Published online Oct 21, 2016. doi: 10.3748/wjg.v22.i39.8831
Peer-review started: July 12, 2016
First decision: August 19, 2016
Revised: September 4, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: October 21, 2016
Processing time: 101 Days and 15.4 Hours
To evaluate annual incidence of low grade dysplasia (LGD) progression to high grade dysplasia (HGD) and/or esophageal adenocarcinoma (EAC) when diagnosis was made by two or more expert pathologists.
Studies evaluating the progression of LGD to HGD or EAC were included. The diagnosis of LGD must be made by consensus of two or more expert gastrointestinal pathologists. Articles were searched in Medline, Pubmed, and Embase. Pooled proportions were calculated using fixed and random effects model. Heterogeneity among studies was assessed using the I2 statistic.
Initial search identified 721 reference articles, of which 53 were selected and reviewed. Twelve studies (n = 971) that met the inclusion criteria were included in this analysis. Among the total original LGD diagnoses in the included studies, only 37.49% reached the consensus LGD diagnosis after review by two or more expert pathologists. Total follow up period was 1532 patient-years. In the pooled consensus LGD patients, the annual incidence rate (AIR) of progression to HGD and or EAC was 10.35% (95%CI: 7.56-13.13) and progression to EAC was 5.18% (95%CI: 3.43-6.92). Among the patients down staged from original LGD diagnosis to No-dysplasia Barrett’s esophagus, the AIR of progression to HGD and EAC was 0.65% (95%CI: 0.49-0.80). Among the patients down staged to Indefinite for dysplasia, the AIR of progression to HGD and EAC was 1.42% (95%CI: 1.19-1.65). In patients with consensus HGD diagnosis, the AIR of progression to EAC was 28.63% (95%CI: 13.98-43.27).
When LGD is diagnosed by consensus agreement of two or more expert pathologists, its progression towards malignancy seems to be at least three times the current estimates, however it could be up to 20 times the current estimates. Biopsies of all Barrett’s esophagus patients with LGD should be reviewed by two expert gastroenterology pathologists. Follow-up strict surveillance programs should be in place for these patients.
Core tip: Current estimates suggest that annual incidence of progression from low grade dysplasia (LGD) to high grade dysplasia and/or esophageal adenocarcinoma is 0.5% to 4% per year. Current estimates are based on diagnosis made by one pathologist. Recent studies indicate that when the diagnosis of LGD is made by two or more expert pathologists, LGD progression is grossly underestimated. When LGD is diagnosed by consensus agreement of two or more expert pathologists, its progression towards malignancy seems to be at least three times the current estimates, however it could be up to 20 times the current estimates. Biopsies of all Barrett’s esophagus patients with LGD should be reviewed by two expert gastroenterology pathologists. Follow-up strict surveillance programs should be in place for these patients.
- Citation: Moole H, Patel J, Ahmed Z, Duvvuri A, Vennelaganti S, Moole V, Dharmapuri S, Boddireddy R, Yedama P, Bondalapati N, Uppu A, Vennelaganti P, Puli S. Progression from low-grade dysplasia to malignancy in patients with Barrett's esophagus diagnosed by two or more pathologists. World J Gastroenterol 2016; 22(39): 8831-8843
- URL: https://www.wjgnet.com/1007-9327/full/v22/i39/8831.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i39.8831
Barrett’s esophagus (BE), first described in 1950, is a condition in which the normal esophageal squamous epithelium is replaced by columnar epithelium in a process known as metaplasia[1]. The development of esophageal adenocarcinoma (EAC) occurs when BE progresses through dysplasia. Dysplasia in BE is classified as either low grade dysplasia (LGD) or high grade dysplasia (HGD). The populations at risk for developing BE include individuals with chronic gastroesophageal reflux disease (GERD), obesity, male gender, have a smoking history, and are Caucasian[2-4].
The epidemiology of BE varies greatly owing to advancements in knowledge, diagnostic approach, and surveillance strategies. BE is prevalent in 10%-20% of patients with GERD, 2%-7% of general population, and incidence is between 23.1 and 32.7 per 100000[5-11]. The risk of progression to malignancy has been found to be lower in Barret’s esophagus without dysplasia[12-14]. Indefinite for dysplasia (IDBE), indicating no clear evidence of dysplasia, poses an additional diagnostic challenge. Increasing dysplasia levels were associated with increased detection of malignancy in a greater number of patients in shorter time intervals[8].
Further epidemiological analysis has been performed in BE patients assessing the incidence of progression from LGD to HGD and/or progression to EAC. The rate of progression is also variable and has been reported to be between 0.5% to 4% per year[10,11]. This wide range of progression incidence is primarily attributed to inter-observer variability in the assessment amongst the reading pathologists. Although LGD has been clearly defined by its histological features, there is a substantial subjective component involved in making the diagnosis. Recent studies indicate the progression of LGD is grossly underestimated. This may be attributed to the expertise of the reading pathologist, in addition to the number of pathologists assessing the biopsies. Given the concern for the risk of progression, accurate assessment of dysplasia is vital for appropriate risk stratification and surveillance strategies. Large statistical analyses have been conducted on the progression of LGD diagnosed by single pathologists. However, there is no statistical analyses in the form of meta-analysis evaluating LGD progression diagnosed by two or more expert pathologists.
The recognition of BE and prevention of progression to esophageal adenocarcinoma is quickly becoming a national public health concern. EAC incidence has more than quadrupled over the last few decades, and is alarmingly becoming a leader for cancer mortality. The aim of this systematic review and meta-analysis was to evaluate annual incidence of LGD progression (to HGD and/or EAC) when the diagnosis was made by two or more expert pathologists. Primary outcomes are to evaluate the annual incidence rate (AIR) of HGD and EAC in patients with LGD, diagnosed by consensus agreement of two or more expert gastroenterology pathologists. Secondary outcomes are to evaluate the AIR of HGD and EAC in patients down staged to No dysplasia in Barrett’s esophagus (NDBE) and Indefinite for dysplasia in Barrett’s esophagus (IDBE) from the original LGD diagnosis. AIR of EAC from HGD was also evaluated. A sub-group meta-analysis was performed on prospective studies only, evaluating the same variables.
Studies that evaluated the progression of LGD to HGD or EAC were included in this analysis. The diagnosis of LGD must be made by consensus agreement of two or more expert gastroenterology pathologists. Studies that evaluated patients with an original diagnosis of EAC were excluded. Patients with prior surgery or procedural interventions for EAC/LGD/HGD management were also excluded. We included both prospective and retrospective studies. Studies without original data, perspective articles, review articles, and expert opinions were excluded from this meta-analysis. Only full text articles, peer reviewed and published in international journals were included in this analysis. If there were duplicate studies, the most complete and latest study was included in this meta-analysis.
The study design was written in accordance to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement. Articles were systematically searched in Medline, PubMed, Ovid journals, EMABSE, Cumulative Index for Nursing and Allied Health Literature, ACP journal club, DARE, International Pharmaceutical Abstracts, old Medline, Medline nonindexed citations, OVID Healthstar, and Cochrane Central Register of Controlled Trials. The search was performed for the years 1966 to June 2016. Abstracts were manually searched in the major gastroenterology journals for the past 3 years. Study authors for the abstracts included in this analysis were contacted when the required data for the outcome measures could not be determined from the publications. The MeSH search headings used were “barrett’s esophagus”, “barrett’s oesophagus”, “low grade dysplasia”, “high grade dysplasia”, “esophageal adenocarcinoma”, “oesophageal adenocarcinoma”. The reference lists of the included studies were manually searched for any relevant publications. Two authors (HM and VM) independently searched and extracted the data into an abstraction form. Any differences were resolved by mutual agreement. If the disagreement persisted, the final decision was made by a third author (SP) after reviewing the relevant information. The agreement between reviewers for the collected data was quantified using the Cohen’s κ[15]. Data was extracted from the selected studies and entered into a standardized data collection form. The following variables were recorded: name and year of study; country where study was performed; type of study; age in years-median; male/female distribution in percentage; total number of patients included; source of patient registry; diagnosis criteria for LGD; esophageal biopsy information (endoscopic details regarding the site of biopsies and pathology details regarding the methods of fixing the biopsy slide); number of patients with original LGD diagnosis (made by a single pathologist); follow-up period in months (median); follow up period in patient years; number of patients with a consensus diagnosis of LGD; number of patients with a consensus diagnosis of HGD at the beginning of study; AIR (expressed as percent) for HGD and EAC in consensus LGD patients; AIR for EAC only in consensus LGD patients; AIR for EAC only in consensus HGD patients; AIR for HGD and EAC in patients down staged to NDBE; AIR for HGD and EAC in patients down staged to IDBE.
For the purpose of this meta-analysis, we have used definitions that are most widely accepted by various gastroenterology organizations and that were used in most of the studies included in this analysis. Barrett’s esophagus was defined as any red colored epithelium visible on endoscopy, above the lower esophageal sphincter/proximal end of gastric folds, biopsies of which reveal intestinal metaplasia. The progression of BE towards malignancy was classified into “no dysplasia”, “indefinite for dysplasia”, “low grade dysplasia”, “high grade dysplasia” and “adenocarcinoma”. This classification is in accordance with Vienna classification of gastrointestinal epithelial neoplasia[16]. Original LGD/HGD diagnosis refers to the patients labelled as having LGD/HGD diagnosed by a single pathologist. Consensus or Confirmed LGD/HGD diagnosis refers to the patients actually having LGD/HGD after two or more expert pathologists reviewed the biopsies and agreed upon the diagnosis.
Clinical trials designed with a control and treatment arms can be assessed for quality of the study. A number of criteria have been used to assess this quality of a study (e.g., randomization, selection bias of the arms in the study, concealment of allocation, and blinding of outcome). Jadad score was used to evaluate the quality of randomized studies. Cochrane Collaborations and the Quality of Reporting of Meta-analysis guidelines were followed to assess the quality of studies[17,18]. Quality of nonrandomized studies included in this meta-analysis was assessed using Newcastle-Ottawa Scale[19].
This meta-analysis was performed by calculating pooled proportions. First the individual study proportion of annual incidence rates of HGD and EAC, consensus LGD diagnosis, etc., was transformed into a quantity using Freeman-Tukey variant of the arcsine square root transformed proportion. The pooled proportion is calculated as the back-transform of the weighted mean of the transformed proportions, using inverse arcsine variance weights for the fixed effects model and DerSimonian-Laird weights for the random effects model[20,21]. Forest plots were drawn to show the point estimates in each study in relation to the summary pooled estimate. The width of the point estimates in the Forest plots indicates the assigned weight to that study. The heterogeneity among studies was tested using I2 statistic and Cochran’s Q test based upon inverse variance weights[22]. I2 of 0–39% was considered as non-significant heterogeneity, 40%-75% as moderate heterogeneity, and 76%-100% as considerable heterogeneity. If P value is > 0.10, it rejects the null hypothesis that the studies are heterogeneous. The effect of publication and selection bias on the summary estimates was tested by both Harbord-Egger bias indicator[23] and Begg-Mazumdar bias indicator[24]. Also, funnel plots were constructed to evaluate potential publication bias[25,26]. Microsoft Excel 2013 software was used to perform statistics for this meta-analysis. Subgroup analysis was performed on prospective studies evaluating the AIR of HGD and EAC in confirmed LGD patients.
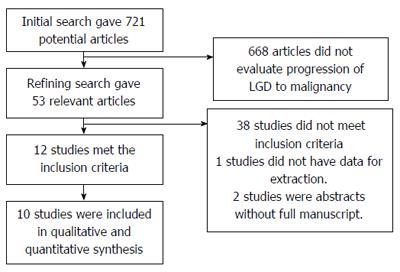
Initial search identified 721 reference articles, in which 53 articles were selected and reviewed. Data was extracted from 12 studies[10,27-37] (n = 971) that evaluated the progression of LGD towards malignancy. All the studies are published as full text articles. Figure 1 shows the flow diagram of search results. All the pooled estimates given are estimates calculated either by fixed or random effects model. Fixed effect model was preferred when heterogeneity was low and random effect model was preferred when heterogeneity was high. Among the 12 studies included in this analysis, seven studies[10,28,30,32-34,36] initially selected patients with LGD diagnosis made by single pathologist, however was reviewed later by two or more expert pathologists to make confirmation diagnosis after a consensus. Remainder of the five studies directly included patient with confirmed diagnosis of LGD/HGD. Eight studies out of the 12 studies were retrospective studies[27-34], the others were prospective studies. Subgroup analysis was performed on all prospective trials[10,35-37]. The included studies were distributed all over the world. Most patient registries of the included studies were specialist center based, except three studies[29,32,36] that included patients from community based cohorts. One study[29] included patients from both specialist center and community based registries. Patients in individual studies were on optimal proton pump inhibitor therapy (PPI) – once or twice daily regimen.
The total number of patients included in this meta-analysis is 971. Nine hundred and seventy-one represents total number of original and confirmed LGD cases. However, the total number of confirmed LGD cases after consensus by two or more expert pathologists is 418, with a predominantly male population (72%). Among the total original LGD diagnoses in the included studies, only 37.49% (95%CI: 25.72-50.1) [I² (inconsistency) = 89.7% (95%CI: 81.2%-93.3%), Egger: bias = 4.66 (95%CI: 1.40-7.91), P = 0.01] reached the consensus LGD diagnosis after review by two or more expert pathologists. Median age of the patients was 65 years. Table 1 shows the baseline characteristics of the studies. Table 2 shows other key characteristics of the individual studies. In summary, the methods used to diagnose confirmed LGD cases in all the studies were mostly similar. Two or more pathologists had to review the biopsies and come to an agreement regarding the diagnosis of LGD. Biopsies were obtained from four quadrants at at-least 2 cm intervals all along the length of Barrett’s esophagus. Hematoxylin Eosin staining of paraffin/formalin/Hollande’s embedded biopsy specimens was used. The P for χ2 heterogeneity for all the pooled accuracy estimates was > 0.10. The agreement between reviewers for the collected data gave a Cohen’s κ value of 1.0. The follow up period in individual studies ranged from 24-96 mo. Median follow up period was 50 mo. Cumulative follow up period in all the included studies was 1532 patient years.
| Ref. | Country | Type | Age: years - Median | Sex: Male% | N LGD - prior to panel review | Follow up - median - months | Follow up - Patient years | N LGD (after panel review) consensus diagnosis | N HGD (after panel review) consensus diagnosis |
| Picardo et al[27] 2015 | Ireland | R | 59 | 67% | NA | 50 | 354.17 | 85 | 60 |
| Duits et al[28] 2014 | Netherlands | R | 63 | 76% | 293 | 39 | 256.75 | 79 | NA |
| von Rahden et al[29] 2008 | Germany | R | 59 | 72% | NA | 24 | 114.00 | 57 | NA |
| Lim et al[30] 2007 | United Kingdom | R | 67 | 80% | 34 | 96 | 112.00 | 14 | 1 |
| Vieth et al[31] 2006 | Germany | R | 61 | 68% | NA | 54 | 85.50 | 19 | 10 |
| Basu et al[32] 2004 | United Kingdom | R | 74 | 74% | 16 | 60 | 50.00 | 10 | 3 |
| Montgomery et al[33] 2001 | United States | R | 65 | 72% | 26 | 24 | 30.00 | 15 | 15 |
| Skacel et al[34] 2000 | United States | R | 67 | 84% | 25 | 26 | 36.83 | 17 | NA |
| Younes et al[35] 2011 | United States | P | NA | NA | NA | 35 | 81.67 | 28 | NA |
| Wani et al[10] 2011 | United States | P | 61 | 85% | 210 | 74 | 252.83 | 41 | NA |
| Curvers et al[36] 2010 | Netherlands | P | 59 | 67% | 147 | 51 | 93.50 | 22 | NA |
| Srivastava et al[37] 2007 | United States | P | 64 | 91% | NA | 25 | 64.58 | 31 | 46 |
| Study | Patient registry | Diagnosis method of LGD | Biopsy details |
| Picardo et al[27] 2015 | Specialist center based registry | Expert pathologist panel: 2 pathologists required to make diagnosis | Four-quadrant biopsies every 1 cm of Barrett's esophagus |
| Duits et al[28] 2014 | Specialist center based registry | Expert pathologists panel: At-least 2 pathologists required to make diagnosis | H&E stained slides of paraffin embedded biopsy specimens |
| von Rahden et al[29] 2008 | Specialist center and Community population based registry | Expert pathologists panel: 3 pathologists required to make diagnosis | Multiple biopsies at different levels of Barrett’s esophagus |
| Lim et al[30] 2007 | Specialist center based registry | Expert pathologists panel: 5 pathologists required to make diagnosis | Four to ten (sometimes more) biopsies taken from Barrett's area. Hematoxylin and eosin staining |
| Vieth et al[31] 2006 | Specialist center based registry | Biopsies assessed twice by two pathologists in a blinded fashion | Four biopsies every 2 cm in relation to the Barrett’s esophagus length |
| Basu et al[32] 2004 | Community based cohort | Experienced gastrointestinal pathologist assessed histological sections, with confirmation by a colleague if high-grade dysplasia or worse was suspected. All cases of low-grade dysplasia were reviewed at a regular gastrointestinal histopathology meeting | 2-cm interval quadrantic biopsies in the entire length of Barrett’s esophagus |
| Montgomery et al[33] 2001 | Specialist center based registry | Expert pathologists panel: 12 pathologists required to make diagnosis - reviewed blindly twice by each pathologist | Multiple biopsies at different levels of Barrett’s esophagus. Submitted biopsy specimen had to show the worst lesion that the patient was known to have at the time of the initial known endoscopy |
| Skacel et al[34] 2000 | Specialist center based registry | Expert pathologists panel: LGD cases were randomized and blindly reviewed by three gastrointestinal pathologists | Four-quadrant biopsies taken using jumbo forceps at intervals of < 2 cm throughout the length of the Barrett’s segment, with additional biopsies of any endoscopic lesions. All biopsy specimens had been fixed in formalin or Hollande’s solution |
| Younes et al[45] 2011 | Specialist center based registry | Expert pathologist panel: 2 pathologists required to make diagnosis | Biopsies from two or more levels in barrett's esophagus. Hematoxylin-eosin–stained sections of formalin-fixed and paraffin-embedded tissue |
| Wani et al[10] 2011 | Specialist center based registry | Consensus diagnosis among two or more pathologists: defined as agreement between the local GI pathologist and expert central pathologists | At least 4 quadrant biopsies every 2 cm with either a standard or jumbo biopsy forceps. Hematoxylin Eosin stained slides of paraffin-embedded biopsy specimens |
| Curvers et al[36] 2010 | Community based cohort | Expert pathologist panel: 2 pathologists required to make diagnosis | All visible abnormalities were sampled, followed by random sampling of the Barrett segment in four quadrants every 2 cm. Hematoxylin and eosin stained slides of paraffin-embedded biopsy specimens |
| Srivastava et al[37] 2007 | Specialist center based registry | Expert pathologists panel: 3 pathologists required to make diagnosis | Four-quadrant endoscopic esophageal mucosal biopsies were obtained at every 1–2 cm. All four-quadrant Hollande’s or formalin fixed biopsies were embedded into one paraffin block and serial 4 µm thick tissue sections were cut and stained with hematoxylin and eosin |
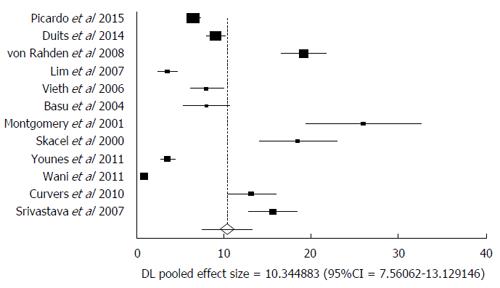
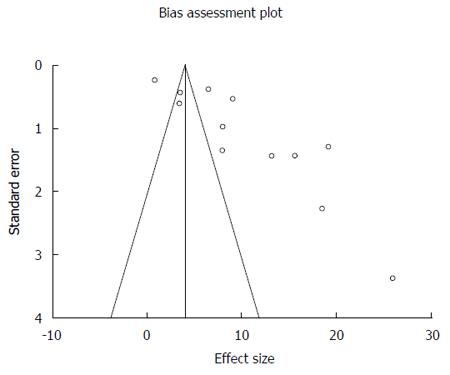
Malignant transformation of LGD includes transformation into HGD and or EAC. In the pooled consensus LGD patients, the AIR of progression to HGD and EAC was 10.35% (95%CI: 7.56-13.13). Bias indicators for this variable were: Begg-Mazumdar: Kendall’s tau b = 0.27, P = 0.25; Egger: bias = 10.22 (95%CI: 5.42-15.03), P = 0.0008. Heterogeneity for this variable was assessed using I² (inconsistency) = 98.4% (95%CI: 98.1%-98.6%). Figure 2 is a forest plot representing the pooled and individual AIR for malignant (HGD and or EAC) transformation in confirmed LGD patients. Figure 3 is a funnel plot assessing the publication bias for same variable. Due to significant heterogeneity among the studies, we have attempted to exclude two studies that were extreme outliers (Wani et al[10] with an AIR 0.84% and Montgomery et al[33] with an AIR 26.7%). AIR for HGD and EAC, calculated after exclusion of these two studies was 10.17 (95%CI: 7.59-12.75). I² (inconsistency) = 96%, Egger: bias = 7.80 (95%CI: 1.67-13.95), P = 0.02.
In pooled patient population, progression of confirmed LGD to EAC only was 5.18% (95%CI: 3.43-6.92). Date for this variable was available only in eight studies[10,27,30-34,37]. Bias indicators for this variable were: Begg-Mazumdar: Kendall’s tau b = 0.79, P = 0.0055; Egger: bias = 7.54 (95%CI: 3.03-12.05) P = 0.0064. I² (inconsistency) = 96.9% (95%CI: 95.9%-97.6%).
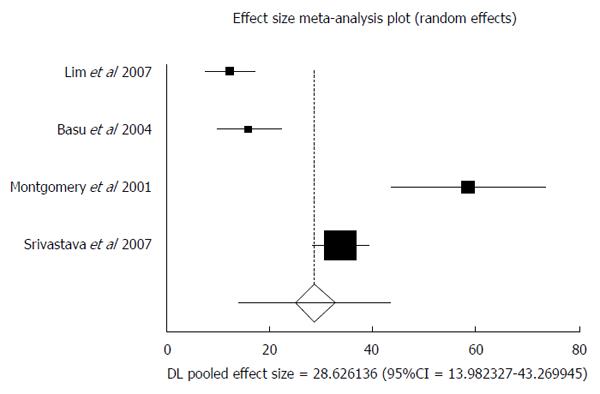
Four studies enrolled confirmed HGD cases into their patient population[30,32,33,37]. These patients were followed up to assess the rate of transformation into EAC. Total patients included in this analysis was 65, with a predominantly male population (74%). Median age of the patients was 65 years. In patients with consensus HGD diagnosis, the AIR of progression to EAC was 28.63 (95%CI: 13.98-43.27). I² (inconsistency) = 95.1%. Egger: bias = 7.65 (95%CI: -20.61-35.92), P = 0.36. Figure 4 is a forest plot representing the pooled and individual AIR for EAC transformation in confirmed HGD patients.
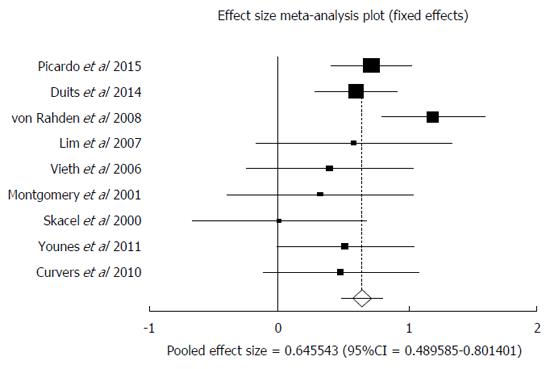
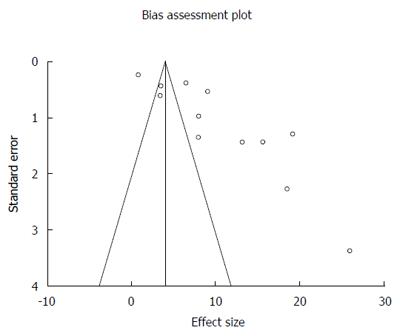
Original diagnosis of LGD, after review by two or more expert pathologists was either confirmed as LGD after consensus or was down staged to one of the two: NDBE or IDBE. In patients down staged to NDBE, data was available from nine studies[27-31,33-35,36]. AIR of malignant transformation (HGD and or EAC) in confirmed NDBE patients after down staging was 0.65% (95%CI: 0.49-0.80). I² (inconsistency) = 38.4%. Egger: bias = -1.94 (95%CI: -4.69-0.83), P = 0.14. Figure 5 is a forest plot representing the pooled and individual AIR for malignant transformation in confirmed NDBE patients. Figure 6 is a funnel plot assessing the publication bias for same variable.
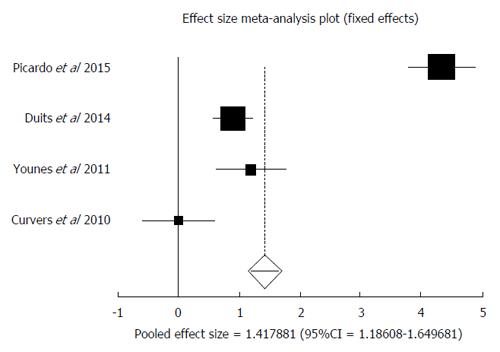
IDBE data was available in four studies[27,28,35,36]. Among the patients down staged to IDBE, the AIR of progression to HGD and or EAC was 1.42% (95%CI: 1.18-1.65). I² (inconsistency) = 97.9%. Egger: bias = 6.48 (95%CI: -59.45-72.41), P = 0.71. Figure 7 is a forest plot representing the pooled and individual AIR for malignant transformation in confirmed IDBE patients.
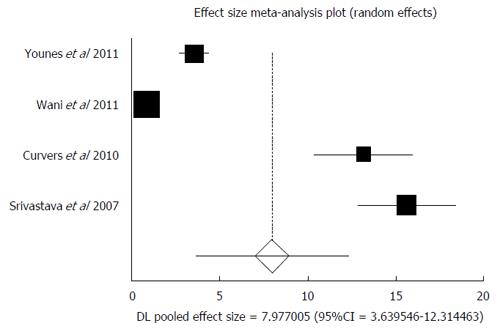
Subgroup analysis was performed on all prospective trials[10,35-37]. Four studies were included in this analysis. The total number of patients included in this subgroup was 122, with a predominantly male population (67%). Median age of the patients was 61 years. In the subgroup analysis of prospective studies, AIR of malignant (HGD and or EAC) transformation in confirmed LGD patients was 7.98 (95%CI: 3.64-12.31). I² (inconsistency) = 98.4%. Egger: bias = 11.49 (95%CI: 7.46-15.53), P = 0.006. Figure 8 is a forest plot representing the pooled and individual AIR for malignant (HGD and or EAC) transformation in confirmed LGD patients.
There is a wide variation in the incidence of adenocarcinoma stemming from Barrett’s esophagus in the current literature. Existing endoscopic surveillance studies show anywhere from 1 in 52 to 1 in 441 patient-years of surveillance. The lack of large prospective studies makes it difficult to determine the efficacy and cost-effectiveness of widespread surveillance programs, and thus there is a large discrepancy between surveillance programs[38-41]. Basu et al[32] conducted a retrospective analysis of the Barrett’s epithelium surveillance database to assess the utility of a surveillance program and to determine the natural incidence of dysplasia in this population. The overall prevalence of LGD in the 5-year period was 7.2%, with an annual incidence of 1.4%. None of these patients progressed to HGD. This study disagreed with the previous study by van Sandick et al[42], who concluded an increased progression to HGD from the initial low grade population. The disagreement may be due to study limitations including small sample size and short duration of follow up in the study by Basu et al[32]. Overall, this study concluded that annual endoscopic surveillance showed little reward, but there are variations among regions[43] and surveillance intervals should also be based on the cancer incidence of that specific region.
Another factor affecting surveillance intervals and the true rate of progression to adenocarcinoma is interobserver variability in grading dysplasia. Outcomes in a study by Montgomery et al[33], support that IDBE and LGD should have identical surveillance and follow up as they had similar frequencies of development of adenocarcinoma, but IDBE was slower to progress than LGD by at least 42 mo. Increasing dysplasia was also associated with increased frequency of ulcers found on exam. The study found that approximately 1 of 50 patients with Barrett’s esophagus without dysplasia progressed to HGD at approximately 6 years. Conversely, those with any grade of dysplasia or evidence of ulceration should be followed up in shorter intervals. They further concluded that criteria for the diagnosis of dysplasia are reliable. Patients with Barrett’s esophagus without dysplasia may be followed with endoscopy and biopsies every few years while those with IDBE, any grade of dysplasia, or evidence of ulceration require shorter follow up intervals.
Dysplasia is diagnosed when epithelial atypia also involves the surface epithelium[44]. In cases where the surface epithelium is denuded, uninvolved, or unable to be evaluated, the grading is changed to indeterminate dysplasia or indefinite for dysplasia (IDBE)[45]. Younes et al[35] demonstrated with statistical significance that patients with an initial biopsy diagnosis of either IDBE-Multifocal, LGD, or LGD-Multifocal were more likely to progress to HGD than those with an initial diagnosis of NDBE or IDBE. Unfortunately, there exists a large breadth of interobserver variability, especially in IDBE, where there are no clear guidelines for diagnosis.
Interobsever variability may result in under-diagnosis, such as in the study by Montgomery et al[33] or in over-diagnosis as demonstrated in the study by Curvers et al[36]. In Curvers et al[36] over 75% of cases with previously diagnosed low grade dysplasia were downgraded to NDBE, which were shown to have an incidence rate of 0.49% per patient year. This is in contrast to the study by Montgomery et al[33] in which pathologists rated IDBE despite marked atypia to avoid over-diagnosis. Also, a true diagnosis of low grade dysplasia, which is defined here as one diagnosed by an expert panel of pathologists, carries a 13.4% incidence of progression per patient year. Though low grade dysplasia may be over-diagnosed, those cases which are confirmed by a consensus diagnosis with a panel of expert pathologists should not be underestimated and warrant close follow-up.
Duits et al[28] conducted a large, retrospective cohort which studied the incidence rates of HGD and EAC in patients diagnosed with LGD by an expert panel of pathologists. Seventy-three percent of the initial community cohort of patients were downgraded to a diagnosis of IDBE or NDBD. When the diagnosis of low grade dysplasia was confirmed by an expert panel, the risk of progression to HGD or EAC was 9.1% annually and had a five-year cumulative progression risk of 33.3%, which was much higher than expected. This study again highlights the initial over-diagnosis of low grade dysplasia. Unfortunately, this leads to an underestimation of the risk to malignant progression in cases of low grade dysplasia which have been confirmed by an expert panel.
Skacel et al[34] further evaluated the degree of interobserver variability in the histological diagnosis of LGD and impact on reported risk of progression to HGD or EAC. Biopsy specimens were reviewed individually by three GI pathologists, blind to the previous diagnosis, and found the individual GI pathologists’ diagnosis did not correlate with disease progression. When at least 2 of the pathologists agreed on the histological diagnosis, there was a statistically significant progression (7 out of 17, P = 0.04) When all 3 pathologists agreed, 4 out of the 5 patients progressed (P = 0.012). Of the 8 patients where there was no agreement between pathologists, 0 of these patients progressed
A study by Weston et al[46] showed 5 out of 62 patients initially diagnosed with LGD progressed, but it is unclear if the initial diagnosis was confirmed by an expert panel[46]. In a study by Reid et al[47], it was noted that a consensus diagnosis of low grade dysplasia suggests an increased risk of progression to high grade dysplasia as compared to identifications where pathologists disagree on the diagnosis.
There has been little evaluation of the extent of LGD as a risk factor for the development of EAC, and studies on HGD as a risk factor were conflicting. Previous studies evaluated the extent of HGD as a risk factor for developing adenocarcinoma in Barrett’s esophagus, but they did not evaluate LGD[48,49]. Srivastava et al[37] conducted a study assessing the total numbers of both LGD and HGD crypts, the extent of dysplasia, and total dysplasia correlated with EAC development. In those who progressed to EAC, the number of dysplastic crypts per patient (115.2) including HGD and LGD, were significantly higher than in nonprogressors (56.2, P = 0.01). When the crypts were stratified by dysplasia grade, the patients who developed EAC had a marginally greater mean number of LGD crypts (93.9) as compared with patients that did not progress (41.2, P = 0.07). Per patient, the mean proportion of LGD crypts were also found to be greater in progressors (46.4% vs 26%, P = 0.037), but more significantly than the mean number of crypts. Interestingly, neither the mean number (P = 0.14) nor the mean proportion (P = 0.20) of HGD crypts per patient was significantly associated with development of EAC. The study concluded that the extent of LGD is a significant risk factor for developing esophageal adenocarcinoma, while the extent of HGD was not found to be an independent risk factor. However, the presence of HGD is associated with a greater relative risk for developing adenocarcinoma.
A registry of patients with Barrett’s esophagus has been found to be beneficial with respect to patient management as well as for identifying populations at greater risk in need of alternate surveillance intervals. The utility of a registry is further increased where surveillance is not cost-effective for all[50]. Esophageal adenocarcinoma has increased drastically in Europe, and is predicted to increase even higher in the near future[51-53]. Picardo et al[27] established a Barrett’s registry in 2008 consisting of 1093 patients from the Republic of Ireland. Of the 73 patients with a diagnosis of LGD with endoscopic follow up beyond 1 year, 46 (65%) had histological regression, 8 progressed to HGD, and 6 to EAC. This study showed that the absolute risk of EAC was higher than reported in whole population studies. Incidence was higher in this study as compared to a Danish population study and another study conducted from the Northern Ireland Barrett’s Register[12,54].
The strengths of this meta-analysis include the high quality methodology of statistical analysis, high quality methodology used in individual studies, relatively greater number of studies that met the inclusion criteria, and large total number of patients included in this analysis (n = 971). This is the first meta-analysis to pool the evidence for AIR of malignancy in confirmed LGD patients.
The limitations of this study include: There was a significant level of heterogeneity among studies included in this analysis. Random effects model was used to calculate pooled effects for most of the variables when the heterogeneity was high. We also performed a pooled subgroup analysis after excluding the outliers, with the intention of reducing the heterogeneity. We were unable to perform financial impact analysis due to the lack of data from the individual studies. The local expertise of pathologists/expert pathologists/gastrointestinal pathologists plays a key role in the outcomes. The variability of their expertise is one of the most significant reasons for heterogeneity among studies. A few other important reasons for heterogeneity among studies could be exclusion of prevalent cases of malignancies, and design of individual studies. There were retrospective studies included in this meta-analysis. In order to mitigate this issue, we have performed a sub-group analysis on prospective studies only. Due to the paucity of data available from individual studies, we were not able to analyze the relation between the length of Barrett’s, extent of dysplasia and progression to malignancy
Since the incidence of esophageal adenocarcinoma is rising, it is vital to focus on all possible preventive measures to halt or slow the progression from BE to EAC. Based on the results of this analysis, the incidence of malignancy in confirmed LGD patients is much higher than the current estimates. For these reasons, it is of paramount importance to confirm the diagnosis of patients labelled as LGD. Once a diagnosis of LGD has been confirmed, these patients would benefit from closer follow-up and surveillance. In patients with LGD, most gastroenterology societies recommend surveillance endoscopy with biopsies every 12 mo. However, with more recent evidence suggesting the true rate of transformation is higher, there may be a role for more frequent endoscopic surveillance or sooner procedural intervention. These questions should be answered with further large prospective trials.
Studies with statistically significant positive results tend to be published and cited. Additionally, smaller studies may show larger treatment effects compared to larger studies. This publication and selection bias may affect the summary estimates. The bias can be estimated using Egger bias indicators and the construction of funnel plots, whose shape can be affected by bias. In the present meta-analysis and systematic review, bias calculations both Egger[23] and Begg-Mazumdar[24] bias indicators showed no statistically significant bias. Furthermore, analysis using funnel plots showed no significant publication bias among the studies included in the present analysis.
Overall, when LGD is diagnosed by consensus agreement of two or more expert pathologists, its progression towards malignancy appears to be at least three times the current estimates, and may be up to 20 times the current estimates. Biopsies of all Barrett’s esophagus patients with LGD should be reviewed by two expert gastroenterology pathologists. Follow-up strict surveillance programs should be in place for these patients. Large prospective studies are required to evaluate if confirmed LGD patients should have follow up surveillance more frequently than every year.
Esophageal adenocarcinoma incidence has more than quadrupled over the last few decades, and is alarmingly becoming a leader for cancer mortality. The recognition of Barretts esophagus and prevention of progression to esophageal adenocarcinoma is quickly becoming a national public health concern.
Recent studies indicate the progression from Barretts esophagus to Low grade dysplasia is grossly underestimated. This may be attributed to the expertise of the reading pathologist, in addition to the number of pathologists assessing the biopsies. Given the concern for the risk of progression, accurate assessment of dysplasia is vital for appropriate risk stratification and surveillance strategies.
Based on the results of this study, when Low grade dysplasia is diagnosed by consensus agreement of two or more expert pathologists, its progression towards malignancy appears to be at least three times the current estimates, and may be up to 20 times the current estimates.
Biopsies of all Barrett’s esophagus patients with low grade dysplasia (LGD) should be reviewed by two expert gastroenterology pathologists. Follow-up strict surveillance programs should be in place for these patients. Large prospective studies are required to evaluate if confirmed LGD patients should have follow up surveillance more frequently than every year.
Barrett’s Esophagus is a condition in which the normal esophageal squamous epithelium is replaced by columnar epithelium in a process known as metaplasia.
In their manuscript the authors performed is a systematic review and meta-analysis to evaluate annual incidence of LGD progression to high grade dysplasia and/or esophageal adenocarcinoma when diagnosis was made by two or more expert pathologists.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Deng B, Francisco G, Gao C, Tang Y, Tarnawski AS S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Barrett NR. Chronic peptic ulcer of the oesophagus and ‘oesophagitis’. Br J Surg. 1950;38:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 655] [Cited by in RCA: 545] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 2. | Corley DA, Kubo A, Levin TR, Block G, Habel L, Rumore G, Quesenberry C, Buffler P. Race, ethnicity, sex and temporal differences in Barrett’s oesophagus diagnosis: a large community-based study, 1994-2006. Gut. 2009;58:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 3. | Shiota S, Singh S, Anshasi A, El-Serag HB. Prevalence of Barrett’s Esophagus in Asian Countries: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2015;13:1907-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Kamat P, Wen S, Morris J, Anandasabapathy S. Exploring the association between elevated body mass index and Barrett’s esophagus: a systematic review and meta-analysis. Ann Thorac Surg. 2009;87:655-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, Vieth M, Stolte M, Talley NJ, Agréus L. Prevalence of Barrett’s esophagus in the general population: an endoscopic study. Gastroenterology. 2005;129:1825-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 644] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 6. | Rex DK, Cummings OW, Shaw M, Cumings MD, Wong RK, Vasudeva RS, Dunne D, Rahmani EY, Helper DJ. Screening for Barrett’s esophagus in colonoscopy patients with and without heartburn. Gastroenterology. 2003;125:1670-1677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 310] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 7. | Hayeck TJ, Kong CY, Spechler SJ, Gazelle GS, Hur C. The prevalence of Barrett’s esophagus in the US: estimates from a simulation model confirmed by SEER data. Dis Esophagus. 2010;23:451-457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | van Soest EM, Dieleman JP, Siersema PD, Sturkenboom MC, Kuipers EJ. Increasing incidence of Barrett’s oesophagus in the general population. Gut. 2005;54:1062-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 9. | Ford AC, Forman D, Reynolds PD, Cooper BT, Moayyedi P. Ethnicity, gender, and socioeconomic status as risk factors for esophagitis and Barrett’s esophagus. Am J Epidemiol. 2005;162:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Wani S, Falk GW, Post J, Yerian L, Hall M, Wang A, Gupta N, Gaddam S, Singh M, Singh V. Risk factors for progression of low-grade dysplasia in patients with Barrett’s esophagus. Gastroenterology. 2011;141:1179-186, 1186.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 11. | Sinh P, Anaparthy R, Young PE, Gaddam S, Thota P, Balasubramanian G, Singh M, Higbee AD, Wani S, Gupta N. Clinical outcomes in patients with a diagnosis of “indefinite for dysplasia” in Barrett’s esophagus: a multicenter cohort study. Endoscopy. 2015;47:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Bhat S, Coleman HG, Yousef F, Johnston BT, McManus DT, Gavin AT, Murray LJ. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 515] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 13. | Chandrasoma P, Wickramasinghe K, Ma Y, DeMeester T. Is intestinal metaplasia a necessary precursor lesion for adenocarcinomas of the distal esophagus, gastroesophageal junction and gastric cardia? Dis Esophagus. 2007;20:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Fitzgerald RC, di Pietro M, Ragunath K, Ang Y, Kang JY, Watson P, Trudgill N, Patel P, Kaye PV, Sanders S. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett’s oesophagus. Gut. 2014;63:7-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 872] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
| 15. | Brennan P, Silman A. Statistical methods for assessing observer variability in clinical measures. BMJ. 1992;304:1491-1494. [PubMed] |
| 16. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1540] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 17. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 12864] [Article Influence: 443.6] [Reference Citation Analysis (1)] |
| 18. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 16734] [Article Influence: 669.4] [Reference Citation Analysis (0)] |
| 19. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses; 2014. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 20. | Stuart A, Ord JK. Kendall’s Advanced Theory of Statistics (6th edition). London: Edward Arnold 1994; . |
| 21. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30309] [Article Influence: 777.2] [Reference Citation Analysis (0)] |
| 22. | Deeks JJ. Systematic reviews of evaluations of diagnostic and screening tests. Systematic Reviews in Health Care. Meta-analysis in context. London: BMJ Books 2001; . |
| 23. | Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443-3457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1699] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 24. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10586] [Cited by in RCA: 12135] [Article Influence: 404.5] [Reference Citation Analysis (0)] |
| 25. | Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101-105. [PubMed] |
| 26. | Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2624] [Article Influence: 109.3] [Reference Citation Analysis (0)] |
| 27. | Picardo SL, O’Brien MP, Feighery R, O’Toole D, Ravi N, O’Farrell NJ, O’Sullivan JN, Reynolds JV. A Barrett’s esophagus registry of over 1000 patients from a specialist center highlights greater risk of progression than population-based registries and high risk of low grade dysplasia. Dis Esophagus. 2015;28:121-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Duits LC, Phoa KN, Curvers WL, Ten Kate FJ, Meijer GA, Seldenrijk CA, Offerhaus GJ, Visser M, Meijer SL, Krishnadath KK. Barrett’s oesophagus patients with low-grade dysplasia can be accurately risk-stratified after histological review by an expert pathology panel. Gut. 2015;64:700-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 29. | von Rahden BH, Stein HJ, Weber A, Vieth M, Stolte M, Rösch T, Schmid RM, Sarbia M, Meining A. Critical reappraisal of current surveillance strategies for Barrett’s esophagus: analysis of a large German Barrett’s database. Dis Esophagus. 2008;21:685-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Lim CH, Treanor D, Dixon MF, Axon AT. Low-grade dysplasia in Barrett’s esophagus has a high risk of progression. Endoscopy. 2007;39:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Vieth M, Schubert B, Lang-Schwarz K, Stolte M. Frequency of Barrett’s neoplasia after initial negative endoscopy with biopsy: a long-term histopathological follow-up study. Endoscopy. 2006;38:1201-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Basu KK, Pick B, de Caestecker JS. Audit of a Barrett’s epithelium surveillance database. Eur J Gastroenterol Hepatol. 2004;16:171-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Montgomery E, Goldblum JR, Greenson JK, Haber MM, Lamps LW, Lauwers GY, Lazenby AJ, Lewin DN, Robert ME, Washington K. Dysplasia as a predictive marker for invasive carcinoma in Barrett esophagus: a follow-up study based on 138 cases from a diagnostic variability study. Hum Pathol. 2001;32:379-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 211] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 34. | Skacel M, Petras RE, Gramlich TL, Sigel JE, Richter JE, Goldblum JR. The diagnosis of low-grade dysplasia in Barrett’s esophagus and its implications for disease progression. Am J Gastroenterol. 2000;95:3383-3387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 252] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 35. | Younes M, Lauwers GY, Ertan A, Ergun G, Verm R, Bridges M, Woods K, Meriano F, Schmulen C, Johnson C. The significance of “indefinite for dysplasia” grading in Barrett metaplasia. Arch Pathol Lab Med. 2011;135:430-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 36. | Curvers WL, ten Kate FJ, Krishnadath KK, Visser M, Elzer B, Baak LC, Bohmer C, Mallant-Hent RC, van Oijen A, Naber AH. Low-grade dysplasia in Barrett’s esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105:1523-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 331] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 37. | Srivastava A, Hornick JL, Li X, Blount PL, Sanchez CA, Cowan DS, Ayub K, Maley CC, Reid BJ, Odze RD. Extent of low-grade dysplasia is a risk factor for the development of esophageal adenocarcinoma in Barrett’s esophagus. Am J Gastroenterol. 2007;102:483-493; quiz 694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Hameeteman W, Tytgat GN, Houthoff HJ, van den Tweel JG. Barrett’s esophagus: development of dysplasia and adenocarcinoma. Gastroenterology. 1989;96:1249-1256. [PubMed] |
| 39. | Van der Veen AH, Dees J, Blankensteijn JD, Van Blankenstein M. Adenocarcinoma in Barrett’s oesophagus: an overrated risk. Gut. 1989;30:14-18. [PubMed] |
| 40. | Williamson WA, Ellis FH, Gibb SP, Shahian DM, Aretz HT, Heatley GJ, Watkins E. Barrett’s esophagus. Prevalence and incidence of adenocarcinoma. Arch Intern Med. 1991;151:2212-2216. [PubMed] |
| 41. | Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett’s esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol. 1997;92:212-215. [PubMed] |
| 42. | van Sandick JW, van Lanschot JJ, Kuiken BW, Tytgat GN, Offerhaus GJ, Obertop H. Impact of endoscopic biopsy surveillance of Barrett’s oesophagus on pathological stage and clinical outcome of Barrett’s carcinoma. Gut. 1998;43:216-222. [PubMed] |
| 43. | Jankowski JA, Provenzale D, Moayyedi P. Esophageal adenocarcinoma arising from Barrett’s metaplasia has regional variations in the west. Gastroenterology. 2002;122:588-590. [PubMed] |
| 44. | Sampliner RE. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol. 2002;97:1888-1895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 500] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 45. | Younes M, Lechago J, Chakraborty S, Ostrowski M, Bridges M, Meriano F, Solcher D, Barroso A, Whitman D, Schwartz J. Relationship between dysplasia, p53 protein accumulation, DNA ploidy, and Glut1 overexpression in Barrett metaplasia. Scand J Gastroenterol. 2000;35:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Weston A, Sharma P, Topalowski M. Low-grade dysplasia in Barrett’s esophagus: Variable fate during long-term prospective follow-up. Gastroenterology. 1999;116:1349A. |
| 47. | Reid BJ, Haggitt RC, Rubin CE, Roth G, Surawicz CM, Van Belle G, Lewin K, Weinstein WM, Antonioli DA, Goldman H. Observer variation in the diagnosis of dysplasia in Barrett’s esophagus. Hum Pathol. 1988;19:166-178. [PubMed] |
| 48. | Buttar NS, Wang KK, Sebo TJ, Riehle DM, Krishnadath KK, Lutzke LS, Anderson MA, Petterson TM, Burgart LJ. Extent of high-grade dysplasia in Barrett’s esophagus correlates with risk of adenocarcinoma. Gastroenterology. 2001;120:1630-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 264] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 49. | Dar MS, Goldblum JR, Rice TW, Falk GW. Can extent of high grade dysplasia in Barrett’s oesophagus predict the presence of adenocarcinoma at oesophagectomy? Gut. 2003;52:486-489. [PubMed] |
| 50. | Provenzale D, Schmitt C, Wong JB. Barrett’s esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 229] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 51. | Cook MB, Chow WH, Devesa SS. Oesophageal cancer incidence in the United States by race, sex, and histologic type, 1977-2005. Br J Cancer. 2009;101:855-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 52. | Steevens J, Botterweck AA, Dirx MJ, van den Brandt PA, Schouten LJ. Trends in incidence of oesophageal and stomach cancer subtypes in Europe. Eur J Gastroenterol Hepatol. 2010;22:669-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 53. | Post PN, Siersema PD, Van Dekken H. Rising incidence of clinically evident Barrett’s oesophagus in The Netherlands: a nation-wide registry of pathology reports. Scand J Gastroenterol. 2007;42:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 54. | Hvid-Jensen F, Pedersen L, Drewes AM, Sørensen HT, Funch-Jensen P. Incidence of adenocarcinoma among patients with Barrett’s esophagus. N Engl J Med. 2011;365:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 978] [Article Influence: 69.9] [Reference Citation Analysis (1)] |