Published online Oct 7, 2016. doi: 10.3748/wjg.v22.i37.8375
Peer-review started: July 12, 2016
First decision: August 8, 2016
Revised: August 18, 2016
Accepted: August 30, 2016
Article in press: August 30, 2016
Published online: October 7, 2016
Processing time: 80 Days and 11.7 Hours
To introduce natural orifice transgastric endoscopic surgery (NOTES) tube ileostomy using pelvis-directed submucosal tunneling endoscopic gastrostomy and endoscopic tube ileostomy.
Six live pigs (three each in the non-survival and survival groups) were used. A double-channeled therapeutic endoscope was introduced perorally into the stomach. A gastrostomy was made using a 2-cm transversal mucosal incision following the creation of a 5-cm longitudinal pelvis-directed submucosal tunnel. The pneumoperitoneum was established via the endoscope. In the initial three operations of the series, a laparoscope was transumbilically inserted for guiding the tunnel direction, intraperitoneal spatial orientation and distal ileum identification. Endoscopic tube ileostomy was conducted by adopting an introducer method and using a Percutaneous Endoscopic Gastrostomy Catheter Kit equipped with the Loop Fixture. The distal tip of the 15 Fr catheter was placed toward the proximal limb of the ileum to optimize intestinal content drainage. Finally, the tunnel entrance of the gastrostomy was closed using nylon endoloops with the aid of a twin grasper. The gross and histopathological integrity of gastrostomy closure and the abdominal wall-ileum stoma tract formation were assessed 1 wk after the operation.
Transgastric endoscopic tube ileostomy was successful in all six pigs, without major bleeding. The mean operating time was 71 min (range: 60-110 min). There were no intraoperative complications or hemodynamic instability. The post-mortem, which was conducted 1-wk postoperatively, showed complete healing of the gastrostomy and adequate stoma tract formation of ileostomy.
Transgastric endoscopic tube ileostomy is technically feasible and reproducible in an animal model, and this technique is worthy of further improvement.
Core tip: A novel technique, natural orifice transgastric endoscopic surgery tube ileostomy, may be successfully performed in a porcine survival model using pelvis-directed submucosal tunneling endoscopic gastrostomy, followed by endoscopic tube ileostomy using an Introducer Kit containing a loop fixture.
- Citation: Shi H, Chen SY, Wang YG, Jiang SJ, Cai HL, Lin K, Xie ZF, Dong FF. Percutaneous transgastric endoscopic tube ileostomy in a porcine survival model. World J Gastroenterol 2016; 22(37): 8375-8381
- URL: https://www.wjgnet.com/1007-9327/full/v22/i37/8375.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i37.8375
Anastomotic leakage is one of the major complications that occurs after anterior resection of the rectum for rectal cancer with a low extraperitoneal colorectal anastomosis[1,2]. With the development of surgery, a defunctioning stoma, colostomy or ileostomy has become a mainstay of proximal fecal diversion to protect against primary colorectal anastomosis, and to reduce the risk and lessen the severity of the sequelae of anastomotic leakage, such as fecal peritonitis and sepsis. However, established conventional defunctioning stomas, including loop colostomy and loop ileostomy, require a second surgical reversal[3], which may cause morbidity and even mortality. Percutaneous tube ileostomy, which is achieved by inserting a tube into the distal ileum through the abdominal wall, has been reported as an alternative that can achieve similar temporary fecal diversion, ensuring greater comfort for the patient and easier management, as well as avoiding the additional surgical procedure required for the closure of a conventional loop-ostomy[4].
Percutaneous tube ileostomy for anastomotic protection is clinically performed by laparotomy or laparoscopy. Natural orifice transluminal endoscopic surgery (NOTES)[5-8] may be an alternative for the execution of the tube ileostomy procedure, as this method is less invasive and is associated with less postoperative pain compared with the two former methods. To date, there is no literature regarding NOTES tube ileostomy. The aim of our study was to demonstrate the feasibility and reproducibility of a novel technique of percutaneous transgastric endoscopic tube ileostomy using an Introducer Kit in a porcine survival model.
Our study was performed on six healthy female domestic pigs weighing between 15 and 20 kg, of which three were enrolled in a non-survival group and three in a 1-wk survival group. All animals were fasted for 24 h prior to surgery. Induction of anesthesia was achieved by intramuscular injection of 100 mg ketamine, 10 mg droperidol and 1 mg atropine, and maintenance of anesthesia was achieved by an intravenous drip of propofol at a dose of 10 mL/h, with endotracheal intubation. The heart rate and oxygen saturation of each animal were monitored during the operation. Animals were maintained in a supine position to allow for optimal access and peritoneal exploration. This study was approved by the Institutional Animal Use and Care Committee of Fujian Provincial Tumor Hospital, Teaching Hospital of Fujian Medical University, Fuzhou, China.
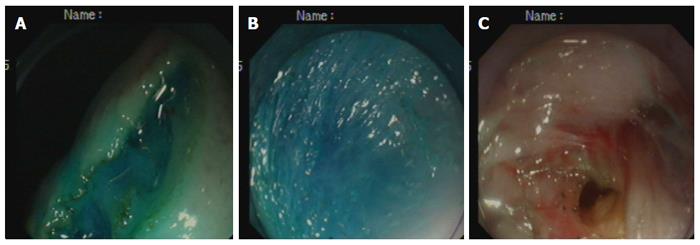
Creation of the gastrostomy by directed submucosal tunneling: A 2-cm transversal mucosal incision was created near the gastroesophageal junction with a dual knife (KD650L; Olympus, Tokyo, Japan), followed by the creation of a 5-cm longitudinal submucosal pelvis-directed tunnel[9,10] (Figure 1A-C). The tunnel ended with a seromuscular incision, and the exit site was selected at the greater curvature of the stomach. A dual-channel therapeutic endoscope (GIF2TQ260M; Olympus) was advanced into the peritoneal cavity through the gastrostomy site. A pelvis-directed tunnel (left lower quadrant abdomen) was considered successful if the exit was in line with the urinary bladder, or the body or left horn of the uterus. The pneumoperitoneum was established via the endoscope. In the initial three operations in the non-survival group, transumbilical laparoscopic visualization was used to guide tunnel direction assessment, intraperitoneal spatial orientation, and subsequent terminal ileum identification.
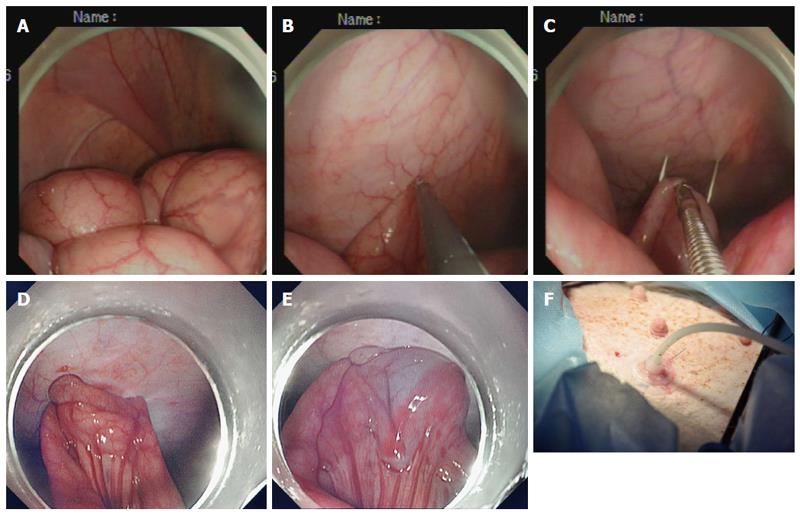
Creation of the ileostomy using the introducer method: A 15 Fr rubber-coated silicone balloon catheter (Cliny PEG Kit; Create Medic, Yokohama, Japan) was used as the tube stoma (Figure 2A-F). The optimal ileostomy site was determined by depressing the left lower abdominal wall with a finger until the tip of the finger was visible intraperitoneally under endoscopic visualization. The pneumoperitoneum was decompressed to bring the distal ileum closer to the parietal peritoneum. After being grasped and held toward the needle, which had penetrated through the anterior abdominal wall, the antimesenteric side of the distal ileum was punctured using a double-lumen loop fixture device under laparoscopic or endoscopic guidance. Two stitches were placed about 2-3 cm apart. An abdominal wall incision was made between the two suture points, and a trocar with a plastic “T” peel-away sheath was introduced into the ileum through the incision site. After removal of the trocar, a 15 Fr catheter was introduced into the plastic sheath. The distal tip of the 15 Fr catheter was placed toward the proximal limb of the ileum to optimize intestinal content drainage. The balloon at the tip of the catheter was inflated with 5 mL sterile water, and the catheter was pulled until the appropriate approximation of the balloon to the abdominal wall was achieved. The peel-away sheath was then removed, and the retaining plate was positioned.
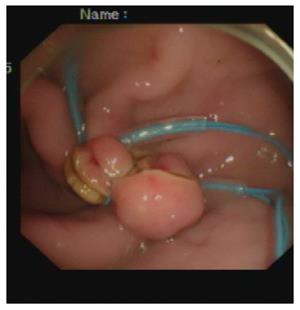
Closure of the gastrostomy: The pneumoperitoneum was evacuated endoscopically, and the dual-channel therapeutic endoscope was withdrawn into the stomach through the gastrostomy site (Figure 3). An endoloop, followed by the twin grasper, was inserted into the gastric cavity through one of the two channels and placed at the gastrostomy side. The gastric tissues adjacent to the mucosal defect were clamped with the twin grasper and dragged into the transparent cap previously mounted on the tip of the endoscope, followed by fully aspirated into the transparent cap, and the bilateral gastric mucosal layers were approximated by tightening the endoloop. If the defect closure was incomplete, more endoloops were used to close the remaining portions. Two or three endoloops were placed to secure the closure.
At 48 h after the procedure, the animals were fed a liquid oral diet for 2 d, followed by a semi-fluid diet for 3 d. Irrigation was performed once daily to keep the tube patent. The animals were monitored for food intake, defecation, infection and other postoperative complications for 1 wk.
Euthanasia was performed immediately in the non-survival group and 1 wk after the procedure in the survival group. Necropsy results, including injury to adjacent organs, vascular bleeding, gross and histopathological evaluation of gastrostomy closure and stoma tract formation of ileostomy, were recorded. Both the healing and formation processes were classified into three phases: inflammatory, proliferative, and remodeling. The inflammatory phase (first) was characterized by accumulation of polymorphonuclear neutrophils and vasodilation; the proliferation phase (second) was characterized by accumulation of macrophages and fibroblasts, angiogenesis, and collagen deposition; and the remodeling phase (third) was characterized by rearrangement of collagen fibers. The microscopic factors of the aforementioned phases were assessed using a semi-quantitative scoring method of 0-3 points, where 0 = absence, 1 = a little, 2 = moderate, and 3 = abundant[11].
The animal protocol was designed to minimize pain or discomfort to the animals. All animals were killed by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection.
Transgastric endoscopic tube ileostomy was successfully performed in all six pigs, without major bleeding. The mean operating time was 71 min (range: 60-110 min). There were no intraoperative complications or hemodynamic instability.
In the non-survival group, the post-mortem showed secure closure of the gastrostomy created by submucosal tunneling and immediate patency of the ileostomy. Neither gastrostomy nor ileostomy leakage were identified on methylene blue evaluation.
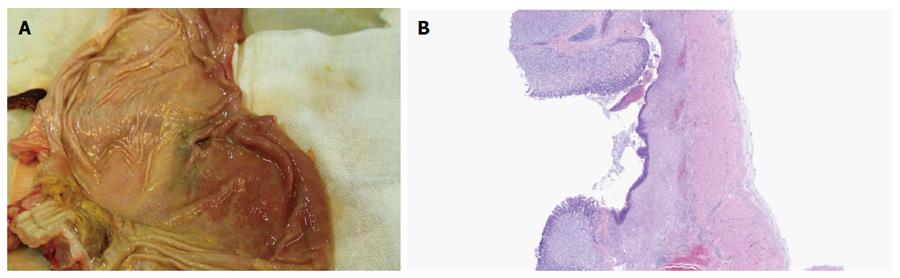
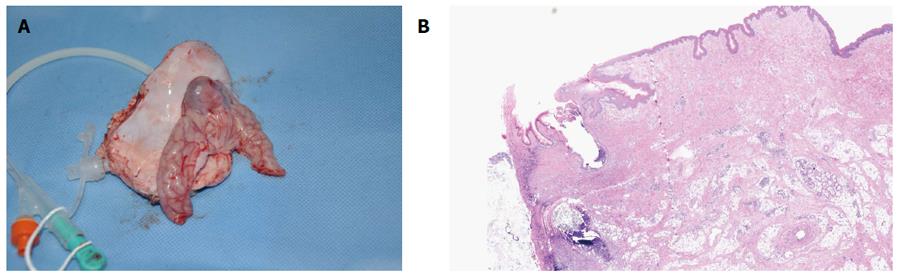
In the survival group, emission of both trans-ileostomy and trans-anal feces were observed. At necropsy, all three gastrostomy sites had completely healed (Figure 4A and B), the tubes were kept patent, and their corresponding ileostomy sites were intact, without evidence of serious infection or significant leakage, as determined by gross assessment. Formation of granulation tissue was observed around the central stoma tract (Figure 5A and B). On postoperative day 7, both the gastrostomy and ileostomy were classified as being in the proliferation phase (Table 1).
| Observation parameters | Pig 1 | Pig 2 | Pig 3 |
| Time required to create gastrostomy (min) | 26 | 21 | 24 |
| Time required to create tube ileostomy (min) | 33 | 31 | 29 |
| Time required to close gastrostomy (min) | 5 | 11 | 7 |
| Complications | |||
| Major bleeding | - | - | - |
| Anastomotic leak | - | - | - |
| Necropsy on postoperative day 7 | |||
| Integrity of gastrostomy closure | The proliferation phase | ||
| Stoma tract formation of the ileostomy | The proliferation phase | ||
To the best of our knowledge, this is the first study addressing NOTES tube ileostomy in a porcine survival model. Safe peritoneal access and secure closure of the access site during NOTES remain significant concerns and must be addressed before its introduction into clinical practice. In our study, the pelvis-directed submucosal tunneling technique was applied to achieve transgastric peritoneal access. Under direct visualization, blood vessels can be clearly identified and precisely coagulated in the submucosal tunnel. Moreover, compared with a direct full-thickness incision, a seromuscular incision may minimize inadvertent injury because the adjacent organs can be visualized through the thin seromuscular layer of the stomach. Furthermore, a designed submucosal tunnel enables in-line endoscope positioning toward predetermined left lower abdominal target locations, without the need for endoscopic retroflexion[10].
As has been reported, the closure of the gastric mucosal incision site with conventional endoscopic clips was easy and secure[9,10]. In the current study, a novel technique of endoloop ligation[12,13], in combination with the dedicated twin grasper, was introduced to facilitate endoscopic closure of the tunnel entrance to the stomach. The Ovesco twin grasper has two jaws that can open and close separately to approximate defect edges[14,15]. Histopathological examination showed satisfactory healing of the submucosal tunnel, thus proving that gastric closure with endoloops is both simple and reliable.
With regards to using tube ileostomy as a diversion procedure, there has been a re-emergence of interest in the use of tube ileostomy to de-function a distal colorectal anastomosis. A systematic review and pooled analysis of seven studies comparing tube with loop ileostomy showed no significant difference in anastomotic leak rate[1]. Tube ileostomy is an alternate diversion procedure that has few complications and is easy to construct and manage compared to conventional loop ileostomy. This procedure can divert the bowel contents and avoid the need for a second operation and its related complications[16-22].
Clinically, there are two methods for placing the tube. One is the transcecal approach, which after anastomosis, an appendectomy or cecostomy is performed, allowing the introduction of the tube into the cecal cavity and then into the terminal ileum via the ileocecal valve. The other method is to place the tube directly into the terminal ileum. In our study, the introducer method via the distal ileum approach was similar to gastropexy[23-29]. The only difference was that, in our study, the antimesenteric side of the distal ileum was sutured to the lower anterior abdominal wall, while gastropexy was achieved by suturing the anterior gastric wall to the anterior abdominal wall before PEG-catheter insertion. Gastropexy may prevent stomal leakage and subsequent peritonitis secondary to early inadvertent percutaneous gastrostomy dislodgement. Similarly, suturing the ileum to the abdominal wall may reduce the risk of peristomal cellulitis before stoma tract maturation. Adhesion of the ileum to the abdominal wall was achieved by placement of two stitches using a double-lumen loop fixture device around a target abdominal stoma site, which has not been previously reported.
The maturation of the stoma tract is critical to percutaneous gastrostomy, and this principle also applies to percutaneous tube ileostomy. Our study showed that the time required for stoma tract formation was about 1 wk after ileostomy, thus providing a safe timeframe for tube removal. Similar results were obtained in human studies, in which the exact timing of tube removal ranged from 7 d to > 3 wk postoperatively[1,30].
In conclusion, this novel technique for performing tube ileostomy exclusively by NOTES is technically feasible and reproducible in an animal model and is worthy of further improvement.
Established conventional defunctioning stomas, loop colostomy and loop ileostomy have become a mainstay of proximal fecal diversion to protect against primary colorectal anastomosis and to reduce the risk and lessen the severity of the sequelae of anastomotic leakage, such as fecal peritonitis and sepsis. Percutaneous tube ileostomy has been reported as an alternative that can achieve similar temporary fecal diversion, ensuring greater comfort for the patient and easier management, as well as avoiding the additional surgical procedure required for the closure of a conventional loop-ostomy.
Percutaneous tube ileostomy for anastomotic protection is clinically performed by laparotomy or laparoscopy. To date, there is no literature regarding the natural orifice transgastric endoscopic surgery (NOTES) tube ileostomy.
A novel technique of percutaneous transgastric endoscopic tube ileostomy may be successfully performed in a porcine survival model, using pelvis-directed submucosal tunneling endoscopic gastrostomy and endoscopic tube ileostomy using an Introducer Kit containing a loop fixture.
This study demonstrates the potential application of percutaneous transgastric endoscopic tube ileostomy using an Introducer Kit for temporary fecal diversion.
A novel NOTES tube ileostomy is performed using pelvis-directed submucosal tunneling endoscopic gastrostomy, following by endoscopic tube ileostomy using the introducer method.
This is an interesting study about the percutaneous transgastric endoscopic tube ileostomy using an Introducer Kit in a porcine survival model. The study is well designed and the results are good discussed.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Higuchi K, Mulvihill S, Yamaoka Y S- Editor: Gong ZM L- Editor: Kerr C E- Editor: Wang CH
| 1. | Nachiappan S, Datta U, Askari A, Faiz O. Tube ileostomy for faecal diversion in elective distal colorectal anastomosis: a systematic review and pooled analysis. Colorectal Dis. 2015;17:665-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Sheng QS, Hua HJ, Cheng XB, Wang WB, Chen WB, Xu JH, Lin JJ. A Modified Spontaneously Closed Defunctioning Tube Ileostomy After Anterior Resection of the Rectum for Rectal Cancer with a Low Colorectal Anastomosis. Indian J Surg. 2016;78:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Markides GA, Wijetunga IU, Brown SR, Anwar S. Meta-analysis of handsewn versus stapled reversal of loop ileostomy. ANZ J Surg. 2015;85:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Bugiantella W, Rondelli F, Mariani L, Boni M, Ermili F, Avenia N, Mariani E. Temporary percutaneous ileostomy for faecal diversion after intestinal resection for acute abdomen in elderly: how to avoid the conventional loop ileostomy. Int J Surg. 2014;12 Suppl 2:S144-S147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Kantsevoy SV, Armengol-Miro JR. Endoscopic Suturing, an Essential Enabling Technology for New NOTES Interventions. Gastrointest Endosc Clin N Am. 2016;26:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Liu BR, Song JT. Submucosal Tunneling Endoscopic Resection (STER) and Other Novel Applications of Submucosal Tunneling in Humans. Gastrointest Endosc Clin N Am. 2016;26:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Yip HC, Chiu PW. Recent advances in natural orifice transluminal endoscopic surgery†. Eur J Cardiothorac Surg. 2016;49 Suppl 1:i25-i30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Patel N, Seneci C, Yang GZ, Darzi A, Teare J. Flexible platforms for natural orifice transluminal and endoluminal surgery. Endosc Int Open. 2014;2:E117-E123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Teoh AY, Chiu PW, Chan SM, Wong TC, Lau JY, Ng EK. Direct incision versus submucosal tunneling as a method of creating transgastric accesses for natural orifice transluminal endoscopic surgery (NOTES) peritoneoscopy: randomized controlled trial. Dig Endosc. 2013;25:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Pauli EM, Haluck RS, Ionescu AM, Rogers AM, Shope TR, Moyer MT, Biswas A, Mathew A. Directed submucosal tunneling permits in-line endoscope positioning for transgastric natural orifice translumenal endoscopic surgery (NOTES). Surg Endosc. 2010;24:1474-1481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Phillips JD, Kim CS, Fonkalsrud EW, Zeng H, Dindar H. Effects of chronic corticosteroids and vitamin A on the healing of intestinal anastomoses. Am J Surg. 1992;163:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 145] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Zheng Z, Jiao G, Wang T, Chen X, Wang B. Ligation-Assisted Endoscopic Enucleation for the Resection of Gastrointestinal Tumors Originating from the Muscularis Propria: Analysis of Efficacy and Facility. Dig Surg. 2016;33:488-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | von Renteln D, Schmidt A, Vassiliou MC, Rudolph HU, Caca K. Endoscopic full-thickness resection and defect closure in the colon. Gastrointest Endosc. 2010;71:1267-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | von Renteln D, Denzer UW, Schachschal G, Anders M, Groth S, Rösch T. Endoscopic closure of GI fistulae by using an over-the-scope clip (with videos). Gastrointest Endosc. 2010;72:1289-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Guo J, Liu Z, Sun S, Liu X, Wang S, Ge N, Wang G, Qi Y. Endoscopic full-thickness resection with defect closure using an over-the-scope clip for gastric subepithelial tumors originating from the muscularis propria. Surg Endosc. 2015;29:3356-3362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Zhou X, Lin C, Chen W, Lin J, Xu J. Completely diverted tube ileostomy compared with loop ileostomy for protection of low colorectal anastomosis: a pilot study. Colorectal Dis. 2014;16:O327-O331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Rondelli F, Balzarotti R, Bugiantella W, Mariani L, Pugliese R, Mariani E. Temporary percutaneous ileostomy versus conventional loop ileostomy in mechanical extraperitoneal colorectal anastomosis: a retrospective study. Eur J Surg Oncol. 2012;38:1065-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Patil V, Vijayakumar A, Ajitha MB, Kumar L S. Comparison between Tube Ileostomy and Loop Ileostomy as a Diversion Procedure. ISRN Surg. 2012;2012:547523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Danielsen AK, Park J, Jansen JE, Bock D, Skullman S, Wedin A, Marinez AC, Haglind E, Angenete E, Rosenberg J. Early Closure of a Temporary Ileostomy in Patients With Rectal Cancer: A Multicenter Randomized Controlled Trial. Ann Surg. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 20. | Salamone G, Licari L, Agrusa A, Romano G, Cocorullo G, Falco N, Tutino R, Gulotta G. Usefulness of ileostomy defunctioning stoma after anterior resection of rectum on prevention of anastomotic leakage A retrospective analysis. Ann Ital Chir. 2016;87:155-160. [PubMed] |
| 21. | Markides GA, Wijetunga I, McMahon M, Gupta P, Subramanian A, Anwar S. Reversal of loop ileostomy under an Enhanced Recovery Programme - Is the stapled anastomosis technique still better than the handsewn technique? Int J Surg. 2015;23:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Brook AJ, Mansfield SD, Daniels IR, Smart NJ. Incisional hernia following closure of loop ileostomy: The main predictor is the patient, not the surgeon. Surgeon. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Maxwell CI, Hilden K, Glasgow RE, Ollerenshaw J, Carlisle JG, Fang JC. Evaluation of gastropexy and stoma tract maturation using a novel introducer kit for percutaneous gastrostomy in a porcine model. JPEN J Parenter Enteral Nutr. 2011;35:630-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Imaeda H, Nakajima K, Hosoe N, Nakahara M, Zushi S, Kato M, Kashiwagi K, Matsumoto Y, Kimura K, Nakamura R. Percutaneous endoscopic gastrostomy under steady pressure automatically controlled endoscopy: First clinical series. World J Gastrointest Endosc. 2016;8:186-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Adachi Y, Akino K, Mita H, Kikuchi T, Yamashita K, Sasaki Y, Arimura Y, Endo T. Systemic Prophylactic Antibiotics for the Modified Introducer Method for Percutaneous Endoscopic Gastrostomy: A Prospective, Randomized, Double-Blind Study. J Clin Gastroenterol. 2016;50:727-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Fox-Alvarez WA, Case JB, Cooke KL, Garcia-Pereira FL, Buckley GJ, Monnet E, Toskich BB. Temporary percutaneous T-fastener gastropexy and continuous decompressive gastrostomy in dogs with experimentally induced gastric dilatation. Am J Vet Res. 2016;77:771-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Milovanovic L, Kennedy SA, Chrea B, Midia M. Safety and Short-Term Complication Rates Using Single-Puncture T-Fastener Gastropexy. J Vasc Interv Radiol. 2016;27:898-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Al-Jazaeri A, Al-Dekhayel M, Al-Saleh N, Al-Turki A, Al-Dhaheri M, Khan S. Guided Transabdominal U-Stitches Gastropexy: A Simplified Technique for Secure Laparoscopic Gastrostomy Tube Insertion. J Laparoendosc Adv Surg Tech A. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Round S, Popovitch C. Prophylactic Gastropexy Incorporating a Gastrotomy Incision in Dogs: A Retrospective Study of 21 Cases (2011-2013). J Am Anim Hosp Assoc. 2016;52:115-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 30. | Hua H, Xu J, Chen W, Zhou X, Wang J, Sheng Q, Lin J. Defunctioning cannula ileostomy after lower anterior resection of rectal cancer. Dis Colon Rectum. 2014;57:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |