Published online Oct 14, 2016. doi: 10.3748/wjg.v22.i38.8568
Peer-review started: June 21, 2016
First decision: July 29, 2016
Revised: August 2, 2016
Accepted: August 23, 2016
Article in press: August 23, 2016
Published online: October 14, 2016
Processing time: 114 Days and 10.4 Hours
To identify risk factors for P1 lesions on small bowel capsule endoscopy (SBCE) and to describe the natural history of anemic patients with such type of lesions.
One hundred patients were consecutively selected for a case-control analysis performed between 37 cases with P1 lesions and 63 controls with negative SBCE. Age, gender, comorbidities and regular medication were collected. Rebleeding, further investigational studies and death were also analyzed during the follow-up.
No significant differences on gender, median age or Charlson index were found between groups. Although no differences were found on the use of proton pump inhibitors, acetylsalicylic acid, anticoagulants or antiplatelet agents, the use of non-steroidal anti-inflammatory drugs (NSAID) was associated with a higher risk of P1 lesions (OR = 12.00, 95%CI: 1.38-104.1). From the 87 patients followed at our center, 39 were submitted to additional studies for investigation of iron-deficiency anemia (IDA), and this was significantly more common in those patients with no findings on SBCE (53.7% vs 30.3%, P = 0.033). A total of 29 patients had at least one rebleeding or IDA recurrence episode and 9 patients died of non-anemia related causes but no differences were found between cases and controls.
P1 lesions are commonly found in patients with IDA submitted to SBCE. The use of NSAID seems to be a risk factor for P1 lesions. The outcomes of patients with P1 lesions do not differ significantly from those with P0 lesions or normal SBCE.
Core tip: Despite the high diagnostic yield of small bowel capsule endoscopy (SBCE) in the study of iron-deficiency anemia (IDA), the clinical relevance and bleeding potential of findings such as red spots or mucosal erosions (P1 lesions) remain uncertain. We found that P1 lesions were commonly found in the SBCE of patients with IDA and their presence was associated with non-steroidal anti-inflammatory drugs use. The outcomes of patients with P1 lesions do not differ significantly from those with P0 lesions or normal SBCE. An algorithm with a stepwise approach to the patients with IDA who are submitted to SBCE is proposed.
- Citation: Cúrdia Gonçalves T, Barbosa M, Rosa B, Moreira MJ, Cotter J. Uncovering the uncertainty: Risk factors and clinical relevance of P1 lesions on small bowel capsule endoscopy of anemic patients. World J Gastroenterol 2016; 22(38): 8568-8575
- URL: https://www.wjgnet.com/1007-9327/full/v22/i38/8568.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i38.8568
Iron-deficiency anemia (IDA) is a highly common medical condition occurring in 2%-5% of adult men and postmenopausal women in developed countries[1]. These patients are often referred to gastroenterologists, accounting for up to 13% of all referrals[1]. Except for premenopausal women in whom the menstrual blood loss is the commonest cause of IDA, blood loss from the gastrointestinal (GI) tract is the most frequent reason for developing IDA. Consequently, the study of the GI tract is the cornerstone in the initial approach of these patients. While upper endoscopy and colonoscopy may reveal the causative lesion in 70%-80% of patients, there is still a reasonable proportion of patients in which these procedures will be unrevealing[2].
Recent guidelines on IDA recommend an empirical trial of iron supplementation before the study of small bowel[1]. Nonetheless, this strategy may delay a definitive diagnosis, which may be unacceptable in some subgroups of patients, particularly those with other associated GI symptoms. With the advent of small bowel capsule endoscopy (SBCE) and device-assisted enteroscopy, in the setting of normal upper and lower GI tract, the study of small bowel should be strongly considered. In fact, in these patients, about 75% of them will have a potentially bleeding lesion in the small bowel which can explain IDA[3]. Due to its wide availability, patient acceptance and safety, SBCE is recommended by current guidelines as a first-line examination, before consideration of other diagnostic modalities[2].
The diagnostic yield of SBCE in patients with IDA is variable among studies, but has been reported to be as high as 66%[4]. Although SBCE may identify different lesions, IDA cannot be equally attributable to all of them. There are certainly some lesions such as angioectasias or tumours, whose bleeding potential is greater than that of scarce red spots, submucosal veins or small bowel diverticula. Saurin et al[5] proposed a classification of lesions in 3 groups according to their bleeding potential: P0 lesions included visible submucosal veins, diverticula without the presence of blood, or nodules without mucosal breaks, which are believed to have no bleeding potential; P1 lesions, such as red spots on the intestinal mucosa or small or isolated erosions, were regarded as having uncertain hemorrhagic potential; and P2 lesions are those considered to have a high potential for bleeding, and include angioectasia, ulcers, tumours or varices. While several studies have focused on the clinical significance of P2 lesions and their risk factors[6,7], little is known about P1 lesions. What are the characteristics of patients with P1 lesions? Are there any risk factors for these lesions? What is the IDA recurrence rate of patients with P1 lesions? What should be the best approach in patients with IDA and P1 lesions found on SBCE? These were some unanswered questions that warranted further investigation.
Therefore, the present study was designed to better characterize patients with P1 lesions, namely their natural history, to identify risk factors for their presence in the small bowel of patients examined by SBCE, and to propose a stepwise approach to these patients.
This was a single-center, retrospective case-control study. All the 222 patients referred to our center for the investigation of IDA between September 2008 and August 2013 were reviewed. Anemia was defined according to the World Health Organization criteria, i.e. hemoglobin level of < 12 g/dL in nonpregnant women and < 13 g/dL in men[1], while IDA was defined when the previous hemoglobin values were associated with a ferritin level of < 15 μg/L[8]. Premenopausal women were cautiously observed by a gynecologist and gynecologic causes for IDA were ruled out previous to SBCE referral. Patients with IDA and P1 lesions on SBCE were considered cases, while those patients with P0 lesions or negative examinations were included in the control group. Patients with P2 lesions were excluded of study analysis. Data from each patient was collected by reviewing medical records and included demographic data such as age and gender, as well as clinical data namely comorbidities and regular medications. The burden of comorbidities was assessed using the Charlson Comorbidity Index[9]. This index estimates the risk of death due to comorbid disease, and includes a total of 22 variables, scored 1, 2, 3 or 6, depending on the risk of dying associated with each one[9]. Relevant medication for analysis included proton pump inhibitors (PPIs), acetylsalicylic acid and other antiplatelet agents, anticoagulants, and non-steroidal anti-inflammatory drugs (NSAIDs). During the follow-up period, the performance of additional diagnostic exams for the study of IDA, IDA recurrence and death were assessed. IDA recurrence was consistently defined as hospitalization due to symptomatic anemia, decrease of > 2 g/dL in the hemoglobin value, transfusion of red blood cells, or melena or hematochezia with non-diagnostic upper endoscopy and colonoscopy, occurring > 30 d after the initial episode. This study was approved by the local Ethical Committee.
After a clear liquid diet in the previous day and a 12 h fasting period, patients were instructed to swallow the capsule (PillCam SB2 or SB3, Given Imaging, Yoqneam, Israel) with a simethicone solution to reduce bubble formation as previously reported[10]. No other specific small bowel preparation was used. To minimize the possibility of incomplete examination, capsule location was assessed using the Real Time Viewer System one hour after the beginning of the exam, and if it was still in the gastric cavity, 10 mg of domperidone were given to the patient[11]. Patients were allowed to drink clear fluids 2 h after the passage of the capsule to the stomach and to have a light snack 4 h from the beginning of the examination. Each SBCE video was analyzed by two SBCE experts (with experience of reporting more than 500 SBCE) at a speed of 12 frames per second. When no consensus was reached regarding the SBCE findings, the video was simultaneously reviewed by both experts so no discrepancy remained. No cases of capsule retention or aspiration were observed.
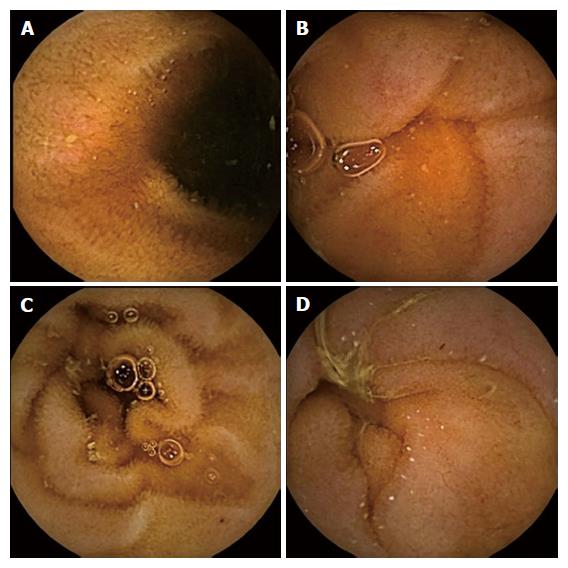
The SBCE findings were classified according to the system reported by Saurin et al[5], which divides the small bowel lesions in three distinct groups: angioectasias, varices, ulcerations and tumours represent P2 lesions; red spots and small or isolated erosions are considered P1 lesions; submucosal veins, diverticula and nodules are included in the P0 lesions group. When none of these findings were found, the SBCE examination was considered negative. Examples of some P1 lesions found on SBCE are shown in Figure 1.
Quantitative data was expressed as mean ± standard deviation. Univariate analysis was performed using the Student’s t test for continuous variables and the χ2 or Fisher’s exact test for categorical variables. A P value of < 0.05 was considered to denote statistical significance. Statistical analysis was performed using the IBM SPSS Statistics for Windows version 20.0 (Armonk, New York, Unites States).
Out of the 222 patients referred to our center for SBCE for the study of IDA from September 2008 until August 2013, 122 had P2 lesions on examination and were excluded from the final analysis. From the remaining 100 patients, 37 were found to have P1 lesions on small bowel (29 had small or isolated erosions, and 8 had red spots) and were included in the case group, while 63 had P0 lesions or negative examinations and were regarded as controls. The baseline characteristics of the analyzed patients are summarized in Table 1. Concerning demographic characteristics, namely mean age and gender, no significant differences were found between cases and controls. P1 lesions were not associated with a heavier burden of comorbidities as shown by the absence of significant differences in the mean Comorbidity Charlson Index between cases and controls. Regarding the regular medication, no differences were found between groups in the consumption of PPI, acetylsalicylic acid, other antiplatelet agents or anticoagulants. Contrarily, the use of NSAID was significantly higher in patients with IDA and P1 lesions (P = 0.01, OR = 12.0, 95%CI: 1.38-104.1).
| Characteristic, n (%) | Cases (n = 37) | Controls (n = 63) | P value |
| Age, mean ± SD | 57.2 ± 15.6 | 55.4 ± 19.5 | 0.609 |
| Female gender | 29 (78.4) | 46 (73.0) | 0.55 |
| Mean Comorbidity Charlson Index, mean ± SD | 4.1 ± 2.8 | 3.9 ± 3.2 | 0.612 |
| PPI | 8 (21.6) | 17 (27.0) | 1.000 |
| Acetylsalicylic acid | 15 (40.5) | 14 (22.2) | 0.051 |
| Other antiplatelet agents | 5 (13.5) | 12 (19.0) | 0.477 |
| Anticoagulants | 9 (24.3) | 8 (12.7) | 0.135 |
| NSAID | 6 (16.2) | 1 (1.6) | 0.01 |
While 13 patients had follow-up intervals shorter than 12 mo and were excluded from this subanalysis, the remaining 87 patients had longer follow-up periods. Globally, the mean follow-up interval was 34.0 ± 16.6 mo (range from 12 to 72 mo). Data related to follow-up of cases and controls, namely its duration, submission to further diagnostic modalities, IDA recurrence and death are presented in Table 2. Thirty-three cases (37.9%) and 54 controls (62.1%) had a follow-up longer than 12 mo. No significant differences were found in the duration of follow-up between groups. The strategy of requiring further diagnostic modalities was significantly more common in the control group (P = 0.033). In general, in the sum of 39 cases and controls submitted to further examinations, a total of 33 upper endoscopies, 37 colonoscopies, 2 SBCE, and 3 99-mTc labeled red-blood cell scintigraphies were performed. Despite a final diagnosis could not be established in 31 (75.8%) patients, a definitive diagnosis was reached in the remaining: 4 patients had colonic angioectasia, 1 patient had Cameron’s lesions, 1 patient had gastric antral vascular ectasia, 1 patient had a duodenal angioectasia, and 1 patient had a benign gastric ulcer. During the follow-up, a total of 29 patients (9 cases and 20 controls) had rebleeding, but no significant differences were found in the rebleeding rate between groups. The mean interval time between SBCE and the rebleeding episode was 17.8 mo. A total of 9 patients (4 cases and 5 controls) died during the follow-up. In all of them the cause of death was not directly attributed to IDA: 3 patients died of sepsis, 2 of terminal cirrhosis, 1 of terminal chronic kidney disease, 1 of terminal heart failure, 1 had hemorrhagic stroke, and 1 had malignant mesothelioma.
| Characteristic, n (%) | Cases (n = 33) | Controls (n = 54) | P value |
| Follow-up duration, mean ± SD | 31.7 ± 17.2 | 38.2 ± 15.9 | 0.075 |
| Further diagnostic examinations | 10 (30.3) | 29 (53.7) | 0.033 |
| IDA recurrence | 9 (21.6) | 20 (37.0) | 0.349 |
| Death | 4 (12.1) | 5 (9.3) | 0.725 |
The role of SBCE in the study of IDA is currently unquestionable, as shown in different international guidelines[2,12]. Despite lacking the potential for therapeutic intervention, due to its safety, acceptance, availability, and diagnostic yield, SBCE is nowadays a first-line procedure for the study of small bowel causes for IDA. The type of lesions that can be found in patients with IDA submitted to SBCE is highly variable and include angioectasia, small bowel tumours, villous atrophy, ulcers, erosions, strictures, varices[13-15]. As the bleeding potential is not the same for all types of lesions, there was a need to classify them according their hemorrhagic potential. The most widely accepted classification system is the one proposed by Saurin et al[5].
P2 lesions are known to have a high bleeding potential, with some studies reporting a rebleeding rate of up to 36.8%[16]. Different studies have also reported several factors that are associated with P2 lesions on SBCE of patients with IDA, namely NSAID and antiplatelet use, higher transfusion requirements, moderate to severe chronic kidney disease, older age, hypertension, hypercholesterolemia, and anticoagulants[6,7,17,18].
While the natural history and risk factors for P2 lesions are relatively well established, the presence of P1 lesions on the SBCE of patients with IDA and its associated uncertainty may pose serious concerns regarding the best management in this clinical scenario.
Regarding the features of patients presenting with P1 lesions, we found that although women were more commonly submitted to SBCE for the study of IDA, P1 lesions had no gender predominance. The mean age of patients in both cases and controls was in the sixth decade of life, reflecting the increasing prevalence of IDA in people older than 50 years old[19]. However, unlike P2 lesions which seem to be more common in older patients, the presence of P1 lesions does not seem to be influenced by age. It is well known that several conditions such as ischemia, collagen vascular diseases, metabolic disorders or systemic inflammatory diseases may be associated with small bowel mucosal damage. In this study we aimed to investigate if any particular condition or a higher comorbidity burden as a whole were responsible for P1 lesions found on SBCE. We found that neither any of the 22 conditions contemplated in the Charlson Comorbidity Index nor the mean Charlson Comorbidity Index value were significantly more common in the case group. Many drugs like NSAID, corticosteroids, digoxin, ferrous salts, immunosuppressors, or enteric-coated potassium have been also linked to small bowel lesions. From the analyzed medication, we found that only NSAID chronic use was significantly more common in patients with P1 lesions and IDA, being associated with a 12 fold increased risk. In fact, it has been reported that up to 75% of NSAID users will have some degree of small bowel mucosal damage, which can lead to anemia or protein loss[20,21]. The proposed mechanism is an increased mucosal permeability induced by NSAID which may lead to inflammation that manifests subsequently as reddened, edematous folds, and areas of denuded villi, petechiae, erosions and ulcers[22]. We had also particular interest in assess the potential association between the use of PPI and P1 lesions. While the gastroprotective effect of PPI is well established, being mediated not only by their anti-secretory properties, but also by inhibition of neutrophil functions and antioxidant actions[23], the effect of PPI in small bowel mucosa is still poorly understood. Some authors advocate that PPI may not be able to repair NSAID-induced small bowel mucosal damage in part because of significant lesion in mechanical barrier function and reduction in epidermal growth factor[24]. Others argue that PPI therapy may even worsen the NSAID-induced injury due to their ability to affect enteric microbial populations[25]. We found no relation between the use of PPI and P1 lesions.
We aimed to characterize the natural history of patients with IDA and P1 lesions on SBCE. According to our results, the mean follow-up time did not differ significantly between cases and controls, suggesting that the presence of P1 lesions on SBCE do not influence the decision to keep the patients under regular surveillance. Regarding IDA recurrence, we found that although no significant differences were found between groups it occurred in about a fifth of patients with P1 lesions and in about a third of patients with P0 lesions or negative SBCE, which is conformable with our previously published results[26]. In fact, it is now known that patients with a negative SBCE (that includes in most studies patients with P1 and P0 lesions) may still be at risk of rebleeding, which can be as high as 50%[27]. Until definitive guidelines about this topic are published, the approach to patients without P2 lesions on SBCE is left to case-by-case decision, but generally relies on one of two strategies: submitting patients to more examinations either to further explore potentially bleeding causes in the GI tract or alternative causes for IDA, or instead assume a “wait and see” policy. In our series, despite a final diagnosis was reached only in a minority of patients who were further studied, the causative lesion for IDA was within reach of conventional upper and lower endoscopic examinations in all of them, a possibility that have been well established in other studies[28,29]. We verified that the number of patients submitted to further diagnostic testing during the follow-up was significantly higher when P0 lesions or no lesions were found on small bowel CE, which means that P1 lesions were often interpreted as lesions that could justify patient’s anemia, not requiring further investigation.
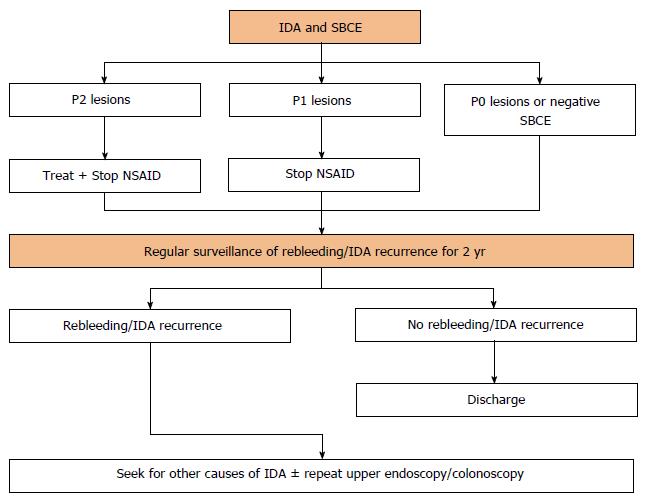
As it was our intention, and according to the presented results, we propose in Figure 2 an algorithm with a stepwise approach to the patients with IDA who are submitted to SBCE. Upon the performance of SBCE for the study of IDA, patients should be classified in one of the 3 groups according to the SBCE findings. On one hand, patients with P2 lesions should receive medical, endoscopic or surgical therapy directed to the specific finding. Additionally, these patients should discontinue NSAID and have their indications for antiplatelets and anticoagulants reassessed as they may be associated with continuous mucosal damage and rebleeding. On the other hand, patients with P1 lesions should be encouraged to stop NSAID use as they are associated with a 12 fold increased risk of small bowel mucosal damage, potentially leading to IDA and protein loss. Independently of the SBCE findings, and as the risk of IDA recurrence is not negligible even in patients with P0 lesions or a negative small bowel CE, patients should receive regular surveillance for rebleeding or IDA recurrence during 2 years without advancing immediately to further investigations. The definition of a 2-year surveillance period is somehow arbitrary, but is in accordance with the current evidence which suggests that most patients will rebleed within the first 2 years after the index event[26]. If rebleeding or IDA recurrence do not occur during the proposed 2-year period, patients may be discharged from the Gastroenterology Clinic. However, if the patient rebleeds or presents recurrent IDA anytime during the follow-up interval, further investigation is suggested. Investigation of alternative causes of IDA such as malnutrition, haematological diseases, chronic liver or renal disease should be thoroughly seek. At this point, if GI bleeding is still strongly suspected, repeat conventional upper endoscopy and colonoscopy may prove valuable as they are cheaper, safer, readily available, and can identify a definitive diagnosis, if not in all, in the great majority of patients. Only when upper endoscopy and colonoscopy are negative, should patients be submitted to other diagnostic modalities such as device-assisted enteroscopy, computed tomography enterography or magnetic resonance enterography.
In conclusion, P1 lesions are commonly found in the SBCE of patients with IDA, particularly after the fifth decade of life. Their presence is associated with NSAID use and may in some cases represent a subtle form of NSAID enteropathy. The outcomes of patients with P1 lesions do not differ significantly from those with P0 lesions or normal SBCE, but keeping these patients under regular surveillance for at least 2 years seems to be a prudent approach. Although immediate further investigation after SBCE may not be warranted for all patients, at least those with recurrent IDA should be investigated for alternative non-gastrointestinal causes of IDA, as P1 lesions in the small bowel seem to have little or no clinical relevance.
Small bowel capsule endoscopy (SBCE) remains a crucial diagnostic instrument for the study of iron-deficiency anemia (IDA). Despite its high diagnostic yield, the clinical relevance and bleeding potential of findings such as red spots or isolated mucosal erosions (P1 lesions) remain uncertain.
The present study was designed to better characterize patients with P1 lesions, namely their natural history, to identify risk factors for their presence in the small bowel of patients examined by SBCE, and to propose a stepwise approach to these patients.
P1 lesions are commonly found in the SBCE of patients with IDA, particularly after the fifth decade of life. Their presence is associated with non-steroidal anti-inflammatory drugs (NSAIDs) use and may in some cases represent a subtle form of NSAID enteropathy. The outcomes of patients with P1 lesions do not differ significantly from those with P0 lesions or normal SBCE. Although immediate further investigation after SBCE may not be warranted for all patients, at least those with recurrent IDA should be investigated for alternative non-gastrointestinal causes of IDA, as P1 lesions in the small bowel seem to have little or no clinical relevance.
An algorithm with a stepwise approach to the patients with IDA who are submitted to SBCE is proposed.
SBCE findings can be classified in 3 groups of lesions according to their bleeding potential: P0 lesions included visible submucosal veins, diverticula without the presence of blood, or nodules without mucosal breaks, which are believed to have no bleeding potential; P1 lesions, such as red spots on the intestinal mucosa or small or isolated erosions, were regarded as having uncertain hemorrhagic potential; and P2 lesions are those considered to have a high potential for bleeding, and include angioectasia, ulcers, tumours or varices.
All to many times researchers are faced with the challenge of how to interpret the significance of a red spot seen on wireless capsule endoscopy but there is a dearth of literature on advising the risk of a rebleeding or how to properly manage the patient. When controlling for the Charleston index, the use of NSIADs appeared to be associated with a higher risk of P1 lesions translating to a 12 fold increased risk. Interestingly, P1 lesions did not have a higher risk of rebleeding whereas P2 lesions have a 36.8% rate. Moreover, from a management perspective, this study enlightens our awareness regarding NSAID use and its relationship to possible rebleeding risk. The algorithm was by the authors also presented very interesting.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Portugal
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Mullin GE, Lakatos PL S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Goddard AF, James MW, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 486] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 2. | Pennazio M, Spada C, Eliakim R, Keuchel M, May A, Mulder CJ, Rondonotti E, Adler SN, Albert J, Baltes P. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2015;47:352-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 560] [Article Influence: 56.0] [Reference Citation Analysis (1)] |
| 3. | Raju GS, Gerson L, Das A, Lewis B. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology. 2007;133:1697-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 4. | Koulaouzidis A, Rondonotti E, Giannakou A, Plevris JN. Diagnostic yield of small-bowel capsule endoscopy in patients with iron-deficiency anemia: a systematic review. Gastrointest Endosc. 2012;76:983-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Saurin JC, Delvaux M, Gaudin JL, Fassler I, Villarejo J, Vahedi K, Bitoun A, Canard JM, Souquet JC, Ponchon T. Diagnostic value of endoscopic capsule in patients with obscure digestive bleeding: blinded comparison with video push-enteroscopy. Endoscopy. 2003;35:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 315] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 6. | Sakai E, Endo H, Taniguchi L, Hata Y, Ezuka A, Nagase H, Yamada E, Ohkubo H, Higurashi T, Sekino Y. Factors predicting the presence of small bowel lesions in patients with obscure gastrointestinal bleeding. Dig Endosc. 2013;25:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Ribeiro I, Pinho R, Rodrigues A, Marqués J, Fernandes C, Carvalho J. Obscure gastrointestinal bleeding: Which factors are associated with positive capsule endoscopy findings? Rev Esp Enferm Dig. 2015;107:334-339. [PubMed] |
| 8. | Guyatt GH, Oxman AD, Ali M, Willan A, McIlroy W, Patterson C. Laboratory diagnosis of iron-deficiency anemia: an overview. J Gen Intern Med. 1992;7:145-153. [PubMed] |
| 9. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [PubMed] |
| 10. | Rosa BJ, Barbosa M, Magalhães J, Rebelo A, Moreira MJ, Cotter J. Oral purgative and simethicone before small bowel capsule endoscopy. World J Gastrointest Endosc. 2013;5:67-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 46] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Cotter J, de Castro FD, Magalhães J, Moreira MJ, Rosa B. Finding the solution for incomplete small bowel capsule endoscopy. World J Gastrointest Endosc. 2013;5:595-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Fisher L, Lee Krinsky M, Anderson MA, Appalaneni V, Banerjee S, Ben-Menachem T, Cash BD, Decker GA, Fanelli RD, Friis C. The role of endoscopy in the management of obscure GI bleeding. Gastrointest Endosc. 2010;72:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 13. | Sidhu PS, McAlindon ME, Drew K, Sidhu R. The Utility of Capsule Endoscopy in Patients under 50 Years of Age with Recurrent Iron Deficiency Anaemia: Is the Juice Worth the Squeeze? Gastroenterol Res Pract. 2015;2015:948574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Muhammad A, Pitchumoni CS. Evaluation of iron deficiency anemia in older adults: the role of wireless capsule endoscopy. J Clin Gastroenterol. 2009;43:627-631. [PubMed] |
| 15. | Muhammad A, Vidyarthi G, Brady P. Role of small bowel capsule endoscopy in the diagnosis and management of iron deficiency anemia in elderly: a comprehensive review of the current literature. World J Gastroenterol. 2014;20:8416-8423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Koh SJ, Im JP, Kim JW, Kim BG, Lee KL, Kim SG, Kim JS, Jung HC. Long-term outcome in patients with obscure gastrointestinal bleeding after negative capsule endoscopy. World J Gastroenterol. 2013;19:1632-1638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Cúrdia Gonçalves T, Magalhães J, Boal Carvalho P, Moreira MJ, Rosa B, Cotter J. Is it possible to predict the presence of intestinal angioectasias? Diagn Ther Endosc. 2014;2014:461602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Boal Carvalho P, Rosa B, Moreira MJ, Cotter J. New evidence on the impact of antithrombotics in patients submitted to small bowel capsule endoscopy for the evaluation of obscure gastrointestinal bleeding. Gastroenterol Res Pract. 2014;2014:709217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Patel KV. Epidemiology of anemia in older adults. Semin Hematol. 2008;45:210-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 229] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 20. | Graham DY, Opekun AR, Willingham FF, Qureshi WA. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55-59. [PubMed] |
| 21. | Caunedo-Alvarez A, Gómez-Rodríguez BJ, Romero-Vázquez J, Argüelles-Arias F, Romero-Castro R, García-Montes JM, Pellicer-Bautista FJ, Herrerías-Gutiérrez JM. Macroscopic small bowel mucosal injury caused by chronic nonsteroidal anti-inflammatory drugs (NSAID) use as assessed by capsule endoscopy. Rev Esp Enferm Dig. 2010;102:80-85. [PubMed] |
| 22. | Maiden L, Thjodleifsson B, Theodors A, Gonzalez J, Bjarnason I. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172-1178. [PubMed] |
| 23. | Higuchi K, Yoda Y, Amagase K, Kato S, Tokioka S, Murano M, Takeuchi K, Umegaki E. Prevention of NSAID-Induced Small Intestinal Mucosal Injury: Prophylactic Potential of Lansoprazole. J Clin Biochem Nutr. 2009;45:125-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Zhang S, Chao GQ, Lu B. Proton pump inhibitors are not the key for therapying non-steroidal anti-inflammatory drugs-induced small intestinal injury. Rheumatol Int. 2013;33:2513-2521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Wallace JL, Syer S, Denou E, de Palma G, Vong L, McKnight W, Jury J, Bolla M, Bercik P, Collins SM. Gastroenterology. 2011;141:1314-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 342] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 26. | Cúrdia Gonçalves T, Dias de Castro F, Moreira MJ, Rosa B, Cotter J. Small bowel capsule endoscopy in obscure gastrointestinal bleeding: normalcy is not reassuring. Eur J Gastroenterol Hepatol. 2014;26:927-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Endo H, Matsuhashi N, Inamori M, Akimoto K, Ohya T, Yanagawa T, Asayama M, Hisatomi K, Teratani T, Fujita K. Rebleeding rate after interventional therapy directed by capsule endoscopy in patients with obscure gastrointestinal bleeding. BMC Gastroenterol. 2008;8:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Vlachogiannakos J, Papaxoinis K, Viazis N, Kegioglou A, Binas I, Karamanolis D, Ladas SD. Bleeding lesions within reach of conventional endoscopy in capsule endoscopy examinations for obscure gastrointestinal bleeding: is repeating endoscopy economically feasible? Dig Dis Sci. 2011;56:1763-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Gilbert D, O’Malley S, Selby W. Are repeat upper gastrointestinal endoscopy and colonoscopy necessary within six months of capsule endoscopy in patients with obscure gastrointestinal bleeding? J Gastroenterol Hepatol. 2008;23:1806-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |