Published online Oct 7, 2016. doi: 10.3748/wjg.v22.i37.8389
Peer-review started: June 2, 2016
First decision: July 12, 2016
Revised: July 27, 2016
Accepted: August 23, 2016
Article in press: August 23, 2016
Published online: October 7, 2016
Processing time: 120 Days and 20.9 Hours
To investigate the expression and prognostic role of programmed death ligand-1 (PD-L1) in locally advanced esophageal squamous cell carcinoma (ESCC).
A total of 200 patients with ESCC who underwent radical esophagectomy with standard lymphadenectomy as the initial definitive treatment in Seoul National University Hospital from December 2000 to April 2013 were eligible for this analysis. Tissue microarrays were constructed by collecting tissue cores from surgical specimens, and immunostained with antibodies directed against PD-L1, p16, and c-Met. Medical records were reviewed retrospectively to assess clinical outcomes. Patients were divided into two groups by PD-L1 status, and significant differences in clinicopathologic characteristics between the two groups were assessed.
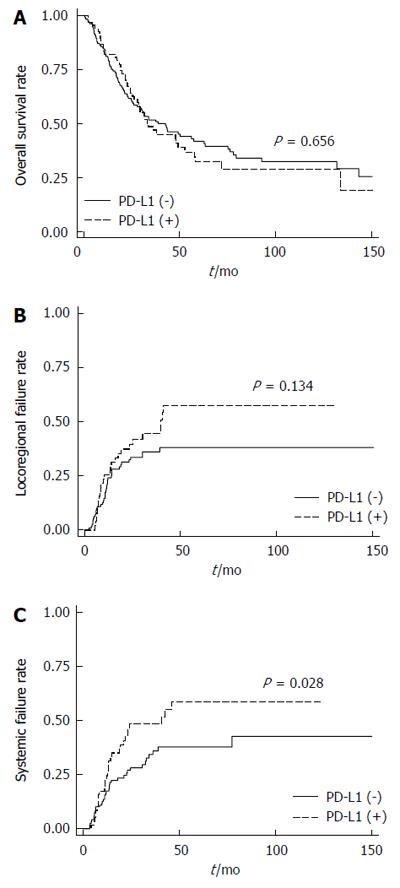
Tumor tissues from 67 ESCC patients (33.5%) were PD-L1-positive. Positive p16 expression was observed in 21 specimens (10.5%). The H-score for c-Met expression was ≥ 50 in 42 specimens (21.0%). Although PD-L1-positivity was not significantly correlated with any clinical characteristics including age, sex, smoking/alcoholic history, stage, or differentiation, H-scores for c-Met expression were significantly associated with PD-L1-positivity (OR = 2.34, 95%CI: 1.16-4.72, P = 0.017). PD-L1 expression was not significantly associated with a change in overall survival (P = 0.656). In contrast, the locoregional relapse rate tended to increase (P = 0.134), and the distant metastasis rate was significantly increased (HR = 1.72, 95%CI: 1.01-2.79, P = 0.028) in patients with PD-L1-positive ESCC compared to those with PD-L1-negative ESCC.
PD-L1 expression is positively correlated with c-Met expression in ESCC. PD-L1 may play a critical role in distant failure and progression of ESCC.
Core tip: The clinical significance of expression of programmed death ligand-1 (PD-L1) in esophageal squamous cell carcinoma (ESCC) has not yet been fully established. We analyzed tissue microarrays of surgical specimen of 200 ESCC patients by immunohistochemistry with antibodies directed against PD-L1, p16, and c-Met. Our results suggest that tumors from approximately one-third of the ESCC patients are positive for PD-L1 expression, and PD-L1 expression is positively correlated with c-Met expression. Although PD-L1 positivity was not found to be associated with survival of ESCC patients, we show that it may play a critical role in distant failure and progression of ESCC.
- Citation: Kim R, Keam B, Kwon D, Ock CY, Kim M, Kim TM, Kim HJ, Jeon YK, Park IK, Kang CH, Kim DW, Kim YT, Heo DS. Programmed death ligand-1 expression and its prognostic role in esophageal squamous cell carcinoma. World J Gastroenterol 2016; 22(37): 8389-8397
- URL: https://www.wjgnet.com/1007-9327/full/v22/i37/8389.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i37.8389
Esophageal cancer is one of the most common gastrointestinal malignancies and is highly prevalent in Asia. Esophageal squamous cell carcinoma (ESCC) represents the most common histological type of esophageal cancer, accounting for more than 90% of all cases in Asian patients[1]. Although the 5-year survival rate for patients with ESCC has improved in recent decades, the prognosis remains unfavorable due to the invasive nature of the disease and the frequency of late diagnosis[2].
An improved understanding of immunobiology has uncovered the interaction between programmed death ligand-1 (PD-L1) and programmed death 1 (PD-1) as one of mechanisms by which cancer cells evade immune surveillance. PD-1 is a negative co-stimulatory factor that inhibits T cell activation when activated by PD-L1 or one of its other ligands[3,4], PD-L1 is a cell surface glycoprotein that belongs to the B7 family and is expressed not only on normal cells, such as T cells, B cells, monocytes, macrophages, and dendritic cells, but also on cancer cells[4-7]. The PD-L1/PD-1 interaction has been found to be associated with poor prognosis and clinical outcomes in various cancers such as non-small cell lung cancer, breast cancer, gastric cancer, soft tissue sarcomas and meningioma[8]; however, its prognostic value is still controversial. Immune checkpoint-blocking agents directed at this interaction have been clinically successful and have been shown to produce a durable clinical response in esophageal cancer patients[9].
Considering the clinical importance of PD-L1, there is great interest in understanding the mechanisms that regulate its expression. PD-L1 upregulation has been reported in human papilloma virus (HPV)-associated malignancies, including uterine, cervical, and head and neck cancers[10-12]. PD-L1 expression may be associated with HPV infection, which represents one of the potential causes of ESCC[13]. Furthermore, c-Met, a receptor tyrosine kinase that is aberrantly activated in numerous human cancers[14], has been shown to promote PD-L1 overexpression in renal cell and pulmonary squamous cell carcinoma[15,16]. However, a comprehensive analysis of the correlation between PD-L1 and c-Met expression in ESCC has not yet been reported.
In this study, we aimed to investigate the expression of PD-L1 in ESCC and explore the correlation between PD-L1 expression and c-Met, as well as p16, a surrogate marker for HPV infection[17]. Additionally, we evaluated the potential for a prognostic role for PD-L1, p16, and c-Met expression in ESCC.
Patients with ESCC who underwent radical esophagectomy with standard lymphadenectomy (two-field or three-field lymphadenectomy) as the initial definitive treatment in Seoul National University Hospital from December 2000 to April 2013 were eligible for this retrospective analysis. All tumor tissues were confirmed to be ESCC through hematoxylin and eosin staining after surgical resection. Tissue microarrays (TMAs; 2 mm in diameter) were constructed by collecting tissue cores from representative intratumoral areas from surgical specimens. The pathologic tumor-node-metastasis (TNM) stage was characterized according to the 7th American Joint Committee on Cancer Guidelines[18].
Treatment decisions were made via a multidisciplinary team-based approach. All patients without clinical evidence of metastatic disease were treated with radial esophagectomy. The type of esophageal resection was dictated by the size, stage, and location of the primary tumor. According to the clinician’s judgment, selected patients were offered neoadjuvant or adjuvant chemotherapy with or without radiation therapy. The chemotherapy regimen was fluoropyrimidine-/taxene-based and was selected based on the performance statuses, comorbidities, and toxicity profiles of individual patients.
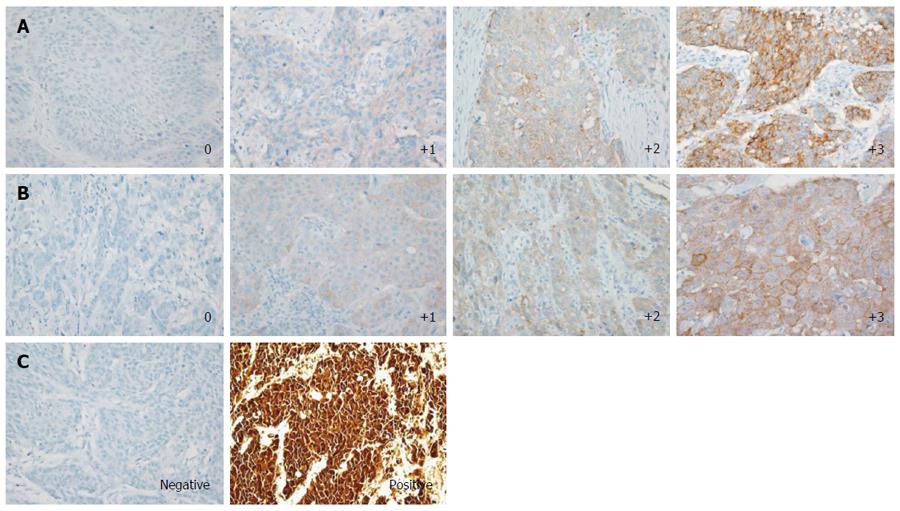
Immunohistochemistry (IHC) was performed using the Benchmark XT automated staining system (Ventana Medical Systems, Tucson, AZ, United States) to estimate the expression of PD-L1, p16, and c-Met. A rabbit anti-PD-L1 (E1L3N) XP® monoclonal antibody (mAb) (Cell Signaling Technology, Danvers, MA, United States), a mouse anti-p16 (E6H4) mAb (Ventana), and a rabbit anti-c-Met (SP44) mAb (Ventana) were used for staining. PD-L1 IHC was evaluated based on the intensity and proportion of membranous staining and/or cytoplasmic staining in tumor cells and was scored as 0 (no or any staining less than 10% of cells), 1+ (weak), 2+ (moderate), or 3+ (strong staining in more than 10% of tumor cells) (Figure 1A). Cases with scores of 1+, 2+, or 3+ were considered to be PD-L1-positive. c-Met expression was analyzed by the membranous and/or cytoplasmic staining pattern and the positivity was evaluated by H-score. The c-Met staining intensity was scored as 0 (none or staining in less than 10% tumor cells), 1 (weak), 2 (moderate), or 3 (strong) based on membranous and/or cytoplasmic staining as previously reported[19,20] (Figure 1B), and each score multiplied by the percentage of cells (0%-100%). Therefore, H-score was ranged from 0 to 300. The median value 50 of c-Met H-score among samples with positive c-Met IHC staining was arbitrarily defined as the cutoff value for c-Met positivity. Samples were considered positive for p16 staining if diffuse strong nuclear and cytoplasmic immunostaining was observed in more than 50% of the tumor cells (Figure 1C)[21]. All slides were blinded with respect to clinical characteristics and outcome and were reviewed and scored by two experienced pathologists (Kwon D and Jeon YK).
The primary objectives of this study were to evaluate PD-L1 expression in ESCC and to evaluate the correlations between PD-L1 expression and other clinicopathologic features, including p16 and c-Met expression. The secondary objective was to assess the prognostic value of PD-L1, p16, and c-Met expression for overall survival (OS) and progression-free survival (PFS). OS was defined as the time from the date of diagnosis until either death due to any cause or the last follow-up date. PFS was defined as the time from the first day of definitive treatment until locoregional/distant relapse or progression, death, or last follow-up. Locoregional relapse refers to regional lymph node metastasis or tumor recurrence at the primary site. OS of patients who received palliative chemotherapy and/or radiotherapy (OSpall) was measured from the date of relapse or surgery (if R0 resection was not achieved) until either death due to any cause or the last follow-up date.
Patients were divided into two groups by PD-L1 status. Significant differences in clinicopathologic characteristics between the two groups were assessed using the Mann-Whitney test for continuous variables and the χ2 (or Fisher’s exact test, if appropriate) for categorical variables. Significant correlations between clinicopatholoic factors and PD-L1-positivity were assessed by logistic regression analysis. Survival analyses were performed using the Kaplan-Meier method and were compared using a log-rank test. Univariate and multivariate analyses using the Cox proportional hazard regression model were applied to determine the hazard ratio (HR) for specific variables with respect to OS and PFS. For all statistical analyses, two-sided P values < 0.05 were considered statistically significant. All statistical analyses were carried out using STATA version 12 (StataCorp LP, College Station, TX, United States).
A total of 200 ESCC patients were included in our analysis. The clinicopathologic characteristics of the patients are summarized in Table 1. Most of the patients (94.0%) were males who ranged in age from 41 to 83 years (median age, 65 years). A majority of the patients were ex/current-smokers (84.9%) or alcohol drinkers (84.3%). All patients underwent radical esophagectomy as an initial definitive treatment, and R0 resection was achieved in 176 patients (88.0%). Twenty patients (10.0%) received neoadjuvant chemotherapy prior to surgery, and 64 patients (32.0%) received adjuvant chemotherapy.
| Characteristics | Total(n = 200) | PD-L1 status | P value | |
| Negative(n = 133) | Positive(n = 67) | |||
| Age, median years (range) | 65 (41-83) | 65 (50-83) | 64 (41-82) | 0.5191 |
| Sex | ||||
| Male | 188 (94.0) | 125 (94.0) | 63 (94.0) | |
| Female | 12 (6.0) | 8 (6.0) | 4 (6.0) | 1.000 |
| Smoking history | 168 (84.9) | 110 (84.0) | 58 (86.6) | 0.630 |
| Alcoholic intake | 166 (84.3) | 110 (84.0) | 56 (84.9) | 0.873 |
| Stage | ||||
| I | 66 (33.0) | 47 (35.3) | 19 (28.4) | |
| II | 59 (29.5) | 41 (30.8) | 18 (26.9) | |
| III | 71 (35.5) | 44 (33.1) | 27 (40.3) | |
| IV | 4 (2.0) | 1 (0.8) | 3 (4.5) | 0.200 |
| Differentiation | ||||
| W/D | 41 (23.0) | 36 (27.1) | 10 (14.9) | |
| M/D | 131 (65.5) | 83 (62.4) | 48 (71.6) | |
| P/D | 23 (11.5) | 14 (10.5) | 9 (13.4) | 0.152 |
| Treatment | ||||
| Surgery alone | 122 (61.0) | 83 (62.5) | 39 (58.2) | |
| Surgery → Adj. | 58 (29.0) | 38 (28.6) | 20 (29.9) | |
| Neoadj. → Surgery | 14 (7.0) | 9 (6.8) | 5 (7.5) | |
| Neoadj. → Surgery → Adj. | 6 (3.0) | 3 (2.3) | 3 (4.5) | 0.927 |
| Surgery results | ||||
| R0 resection | 176 (88.0) | 121 (91.0) | 55 (82.1) | |
| R1, R2 resection | 24 (12.0) | 12 (9.0) | 12 (17.9) | 0.068 |
| p16 | ||||
| Negative | 179 (89.5) | 121 (91.0) | 58 (86.6) | |
| Positive | 21 (10.5) | 12 (9.0) | 9 (13.4) | 0.616 |
| H-score | ||||
| < 50 | 158 (79.0) | 112 (84.2) | 46 (68.7) | |
| ≥ 50, < 100 | 31 (15.5) | 16 (12.0) | 15 (22.4) | |
| ≥ 100, < 200 | 11 (5.5) | 5 (3.8) | 6 (9.0) | 0.036 |
| Follow-up duration, median months (range) | 33.2 (0.6-178.7) | 33.9 (0.6-176.7) | 31.7 (2.3-178.7) | 0.7901 |
IHC was performed to assess PD-L1, p16, and c-Met expression in surgical specimens collected from a total of 200 ESCC patients (Table 1). Tumor tissues from 67 patients (33.5%) were PD-L1-positive, and the remaining specimens (133 patients, 66.5%) were PD-L1-negative. PD-L1-positivity was not significantly correlated with any clinical characteristics, including age, sex, smoking/alcoholic history, stage, or differentiation (Table 1). A total of 21 samples were positive for p16 expression (10.5%), 12 of which were PD-L1-negative and 9 of which were PD-L1-positive. The c-Met H-scores were ≥ 50 in 42 of 200 samples (21.0%). Of these cases, 21 were PD-L1-negative, and the remaining 21 were PD-L1-positive.
The factors associated with PD-L1 expression were investigated by univariate and multivariate analyses using a logistic regression model (Table 2). Most clinical characteristics, including age, sex, smoking/alcoholic history, carcinoembryonic antigen (CEA) level, TNM stage and neoadjuvant chemotherapy were not significantly associated with PD-L1 expression. Moderately or poorly differentiated ESCC tended to be PD-L1-positive compared to well-differentiated ESCC in both univariate (P = 0.058) and multivariate analysis (P = 0.080). PD-L1 expression was not significantly associated with p16 expression (P = 0.340), but elevated c-Met expression (H-score ≥ 50) was significantly associated with PD-L1-positivity compared to lower c-Met expression (H-score < 50) (OR = 2.34, 95%CI: 1.16-4.72, P = 0.017 in multivariate analysis).
| Factors | Ref. | OR (95%CI) | P value |
| Univariate analysis | |||
| Age1 | 0.98 (0.94-1.02) | 0.323 | |
| Sex | Male vs Female | 1.01 (0.29-3.48) | 0.99 |
| Smoking | Yes vs No | 1.23 (0.53-2.86) | 0.63 |
| Alcohol | Yes vs No | 1.07 (0.47-2.42) | 0.873 |
| CEA1 | 0.86 (0.66-1.11) | 0.234 | |
| TNM stage | III/IV vs I/II | 1.59 (0.87-2.89) | 0.133 |
| Differentiation | M/D or P/D vs W/D | 2.12 (0.98-4.58) | 0.058 |
| Neoadj. | Yes vs No | 1.37 (0.53-3.53) | 0.517 |
| p16 | Positive vs Negative | 1.56 (0.62-3.92) | 0.34 |
| c-Met H-score | ≥ 50 vs < 50 | 2.43 (1.21-4.88) | 0.012 |
| Multivariate analysis | |||
| Differentiation | M/D or P/D vs W/D | 2.01 (0.92-4.40) | 0.08 |
| c-Met H-score | ≥ 50 vs < 50 | 2.34 (1.16-4.72) | 0.017 |
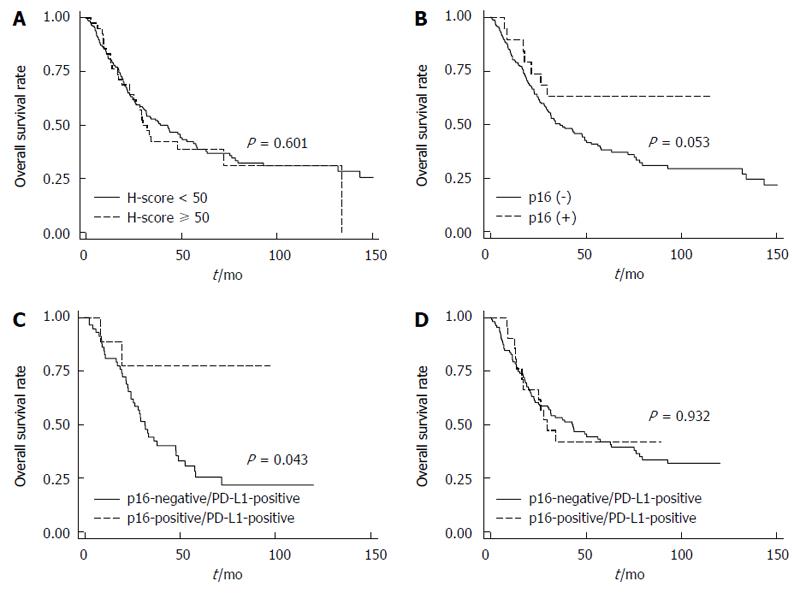
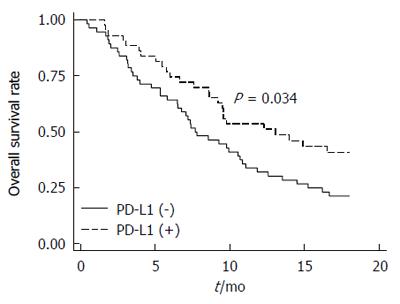
In our cohort of ESCC patients, there was no significant difference in the OS (P = 0.656) according to PD-L1 expression (Figure 2A). Modifying the threshold for PD-L1-positivity by IHC score (e.g., 1+, 2+ or 3+) also did not yield a significant difference (data not shown). However, the locoregional relapse rate tended to increase (P = 0.134; Figure 2B), and the distant metastasis rate was significantly increased in patients with PD-L1-positive ESCC compared to those with PD-L1-negative ESCC (HR = 1.72, 95%CI: 1.06-2.79, P = 0.028; Figure 2C). To investigate the prognostic factors for OS in ESCC, univariate and multivariate Cox regression analyses were carried out (Table 3). There was no significant difference in OS according to c-Met expression (P = 0.601; Figure 3A). However, there was a trend toward improved OS in patients with p16-positive ESCC compared to those with p16-negative ESCC in univariate analysis (HR = 0.49; 95%CI: 0.24-1.01, P = 0.053; Figure 3B) and multivariate analysis (HR = 0.51, 95%CI: 0.25-1.05, P = 0.069). This tendency became statistically significant in PD-L1-positive ESCC patients (HR = 0.23, 95%CI: 0.06-0.96, P = 0.043; Figure 3C), but not in PD-L1-negative ESCC patients (P = 0.932; Figure 3D). Interestingly, the OSpall was significantly better in patients with PD-L1-positive ESCC compared to those with PD-L1-negatvie ESCC (HR = 0.59, 95%CI: 0.36-0.96, P = 0.034; Figure 4).
| Factors | Ref. | HR (95%CI) | P value |
| Univariate analysis | |||
| Age1 | 1.03 (1.00-1.05) | 0.023 | |
| Sex | Male vs Female | 6.29 (1.55-25.4) | 0.010 |
| Smoking | Yes vs No | 1.36 (0.80-2.33) | 0.261 |
| Alcohol | Yes vs No | 1.30 (0.80-2.13) | 0.286 |
| CEA1 | 1.07 (0.95-1.20) | 0.269 | |
| TNM stage | III/IV vs I/II | 2.77 (1.97-3.90) | < 0.001 |
| Differentiation | M/D or P/D vs W/D | 1.23 (0.82-1.85) | 0.308 |
| Neoadj. | Yes vs No | 1.70 (1.04-2.78) | 0.032 |
| Adj. | Yes vs No | 1.73 (1.23-2.45) | 0.002 |
| Operation result | R1/R2 vs R0 | 3.53 (2.25-5.52) | < 0.001 |
| p16 | Positive vs Negative | 0.49 (0.24-1.01) | 0.053 |
| c-Met H-score | ≥ 50 vs < 50 | 1.12 (0.73-1.72) | 0.601 |
| Multivariate analysis | |||
| Age1 | 1.03 (1.01-1.06) | 0.001 | |
| Sex | Male vs Female | 4.31 (1.06-17.6) | 0.042 |
| TNM stage | III/IV vs I/II | 2.52 (1.64-3.87) | < 0.001 |
| Neoadj. | Yes vs No | 1.26 (0.73-2.19) | 0.405 |
| Adj. | Yes vs No | 0.91 (0.58-1.44) | 0.685 |
| Operation result | R1/R2 vs R0 | 2.53 (1.48-4.32) | 0.001 |
| p16 | Positive vs Negative | 0.51 (0.25-1.05) | 0.069 |
In this study, we report that approximately one-third of ESCC cases were positive for expression of PD-L1, and that PD-L1 expression was positively correlated with c-Met-positivity. Although PD-L1-positivity appeared to have no prognostic value for OS, it was associated with increased rate of distant failure. Expression of p16, a marker for HPV infection, was not correlated with the PD-L1 expression.
Because the PD-L1/PD-1 interaction has a negative regulatory function in T cell activation, cancer patients with elevated PD-L1 expression often exhibit a poor prognosis and clinical outcomes[8]. With respect to ESCC, Ohigashi et al[22] analyzed data from 41 patients with ESCC and showed that PD-L1 can serve as a prognostic biomarker for ESCC. Similarly, Chen et al[23] reported that PD-L1 expression was significantly associated with patient survival by analyzing 99 patients with ESCC. In contrast, we found no significant difference in survival of ESCC patients according to PD-L1 expression. The discrepancies among these studies can be partially explained by variations in the antibodies used for detection, as well as differing IHC cut-off definitions for PD-L1-positivity. ESCC specimens with any positive immunohistochemial staining using the rabbit anti-PD-L1 (E1L3N) XP® mAb were considered positive in our analysis. However, Ohigashi et al[22] performed immunostaining with a mouse anti-PD-L1 immunoglobulin G1 mAb (MIH1) and considered specimens with ≥ 10% PD-L1-positive tumor cells to be positive. In contrast, Chen et al[23] used the H-score method to assess PD-L1 immunostaining with a rabbit anti-human PD-L1 mAb (NBP1-03220). In addition to these variations in technique, differences in the multidisciplinary approach to treatment, such as use of palliative chemotherapy, radiotherapy, and supportive care might have also influenced OS. Furthermore, differences in tissue preparation and processing variability could have confounded results regarding the use of PD-L1 IHC as a prognostic marker for ESCC.
c-Met is one of the most important cancer-associated receptor tyrosine kinases and is activated through binding to its specific ligand, HGF[24]. c-Met has been reported to be involved in the development of a number of human primary tumors, such as gastric, breast, colorectal, liver, and rectal cancers[25]. Activation of the receptor enhances oncogenesis through a wide range of mechanisms, including the promotion of tumor cell invasiveness, angiogenesis, and the epithelial-to-mesenchymal transition[24,26]. Based on its many roles in regulation of pro-oncogenic pathways, we hypothesized that c-Met modulates PD-L1 expression in ESCC. Consistent with this hypothesis, c-Met-induced signaling has been reported to lead to PD-L1 overexpression in renal cancer cells[15]. Our analysis demonstrated that c-Met expression is significantly correlated with PD-L1-positivity in ESCC. However, in agreement with a previous report[27], c-Met overexpression had no prognostic value for OS[27]. Thus, the biological impact of the correlation between PD-L1 and c-Met expression should be further investigated in future studies.
HPV infection is one of the risk factors associated with esophageal cancer, oropharyngeal squamous cell carcinoma, and cervical cancer[13,28-30]. Previous reports have shown that HPV-positive tumors exhibit high PD-L1 expression compared to HPV-negative tumors[10-12]. With respect to ESCC, we did not observe any significant correlation between expression of PD-L1 and that of p16, a surrogate marker for HPV infection[17]. However, we did find that p16 expression has potential prognostic value for ESCC. This result was similar to that observed in patients with oropharyngeal squamous cell carcinoma[30,31]. Interestingly, the prognostic value of p16 expression was more significant in patients with PD-L1-positive ESCC.
There are several limitations that must be considered regarding the findings of this study. First, the retrospective design could bias the results. Second, there is no standard IHC threshold definition for PD-L1, p16 and c-Met positivity, and our definition was arbitrary. Therefore, one should be cautious when generalizing the results of our analysis. Third, we used p16 IHC as a surrogate marker instead of detecting HPV DNA to assess HPV infection. Fourth, although we identified a positive correlation between PD-L1 and c-Met expression, the biological meaning of the correlation was not investigated in this study. Therefore, further follow-up studies including external validation are needed. Nevertheless, the present study represents the largest retrospective study to date with sufficient statistical power to assess PD-L1 expression and its prognostic implications in ESCC patients. Furthermore, the correlation between PD-L1 and c-Met expression in ESCC identified in this study is a novel finding.
In conclusion, approximately one-third of the ESCC patient samples analyzed were positive for PD-L1 expression, and PD-L1 expression was positively correlated with c-Met expression. Although PD-L1-positivity was not found to be associated with the prognosis of ESCC patients, it may play a critical role in distant metastasis and progression of ESCC. Because most cancer-related deaths are associated with distant metastasis, our findings provide a rationale for immunotherapy targeting PD-L1 for ESCC.
We thank our database manager Ju Yon Kim for her accurate data management. We would like to thank BioMed Proofreading LLC for English editing.
Recently, an improved understanding of immunobiology has uncovered the interaction between programmed death ligand-1 (PD-L1) and programmed death 1 (PD-1) as one of mechanisms by which cancer cells evade immune surveillance. The prognostic role of this interaction is controversial, and the mechanisms that regulate the expression of PD-L1 are obscure. There has been relatively scarce study examining the clinical meaning of PD-L1/PD-1 interaction in esophageal cancer, which is one of the most common gastrointestinal malignancies and is highly prevalent in Asia.
PD-L1 upregulation has been reported in human papilloma virus-associated malignancies such as esophageal squamous cell carcinoma (ESCC), and c-Met has been shown to promote PD-L1 overexpression in some type of cancers. However, a comprehensive analysis of the correlation between PD-L1 and c-Met expression in ESCC has not yet been reported.
This study revealed that PD-L1 play a critical role in distant failure and progression of ESCC. In addition, this study found that PD-L1 expression is positively correlated with c-Met expression, which is a novel finding.
PD-L1-positivity may play a critical role in distant metastasis and progression of ESCC. Because most cancer-related deaths are associated with distant metastasis, this finding provides a rationale for immunotherapy targeting PD-L1 for ESCC.
PD-1 is a negative co-stimulatory factor that inhibits T cell activation when activated by PD-L1 or one of its other ligands. PD-L1 is a cell surface glycoprotein that belongs to the B7 family and is expressed not only on normal cells, such as T cells, B cells, monocytes, macrophages, and dendritic cells, but also on cancer cells.
The article focuses on the role of PD-L1 and another two markers, the authors are looking for new and different correlations.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ananiev J S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25531] [Article Influence: 1823.6] [Reference Citation Analysis (7)] |
| 2. | Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 997] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 3. | Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2838] [Cited by in RCA: 3533] [Article Influence: 153.6] [Reference Citation Analysis (0)] |
| 4. | Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027-1034. [PubMed] |
| 5. | Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 1958] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 6. | Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2021] [Cited by in RCA: 2238] [Article Influence: 93.3] [Reference Citation Analysis (0)] |
| 7. | Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839-846. [PubMed] |
| 8. | Sui X, Ma J, Han W, Wang X, Fang Y, Li D, Pan H, Zhang L. The anticancer immune response of anti-PD-1/PD-L1 and the genetic determinants of response to anti-PD-1/PD-L1 antibodies in cancer patients. Oncotarget. 2015;6:19393-19404. [PubMed] |
| 9. | Doi T, Piha-Paul SA, Jalal SI, Mai-Dang H, Yuan S, Koshiji M, Csiki I, Bennouna J. Pembrolizumab (MK-3475) for patients (pts) with advanced esophageal carcinoma: Preliminary results from KEYNOTE-028. ASCO Meet Abstr. 2015;33 suppl 15:4010. |
| 10. | Lyford-Pike S, Peng S, Young GD, Taube JM, Westra WH, Akpeng B, Bruno TC, Richmon JD, Wang H, Bishop JA. Evidence for a role of the PD-1: PD-L1 pathway in immune resistance of HPV-associated head and neck squamous cell carcinoma. Cancer Res. 2013;73:1733-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 569] [Cited by in RCA: 608] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 11. | Yang W, Song Y, Lu YL, Sun JZ, Wang HW. Increased expression of programmed death (PD)-1 and its ligand PD-L1 correlates with impaired cell-mediated immunity in high-risk human papillomavirus-related cervical intraepithelial neoplasia. Immunology. 2013;139:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 2013;73:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 492] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 13. | Syrjänen KJ. HPV infections and oesophageal cancer. J Clin Pathol. 2002;55:721-728. [PubMed] |
| 14. | Gherardi E, Birchmeier W, Birchmeier C, Vande Woude G. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12:89-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1057] [Cited by in RCA: 1151] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 15. | Balan M, Mier y Teran E, Waaga-Gasser AM, Gasser M, Choueiri TK, Freeman G, Pal S. Novel roles of c-Met in the survival of renal cancer cells through the regulation of HO-1 and PD-L1 expression. J Biol Chem. 2015;290:8110-8120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Kim MY, Koh J, Kim S, Go H, Jeon YK, Chung DH. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer. 2015;88:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 17. | Shi W, Kato H, Perez-Ordonez B, Pintilie M, Huang S, Hui A, O’Sullivan B, Waldron J, Cummings B, Kim J. Comparative prognostic value of HPV16 E6 mRNA compared with in situ hybridization for human oropharyngeal squamous carcinoma. J Clin Oncol. 2009;27:6213-6221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 18. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6445] [Article Influence: 429.7] [Reference Citation Analysis (0)] |
| 19. | Lee HE, Kim MA, Lee HS, Jung EJ, Yang HK, Lee BL, Bang YJ, Kim WH. MET in gastric carcinomas: comparison between protein expression and gene copy number and impact on clinical outcome. Br J Cancer. 2012;107:325-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Yan B, Lim M, Zhou L, Kuick CH, Leong MY, Yong KJ, Aung L, Salto-Tellez M, Chang KT. Identification of MET genomic amplification, protein expression and alternative splice isoforms in neuroblastomas. J Clin Pathol. 2013;66:985-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Cao F, Han H, Zhang F, Wang B, Ma W, Wang Y, Sun G, Shi M, Ren Y, Cheng Y. HPV infection in esophageal squamous cell carcinoma and its relationship to the prognosis of patients in northern China. ScientificWorldJournal. 2014;2014:804738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947-2953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 638] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 23. | Chen L, Deng H, Lu M, Xu B, Wang Q, Jiang J, Wu C. B7-H1 expression associates with tumor invasion and predicts patient’s survival in human esophageal cancer. Int J Clin Exp Pathol. 2014;7:6015-6023. [PubMed] |
| 24. | Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1992] [Cited by in RCA: 2085] [Article Influence: 99.3] [Reference Citation Analysis (0)] |
| 25. | Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 657] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 26. | Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 421] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 27. | Mesteri I, Schoppmann SF, Preusser M, Birner P. Overexpression of CMET is associated with signal transducer and activator of transcription 3 activation and diminished prognosis in oesophageal adenocarcinoma but not in squamous cell carcinoma. Eur J Cancer. 2014;50:1354-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Naucler P, Chen HC, Persson K, You SL, Hsieh CY, Sun CA, Dillner J, Chen CJ. Seroprevalence of human papillomaviruses and Chlamydia trachomatis and cervical cancer risk: nested case-control study. J Gen Virol. 2007;88:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Chang F, Syrjänen S, Shen Q, Cintorino M, Santopietro R, Tosi P, Syrjänen K. Evaluation of HPV, CMV, HSV and EBV in esophageal squamous cell carcinomas from a high-incidence area of China. Anticancer Res. 2000;20:3935-3940. [PubMed] |
| 30. | Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5387] [Cited by in RCA: 4959] [Article Influence: 330.6] [Reference Citation Analysis (0)] |
| 31. | Fakhry C, Westra WH, Li S, Cmelak A, Ridge JA, Pinto H, Forastiere A, Gillison ML. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2023] [Cited by in RCA: 2091] [Article Influence: 123.0] [Reference Citation Analysis (0)] |