ultrasound in HAT has been reported to be between 75% and 100%[26-31], and HAT can be detected even before the clinical symptoms appear[31]. However, Doppler ultrasound may yield false positive or false negative results. False positive results are mainly due to reduced hepatic arterial flow caused by hypotension, small hepatic artery caliber, early postoperative vasospasm, rejection reaction, improper adjustment of ultrasound machine or scanning. Reported false-positive rates are relatively high[26-31] and Hom et al[30] reported the false-positive rate even reaching as high as 75%. False negative results arise mainly from collateral circulation, with a reported false-negative rate of 7%-29%[29,31].
With the use of micro-bubble contrast agent, a blood pool tracer, CEUS can significantly improve the visualization of blood vessels and reveal perfusion of the liver parenchyma[18-20]. The diagnosis of HAT can be established when there is no contrast agent filling in the hepatic artery during the arterial phase on CEUS (Figure 1B). The sensitivity, specificity, and accuracy of CEUS in diagnosing HAT were 96%-100%[26,30,32]. Therefore, CEUS should be performed immediately to confirm HAT when it is suspected by Doppler ultrasound, which may reduce the use of angiography[32,33]. Additionally, the scanning time can be remarkably shortened[34]. The patient prognosis is influenced by the presence or absence of abundant collateral circulation[35]. Remarkably, CEUS is able to reveal the hepatic artery collateral circulation due to its high sensitivity to low velocity blood flow, thus providing reliable imaging information. Moreover, infarcted areas following HAT can also be identified on CEUS. This technique demonstrates absent enhancement in the arterial phase and hypo-enhancement in the portal and the late phases, occasionally with ‘branch-like’ portal venous distribution.
HAT-induced ischemia initially affects the bile ducts because bile ducts are supplied only by the hepatic artery and the biliary epithelium is more sensitive to ischemic injury than hepatocytes[36]. Biliary ischemia may lead to biliary necrosis, cast formation, abscesses, non-anastomotic bile leak and bilomas[36-37]. Conventional ultrasound plays an important role in the detection of bile duct complications as a first screening modality[37]. Recently, CEUS has been used to show the perfusion of the hilar bile ducts, facilitating the early diagnosis of biliary complications[36,38,39].
HAS occurs primarily at the anastomotic site with an incidence rate 5%-11%[6,8-10]. The causes are diverse and mainly include surgical factors, clamp injury, intimal trauma caused by perfusion catheters, disrupted vasa vasorum leading to ischemia of the arterial ends and rejection[8-11]. According to the diameter narrowing rates, HAS can be classified as mild stenosis (narrowing rate < 50%), moderate stenosis (narrowing rate 50%-75%) and severe stenosis (narrowing rate > 75%)[40]. Mild stenosis, which does not induce hemodynamic disorders of the hepatic artery or graft ischemia, presents no significant Doppler abnormalities. In contrast, moderate and severe stenosis, which may result in graft complications such as biliary ischemia, hepatic dysfunction or even hepatic failure, may have abnormal artery blood flow on Doppler imaging[26,28,40]. The diagnosis of HAS on Doppler ultrasound is based on a focal increased blood flow velocity greater than 200 cm/s at the extrahepatic artery or the tardus-parvus waveform at the intra-hepatic arteries, with a sensitivity of 72%-97% and a specificity of 64%-99.1%[28,40-42]. Some studies took a SAT threshold of 100 ms as the diagnostic criterion for HAS, resulting in an increased specificity[28,42]. However, it is difficult to observe the high-velocity at the deep-situated hepatic artery, and velocity measurements are not always accurate because of the difficulty in obtaining the appropriate Doppler angle and accurate gate placement. Tardus-parvus waveform is regarded as an excellent diagnostic parameter for HAS, but it is not a specific finding; this waveform can also be found in long-term HAT accompanied with collateral vessel formation, portal vein thrombosis, and atherosclerotic disease, resulting in a false-positive diagnosis[43]. Park et al[44] reported that the combination of the tardus-parvus pattern and an optimal peak systolic velocity cutoff greatly improved the positive predictive value and reduced the false positive rate for the diagnosis of HAS. Vit et al[41] concluded that an increased SAT value is more reliable than the RI. CEUS has been gradually used in the diagnosis of HAS in recent years. It provides direct visualization of the hepatic artery and possible stenosis, as well as the collateral circulation. On CEUS, stenoses are manifested as focal stenosis at the anastomoses (most frequently found), intra- or extra-hepatic arterial beaded or segmental stenosis, and diffuse tapering stenosis (less commonly found). However, the value of CEUS in diagnosing HAS remains controversial. Some scholars believed that CEUS has a high diagnostic value with an accuracy of 91.5%[43], while some[40] claimed that the diagnostic value of CEUS is limited in diagnosing HAS.
HAP is associated with high morbidity and mortality, and has an incidence of 0.3%-1%[45-47]. The clinical manifestations are diverse and include fever, bile leak, hepatic dysfunction, abdominal pain, hematemesis, melena, anemia, hypotension and jaundice. Some patients are asymptomatic and identified incidentally during routine examinations[48,49]. Intra-abdominal or gastrointestinal hemorrhages from rupture of HAP are the most severe and life-threatening presentations[50,51]. Early diagnosis and treatment are critical for graft salvage. Gray-scale ultrasound and Doppler ultrasound are regarded as the primary imaging techniques for the diagnosis and follow-up of HAP. When a focal cystic lesion involving the hepatic artery was detected and Doppler ultrasound showed a pulsatile wave pattern, the diagnosis of HAP is suspected. CEUS may be used to further confirm the diagnosis[27,41,52].
SASS, first described in 1992 by Langer et al[53], is not a well-recognized arterial complication following liver transplantation. SASS is described as the phenomenon of hepatic arterial hypo-perfusion due to “stealing” or diverting of blood from the hepatic artery to the dilated splenic artery. The incidence of SASS has been reported to be 3%-8%[54-56]. Clinically, the symptoms of SASS are non-specific, including liver function impairment and biliary injury, which can cause severe graft ischemia without timely management. Celiac angiography is the “gold standard” for the diagnosis of SASS. Recognition of the ultrasonographic indicators of SASS is imperative for an early diagnosis. When a high-resistance waveform (RI > 0.8) with low diastolic flow in the intra-hepatic and extra-hepatic arteries was detected on Doppler ultrasound, accompanied by dilated splenic arterial and splenomegaly, SASS should be highly suspected. And portal hyper-perfusion is another important feature of SASS[56-59].
Portal vein complications primarily include PVT, PVS and phlebangioma. The incidence of PVT and/or PVS is 1%-12.5%[60-62], while phlebangioma is rare. Yerdel et al[62] found an extremely high incidence of portal vein complications (12.5%) in male patients, patients with a history of severe portal hypertension or thrombosis preoperatively and patients who received treatment for portal hypertension such as sclerotherapy, transjugular intrahepatic portosystemic shunt, portocaval shunt, splenectomy, and splenic embolization. Because the portal vein accounts for 70%-80% of the hepatic blood supply, complications in this vessel will result in severe damage to the liver function. Therefore, postoperative monitoring is extremely important.
The duration and range of thrombosis affects the clinical manifestations of PVT. In the early stage, PVT may lead to liver function impairment complicated with prolonged prothrombin time, portal hypertension, variceal bleeding, intestinal edema, and massive ascites.
It is not difficult to diagnose PVT using gray-scale and Doppler ultrasound. The findings include absence or filling defect of blood flow. Although conventional ultrasound provides an ideal specificity ranging from 95% to 100% in diagnosing PVT[27,30,62,63], it is difficult to identify the duration and range of thrombosis. Nevertheless, conventional ultrasound still has the following limitations: (1) the portal vein may not be displayed clearly due to obesity, flatulence and ascites, and (2) color Doppler ultrasound is insensitive to portal blood flow that is deeply located, perpendicular to the acoustic beam, or of low velocity. CEUS greatly improved the detection of this defect, with a high diagnostic accuracy (97%-100%)[33,64-66]. Previous studies have indicated that the diagnostic validity of CEUS was comparable to that of MRI, CT or angiography[65,66]. Additionally, CEUS shortened the study time remarkably. With CEUS, thromboembolism is characterized by the absence of blood perfusion inside the thrombus as follows: (1) when the vessel is not completely occluded by the thrombus, micro-bubble bypass or filling defects are observed; and (2) when the vessel is completely occluded, contrast agent filling is constantly absent in the blood vessel[64].
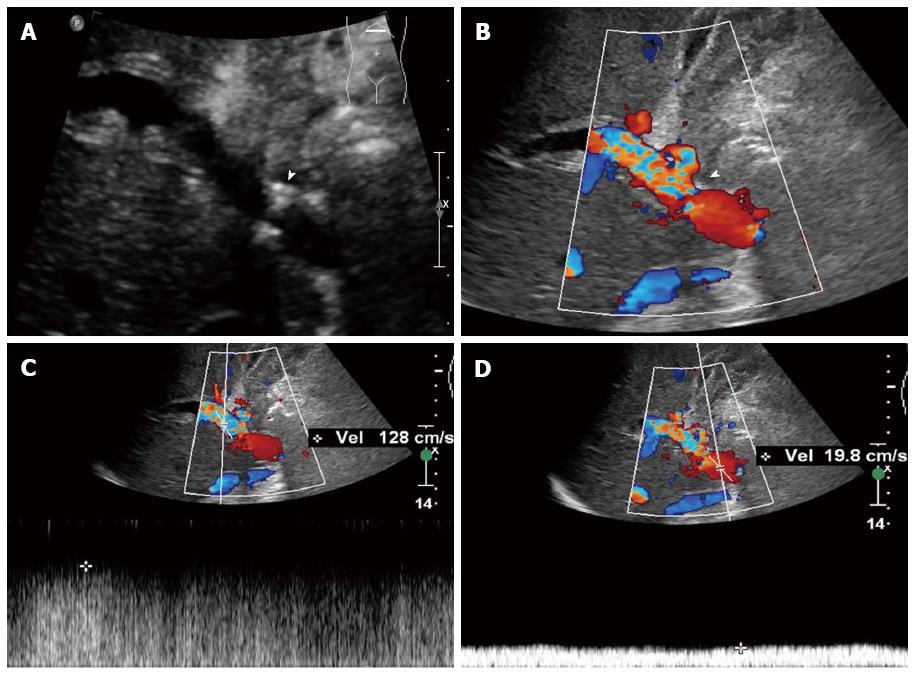
PVS is found mainly at the site of the anastomosis. During LDLT, vessel caliber mismatch is often encountered between the donor and recipient portal vein. Therefore, mild anastomotic stenosis is common but does not affect hemodynamics and liver function. When PVS is severe, symptoms of graft dysfunction and acute liver failure may occur, complicated with portal hypertension and ascites. Stenoses greater than 50% were considered hemodynamically significant[67]. To date, there is still no consensus on the ultrasound diagnostic criteria for post-LDLT PVS. According to our experience, the criteria for PVS include: regional stenosis with a diameter < 2.5 mm (Figure 2A), blood flow aliasing and acceleration at the stenotic site (Figure 2B), blood flow velocity > 150 cm/s at the stenotic site, or velocity ratio ≥ 4:1 between stenotic and pre-stenotic flow (Figure 2C and D), and signs of portal hypertension, such as splenomegaly, ascites or collateral circulation formation[26]. Mullan et al[67] proposed a maximal blood velocity > 80 cm/s at the stenotic segment of the portal vein as the diagnostic criterion for PVS, with a sensitivity of up to 100% and a specificity of 84%. Chong et al[68] regarded a maximal blood velocity > 125 cm/s at the stenotic segment of the portal vein as the diagnostic criterion for PVS, and although the sensitivity was only 73%, the specificity was 95%. It is noteworthy that turbulence and high-speed flow may appear at the anastomosis during the early postoperative period and the blood velocity will decrease with time.
Sometimes, the portal vein may be distorted due to surgical factors or portal vein enlargement after LDLT. Consequently, it is difficult to visualize the portal vein directly by gray-scale ultrasound. The Doppler ultrasound, constrained by color gain, as well as direction and angle adjustment of the acoustic beam, may lack the diagnostic accuracy. Aided by the micro-bubble contrast agent, visualization of blood vessels is greatly improved on CEUS, which provides accurate information of the specific position and degree of stenosis of PVS[30,33,34]. Moreover, for severe PVS and complete portal vein occlusion, which are often difficult to differentiate using conventional ultrasound, CEUS can improve the visualization of residual lumen of the stenotic portal vein through the dynamic display of micro-bubble contrast agent filling condition, thus facilitating the correct diagnosis.
In LDLT, because the graft is part of the liver of the recipient, a hyperkinetic circulation usually persists during the early postoperative period and is associated with the increases of portal venous flow (PVF) and its velocity. These changes play important roles in liver regeneration, but persistently high PVF and portal venous pressure (PVP) values may induce mechanical vascular injury, which may lead to poor graft function and small-for-size syndrome[69-73]. If PVF is greater than 250 or 300 mL/min per 100 g, and PVP is greater than 15 or 20 mmHg, PVF modulation is necessary to alleviate graft over-perfusion and early graft dysfunction[69,71,73].
Reconstruction of the outflow tract during LDLT is complex. In an adult right lobe LDLT, the right hepatic veins are preserved in the right lobes of the graft liver. As for preservation of the middle hepatic vein and reconstruction of the other blood vessels, decisions should be made in accordance with the specific individual conditions. Currently, in consideration of the safety of the donor, the middle hepatic vein is often preserved in the donor. Therefore, to ensure adequate drainage, reconstruction of the inferior right hepatic vein and thick tributaries of the middle hepatic vein V5 and/or V8 is usually required. Therefore, for an adult right lobe LDLT without the middle hepatic vein, the right hepatic vein, inferior right hepatic vein, tributaries of the middle hepatic vein, and bridging veins may serve as outflow tracts of the liver. Outflow tract obstruction leads to congestions in the drained area. Mild congestion may manifest no significant clinical symptoms, while severe congestion can result in effective liver volume reduction, which may also cause small-for-size syndrome and liver failure. Therefore, postoperative monitoring of the outflow tracts is extremely important[74-76].
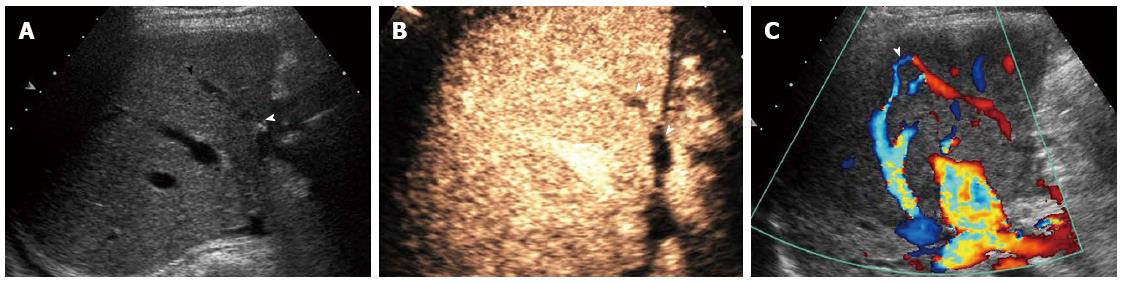
Ultrasound is a commonly used method for outflow tract monitoring. Gray-scale ultrasound can reveal the inferior vena cava, hepatic vein, inferior right hepatic vein, and some thick bridging veins (Figure 3A), and sometimes congestion may be revealed preliminarily by sonographic changes in the liver parenchyma. Doppler ultrasound can reveal the blood flow of hepatic veins and bridging veins, while CEUS can further visualize the vessels mentioned above and provide more accurate information as well as perfusion in the liver parenchyma.
Hepatic vein stenosis (HVS) is a relatively uncommon complication after LDLT with an incidence rate of 0.5%-3.2%[77,78]. Hepatic vein thrombosis is rare but is not difficult to diagnose by conventional ultrasound. Early-stage HVS is caused by surgical factors such as improper fixation, distortion, or shifting of the graft, while late HVS may be induced by intimal hyperplasia or perianastomotic fibrosis. The clinical manifestations are usually non-specific, some may be characterized by congestion of the liver parenchyma with abnormal laboratory test results, ascites, and pleural effusions. There can even be graft loss if it is not managed in a timely manner[76]. The diagnostic criteria for HVS using Doppler ultrasound remain controversial. The normal spectrum of the hepatic vein is a triphasic waveform reflecting the cardiac cycle, but after transplantation the waveform is often biphasic even without any other signs or symptoms of outflow obstruction. HVS should be considered when a significant stenosis is revealed by the gray-scale ultrasound or a high-speed blood flow disorder appears at the stenosis. The ratio of stenotic to pre-stenotic blood flow velocity is greater than 3-4:1 with a flat hepatic venous wave and slow or even reversed blood flow at the distal-stenotic segment[26]. Ko et al[79] reported that if the blood flow persists as a monophasic waveform, substantial HVS should be suspected, but a persistent triphasic wave pattern can exclude the possibility of substantial stenosis.
Although Doppler ultrasound is generally used for monitoring hepatic venous obstruction, it is associated with a relatively high false-positive rate because of the use of non-specific parameters[78,79]. Additionally, the assessment of waveform patterns is somewhat subjective because venous phasicity is a continuum and there is no clear-cut distinction between monophasic and biphasic wave patterns[68]. In contrast to Doppler ultrasound, CEUS may be more capable of visualizing the trunk of the hepatic vein, possible sites of stenosis, and the congestion areas. A cutoff value of the pressure gradient between the inferior vena cava and the hepatic vein is also used to diagnose hepatic venous obstruction[80-82]. Most studies have regarded a pressure gradient of 5-10 mmHg as the diagnostic criteria for substantial HVS, and Hwang et al[78] adopted a pressure gradient threshold of 6 mmHg for diagnosing HVS, with a sensitivity of 86.7% and a specificity of 68%. Therefore, depending on the clinical manifestations, ultrasound combined with a pressure gradient for the hepatic vein and inferior vena cava can be performed to make an accurate diagnosis of HVS.
As a common complication after LDLT, bridging vein occlusion occurs mainly because: (1) bridging veins are located at the surface of the section of the liver, making them susceptible to abdominal pressure; (2) blood volume of the drained area is small; and (3) angulations are formed at the anastomoses between the bridging veins and middle hepatic vein branches[83-86]. Bridging vein occlusion may result in focal congestion of the drained area or extensive ramus communicans between the middle hepatic vein branches and the right hepatic vein, through which the blood flow is drained to the right anterior hepatic lobe. Due to their small vessel caliber and deep location, bridging veins are difficult to detect by gray-scale and Doppler ultrasonography in most cases, whereas CEUS, which allows better bridging vein imaging, serves as a superior method for observing the bridging vein as well as intrahepatic venous collateral formation (Figure 3B and C)[87-89].
Both conventional gray-scale and Doppler ultrasound play important roles in vascular monitoring after LDLT. CEUS can reveal the microcirculation of the liver graft and greatly improve the visualization of blood vessels, providing a novel and effective mean for the detection and evaluation of post-LDLT vascular complications. However, further investigations are required to clarify issues such as (1) the diagnostic criteria, intervention timing and indications of HAS, and the impact of HAS and hepatic artery curvature on perfusion of the liver parenchyma; (2) the diagnostic criteria, intervention timing and indications of PVS, and the impact of portal venous perfusion on the liver regeneration; and (3) the diagnostic criteria, intervention timing and indications for outflow tract obstruction, and the impact of outflow tract obstruction on liver function and regeneration. The development and application of ultrasonic elastography, three-dimensional CEUS, ultrasound perfusion imaging and molecular imaging will offer a better understanding of the pathophysiology of the liver graft and the occurrence and development of vascular diseases, which will further improve the ultrasonographic diagnosis and the prognostic evaluation.
P- Reviewer: Meshikhes AW S- Editor: Gong ZM L- Editor: Ma JY E- Editor: Zhang DN
| 1. | Abbasoglu O. Liver transplantation: yesterday, today and tomorrow. World J Gastroenterol. 2008;14:3117-3122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | Eghtesad B, Aucejo F. Liver transplantation for malignancies. J Gastrointest Cancer. 2014;45:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Mehrabi A, Fonouni H, Müller SA, Schmidt J. Current concepts in transplant surgery: liver transplantation today. Langenbecks Arch Surg. 2008;393:245-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Smith B. Segmental liver transplantation from a living donor. J Pediatr Surg. 1969;4:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 5. | Fan ST. Live donor liver transplantation in adults. Transplantation. 2006;82:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Khalaf H. Vascular complications after deceased and living donor liver transplantation: a single-center experience. Transplant Proc. 2010;42:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Azzam AZ, Tanaka K. Management of vascular complications after living donor liver transplantation. Hepatogastroenterology. 2012;59:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Steinbrück K, Enne M, Fernandes R, Martinho JM, Balbi E, Agoglia L, Roma J, Pacheco-Moreira LF. Vascular complications after living donor liver transplantation: a Brazilian, single-center experience. Transplant Proc. 2011;43:196-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Pérez-Saborido B, Pacheco-Sánchez D, Barrera-Rebollo A, Asensio-Díaz E, Pinto-Fuentes P, Sarmentero-Prieto JC, Rodríguez-Vielba P, Martínez-Díaz R, Gonzalo-Martín M, Rodríguez M. Incidence, management, and results of vascular complications after liver transplantation. Transplant Proc. 2011;43:749-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Jiang XZ, Yan LN, Li B, Zhao JC, Wang WT, Li FG, Wen TF, Ma YK, Zeng Y, Xu MQ. Arterial complications after living-related liver transplantation: single-center experience from West China. Transplant Proc. 2008;40:1525-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Berrocal T, Parrón M, Alvarez-Luque A, Prieto C, Santamaría ML. Pediatric liver transplantation: a pictorial essay of early and late complications. Radiographics. 2006;26:1187-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Xiao L, Li F, Wei B, Li B, Tang CW. Small-for-size syndrome after living donor liver transplantation: successful treatment with a transjugular intrahepatic portosystemic shunt. Liver Transpl. 2012;18:1118-1120. [PubMed] |
| 13. | Low G, Crockett AM, Leung K, Walji AH, Patel VH, Shapiro AM, Lomas DJ, Coulden RA. Imaging of vascular complications and their consequences following transplantation in the abdomen. Radiographics. 2013;33:633-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Singh AK, Nachiappan AC, Verma HA, Uppot RN, Blake MA, Saini S, Boland GW. Postoperative imaging in liver transplantation: what radiologists should know. Radiographics. 2010;30:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Roberts JH, Mazzariol FS, Frank SJ, Oh SK, Koenigsberg M, Stein MW. Multimodality imaging of normal hepatic transplant vasculature and graft vascular complications. J Clin Imaging Sci. 2011;1:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Caiado AH, Blasbalg R, Marcelino AS, da Cunha Pinho M, Chammas MC, da Costa Leite C, Cerri GG, de Oliveira AC, Bacchella T, Machado MC. Complications of liver transplantation: multimodality imaging approach. Radiographics. 2007;27:1401-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Crossin JD, Muradali D, Wilson SR. US of liver transplants: normal and abnormal. Radiographics. 2003;23:1093-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 18. | Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D’Onofrio M. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 498] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 19. | Krix M, Jenne JW. [Ultrasound contrast agents. Pharmaceutical drug safety and bioeffects]. Radiologe. 2007;47:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Jakobsen JA, Oyen R, Thomsen HS, Morcos SK. Safety of ultrasound contrast agents. Eur Radiol. 2005;15:941-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Vaidya S, Dighe M, Kolokythas O, Dubinsky T. Liver transplantation: vascular complications. Ultrasound Q. 2007;23:239-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Yang Y, Zhao JC, Yan LN, Ma YK, Huang B, Yuan D, Li B, Wen TF, Wang WT, Xu MQ. Risk factors associated with early and late HAT after adult liver transplantation. World J Gastroenterol. 2014;20:10545-10552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 23. | Warner P, Fusai G, Glantzounis GK, Sabin CA, Rolando N, Patch D, Sharma D, Davidson BR, Rolles K, Burroughs AK. Risk factors associated with early hepatic artery thrombosis after orthotopic liver transplantation - univariable and multivariable analysis. Transpl Int. 2011;24:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Proposito D, Loinaz Segurola C, Garcia Garcìa I, Jimènez C, Gonzalez Pinto I, Gomez Sanz R, De La Cruz J, Moreno Gonzàlez E. [Assessment of risk factors in the incidence of hepatic artery thrombosis in a consecutive series of 687 liver transplantations]. Ann Ital Chir. 2001;72:187-205. [PubMed] |
| 25. | Kok T, Haagsma EB, Klompmaker IJ, Zwaveling JH, Peeters PM, Bijleveld CM, Meerman L, Slooff MJ. Doppler ultrasound of the hepatic artery and vein performed daily in the first two weeks after orthotopic liver transplantation. Useful for the diagnosis of acute rejection? Invest Radiol. 1996;31:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Luo Y, Fan YT, Lu Q, Li B, Wen TF, Zhang ZW. CEUS: a new imaging approach for postoperative vascular complications after right-lobe LDLT. World J Gastroenterol. 2009;15:3670-3675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Tamsel S, Demirpolat G, Killi R, Aydin U, Kilic M, Zeytunlu M, Parildar M, Oran I, Ucar H. Vascular complications after liver transplantation: evaluation with Doppler US. Abdom Imaging. 2007;32:339-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Dodd GD, Memel DS, Zajko AB, Baron RL, Santaguida LA. Hepatic artery stenosis and thrombosis in transplant recipients: Doppler diagnosis with resistive index and systolic acceleration time. Radiology. 1994;192:657-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 232] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 29. | Nolten A, Sproat IA. Hepatic artery thrombosis after liver transplantation: temporal accuracy of diagnosis with duplex US and the syndrome of impending thrombosis. Radiology. 1996;198:553-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 91] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Hom BK, Shrestha R, Palmer SL, Katz MD, Selby RR, Asatryan Z, Wells JK, Grant EG. Prospective evaluation of vascular complications after liver transplantation: comparison of conventional and microbubble contrast-enhanced US. Radiology. 2006;241:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | García-Criado A, Gilabert R, Nicolau C, Real I, Arguis P, Bianchi L, Vilana R, Salmerón JM, García-Valdecasas JC, Brú C. Early detection of hepatic artery thrombosis after liver transplantation by Doppler ultrasonography: prognostic implications. J Ultrasound Med. 2001;20:51-58. [PubMed] |
| 32. | Lu Q, Zhong XF, Huang ZX, Yu BY, Ma BY, Ling WW, Wu H, Yang JY, Luo Y. Role of contrast-enhanced ultrasound in decision support for diagnosis and treatment of hepatic artery thrombosis after liver transplantation. Eur J Radiol. 2012;81:e338-e343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Sidhu PS, Marshall MM, Ryan SM, Ellis SM. Clinical use of Levovist, an ultrasound contrast agent, in the imaging of liver transplantation: assessment of the pre- and post-transplant patient. Eur Radiol. 2000;10:1114-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Herold C, Reck T, Ott R, Schneider HT, Becker D, Schuppan D, Hahn EG. Contrast-enhanced ultrasound improves hepatic vessel visualization after orthotopic liver transplantation. Abdom Imaging. 2001;26:597-600. [PubMed] |
| 35. | Fouzas I, Sklavos A, Bismpa K, Paxiadakis I, Antoniadis N, Giakoustidis D, Katsiki E, Tatsou N, Mouloudi E, Karapanagiotou A. Hepatic artery thrombosis after orthotopic liver transplantation: 3 patients with collateral formation and conservative treatment. Transplant Proc. 2012;44:2741-2744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Fontanilla T, Noblejas A, Cortes C, Minaya J, Mendez S, Van den Brule E, Hernando CG, Alfageme M, Baños I, Aguirre E. Contrast-enhanced ultrasound of liver lesions related to arterial thrombosis in adult liver transplantation. J Clin Ultrasound. 2013;41:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Potthoff A, Hahn A, Kubicka S, Schneider A, Wedemeyer J, Klempnauer J, Manns M, Gebel M, Boozari B. Diagnostic value of ultrasound in detection of biliary tract complications after liver transplantation. Hepat Mon. 2013;13:e6003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Spârchez Z, Radu P. Role of contrast enhanced ultrasound in the assessment of biliary duct disease. Med Ultrason. 2014;16:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Ren J, Zheng BW, Wang P, Liao M, Zheng RQ, Lu MD, Lu Y, Zeng J, Zhang YL. Revealing impaired blood supply to the bile ducts on contrast-enhanced ultrasound: a novel diagnosis method to ischemic-type biliary lesions after orthotropic liver transplantation. Ultrasound Med Biol. 2013;39:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 40. | Sidhu PS, Ellis SM, Karani JB, Ryan SM. Hepatic artery stenosis following liver transplantation: significance of the tardus parvus waveform and the role of microbubble contrast media in the detection of a focal stenosis. Clin Radiol. 2002;57:789-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Vit A, De Candia A, Como G, Del Frate C, Marzio A, Bazzocchi M. Doppler evaluation of arterial complications of adult orthotopic liver transplantation. J Clin Ultrasound. 2003;31:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 42. | Platt JF, Yutzy GG, Bude RO, Ellis JH, Rubin JM. Use of Doppler sonography for revealing hepatic artery stenosis in liver transplant recipients. AJR Am J Roentgenol. 1997;168:473-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Zheng RQ, Mao R, Ren J, Xu EJ, Liao M, Wang P, Lu MQ, Yang Y, Cai CJ, Chen GH. Contrast-enhanced ultrasound for the evaluation of hepatic artery stenosis after liver transplantation: potential role in changing the clinical algorithm. Liver Transpl. 2010;16:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Park YS, Kim KW, Lee SJ, Lee J, Jung DH, Song GW, Ha TY, Moon DB, Kim KH, Ahn CS. Hepatic arterial stenosis assessed with doppler US after liver transplantation: frequent false-positive diagnoses with tardus parvus waveform and value of adding optimal peak systolic velocity cutoff. Radiology. 2011;260:884-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 45. | Maggi U, Dondossola D, Consonni D, Gatti S, Arnoldi R, Bossi M, Rossi G. Visceral artery aneurysms in liver transplant candidates and in patients after liver transplantation. PLoS One. 2011;6:e29544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Leelaudomlipi S, Bramhall SR, Gunson BK, Candinas D, Buckels JA, McMaster P, Mirza DF, Mayer AD. Hepatic-artery aneurysm in adult liver transplantation. Transpl Int. 2003;16:257-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Asonuma K, Ohshiro H, Izaki T, Okajima H, Ueno M, Kodera A, Inomata Y. Rescue for rare complications of the hepatic artery in living donor liver transplantation using grafts of autologous inferior mesenteric artery. Transpl Int. 2004;17:639-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Luo X, Tan S, Wang J, Qian C. Upper gastrointestinal hemorrhage from hepatic artery pseudoaneurysm secondary to trauma: a case report. Med Princ Pract. 2010;19:493-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Maleux G, Pirenne J, Aerts R, Nevens F. Case report: hepatic artery pseudoaneurysm after liver transplantation: definitive treatment with a stent-graft after failed coil embolisation. Br J Radiol. 2005;78:453-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Marion-Audibert AM, Mesnil A, Guillet M, Rode A, Mabrut JY, Garbit V, Lepoutre-Dujardin E, Pere-Verge D, Baulieux J, Souquet JC. [Pseudoaneurysm of the hepatic artery: rare complication of chronic pancreatitis]. Gastroenterol Clin Biol. 2008;32:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 51. | Marshall MM, Muiesan P, Srinivasan P, Kane PA, Rela M, Heaton ND, Karani JB, Sidhu PS. Hepatic artery pseudoaneurysms following liver transplantation: incidence, presenting features and management. Clin Radiol. 2001;56:579-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 52. | Kim HJ, Kim KW, Kim AY, Kim TK, Byun JH, Won HJ, Shin YM, Kim PN, Ha HK, Lee SG. Hepatic artery pseudoaneurysms in adult living-donor liver transplantation: efficacy of CT and Doppler sonography. AJR Am J Roentgenol. 2005;184:1549-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Langer R, Langer M, Scholz A, Felix R, Neuhaus P, Keck H. [The splenic steal syndrome and the gastroduodenal steal syndrome in patients before and after liver transplantation]. Aktuelle Radiol. 1992;2:55-58. [PubMed] |
| 54. | Sevmis S, Boyvat F, Aytekin C, Gorur SK, Karakayali H, Moray G, Haberal M. Arterial steal syndrome after orthotopic liver transplantation. Transplant Proc. 2006;38:3651-3655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 55. | Quintini C, Hirose K, Hashimoto K, Diago T, Aucejo F, Eghtesad B, Vogt D, Pierce G, Baker M, Kelly D. “Splenic artery steal syndrome” is a misnomer: the cause is portal hyperperfusion, not arterial siphon. Liver Transpl. 2008;14:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Nüssler NC, Settmacher U, Haase R, Stange B, Heise M, Neuhaus P. Diagnosis and treatment of arterial steal syndromes in liver transplant recipients. Liver Transpl. 2003;9:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 57. | Sanyal R, Shah SN. Role of imaging in the management of splenic artery steal syndrome. J Ultrasound Med. 2009;28:471-477. [PubMed] |
| 58. | Uslu N, Aslan H, Tore HG, Moray G, Karakayali H, Boyvat F, Arslan G, Haberal M. Doppler ultrasonography findings of splenic arterial steal syndrome after liver transplant. Exp Clin Transplant. 2012;10:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 59. | Mogl MT, Nüssler NC, Presser SJ, Podrabsky P, Denecke T, Grieser C, Neuhaus P, Guckelberger O. Evolving experience with prevention and treatment of splenic artery syndrome after orthotopic liver transplantation. Transpl Int. 2010;23:831-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Egawa H, Tanaka K, Kasahara M, Takada Y, Oike F, Ogawa K, Sakamoto S, Kozaki K, Taira K, Ito T. Single center experience of 39 patients with preoperative portal vein thrombosis among 404 adult living donor liver transplantations. Liver Transpl. 2006;12:1512-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 61. | Perkins JD. Incidence of portal vein complications following liver transplantation. Liver Transpl. 2008;14:1813-1815. [PubMed] |
| 62. | Yerdel MA, Gunson B, Mirza D, Karayalçin K, Olliff S, Buckels J, Mayer D, McMaster P, Pirenne J. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation. 2000;69:1873-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 525] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 63. | Jia YP, Lu Q, Gong S, Ma BY, Wen XR, Peng YL, Lin L, Chen HY, Qiu L, Luo Y. Postoperative complications in patients with portal vein thrombosis after liver transplantation: evaluation with Doppler ultrasonography. World J Gastroenterol. 2007;13:4636-4640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Rossi S, Rosa L, Ravetta V, Cascina A, Quaretti P, Azzaretti A, Scagnelli P, Tinelli C, Dionigi P, Calliada F. Contrast-enhanced versus conventional and color Doppler sonography for the detection of thrombosis of the portal and hepatic venous systems. AJR Am J Roentgenol. 2006;186:763-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 90] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 65. | Rennert J, Dornia C, Georgieva M, Roehrl S, Fellner C, Schleder S, Stroszczynski C, Jung EM. Identification of early complications following liver transplantation using contrast enhanced ultrasound (CEUS). First results. J Gastrointestin Liver Dis. 2012;21:407-412. [PubMed] |
| 66. | Clevert DA, Stickel M, Minaifar N, Löhe F, Graeb C, Jauch KW, Reiser M. Contrast-enhanced ultrasound in liver transplant: first results and potential for complications in the postoperative period. Clin Hemorheol Microcirc. 2009;43:83-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Mullan CP, Siewert B, Kane RA, Sheiman RG. Can Doppler sonography discern between hemodynamically significant and insignificant portal vein stenosis after adult liver transplantation? AJR Am J Roentgenol. 2010;195:1438-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Chong WK, Beland JC, Weeks SM. Sonographic evaluation of venous obstruction in liver transplants. AJR Am J Roentgenol. 2007;188:W515-W521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Wu TJ, Dahiya D, Lee CS, Lee CF, Chou HS, Chan KM, Lee WC. Impact of portal venous hemodynamics on indices of liver function and graft regeneration after right lobe living donor liver transplantation. Liver Transpl. 2011;17:1035-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Maetani Y, Itoh K, Egawa H, Shibata T, Ametani F, Kubo T, Kiuchi T, Tanaka K, Konishi J. Factors influencing liver regeneration following living-donor liver transplantation of the right hepatic lobe. Transplantation. 2003;75:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 71. | Gondolesi GE, Florman S, Matsumoto C, Huang R, Fishbein TM, Sheiner PA, Schwartz ME, Emre S, Thung S, Shapiro R. Venous hemodynamics in living donor right lobe liver transplantation. Liver Transpl. 2002;8:809-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 72. | Yagi S, Iida T, Taniguchi K, Hori T, Hamada T, Fujii K, Mizuno S, Uemoto S. Impact of portal venous pressure on regeneration and graft damage after living-donor liver transplantation. Liver Transpl. 2005;11:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 73. | Jiang SM, Zhou GW, Zhang R, Peng CH, Yan JQ, Wan L, Shen C, Chen H, Li QY, Shen BY. Role of splanchnic hemodynamics in liver regeneration after living donor liver transplantation. Liver Transpl. 2009;15:1043-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 74. | Yu PF, Wu J, Zheng SS. Management of the middle hepatic vein and its tributaries in right lobe living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2007;6:358-363. [PubMed] |
| 75. | Liu CL, Fan ST. Adult-to-adult live-donor liver transplantation: the current status. J Hepatobiliary Pancreat Surg. 2006;13:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 76. | Darcy MD. Management of venous outflow complications after liver transplantation. Tech Vasc Interv Radiol. 2007;10:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 77. | Akun E, Yaprak O, Killi R, Balci NC, Tokat Y, Yuzer Y. Vascular complications in hepatic transplantation: single-center experience in 14 years. Transplant Proc. 2012;44:1368-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 78. | Hwang HJ, Kim KW, Jeong WK, Song GW, Ko GY, Sung KB, Shin YM, Kim PN, Ha TY, Moon DB. Right hepatic vein stenosis at anastomosis in patients after living donor liver transplantation: optimal Doppler US venous pulsatility index and CT criteria--receiver operating characteristic analysis. Radiology. 2009;253:543-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 79. | Ko EY, Kim TK, Kim PN, Kim AY, Ha HK, Lee MG. Hepatic vein stenosis after living donor liver transplantation: evaluation with Doppler US. Radiology. 2003;229:806-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Rossi AR, Pozniak MA, Zarvan NP. Upper inferior vena caval anastomotic stenosis in liver transplant recipients: Doppler US diagnosis. Radiology. 1993;187:387-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Raby N, Karani J, Thomas S, O’Grady J, Williams R. Stenoses of vascular anastomoses after hepatic transplantation: treatment with balloon angioplasty. AJR Am J Roentgenol. 1991;157:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 131] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 82. | Borsa JJ, Daly CP, Fontaine AB, Patel NH, Althaus SJ, Hoffer EK, Winter TC, Nghiem HV, McVicar JP. Treatment of inferior vena cava anastomotic stenoses with the Wallstent endoprosthesis after orthotopic liver transplantation. J Vasc Interv Radiol. 1999;10:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Kim BW, Park YK, Paik OJ, Lee BM, Wang HJ, Kim MW. Effective anatomic reconstruction of the middle hepatic vein in modified right lobe graft living donor liver transplantation. Transplant Proc. 2007;39:3228-3233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Wu J, Wang W, Zhang M, Shen Y, Liang T, Yu P, Xu X, Yan S, Zheng S. Reconstruction of middle hepatic vein in living donor liver transplantation with modified right lobe graft: a single center experience. Transpl Int. 2008;21:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Shin JH, Sung KB, Yoon HK, Ko GY, Kim KW, Lee SG, Hwang S, Ahn CS, Kim KH, Moon DB. Endovascular stent placement for interposed middle hepatic vein graft occlusion after living-donor liver transplantation using right-lobe graft. Liver Transpl. 2006;12:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 86. | Yan L, Wu H, Chen Z, Luo Y, Lu Q, Zhang Z, Zhao J, Wang W, Ma Y, Wen T. Intrahepatic venous collaterals formation following outflow block in adult-to-adult living donor liver transplantation. J Surg Res. 2008;146:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Kim SY, Kim KW, Lee SS, Song GW, Hwang S, Kim PN, Lee SG. Doppler sonography to diagnose venous congestion in a modified right lobe graft after living donor liver transplantation. AJR Am J Roentgenol. 2008;190:1010-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 88. | Kaneko T, Sugimoto H, Hirota M, Kure S, Kiuchi T, Nakao A. Intrahepatic venous anastomosis formation of the right liver in living donor liver transplantation: evaluations by Doppler ultrasonography and pulse-inversion ultrasonography with Levovist. Surgery. 2005;138:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 89. | Park YS, Kim KW, Kim SY, Lee SJ, Lee J, Kim JH, Lee JS, Kim HJ, Song GW, Hwang S. Obstruction at middle hepatic venous tributaries in modified right lobe grafts after living-donor liver Transplantation: diagnosis with contrast-enhanced US. Radiology. 2012;265:617-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |