Published online Oct 21, 2016. doi: 10.3748/wjg.v22.i39.8779
Peer-review started: November 11, 2015
First decision: December 21, 2015
Revised: January 22, 2016
Accepted: March 18, 2016
Article in press: March 18, 2016
Published online: October 21, 2016
Processing time: 344 Days and 22.9 Hours
To investigate the role of macrophage colony-stimulating factor (M-CSF) in patients with hepatocellular carcinoma (HCC) after surgery.
Expression of M-CSF, distribution of M2 macrophages (MΦs), and angiogenesis were assessed in the liver, including tumors and peritumoral liver tissues. The prognostic power of these factors was assessed. Mouse isolated hepatic MΦs or monocytes were cultured with media containing M-CSF. The concentration of vascular endothelial growth factor (VEGF) in media was assessed. Furthermore, the role of the M-CSF-matured hepatic MΦs on proliferation of the vascular endothelial cell (VEC) was investigated.
A strong correlation between the expressions of M-CSF and CD163 was observed in the peritumoral area. Also, groups with high density of M-CSF, CD163 or CD31 showed a significantly shorter time to recurrence (TTR) than low density groups. Multivariate analysis revealed the expression of M-CSF or hepatic M2MΦs in the peritumoral area as the most crucial factor responsible for shorter TTR. Moreover, the expression of M-CSF and hepatic M2MΦs in the peritumoral area had better predictable power of overall survival. Values of VEGF in culture media were significantly greater in the hepatic MΦs compared with the monocytes. Proliferation of the VEC was greatest in the cells co-cultured with hepatic MΦs when M-CSF was present in media.
M-CSF increases hepatocarcinogenesis, most likely by enhancing an angiogenic factor derived from hepatic MΦ and could be a useful target for therapy against HCC.
Core tip: This study was designed to investigate the role of macrophage colony-stimulating factor (M-CSF) in patients with hepatocellular carcinoma (HCC) after resection. Groups with high density of M-CSF, CD163 or CD31 showed a significantly shorter time to recurrence (TTR) than low density groups. Multivariate analysis revealed the expression of M-CSF or hepatic M2MΦs in the peritumoral area as the most crucial factor responsible for shorter TTR. The expression of M-CSF and hepatic M2MΦs in the peritumoral area had better power for the prediction of overall survival. Thus, M-CSF could be a useful target for therapy against HCC.
- Citation: Kono H, Fujii H, Furuya S, Hara M, Hirayama K, Akazawa Y, Nakata Y, Tsuchiya M, Hosomura N, Sun C. Macrophage colony-stimulating factor expressed in non-cancer tissues provides predictive powers for recurrence in hepatocellular carcinoma. World J Gastroenterol 2016; 22(39): 8779-8789
- URL: https://www.wjgnet.com/1007-9327/full/v22/i39/8779.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i39.8779
Hepatocellular carcinoma (HCC) is a globally common cancer, resulting over one million deaths every year[1]. The etiology of HCC is strongly associated with liver cirrhosis due to alcohol abuse and hepatitis viral infection, exposure to aflatoxin B1 and various metabolic liver diseases[2]. The mechanisms of HCC are mainly still unknown.
Although liver resection improves overall survival in patients with HCC[3], a high rate of postoperative recurrence is still one critical problem, including intrahepatic metastasis (IM) or multicentric (MC) recurrence. Biomarkers, which derived from carcinoma cells or tumor-associated fibrotic tissues, have been studied[4]; however, results have not been well elucidated. Previously, the oncogenesis of HCC has been predominantly investigated in terms of oncogenic factors above mentioned. Alternatively, chronic inflammation is also strongly linked to mechanisms of initiation or progression of carcinoma by increasing production of reactive oxygen species and inflammatory cytokines from inflammatory cells, such as hepatic macrophages (MΦ), “Kupffer cells (KC)”, and infiltrating neutrophils into the liver[5,6]. Previously, we reported a relationship between chronic inflammation caused by hepatitis viral infection and DNA damage due to oxidative stress in patients with HCC. Furthermore, it was also reported that the period of post-operative recurrence was much shorter in patients with high oxidative stress compared with patients who had low oxidative stress in HCV-infected liver[7]. In addition to the oncogenic factors, the microenvironment is also an important factor as the soil for initiation and progression of HCC. Budhu et al[8] reported that metastasis of HCC was associated with an immune system in the liver, suggesting that liver tissues surrounding tumors have an effect on prognosis of HCC.
Macrophages, one of the predominant infiltrating cell types into the tumor[9], are attracted by chemokines[4]. When MΦs are activated, they can eliminate malignant cells or draw out reactions causing tissue destruction[10]. Although the significant role of macrophage colony-stimulating factors (M-CSF) and tumor-infiltrating MΦs in IM of HCC was already reported by Budhu et al[8], the postoperative prognostic significance has not been cleared. Results from the most recent report suggest expression of M-CSF was associated with poor survival after curative liver resection in patients with HCC, emphasizing the important role of the microenvironment in the intrahepatic recurrence of HCC[11]. M-CSF can also induce production of Th2 cytokines[12,13] and some growth factors by the monocyte, which are essential for cell growth and invasion of tumor[14]. Indeed, M-CSF induces vascular endothelial growth factor (VEGF) production and angiogenesis in monocytes. Furthermore, suppression of M-CSF decreased the infiltration of MΦs and suppressed tumor progression. On the other hand, hyper expression of M-CSF or treatment of recombinant M-CSF enhanced infiltration of MΦs, which is associated with growth of tumor cells and angiogenesis[15-17].
Based on these results, it was hypothesized that M-CSF expression in non-tumoral liver tissues may enhance recruitment of M2MΦ and angiogenesis, and enhance growth of tumor cells. This may increase the rate of recurrence of HCC, leading to a poor overall survival. Furthermore, in this study, the role of M-CSF on production of VEGF by the hepatic MΦs and the proliferation of vascular endothelial cells (VEC) were investigated in vitro.
This is a retrospective single-center, open-label study. From July 2000 to June 2008, consecutive patients in the University of Yamanashi Hospital (Yamanashi, Japan) who (1) were diagnosed as having HCC; and (2) underwent curative resection were enrolled in this study (Table 1).
| Clinicopathological features | |
| Patients | n = 77 |
| Gender | male : female = 38 : 10 |
| Age (yr) | mean = 66.6 (44-85) |
| Virus infection | HCV : HBV : NBNC = 72 : 4 : 1 |
| UICC-TNM classification | I : II : IIIA : IIIB = 41 : 32 : 3 : 1 |
| Tumor number | single : multiple : unknown = 48 : 28 : 1 |
| Tumor size (cm) | mean = 3.0 (1.0-9.5) |
| Portal vein invasion | yes : no : unknown = 7 : 68 : 2 |
| Tumor differentiation | well : mod : poor : unknown = 18 : 44 : 13 : 2 |
| Number of platelet (× 104) | mean = 13.4 (3.8-28.1) |
| Alanine aminotransferase (ALT) (U/L) | mean = 50.9 (16-207) |
| Total bilirubin (mg/dL) | mean = 0.9 (0.3-1.9) |
| Prothrombin activity (%) | mean = 77.1 (33.0-104.0) |
| Indocyanine green R15 (%) | mean = 19.1 (6.0-44.4) |
| Alpha-fetoprotein (ng/mL) | mean = 389.6 (1.8-602.6) |
All patients were observed until December 2013. The presence and identification of the hepatitis virus was determined by one or more of the following techniques: (1) the presence of anti-HCV and anti-HBV reactive serum proteins; (2) reverse transcription-PCR for serum HCV-RNA; or (3) the branched DNA-HCV probe assay. Following liver resection patients returned to the ambulatory care clinic for additional tests monthly. Serum α-fetoprotein levels were measured every month. In addition, ultrasonography and computed tomography (CT) of the liver were performed every 2 and 4 mo, respectively. Informed consent was obtained from all subjects who participated in the study, and the study was approved by the Institutional Board on Ethics for Human Science at the University of Yamanashi. All available clinical data for the patients enrolled in this study are summarized in Table 1. Overall survival (OS) was defined as the interval between the dates of surgery and death. Disease-free survival (DFS) was defined as the interval between the dates of surgery and recurrence; if recurrence was not diagnosed, patients were censored on the date of death or the last follow-up.
For the in vitro study, male C57BL/6 mice (8-10 wk of age, obtained from Jackson Laboratories, Bar Harbor, ME), were housed in a clean, temperature-controlled environment with a 12-h light-dark cycle and were given free access to regular laboratory chow diet and water for several days. All animals received humane care, and the study protocols were approved by the Committee of Laboratory Animals at University of Yamanashi according to institutional guidelines.
Sections of tumor and surrounding liver tissues were collected at the time of the operation. Then, sections were fixed in formalin (10%), dehydrated in absolute ethanol and embedded in paraffin. Serial sections (5 µm thick) were prepared for further immunohistochemical analysis.
Paraffin-embedded serial sections of liver tissues were stained immunohistochemically with anti-M-CSF (Santa Cruz Biotechnology, Santa Cruz, CA), anti-CD68 (DAKO, Kyoto, Japan), anti-CD163 (Abcam, Cambridge, United Kingdom), or anti-CD31 (Abcam) antibodies. Briefly, after deparaffinization and rehydration, the antigen retrieval procedure was applied by heating the slides in 0.1 mol/L citrate buffer (pH 6.0) for 10 min. The sections were then incubated first with 0.3% H2O2 in distilled water for 5 min to block endogenous peroxidase and then incubated with one of the following antibodies: monoclonal mouse antihuman CD68 (diluted to 1:200), CD163 (diluted to 1:200), CD31 (diluted to 1:200) , and M-CSF (diluted to 1:100) at 4 °C overnight. Biotinylated secondary antibody conjugated with avidin-biotin horseradish peroxidase (Dako Envison kit/HRP; Dako, Kyoto, Japan) and 3’,3’-diaminobenzidine tetrahydrochloride were used for standard avidin biotinylated peroxidase detection. Quantitation of cells that stained positive for CD68, CD163, CD31 and M-CSF was performed using image analysis software (Scion Image; Scion Corp., Frederick, MD) by evaluating five different fields (magnification: × 400) for areas positively stained and expressed as a percentage of the total area.
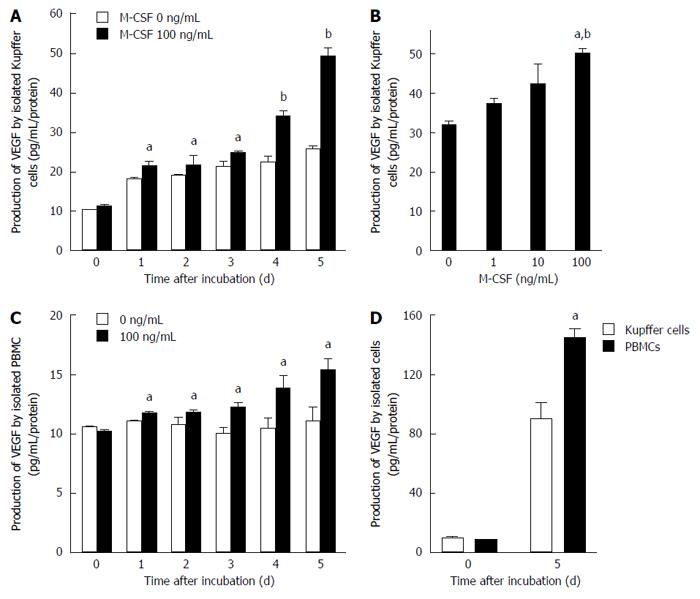
Kupffer cells were isolated by counterflow centrifugal elution as described in our previous work with some modifications[18]. Briefly, nonparenchymal cells were isolated by collagenase digestion and differential centrifugation using Nycodenz (Nycomed Pharma AS, Oslo, Norway) as described elsewhere[19]. Furthermore, the peripheral blood monocytes were isolated by the centrifugation method using magnetic beads EasySep (Tokyo, Japan). These cells were incubated in media containing recombinant mouse M-CSF (100 ng/mL) (Sigma) for a designated time period (from 1 to 5 d). In another set of experiments, isolated KCs were incubated with different doses of M-CSF (0, 1 ng, 10 ng, and 100 ng/mL) in media for 5 d. The concentration of VEGF in media was measured by an ELISA kit (R&D Systems, Minneapolis, MN).
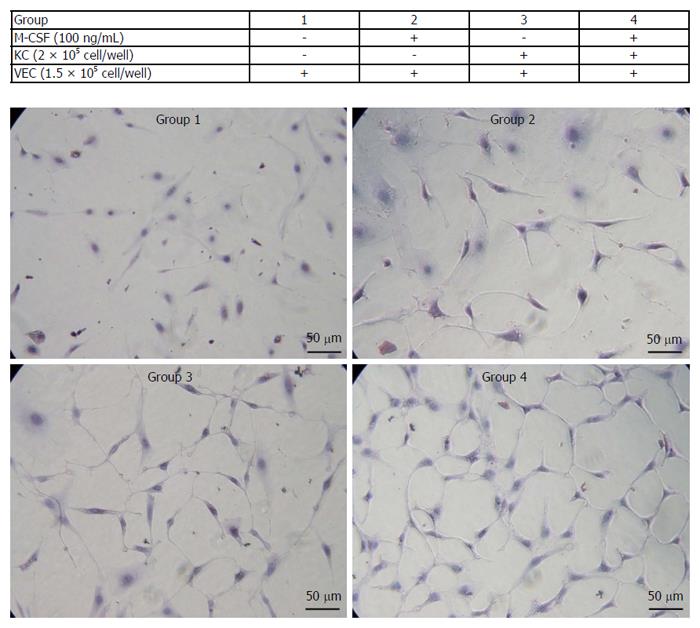
The effects of M-CSF and the KCs on the proliferation of the VECs were investigated. The KCs were isolated by collagenase digestion and the centrifugation method above mentioned, and the VECs were isolated from the aorta. The VECs were seeded on the bottom chamber of a trans-well chamber (Corning Inc., Corning, NY) and the hepatic macrophages were seeded on the upper chamber. These cells were incubated with the media containing M-CSF (mouse recombinant M-CSF; 100 ng/mL) (Sigma, St. Louis, MO) for 4 d and proliferation of the VECs was assessed.
Analysis was performed using SPSS 13.0 for Windows (SPSS Inc., Chicago IL). The Pearson χ2 test or Fisher’s exact test was used to compare qualitative variables, and quantitative variables were analyzed by the t test or Pearson’s correlation test. Kaplan-Meier analysis was used to determine the survival (time to recurrence and overall survival). Receiver operating characteristic (ROC) curve analysis was used to determine the predictive value of the parameters. For the univariate analysis, linear regression, or Log-rank test statistical procedures were used to assess which endpoints could be used for predicting the prognosis of HCC patients after surgery. For the multivariate analysis, the Cox proportional hazard model was used to calculate hazard ratio and the P value of each parameter. P < 0.05 was considered statistically significant.
The clinical characteristics of HCC patients in this study are shown in Table 1. Experiments were performed using resected liver specimens including HCC and tumor free non-cancerous liver tissue by surgery.
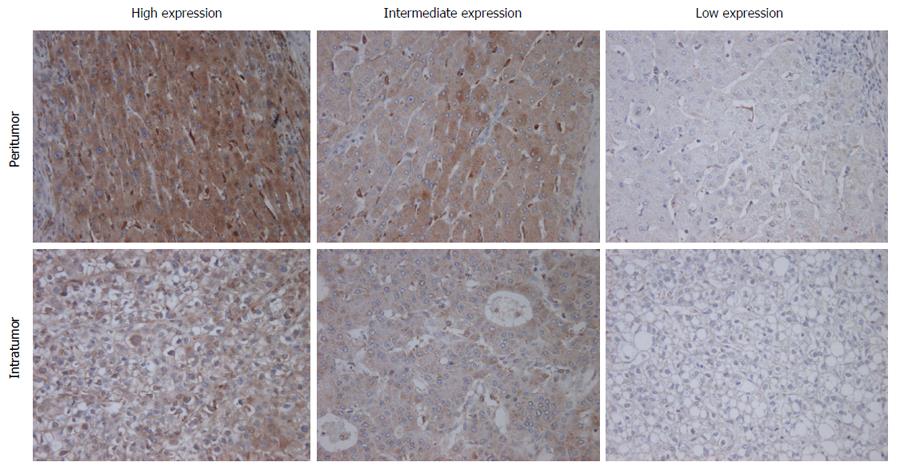
Immunohistochemical staining for M-CSF was performed as described in the Patients and Methods section. Immunohistochemical staining for M-CSF was observed in the cytoplasm of both tumor cells and hepatocytes (Figure 1). On the other hand, most of the non-parenchymal cells were negatively stained.
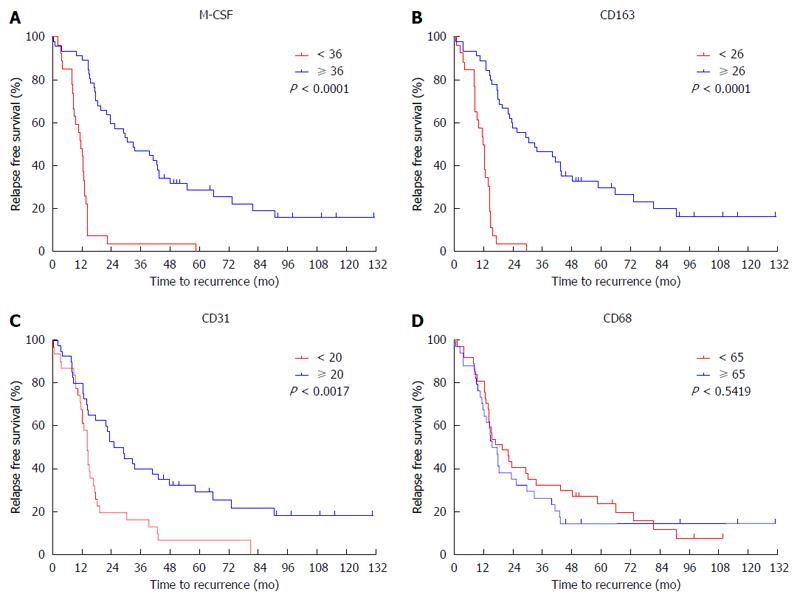
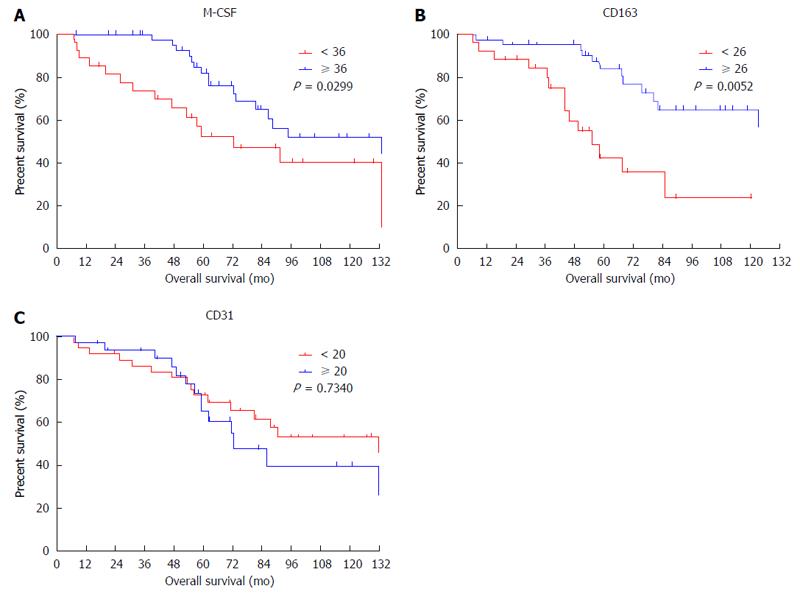
The expression of M-CSF in intratumoral tissues was not correlated with DFS and overall survival rate (OS) (data not shown), consistent with the previous report[11]. On the other hand, in peritumoral tissues, DFS and OS were significantly shorter in the high expression group than in the low expression group (Figures 2 and 3), consistent with the previous reports[11].
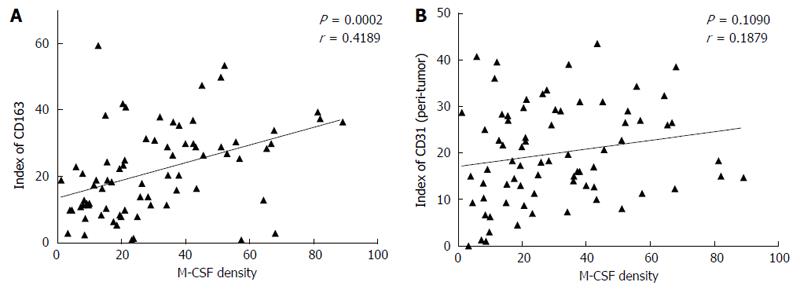
Immunohistochemical staining for CD68 (a marker of the M1MΦs and/or the monocytes), CD163 (a marker of the M2MΦs), and CD31 (a marker of the vascular endothelial cells) was performed as described in the Patients and Methods section. A positive correlation was observed between the expression of M-CSF and CD31 in the peritumoral liver tissues, but not between M-CSF and CD163 (Figure 4), or CD68 (data not shown).
To determine the cutoff value of the M-CSF positive area, an ROC curve was drawn using recurrence within one year. Patients with a high expression of CD31 or CD163 in peritumoral tissues showed significantly poorer DFS compared with patients with low expression of these markers (Figures 2 and 3). Furthermore, patients with high expression of CD163 in peritumoral tissues showed significantly poorer OS compared with patients with low expression of these markers. Although positive staining for CD68 was detected in peritumoral tissues, the number of CD68-positive cells was not correlated with DFS (Figure 2) or OS (data not shown).
According to the ROC curve, the ideal cutoff value of the densities of M-CSF, CD163, and CD31 were 36, 26, and 20, respectively. The median DFS was 12.3 mo for patients with high expression of M-CSF in peritumoral tissues, which was significantly shorter compared with the median DFS for patients with low expression of M-CSF (43.3 mo). Furthermore, the median survival times for the high CD163 density and the low density group were 12.2 and 32.0 mo, respectively. The median survival times for the high CD31 density and the low density group were 13.6 and 37.3 mo, respectively.
The univariate analysis of the prognostic value for various factors assessed in the present study is shown in Table 2.
| Factors | Time to recurrence | Overall survival | ||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
| P value | Hazard ratio | 95%CI | P value | P value | Hazard ratio | 95%CI | P value | |
| Age: ≥ 60 yr vs < 60 yr | 0.240 | NA | 0.523 | NA | ||||
| Gender: female vs male | 0.760 | NA | 0.111 | NA | ||||
| Indx of α-SMA: ≥ 12 vs < 12 | 0.751 | NS | 0.423 | NS | ||||
| Indx of CD163: ≥ 26 vs < 26 | < 0.001 | 4.517 | 1.879-10.857 | 0.001 | < 0.001 | 1.086 | 1.039-1.135 | < 0.001 |
| M-CSF density: ≥ 36 vs < 36 | < 0.001 | 4.826 | 2.045-11.389 | < 0.001 | 0.037 | NS | ||
| Indx of CD31: ≥ 20 vs < 20 | 0.002 | NS | 0.020 | NS | ||||
| AFP: ≥ 100 vs < 100 | 0.232 | NA | 0.785 | NA | ||||
| AFP-L3%: ≥ 10 vs < 10 | 0.827 | NA | 0.900 | NA | ||||
| PIVKA-II: ≥ 100 vs < 100 | 0.841 | NA | 0.325 | NA | ||||
| Tumor size: ≥ 2 cm vs < 2 cm | 0.185 | NA | 0.847 | NA | ||||
| Tumor number: multiple vs single | 0.447 | NA | 0.038 | NS | ||||
| Tumor differentiation: | 0.379 | NA | 0.745 | NA | ||||
| well vs mod. vs poor | ||||||||
| Fibrosis score: 1, 2, 3 vs 4 | 0.755 | NA | 0.015 | 0.002 | ||||
| TNM stage: I vs II vs IIIa | 0.020 | NS | 0.173 | NA | ||||
In clinicopathologic factors, the size of the tumor, the clinical disease stage, serum ALT and AFP levels are useful to assess recurrence of HCC after surgery. In the present study, there were significant differences in the univariate analysis of DFS between the UICC-TNM Stages (Stage I vs Stages II and III) (Table 2). Furthermore, there were significant differences in the univariate analysis of OS between the tumor number (single vs multiple) and the fibrosis score (1-3 vs 4). In multivariate analysis of the prognostic power, the fibrosis score was a factor for predicting the length of overall survival in patients with HCC (Table 2).
M-CSF density, CD163 index, and CD31 index in peritumoral liver tissues appear to be risk factors for DFS by univariate analysis (Table 2). When each of these markers was included in the multivariate analysis, M-CSF density and CD163 index were found to be the only significant predictors for DFS. Alternatively, peritumoral M-CSF density, CD163 index, and CD31 index appear to be risk factors for OS by univariate analysis. Furthermore, when each of these markers was included in the multivariate analysis, the CD163 index was found to be the only significant predictor for OS.
The production of VEGF by isolated KCs increased in a dose- and time-dependent manner after incubation with M-CSF in media (Figure 5). Furthermore, the production by isolated monocytes also increased in a time dependent manner by stimulation with M-CSF (Figure 5). Although the production of VEGF increased in both isolated KCs and monocytes incubated with M-CSF, production was significantly greater in the KC compared with the monocytes (Figure 5).
Cell proliferation of isolated VECs markedly increased in cells treated with M-CSF compared with those that were not treated with M-CSF in media (Figure 6). Importantly, among the groups studied, the proliferation was greatest in the M-CSF-treated VECs co-cultured with the KCs.
It was previously reported that overexpression of M-CSF is observed in tumor tissues in various human cancers and is related with poor survival[20-25]. It was also reported that M-CSF is predominantly detected in peritumoral liver tissues[8]. Furthermore, it was reported from this laboratory that the incidence of chemically-induced HCC was reduced in M-CSF deficient mice (KO) compared with their littermates (WT) by inhibiting the expression of M2 MΦ and angiogenesis in peritumor liver tissues[26]. In the present study, the high expression of M-CSF in peritumoral tissues was correlated with a high incidence of HCC, including MC recurrence and IM, and poor prognosis after curative resection (Figures 2 and 4), consistent with the previous report[11]. Thus, M-CSF is involved in hepatic carcinogenesis and its progression. Based on these reports, this study investigated whether M-CSF induces hepatic carcinogenesis, most likely by enhancing angiogenesis by M-CSF-induced hepatic macrophages.
In the present study, expression of M-CSF differed in intratumoral tissues and peritumoral liver tissues, suggesting that the expression level of M-CSF differs in each individual. Importantly, the level of M-CSF expression did not correlate with any pathophysiologic factors. Therefore, the expression of M-CSF may be associated with other genetic factors in each patient[11]. Indeed, Okamoto et al[27] reported that a specific gene profile in non-tumoral tissue predicted MC recurrence or IM of HCC. Alternatively, placental[28,29] and endothelial cells[30] excreted M-CSF under hypoxic conditions. Based on this result, distribution of M-CSF may be caused by hypoxia in hepatocytes due to compression by the primary tumor. A tumor itself also enhances acute and chronic inflammation, causes cytokine production and attracts MΦs to peritumoral liver tissues. The previous study indicated that implanted or spontaneous tumors induced vascular endothelial growth factor in peritumoral tissue that was much greater than in the intratumoral tissue[31]. Furthermore, angiogenesis was greater in peritumoral tissues than in the intratumoral tissues in chemically-induced HCC in mice[26]. Taken together, the expression of M-CSF in peritumoral hepatocytes may be associated with the genetic heterogeneity in each individual or inflammatory factors in the liver.
The high rate of IM and MC recurrence after complete resection indicated that the non-tumoral hepatic microenvironment plays a key role in tumor initiation and progression. Previous investigations of causes of HCC were mainly focused on factors of tumor initiation as “seeds.” On the other hand, few studies that focus on hepatic microenvironmental factors as “soil” were reported. Indeed, Ezaki et al[32,33] reported that the hyper expression of thymidine phosphorylase in the peritumoral liver tissues was correlated with the higher incidence of postoperative recurrence of HCC. Furthermore, Yu et al[34] reported that vascular density was higher in peritumoral tissues compared with that in intratumoral tissue, leading to increased expression of VEGF and hypoxia inducible factor-1 in peritumoral liver tissues. Moreover, it was reported that immunological features in the peritumoral tissue predicted venous metastases in HCC[35]. In this study, the high density of M-CSF in peritumoral liver tissues was correlated with a high incidence of HCC and prognosis, consistent with the previous report[11] (Figures 2 and 3). Taken together, it is concluded that the peritumoral microenvironment, as “the soil,” is also important in understanding the mechanism of incidence of HCC. Furthermore, previous studies and this study suggest that postoperative adjuvant therapies could target not only the subclinical carcinoma cells, but also the microenvironment in the liver.
The union of M-CSF expression, population of M2MΦs and angiogenesis in peritumoral tissues had a better predictable power for prognosis in HCC (Figures 2 and 3, and Table 2). The number of MΦs was greater in peritumoral tissue than in intratumoral tissue, consistent with results in previous reported studies[35,36]. In the present study, expression of M-CSF, M2MΦs and angiogenesis in the peritumoral liver tissue was correlated with DFS after surgery (Figure 2). The role of microenvironments in intratumoral tissues and peritumoral tissues may be different in initiation and progression of HCC. Expression of M-CSF, CD31 and M2MΦs in intratumoral tissues may be involved in promoting the dissemination of the cancer cells[37-39]. M-CSF and M2MΦs in the peritumoral tissues are involved in accelerating colonization and growth of disseminated tumor cells, leading to micrometastasis, since M-CSF and M2 MΦ in the tumor is eliminated by operation. Therefore, as the defense to inhibit growth of the tumor, the peritumoral liver tissue, which supplies M-CSF to the tumor[31], plays a pivotal role by providing a fruitful soil for micrometastasis of HCC as “seeds”. The role of the MΦs in providing a fertile soil as the metastasis niche has also been reported[40]. In this study, the number of M2MΦs in the peritumoral tissues was correlated with the expression of M-CSF in the peritumoral tissues and was linked with DFS and OS (Figures 2 and 3). Thus, M-CSF-induced M2MΦs may be involved in DFS. In addition to this result, the number of M2MΦs and the expression of angiogenic factors were also positively correlated. Importantly, VEGF production by isolated hepatic MΦ was increased by M-CSF stimulation (Figure 5) and proliferation of isolated VEC was greatest in the cells incubated with M-CSF-stimulated hepatic MΦ in the presence of M-CSF in media (Figure 6). Thus, M-CSF-induced M2MΦs could be involved in progression of HCC by inducing angiogenesis.
Undoubtedly, M-CSF and M2MΦ could be suitable targets for adjuvant therapy after surgery, since in the present study the expression of M-CSF and the number of M2MΦs increased in the peritumoral tissues, hepatic MΦs incubated with M-CSF in media produced VEGF, and the proliferation was greatest in the M-CSF-treated VECs co-cultured with the KCs among the groups studied. Thus, this study indicates that M-CSF expressed in the peritumoral liver tissue could predict the recurrence of patients with HCC after surgery and also indicates that the remnant liver may play an important role in recurrence and metastasis. Therefore, evaluation of M-CSF is a useful result that can be easily assessed in specimens collected during surgery.
Expression of macrophage colony-stimulating factor (M-CSF) was associated with hepatocellular carcinoma (HCC) progression, disease recurrence, and poor survival after hepatectomy, highlighting the importance of the peritumoral tissue environment in the intrahepatic recurrence of HCC.
In this study, the authors investigated the relationship between the M-CSF-matured hepatic macrophage and the prognosis of HCC after resection.
In this manuscript, the major point is that vascular endothelial growth factor derived from the M-CSF-matured hepatic macrophage plays a pivotal role in the prognosis of HCC.
M-CSF or its receptor could be a new molecular target for therapy against HCC.
The present study indicates that M-CSF expressed in peritumoral liver tissues can predict recurrence in patients with HCC after surgery and also implies that the remnant liver may play a key role in recurrence and metastasis. Therefore, evaluation of M-CSF is a useful result that can be easily assessed in specimens collected during liver tumor resection.
In this paper, the authors aimed at investigating the role of M-CSF in patients with HCC after resection. They analyze the prognostic value of MCSF, CD163, CD31 and other clinicopathologic parameters for patients’ disease-free survival (DFS) with HCC and find M-CFS in non-cancer tissues can act as a predictor for patients DFS after resection.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Xu Y S- Editor: Qi Y L- Editor: O’Neill M E- Editor: Wang CH
| 1. | Inoue H, Seitz HK. Viruses and alcohol in the pathogenesis of primary hepatic carcinoma. Eur J Cancer Prev. 2001;10:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Kountouras J, Lygidakis NJ. New epidemiological data on liver oncogenesis. Hepatogastroenterology. 2000;47:855-861. [PubMed] |
| 3. | Tang ZY, Ye SL, Liu YK, Qin LX, Sun HC, Ye QH, Wang L, Zhou J, Qiu SJ, Li Y. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 361] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 4. | Wiktor-Jedrzejczak W, Gordon S. Cytokine regulation of the macrophage (M phi) system studied using the colony stimulating factor-1-deficient op/op mouse. Physiol Rev. 1996;76:927-947. [PubMed] |
| 5. | Koike K, Miyoshi H. Oxidative stress and hepatitis C viral infection. Hepatol Res. 2006;34:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3280] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 7. | Maki A, Kono H, Gupta M, Asakawa M, Suzuki T, Matsuda M, Fujii H, Rusyn I. Predictive power of biomarkers of oxidative stress and inflammation in patients with hepatitis C virus-associated hepatocellular carcinoma. Ann Surg Oncol. 2007;14:1182-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, Kammula US, Chen Y, Qin LX, Tang ZY. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10:99-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 650] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 9. | Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 735] [Cited by in RCA: 742] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 10. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5744] [Article Influence: 239.3] [Reference Citation Analysis (0)] |
| 11. | Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ, Wu WZ, Wang L, Tang ZY, Sun HC. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2008;26:2707-2716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 461] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 12. | Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc Natl Acad Sci USA. 2004;101:4560-4565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 750] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 13. | Smith W, Feldmann M, Londei M. Human macrophages induced in vitro by macrophage colony-stimulating factor are deficient in IL-12 production. Eur J Immunol. 1998;28:2498-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Zins K, Abraham D, Sioud M, Aharinejad S. Colon cancer cell-derived tumor necrosis factor-alpha mediates the tumor growth-promoting response in macrophages by up-regulating the colony-stimulating factor-1 pathway. Cancer Res. 2007;67:1038-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064-5066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 347] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 16. | Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1056] [Cited by in RCA: 1123] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 17. | Paulus P, Stanley ER, Schäfer R, Abraham D, Aharinejad S. Colony-stimulating factor-1 antibody reverses chemoresistance in human MCF-7 breast cancer xenografts. Cancer Res. 2006;66:4349-4356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Kono H, Fujii H, Asakawa M, Yamamoto M, Maki A, Matsuda M, Rusyn I, Matsumoto Y. Functional heterogeneity of the kupffer cell population is involved in the mechanism of gadolinium chloride in rats administered endotoxin. J Surg Res. 2002;106:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Kono H, Enomoto N, Connor HD, Wheeler MD, Bradford BU, Rivera CA, Kadiiska MB, Mason RP, Thurman RG. Medium-chain triglycerides inhibit free radical formation and TNF-alpha production in rats given enteral ethanol. Am J Physiol Gastrointest Liver Physiol. 2000;278:G467-G476. [PubMed] |
| 20. | Kacinski BM. CSF-1 and its receptor in ovarian, endometrial and breast cancer. Ann Med. 1995;27:79-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2416] [Cited by in RCA: 2562] [Article Influence: 122.0] [Reference Citation Analysis (0)] |
| 22. | Smith HO, Anderson PS, Kuo DY, Goldberg GL, DeVictoria CL, Boocock CA, Jones JG, Runowicz CD, Stanley ER, Pollard JW. The role of colony-stimulating factor 1 and its receptor in the etiopathogenesis of endometrial adenocarcinoma. Clin Cancer Res. 1995;1:313-325. [PubMed] |
| 23. | Mroczko B, Groblewska M, Wereszczyńska-Siemiatkowska U, Okulczyk B, Kedra B, Łaszewicz W, Dabrowski A, Szmitkowski M. Serum macrophage-colony stimulating factor levels in colorectal cancer patients correlate with lymph node metastasis and poor prognosis. Clin Chim Acta. 2007;380:208-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Mroczko B, Groblewska M, Wereszczynska-Siemiatkowska U, Kedra B, Konopko M, Szmitkowski M. The diagnostic value of G-CSF measurement in the sera of colorectal cancer and adenoma patients. Clin Chim Acta. 2006;371:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Groblewska M, Mroczko B, Wereszczyńska-Siemiatkowska U, Myśliwiec P, Kedra B, Szmitkowski M. Serum levels of granulocyte colony-stimulating factor (G-CSF) and macrophage colony-stimulating factor (M-CSF) in pancreatic cancer patients. Clin Chem Lab Med. 2007;45:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Hara M, Kono H, Furuya S, Hirayama K, Tsuchiya M, Fujii H. Macrophage colony-stimulating factor plays a pivotal role in chemically induced hepatocellular carcinoma in mice. Hepatol Res. 2014;44:798-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Okamoto M, Utsunomiya T, Wakiyama S, Hashimoto M, Fukuzawa K, Ezaki T, Hanai T, Inoue H, Mori M. Specific gene-expression profiles of noncancerous liver tissue predict the risk for multicentric occurrence of hepatocellular carcinoma in hepatitis C virus-positive patients. Ann Surg Oncol. 2006;13:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Hayashi M, Ohkura T, Inaba N. Elevation of serum macrophage colony-stimulating factor before the clinical manifestations of preeclampsia. Am J Obstet Gynecol. 2003;189:1356-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Hayashi M, Ohkura T, Inaba N. Increased levels of serum macrophage colony-stimulating factor before the onset of preeclampsia. Horm Metab Res. 2003;35:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Krishnaswamy G, Kelley J, Yerra L, Smith JK, Chi DS. Human endothelium as a source of multifunctional cytokines: molecular regulation and possible role in human disease. J Interferon Cytokine Res. 1999;19:91-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 247] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 31. | Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 692] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 32. | Ezaki T, Ikegami T, Ishida T, Aimitsu S, Mori M, Fujihara M. Significance of thymidine phosphorylase in HCC with chronic liver disease for long-term postoperative recurrence. J Surg Oncol. 2003;83:173-19; discussion 179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Ezaki T, Ikegami T, Maeda T, Yamada T, Ishida T, Hashizume M, Maehara Y. Prognostic value of thymidine phosphorylase activity in liver tissue adjacent to hepatocellular carcinoma. Int J Clin Oncol. 2005;10:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Yu D, Zhuang L, Sun X, Chen J, Yao Y, Meng K, Ding Y. Particular distribution and expression pattern of endoglin (CD105) in the liver of patients with hepatocellular carcinoma. BMC Cancer. 2007;7:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Liu K, He X, Lei XZ, Zhao LS, Tang H, Liu L, Lei BJ. Pathomorphological study on location and distribution of Kupffer cells in hepatocellular carcinoma. World J Gastroenterol. 2003;9:1946-1949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Cervello M, Foderàa D, Florena AM, Soresi M, Tripodo C, D’Alessandro N, Montalto G. Correlation between expression of cyclooxygenase-2 and the presence of inflammatory cells in human primary hepatocellular carcinoma: possible role in tumor promotion and angiogenesis. World J Gastroenterol. 2005;11:4638-4643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1362] [Article Influence: 71.7] [Reference Citation Analysis (0)] |
| 38. | Bockhorn M, Jain RK, Munn LL. Active versus passive mechanisms in metastasis: do cancer cells crawl into vessels, or are they pushed? Lancet Oncol. 2007;8:444-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 39. | Gupta GP, Massagué J. Cancer metastasis: building a framework. Cell. 2006;127:679-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2932] [Cited by in RCA: 3198] [Article Influence: 168.3] [Reference Citation Analysis (0)] |
| 40. | Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2613] [Cited by in RCA: 2385] [Article Influence: 119.3] [Reference Citation Analysis (0)] |