Published online Jan 28, 2016. doi: 10.3748/wjg.v22.i4.1523
Peer-review started: May 5, 2015
First decision: September 29, 2015
Revised: October 14, 2015
Accepted: November 19, 2015
Article in press: November 19, 2015
Published online: January 28, 2016
Processing time: 267 Days and 14.5 Hours
Acute liver failure is a critical medical condition defined as rapid development of hepatic dysfunction associated with encephalopathy. The prognosis in these patients is highly variable and depends on the etiology, interval between jaundice and encephalopathy, age, and the degree of coagulopathy. Determining the prognosis for this population is vital. Unfortunately, prognostic models with both high sensitivity and specificity for prediction of death have not been developed. Liver transplantation has dramatically improved survival in patients with acute liver failure. Still, 25% to 45% of patients will survive with medical treatment. The identification of patients who will eventually require liver transplantation should be carefully addressed through the combination of current prognostic models and continuous medical assessment. The concerns of inaccurate selection for transplantation are significant, exposing the recipient to a complex surgery and lifelong immunosuppression. In this challenging scenario, where organ shortage remains one of the main problems, alternatives to conventional orthotopic liver transplantation, such as living-donor liver transplantation, auxiliary liver transplant, and ABO-incompatible grafts, should be explored. Although overall outcomes after liver transplantation for acute liver failure are improving, they are not yet comparable to elective transplantation.
Core tip: Acute liver failure is the most dramatic clinical situation in which liver transplantation is performed. In this manuscript, we describe the timing and benefits of this procedure by analyzing the different prognostic scores and surgical techniques.
- Citation: Mendizabal M, Silva MO. Liver transplantation in acute liver failure: A challenging scenario. World J Gastroenterol 2016; 22(4): 1523-1531
- URL: https://www.wjgnet.com/1007-9327/full/v22/i4/1523.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i4.1523
Acute liver failure (ALF) is characterized by a rapid deterioration of the liver function (international normalized ratio ≥ 1.5) and the development of hepatic encephalopathy within 26 wk of jaundice in a patient with no previous history of liver disease[1]. ALF accounts for 8% of indications for liver transplantation (LT) in Europe and 7% in the United States[2,3]. Globally, viral hepatitis infections are probably responsible for the majority of cases of ALF. Hepatitis A and E are common in developing countries, while hepatitis B is a common cause in some Asian and South American countries[4-6]. In developed countries, drug-induced liver injury, especially with paracetamol, accounts for approximately 50% of cases[7]. Before the era of LT, ALF mortality rates ranged between 80% and 85%[8]. Advances in the field of critical care management and LT, however, have dramatically improved survival outcomes for patients with ALF[9,10]. Current LT results are especially good considering the emergency context of the surgical indication, with one- and five-year patient survival rates of 80% and 75%, respectively[9,10]. Nevertheless, LT candidate selection in the ALF setting must be carefully addressed. Risks of emergency transplantation in patients with evolving or established multiple organ failure (MOF) must be balanced against survival with continued medical supportive care alone. In this review, we discuss current decision-making strategies used to indicate LT in this challenging clinical scenario.
Several prognostic evaluation systems use different variables correlating with outcome in ALF patients to identify patients with high likelihood of mortality[11-15] (Table 1). When evaluating the accuracy of a prognostic score to identify those patients who will die if an LT is not performed, one should consider the positive and negative predictive values. The positive predictive value is the probability that a positive prognostic test will truly reflect the need for an LT. On the other hand, the negative predictive value will describe which patients will survive without LT. Four variables are considered key determinants to assess prognosis: etiology, interval between jaundice and encephalopathy, age, and synthetic markers of disease severity[16,17]. Most importantly, prognostic models need to be able to capture critical data at crucial time points during the course of disease[18]. Many prognostic models are used worldwide, most based on historic cohorts of patients not receiving a transplant. Although details among the systems differ, they share many common variables (Table 2). Unfortunately, prognostic models have limitations, and their predictive accuracies vary[16,19].
| Ref. | n | Etiologies | Parameters | Comments | |
| Laboratory | Clinical | ||||
| Bernuau et al[8] (1986) | 115 | Hepatitis B | Factor V levels | Age | Clichy criteria |
| O´Grady et al[11] (1989) | 332 | Non-paracetamol | Bilirubin, INR | Age, etiology, jaundice to encephalopathy > 7 | First model to differentiate between paracetamol-induced and other etiologies |
| 431 | Paracetamol | Arterial pH, Creatinine, INR, grade 3-4 encephalopathy | |||
| Bismuth et al[63] (1996) | 139 | All patients | Factor V levels | Age, grade 3-4 encephalopathy | |
| Mitchell et al[30] (1998) | 102 | Paracetamol | APACHE II | APACHE II score > 15: sensitivity 82%, specificity 98%; similar to KCC | |
| Schmidt et al[37] (2002) | 125 | Paracetamol | Serum phosphate > 1.2 mmol/L | Applicable from day 2-4 after overdose; sensitivity 89%, specificity 100%; superior to KCC | |
| Bernal et al[24] (2002) | 210 | Paracetamol | Lactate | Addition of post resuscitation lactate to KCC improved sensitivity | |
| Larson et al[46] (2005) | 275 | Paracetamol | APACHE II | APACHE II score > 20: sensitivity 68%, specificity 87%; superior to KCC | |
| Ganzert et al[44] (2005) | 198 | Amanita phalloides | Prothrombin time < 25%, creatinine > 1.2 mg/dL | Applicable from day 3 after ingestion; sensitivity 100%, specificity 98% | |
| Schmidt et al[36] (2005) | 239 | Paracetamol | α-fetoprotein | Dynamic α-fetoprotein measurement | |
| Schiødt et al[41] (2005) | 252 | All patients | Actin-free Gc-globulin | Cutoff level 40 mL/L; similar prognostic information as KCC in a single measurement admission | |
| Taylor et al[45] (2006) | 29 | Hepatitis A | ALT ≤ 2600 IU/L, creatinine ≥ 2.0 mg/dL | Intubation, vasopressors requirement | Superior to MELD score and KCC |
| Schiødt et al[35] (2007) | 206 | All patients | α-fetoprotein ratio day 1 and 3 | Ratio ≥ 1 indicated better prognosis | |
| Antoniades et al[40] (2006) | 70 | Paracetamol | Monocyte HLA-DR ≤ 15% | ||
| Yantorno et al[13] (2007) | 64 | Non-paracetamol | MELD score | MELD superior to KCC and Clichy criteria | |
| Dhiman et al[32] (2007) | 144 | Acute viral hepatitis | Creatinine ≥ 1.5 mg/dL, prothrombin time ≥ 35 s | Age ≥ 50, jaundice to encephalopathy > 7, cerebral edema, grade 3-4 encephalopathy | Presence of any of three variables superior to KCC and MELD score |
| Schimdt and Larsen[31] (2007) | 460 | Paracetamol | Serial MELD score | MELD score did not provide more information than KCC or INR alone | |
| Escudié et al[43] (2007) | 27 | Amanita phalloides | INR > 6 at day 4 | Ingestion diarrhea interval < 8 h | Encephalopathy not needed to decide transplantation |
| Volkmann et al[38] (2008) | 70 | All patients | Caspase activation (measured by Cytokeratin 18 fragments, M30 and M65) | Caspase activity might predict spontaneous recovery | |
| Mochida et al[15] (2008) | 698 | All patients | Prothrombin time < 10%, bilirubin ≥ 18 mg/dL | Age ≥ 45, jaundice to encephalopathy ≥ 11 d | Re-evaluates within 5 d if patient remains alive and liver transplantation was not performed |
| Hadem et al[14] (2008) | 102 | All patients | Bilirubin, lactate | Etiology | Bile score, better prognostic accuracy than MELD score or KCC |
| Bechmann et al[33] (2010) | 68 | All patients | Cytokeratin 18 (M65), creatinine, INR | MELD-M65 score | |
| Westbrook et al[42] (2010) | 54 | Pregnancy-related | Lactate ≥ 2.8 mg/dL | Encephalopathy | Sensitivity 90%, specificity 86%; superior to KCC |
| Cholongitas et al[28] (2012) | 125 | Paracetamol | SOFA score, APACHE II score, KCC, MELD | SOFA score was superior to KCC, MELD and APACHE II | |
| Rutherford et al[34] (2012) | 500 | All patients | INR, bilirubin, phosphorus ≥ 3.7 mg/dL, log10 M30 | Encephalopathy grade | ALFSG index sensitivity 86% and specificity 65%; superior to MELD score and KCC |
| Mendizabal et al[6] (2014) | 154 | Non-paracetamol | MELD score | MELD superior to KCC and Clichy criteria | |
| Clinical/demographic | Serologic | |
| Age | Bilirubin | Phosphate |
| Encephalopathy | Creatinine | Ketone body ratio |
| Etiology | INR/factor V | α-fetoprotein |
| Cerebral edema | Lactate | Cell death markers |
| Jaundice to encephalopathy interval | pH | (M30, M65) |
| Mechanical ventilation | Gc globulin | Monocyte HLA-DR |
| Vasopressors requirement | ||
| Functional/physiologic | Morphologic | |
| APACHE II | Hepatocyte necrosis | |
| Hepatic artery resistance index changes | Liver volume | |
Described in 1989, the King’s College Hospital criteria (KCC) are amongst the most common set of tests applied for patient selection, and were the first to distinguish between paracetamol-induced and other ALF etiologies[11]. The criteria have a clinically acceptable specificity, in that patients fulfilling the criteria are very likely to die if they do not receive transplants. Conversely, sensitivity is less, as a certain number of patients not meeting criteria do not survive[20,21]. A recent meta-analysis from 18 studies analyzing KCC performance in patients with nonparacetamol-induced ALF, totaling 1105 patients, reported an overall sensitivity of 68% and a specificity of 82%[22]. Interestingly, specificity increased to 88% when criteria were applied dynamically, and to 93% in patients with more advanced encephalopathy. Sensitivity fell in studies published after 2005, suggesting modern medical management of ALF may affect KCC performance[22]. Two separate meta-analyses studied paracetamol-induced ALF reporting an overall sensitivity of 58%-69% and specificity of 92%-94%[20,23]. Again, sensitivity was considered to be low as a result of non-dynamic application of the criteria[23]. The model has been refined and both specificity and sensitivity improved, for example, with the inclusion of lactate[24]. In a cohort of patients with paracetamol-induced ALF, the addition of the postresuscitation lactate concentration to the established KCC increased the ability to identify patients unlikely to survive unless they received transplants[24]. The rationale for including lactate as a prognostic marker is based on the fact that hyperlactatemia reflects not only systemic tissue dysfunction, but, most importantly, substantially decreased hepatic clearance of lactate. Two significant points to remember regarding KCC application are: first, its use is clearly most effective in patients with high-grade encephalopathy; and second, KCC were not formulated as part of a static model, but rather as a dynamic evaluation system, and therefore, their most effective application will arise from continued patient monitoring.
The Clichy criteria were described in 1986, and originated from the study of a cohort of 115 patients with fulminant hepatitis B[12]. The model is based on decreased levels of factor V, age, and the presence of grade 3-4 encephalopathy, with a positive predictive value of 82% and a negative predictive value of 98%[12]. Validation studies have shown the Clichy criteria to be not only less accurate than originally reported, but also less accurate than KCC for predicting outcome[25,26]. Seeking a better alternative to the KCC and Clichy criteria, the following scores were also evaluated: the Sequential Organ Failure Assessment (SOFA), and the Acute Physiology and Chronic Health Evaluation II (APACHE II). The rationale of evaluating non-liver-specific scores is based on the uncontrolled inflammatory response observed in patients with ALF. The SOFA and APACHE II scores are strongly associated with MOF and blood lactate levels[27]. In a retrospective study that included 125 patients with paracetamol-induced ALF, assessment of the prognosis was performed with KCC, Model for End-Stage Liver Disease (MELD), SOFA, and APACHE II[28]. The SOFA score performed better than the other prognostic scores with an area under the curve of 0.79, sensitivity of 67%, and specificity of 80%. Interestingly, temporal changes in SOFA score were also evaluated. A SOFA score > 6 by 72 h or > 7 by 96 h post-paracetamol overdose predicted death/transplantation with negative predictive values of 97% and 99%, respectively[29]. The APACHE II model has similar sensitivity and specificity to the KCC, but cannot be applied as early after admission[30].
More recently, interest has been focused on application of the MELD score to predict ALF outcomes in both paracetamol-induced and nonparacetamol-induced groups. However, a consistent advantage has not been conclusively demonstrated[6,13,19,31,32]. In order to improve the predictive value of MELD scores, a prognostic score substituting M65 (a cell death-associated marker) for bilirubin was described[33]. Fatal outcome predictability with the MELD-M65 score was superior to both classic MELD and KCC[33]. Characterization of cell death-related serum factors were further explored. The Acute Liver Failure Study Group proposed an index based on a composite of different clinical markers on admission (international normalized ratio, coma grade, bilirubin, and phosphorus level) with an additional apoptosis marker: M30[34]. This index outperformed MELD and KCC, improving sensitivity to 86%, but reducing specificity to only 65%. Markers of cell death or apoptosis are encouraging, though their use is technically more complex, and published results still need to be confirmed in further studies. Alternative prognostic variables have also been suggested to improve the selection of transplant candidates. A wide variety of blood markers have been proposed including: α-fetoprotein[35,36], serum phosphate[37], apoptosis and necrosis markers[38,39], monocyte HLA-DR expression[40], and Gc-globulin levels[41]. However, widespread clinical application of these blood markers and other prognostic scores are limited due to lack of external validation and general availability[42-45].
Ideally, prognostic models should be simple, accurate, and rapidly measured with high positive and negative predictive values, which should not lower specificity. In order to improve LT candidate selection, prognostic models should be applied continuously over time, as part of overall clinical monitoring by experienced multidisciplinary transplant teams.
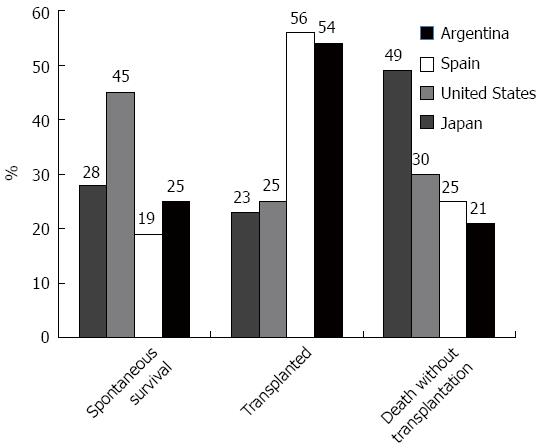
In the transplant era, overall survival in patients with ALF underwent considerable improvement. Still, nearly 30% of patients with ALF die[6,9,10]. Reasons restricting transplantation of more patients include: organ shortage, clinical complications, substance abuse issues, or involvement of other organ systems (i.e., heart failure, malignancy). Outcomes differ between regions mostly due to differences in etiology and to access to a suitable organ (Figure 1). In Argentina, for example, we reported a large ALF series with no cases of paracetamol toxicity, in clear contrast to series from the United States and the United Kingdom[6,7,9]. ALF associated with paracetamol toxicity has not only a better prognosis than most other etiologies, but is often associated with concomitant psychosocial issues, explaining why general transplantation rates for ALF in the Unites States are lower than in Argentina, at 24% and 54%, respectively[6,7,46].
One-year survival following LT in ALF patients ranges between 74% and 84%. These results are worse than those of patients grafted for other indications[7,10,47]. In spite of this fact, outcomes remain better compared to a 64% one-year survival described in patients in ICU immediately prior to LT, and to 54% observed in patients on mechanical ventilation at the time of organ allocation[7,47]. Most deaths occur in the first three months after surgery, from neurologic complications, MOF, or sepsis[10,48-50]. Great efforts have been made to determine which factors affect outcome (Table 3). Those better predicting survival without transplant are not the same as those predicting survival after LT. Graft quality and recipient clinical condition were described as the most relevant factors influencing transplant outcome in this setting[10,50,51]. Multivariate analysis of variables in the United Network for Organ Sharing (UNOS) database identified four factors associated with poor outcome: recipient age > 50 years, history of life support, body mass index ≥ 30 kg/m2, and serum creatinine > 2.0 mg/dL[50]. Five-year survival ranged from 47% for those with all four variables, to 83% for those with none. Limitations identified were that the high-risk group accounted for only 2% of the study population, that cumulative effects of adverse graft factors were not considered, and that the analysis included patients who underwent LT, exclusively.
| Demographic | Graft-related | Clinical |
| Age > 45 yr | Donor > 60 yr | Vasopressors |
| Male sex | ABO mismatch | Creatinine |
| Non-viral etiology | Steatosis | Body mass index ≥ 30 kg/m2 |
| Reduced liver |
Data from the European Liver Transplant Registry (ELTR) were analyzed, including 4903 patients with ALF[10]. Despite certain limitations of the multivariate model, authors identified major risk factors with a detrimental impact on post-LT mortality as: use of reduced-sized organs, recipient age > 50 years, male sex, donor age > 60 years, and incompatible ABO group matching. A prognostic model constructed for patients > 50 years of age foreseeably indicated that presence of multiple risk factors (ABO incompatibility, male recipient, use of partial graft, and donor age > 60 years) had negative impact both on patient and graft survival. In this model, a male patient over 50 years of age receiving a graft from a donor older than 60 years, for example, would have an estimated 57% risk of death or graft loss at one year. Limitations of the ELTR database relate to insufficient information on pretransplant renal function, encephalopathy grade, brain edema, and/or mechanical ventilation, all of which could improve LT prognosis assessment.
King’s College Hospital presented their experience in 310 patients with ALF over a ten-year period[51]. Four variables associated with 90-d post-LT mortality were observed, namely: recipient age > 45 years, vasopressor requirement, transplantation era, and use of high-risk grafts. The latter were defined by the presence of any two of the following: donor age > 60 years, liver steatosis, ABO non-identical match, and use of non-whole graft. Interestingly, older recipient age presented the strongest link to increased mortality; 90-d survival was only 47%, compared to 80% in the younger cohort. An age-related reduction in physiologic hepatic reserve was proposed to explain the higher mortality rate observed in this group[51].
Based on pre-LT evaluation of the factors described, transplant teams should attempt to establish when an outcome might be unacceptable. Recipient and donor factors associated with poor post-LT outcomes are similar in transplants performed electively or secondary to ALF. Particular causes of graft failure or patient death could potentially explain the gap in survival rates in ALF compared to other LT indications, especially during the first three months following surgery. For example, multisystem disorder triggered by ALF, as well as marked activation of systemic inflammatory response, can extend into the post-transplant period. In UNOS and ELTR reviews of databases, infection was the most common cause of mortality following LT for ALF (24% and 18%, respectively). Remarkably, in the UNOS data, almost 22% of infectious complications were associated with fungal infections. Neurologic complications were reported as the second-most common cause of death following transplantation (13%). Fortunately, with better intensive care management of these critical patients, intracranial hypertension incidence has fallen dramatically, coinciding with survival improvement observed over time[9,10]. Another important and alarming issue is death or graft failure related to psychosocial problems. According to the ELTR, patients transplanted for paracetamol overdose present ten times higher rates of death or graft failure, resulting from suicide or lack of compliance, than patients transplanted for other etiologies[10]. The finding is even more alarming if we consider that in Europe, transplantation for paracetamol-induced ALF has increased sevenfold, from 2% (1973-1978) to 14.1% (2004-2008)[10]. Patients with ALF due to paracetamol need very close post-transplant monitoring, including improved psychologic and social patient care.
In this challenging scenario in which patients with ALF quickly deteriorate and organ shortage remains one of the main problems, risk of mortality while on the waiting list should be weighed against risk of complications or failure resulting from use of an alternative graft. Different LT procedures can be selected depending on donor organ availability, including use of: deceased organ donor, living-donor liver transplantation (LDLT), auxiliary liver transplant, and variable ABO status (Table 4).
LDLT: LDLT provides an alternative source of grafts to overcome the problem of organ shortage, accounting for up to 4% of LTs in the United States. However, this figure could account for more than 90% of LTs in some Asian countries[47,52,53]. The indication in the pediatric ALF population is well established, and in experienced centers, patient and graft outcomes are similar to those of conventional cadaver donor transplants[54,55]. ALF is the indication for transplantation in only 1% of patients evaluated for LDLT in the United States[56]. This contrasts sharply with LDLT indication for ALF in Asia, where different groups report rates between 6% and 15%[57,58]. Use of living-donor grafts in against-the-clock emergency settings is often complicated by insufficient time to assess potential donor’s spontaneous willingness to donate or for transplant teams to evaluate important ethical and medical issues. Pressing donor evaluation raises special concerns regarding possible donor coercion. Potential consequences of expedited donor evaluation could increase donor postoperative complication rates and worsen psychosocial problems. Transplant centers with great expertise in LDLT report living-donor complication rates in ALF to be 34%, similar to those of other indications.
Graft size is a crucial variable in LDLT. Graft-to-recipient weight ratios < 0.8% are generally associated with poor outcome[59]. The optimal graft-to-recipient weight ratio appears to be closer to 1.0%. However, some authors believe smaller grafts can still be used in ALF, given that this is an acute condition and most patients do not have portal hypertension[60]. In countries where wait-list mortality rates are high or access to deceased donors is limited, LDLT allows better control over surgical procedure timing[61]. Additionally, once donor evaluation is completed, LDLT can be performed at the first sign of patient decompensation using a good quality graft.
Auxiliary transplantation: Auxiliary liver transplantation is an attractive alternative to total transplantation. A partial left or right donor lobe is used, acting as temporary support to replace the damaged recipient liver, while all or part of the native liver remains in situ. The partial graft can be placed below the native liver (heterotopic) or replacing the resected right or left native lobe (auxiliary transplantation). Increased incidence of portal vein thrombosis or primary nonfunction has been observed with heterotopic transplantation compared to auxiliary partial or standard LT[62]. Auxiliary transplantation provides temporary support of liver function until spontaneous regeneration and recovery of the native liver occurs, at which time immunosuppressive treatment is withdrawn and the implant atrophies or is removed. The surgical procedure is challenging because it requires partial native liver resection in a critically ill patient and complex vascular reconstruction. Initial reports of auxiliary LT showed relatively high anastomotic complications and retransplantation rates, though recent outcomes have improved substantially[63-65]. In Europe, auxiliary transplantation peaked at 4% between 1994 and 1998, but has since fallen to only 1.9% in 2004-2009[10]. Optimal indications for auxiliary transplantation include patient age < 40 years, excellent temporary liver graft, and hemodynamic stability. However, it is difficult to predict which patients will present regeneration of the native liver. This appears to be the case in those with hyperacute presentation and viral or paracetamol etiology, as well as certain histology subtypes (diffuse pattern, map-like necrosis) and timing of hepatectomy[65,66].
ABO-incompatible graft: Length of time on the waiting list also influences policies related to use of ABO incompatible grafts. Early results with ABO-incompatible LTs were disappointing because of increased risk of severe cellular and humoral graft rejection, biliary complications, and vascular thrombosis. Recent analysis of the ELTR showed the rate of graft loss at three months doubled in ABO-mismatched grafts used during emergency transplantation[10]. Different strategies have been implemented to improve results with ABO-incompatible livers and other grafts. Toso et al[67] reported acceptable graft and patient survival in 14 patients using a quadruple immunosuppressive regimen without splenectomy; 64% and 56% of ABO-incompatible grafts remained functional after one and five years, respectively. Recent approaches have been described with promising results. Paul Brousse Hospital reported three patients treated with antigen-specific immunoadsorption and a quadruple immunosuppressive regimen combined or not with anti-CD20 humanized monoclonal antibodies (rituximab)[68]. Keio University proposed a complex but successful protocol for ABO-incompatible LDLTs that included multiple perioperative plasmaphereses together with rituximab, splenectomy, and triple systemic immunosuppression. In addition, portal vein-infusion therapy was administered after transplant with methylprednisolone, prostaglandin E1, and gabexate mesylate[69]. Thirteen adult patients underwent ABO-incompatible LDLTs under this protocol, for which authors reported a three-year survival of 76%, almost identical to that of ABO-compatible cases[69]. Regardless of these promising results, close monitoring of the patient’s immune status and adjustment of immunosuppression need to be implemented, as infection remains the major cause of morbidity and mortality. The protocols described should be viewed as important treatment options in select adult patients with ALF. However, they are very complex and require maximum expertise. Controversy remains as to whether ABO-compatible or -incompatible LT can really present similar post-transplant outcomes.
Despite progressive and constant improvement in ALF survival after LT, high mortality and graft loss rates persist, especially within the first three months post-transplant. Current prognostic models are helpful in identifying individuals who will need LT; nonetheless, fine-tuning of these scores is needed to improve identification in patients who would benefit from transplantation. Newer technologies are being developed and enhanced to improve survival. Different extracorporeal support devices have been advocated to supplant liver function in patients with ALF, either to improve native liver regeneration or to stabilize patients before transplantation. However, conclusive evidence has not been reported[70,71]. Mesenchymal stem cell infusion also appears promising, but several problems remain in relation to use of this therapy, including conflicting data on the potential risk of malignant transformation, as well as degree of liver engraftment and their long-term efficacy[72].
With increasing knowledge on encephalopathy pathogenesis, hepatic regeneration, and mechanisms of liver cell injury, outcomes should continue to improve. Early referral to a transplant center and prompt treatment of patients with worsening liver failure remain, however, the backbone behind outcome improvement.
We are grateful to Podestá C for her review and assistance with editing this paper.
P- Reviewer: Bramhall S, Cholongitas EC, Dehghani SM S- Editor: Qi Y L- Editor: Filipodia E- Editor: Zhang DN
| 1. | Lee WM, Stravitz RT, Larson AM. Introduction to the revised American Association for the Study of Liver Diseases Position Paper on acute liver failure 2011. Hepatology. 2012;55:965-967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 355] [Article Influence: 27.3] [Reference Citation Analysis (35)] |
| 2. | Adam R, Karam V, Delvart V, O’Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 643] [Article Influence: 49.5] [Reference Citation Analysis (2)] |
| 3. | Available from: http://optn.transplant.hrsa.gov/ar2009. |
| 4. | Jilani N, Das BC, Husain SA, Baweja UK, Chattopadhya D, Gupta RK, Sardana S, Kar P. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J Gastroenterol Hepatol. 2007;22:676-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Oketani M, Ido A, Tsubouchi H. Changing etiologies and outcomes of acute liver failure: A perspective from Japan. J Gastroenterol Hepatol. 2011;26 Suppl 1:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Mendizabal M, Marciano S, Videla MG, Anders M, Zerega A, Balderramo DC, Chan D, Barrabino M, Gil O, Mastai R. Changing etiologies and outcomes of acute liver failure: perspectives from 6 transplant centers in Argentina. Liver Transpl. 2014;20:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Lee WM. Etiologies of acute liver failure. Semin Liver Dis. 2008;28:142-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Bernuau J, Rueff B, Benhamou JP. Fulminant and subfulminant liver failure: definitions and causes. Semin Liver Dis. 1986;6:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 398] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Bernal W, Hyyrylainen A, Gera A, Audimoolam VK, McPhail MJ, Auzinger G, Rela M, Heaton N, O’Grady JG, Wendon J. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013;59:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 306] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 10. | Germani G, Theocharidou E, Adam R, Karam V, Wendon J, O’Grady J, Burra P, Senzolo M, Mirza D, Castaing D. Liver transplantation for acute liver failure in Europe: outcomes over 20 years from the ELTR database. J Hepatol. 2012;57:288-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | O’Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439-445. [PubMed] |
| 12. | Bernuau J, Goudeau A, Poynard T, Dubois F, Lesage G, Yvonnet B, Degott C, Bezeaud A, Rueff B, Benhamou JP. Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology. 1986;6:648-651. [PubMed] |
| 13. | Yantorno SE, Kremers WK, Ruf AE, Trentadue JJ, Podestá LG, Villamil FG. MELD is superior to King’s college and Clichy’s criteria to assess prognosis in fulminant hepatic failure. Liver Transpl. 2007;13:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 14. | Hadem J, Stiefel P, Bahr MJ, Tillmann HL, Rifai K, Klempnauer J, Wedemeyer H, Manns MP, Schneider AS. Prognostic implications of lactate, bilirubin, and etiology in German patients with acute liver failure. Clin Gastroenterol Hepatol. 2008;6:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Mochida S, Nakayama N, Matsui A, Nagoshi S, Fujiwara K. Re-evaluation of the Guideline published by the Acute Liver Failure Study Group of Japan in 1996 to determine the indications of liver transplantation in patients with fulminant hepatitis. Hepatol Res. 2008;38:970-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | O’Grady J. Timing and benefit of liver transplantation in acute liver failure. J Hepatol. 2014;60:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 17. | Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 845] [Article Influence: 70.4] [Reference Citation Analysis (2)] |
| 18. | O’Grady JG. Prognostication in acute liver failure: a tool or an anchor? Liver Transpl. 2007;13:786-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Polson J. Assessment of prognosis in acute liver failure. Semin Liver Dis. 2008;28:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Bailey B, Amre DK, Gaudreault P. Fulminant hepatic failure secondary to acetaminophen poisoning: a systematic review and meta-analysis of prognostic criteria determining the need for liver transplantation. Crit Care Med. 2003;31:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Bernal W, Wendon J, Rela M, Heaton N, Williams R. Use and outcome of liver transplantation in acetaminophen-induced acute liver failure. Hepatology. 1998;27:1050-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 117] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | McPhail MJ, Wendon JA, Bernal W. Meta-analysis of performance of Kings’s College Hospital Criteria in prediction of outcome in non-paracetamol-induced acute liver failure. J Hepatol. 2010;53:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Craig DG, Ford AC, Hayes PC, Simpson KJ. Systematic review: prognostic tests of paracetamol-induced acute liver failure. Aliment Pharmacol Ther. 2010;31:1064-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Bernal W, Donaldson N, Wyncoll D, Wendon J. Blood lactate as an early predictor of outcome in paracetamol-induced acute liver failure: a cohort study. Lancet. 2002;359:558-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 321] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 25. | Choi WC, Arnaout WC, Villamil FG, Demetriou AA, Vierling JM. Comparison of the applicability of two prognostic scoring systems in patients with fulminant hepatic failure. Korean J Intern Med. 2007;22:93-100. [PubMed] |
| 26. | Pauwels A, Mostefa-Kara N, Florent C, Lévy VG. Emergency liver transplantation for acute liver failure. Evaluation of London and Clichy criteria. J Hepatol. 1993;17:124-127. [PubMed] |
| 27. | Craig DG, Reid TW, Martin KG, Davidson JS, Hayes PC, Simpson KJ. The systemic inflammatory response syndrome and sequential organ failure assessment scores are effective triage markers following paracetamol (acetaminophen) overdose. Aliment Pharmacol Ther. 2011;34:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 28. | Cholongitas E, Theocharidou E, Vasianopoulou P, Betrosian A, Shaw S, Patch D, O’Beirne J, Agarwal B, Burroughs AK. Comparison of the sequential organ failure assessment score with the King’s College Hospital criteria and the model for end-stage liver disease score for the prognosis of acetaminophen-induced acute liver failure. Liver Transpl. 2012;18:405-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Craig DG, Reid TW, Wright EC, Martin KG, Davidson JS, Hayes PC, Simpson KJ. The sequential organ failure assessment (SOFA) score is prognostically superior to the model for end-stage liver disease (MELD) and MELD variants following paracetamol (acetaminophen) overdose. Aliment Pharmacol Ther. 2012;35:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Mitchell I, Bihari D, Chang R, Wendon J, Williams R. Earlier identification of patients at risk from acetaminophen-induced acute liver failure. Crit Care Med. 1998;26:279-284. [PubMed] |
| 31. | Schmidt LE, Larsen FS. MELD score as a predictor of liver failure and death in patients with acetaminophen-induced liver injury. Hepatology. 2007;45:789-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 32. | Dhiman RK, Jain S, Maheshwari U, Bhalla A, Sharma N, Ahluwalia J, Duseja A, Chawla Y. Early indicators of prognosis in fulminant hepatic failure: an assessment of the Model for End-Stage Liver Disease (MELD) and King’s College Hospital criteria. Liver Transpl. 2007;13:814-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 33. | Bechmann LP, Jochum C, Kocabayoglu P, Sowa JP, Kassalik M, Gieseler RK, Saner F, Paul A, Trautwein C, Gerken G. Cytokeratin 18-based modification of the MELD score improves prediction of spontaneous survival after acute liver injury. J Hepatol. 2010;53:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 34. | Rutherford A, King LY, Hynan LS, Vedvyas C, Lin W, Lee WM, Chung RT. Development of an accurate index for predicting outcomes of patients with acute liver failure. Gastroenterology. 2012;143:1237-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 35. | Schiødt FV, Bangert K, Shakil AO, McCashland T, Murray N, Hay JE, Lee WM. Predictive value of actin-free Gc-globulin in acute liver failure. Liver Transpl. 2007;13:1324-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Schmidt LE, Dalhoff K. Alpha-fetoprotein is a predictor of outcome in acetaminophen-induced liver injury. Hepatology. 2005;41:26-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 37. | Schmidt LE, Dalhoff K. Serum phosphate is an early predictor of outcome in severe acetaminophen-induced hepatotoxicity. Hepatology. 2002;36:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 38. | Volkmann X, Anstaett M, Hadem J, Stiefel P, Bahr MJ, Lehner F, Manns MP, Schulze-Osthoff K, Bantel H. Caspase activation is associated with spontaneous recovery from acute liver failure. Hepatology. 2008;47:1624-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Bechmann LP, Marquitan G, Jochum C, Saner F, Gerken G, Canbay A. Apoptosis versus necrosis rate as a predictor in acute liver failure following acetaminophen intoxication compared with acute-on-chronic liver failure. Liver Int. 2008;28:713-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Antoniades CG, Berry PA, Davies ET, Hussain M, Bernal W, Vergani D, Wendon J. Reduced monocyte HLA-DR expression: a novel biomarker of disease severity and outcome in acetaminophen-induced acute liver failure. Hepatology. 2006;44:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 41. | Schiødt FV, Rossaro L, Stravitz RT, Shakil AO, Chung RT, Lee WM. Gc-globulin and prognosis in acute liver failure. Liver Transpl. 2005;11:1223-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Westbrook RH, Yeoman AD, Joshi D, Heaton ND, Quaglia A, O’Grady JG, Auzinger G, Bernal W, Heneghan MA, Wendon JA. Outcomes of severe pregnancy-related liver disease: refining the role of transplantation. Am J Transplant. 2010;10:2520-2526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Escudié L, Francoz C, Vinel JP, Moucari R, Cournot M, Paradis V, Sauvanet A, Belghiti J, Valla D, Bernuau J. Amanita phalloides poisoning: reassessment of prognostic factors and indications for emergency liver transplantation. J Hepatol. 2007;46:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 44. | Ganzert M, Felgenhauer N, Zilker T. Indication of liver transplantation following amatoxin intoxication. J Hepatol. 2005;42:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Taylor RM, Davern T, Munoz S, Han SH, McGuire B, Larson AM, Hynan L, Lee WM, Fontana RJ. Fulminant hepatitis A virus infection in the United States: Incidence, prognosis, and outcomes. Hepatology. 2006;44:1589-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 46. | Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, Schiødt FV, Ostapowicz G, Shakil AO. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1280] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 47. | Freeman RB, Steffick DE, Guidinger MK, Farmer DG, Berg CL, Merion RM. Liver and intestine transplantation in the United States, 1997-2006. Am J Transplant. 2008;8:958-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 48. | Tessier G, Villeneuve E, Villeneuve JP. Etiology and outcome of acute liver failure: experience from a liver transplantation centre in Montreal. Can J Gastroenterol. 2002;16:672-676. [PubMed] |
| 49. | Farmer DG, Anselmo DM, Ghobrial RM, Yersiz H, McDiarmid SV, Cao C, Weaver M, Figueroa J, Khan K, Vargas J. Liver transplantation for fulminant hepatic failure: experience with more than 200 patients over a 17-year period. Ann Surg. 2003;237:666-675; discussion 675-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 112] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 50. | Barshes NR, Lee TC, Balkrishnan R, Karpen SJ, Carter BA, Goss JA. Risk stratification of adult patients undergoing orthotopic liver transplantation for fulminant hepatic failure. Transplantation. 2006;81:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 51. | Bernal W, Cross TJ, Auzinger G, Sizer E, Heneghan MA, Bowles M, Muiesan P, Rela M, Heaton N, Wendon J. Outcome after wait-listing for emergency liver transplantation in acute liver failure: a single centre experience. J Hepatol. 2009;50:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 52. | Yamashiki N, Sugawara Y, Tamura S, Nakayama N, Oketani M, Umeshita K, Uemoto S, Mochida S, Tsubouchi H, Kokudo N. Outcomes after living donor liver transplantation for acute liver failure in Japan: results of a nationwide survey. Liver Transpl. 2012;18:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | de Villa VH, Lo CM, Chen CL. Ethics and rationale of living-donor liver transplantation in Asia. Transplantation. 2003;75:S2-S5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 54. | Oh SH, Kim KM, Kim DY, Kim Y, Song SM, Lee YJ, Park SJ, Yoon CH, Ko GY, Sung KB. Improved outcomes in liver transplantation in children with acute liver failure. J Pediatr Gastroenterol Nutr. 2014;58:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Farmer DG, Venick RS, McDiarmid SV, Duffy JP, Kattan O, Hong JC, Vargas J, Yersiz H, Busuttil RW. Fulminant hepatic failure in children: superior and durable outcomes with liver transplantation over 25 years at a single center. Ann Surg. 2009;250:484-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 56. | Campsen J, Blei AT, Emond JC, Everhart JE, Freise CE, Lok AS, Saab S, Wisniewski KA, Trotter JF. Outcomes of living donor liver transplantation for acute liver failure: the adult-to-adult living donor liver transplantation cohort study. Liver Transpl. 2008;14:1273-1280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Lo CM, Fan ST, Liu CL, Yong BH, Wong Y, Lau GK, Lai CL, Ng IO, Wong J. Lessons learned from one hundred right lobe living donor liver transplants. Ann Surg. 2004;240:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 58. | Morioka D, Egawa H, Kasahara M, Ito T, Haga H, Takada Y, Shimada H, Tanaka K. Outcomes of adult-to-adult living donor liver transplantation: a single institution’s experience with 335 consecutive cases. Ann Surg. 2007;245:315-325. [PubMed] |
| 59. | Uemoto S, Inomata Y, Sakurai T, Egawa H, Fujita S, Kiuchi T, Hayashi M, Yasutomi M, Yamabe H, Tanaka K. Living donor liver transplantation for fulminant hepatic failure. Transplantation. 2000;70:152-157. [PubMed] |
| 60. | Ikegami T, Taketomi A, Soejima Y, Maehara Y. Feasibility of adult-to-adult living donor liver transplantation for acute liver failure. Liver Transpl. 2009;15:117-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 61. | Lo CM. Living donor liver transplantation for acute liver failure: no other choice. Liver Transpl. 2012;18:1005-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 62. | van Hoek B, de Boer J, Boudjema K, Williams R, Corsmit O, Terpstra OT. Auxiliary versus orthotopic liver transplantation for acute liver failure. EURALT Study Group. European Auxiliary Liver Transplant Registry. J Hepatol. 1999;30:699-705. [PubMed] |
| 63. | Bismuth H, Azoulay D, Samuel D, Reynes M, Grimon G, Majno P, Castaing D. Auxiliary partial orthotopic liver transplantation for fulminant hepatitis. The Paul Brousse experience. Ann Surg. 1996;224:712-724; discussion 724-726. [PubMed] |
| 64. | Dokmak S, Aussilhou B, Durand F, Paradis V, Belghiti J. Complete spontaneous liver graft disappearance after auxiliary liver transplantation. Hepatology. 2014;60:1104-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Durand F, Belghiti J, Handra-Luca A, Francoz C, Sauvanet A, Marcellin P, Farges O, Bernuau J, Valla D. Auxiliary liver transplantation for fulminant hepatitis B: results from a series of six patients with special emphasis on regeneration and recurrence of hepatitis B. Liver Transpl. 2002;8:701-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Quaglia A, Portmann BC, Knisely AS, Srinivasan P, Muiesan P, Wendon J, Heneghan MA, O’Grady JG, Samyn M, Hadzic D. Auxiliary transplantation for acute liver failure: Histopathological study of native liver regeneration. Liver Transpl. 2008;14:1437-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | Toso C, Al-Qahtani M, Alsaif FA, Bigam DL, Meeberg GA, James Shapiro AM, Bain VG, Kneteman NM. ABO-incompatible liver transplantation for critically ill adult patients. Transpl Int. 2007;20:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Saliba F, Ichaï P, Azoulay D, Habbouchi H, Antonini T, Sebagh M, Adam R, Castaing D, Samuel D. Successful long-term outcome of ABO-incompatible liver transplantation using antigen-specific immunoadsorption columns. Ther Apher Dial. 2010;14:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Tanabe M, Kawachi S, Obara H, Shinoda M, Hibi T, Kitagawa Y, Wakabayashi G, Shimazu M, Kitajima M. Current progress in ABO-incompatible liver transplantation. Eur J Clin Invest. 2010;40:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 70. | Saliba F, Camus C, Durand F, Mathurin P, Letierce A, Delafosse B, Barange K, Perrigault PF, Belnard M, Ichaï P. Albumin dialysis with a noncell artificial liver support device in patients with acute liver failure: a randomized, controlled trial. Ann Intern Med. 2013;159:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 71. | Grodzicki M, Kotulski M, Leonowicz D, Zieniewicz K, Krawczyk M. Results of treatment of acute liver failure patients with use of the prometheus FPSA system. Transplant Proc. 2009;41:3079-3081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 72. | Volarevic V, Nurkovic J, Arsenijevic N, Stojkovic M. Concise review: Therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells. 2014;32:2818-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |