Published online Oct 7, 2016. doi: 10.3748/wjg.v22.i37.8349
Peer-review started: June 24, 2016
First decision: August 8, 2016
Revised: August 18, 2016
Accepted: September 8, 2016
Article in press: September 8, 2016
Published online: October 7, 2016
Processing time: 99 Days and 20.1 Hours
To investigate the roles of the neuropeptides vasoactive intestinal peptide (VIP), substance P (SP), and calcitonin gene-related peptide (CGRP) in chronic gastritis and duodenitis in children.
Biopsy samples from the gastric and duodenal mucosa of 52 patients and 30 control subjects were obtained. Samples were taken for pathological examination, immunohistochemical staining, enzyme activity measurements and quantitative measurements of tissue peptide levels.
We observed differential effects of the disease on peptide levels, which were somewhat different from previously reported changes in chronic gastritis in adults. Specifically, SP was increased and CGRP and VIP were decreased in patients with gastritis. The changes were more prominent at sites where gastritis was severe, but significant changes were also observed in neighboring areas where gastritis was less severe. Furthermore, the degree of changes was correlated with the pathological grade of the disease. The expression of CD10, the enzyme primarily involved in SP hydrolysis, was also decreased in patients with duodenitis.
Based on these findings, we propose that decreased levels of VIP and CGRP and increased levels of SP contribute to pathological changes in gastric mucosa. Hence, new treatments targeting these molecules may have therapeutic and preventive effects.
Core tip: The etiology and pathogenesis of childhood gastritis are not entirely known. The lamina propria of the gastrointestinal tract includes sensory neuropeptides that regulate gastric blood flow, local inflammatory responses and healing processes. Vasoactive intestinal peptide (VIP), substance P (SP), and calcitonin gene-related peptide (CGRP) are such neuropeptides, and their roles in chronic childhood gastritis are not known. In this study, we investigated the changes in neuropeptides in childhood gastritis and duodenitis. Disturbances in the neuropeptide content in gastric mucosa may cause gastritis. On the basis of our findings, we propose that decreased levels of VIP and CGRP and increased levels of SP may contribute to pathological changes in gastric mucosa. New treatments targeting these molecules may have therapeutic and preventive effects.
- Citation: Islek A, Yilmaz A, Elpek GO, Erin N. Childhood chronic gastritis and duodenitis: Role of altered sensory neuromediators. World J Gastroenterol 2016; 22(37): 8349-8360
- URL: https://www.wjgnet.com/1007-9327/full/v22/i37/8349.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i37.8349
Inflammation of the gastric mucosa is an important cause of chronic abdominal pain in children. Neither the gastric mucosa’s protective mechanisms nor the pathogenesis of gastritis have been fully elucidated. Increases in gastric acid secretion and Helicobacter pylori (H. pylori) infections are the most important factors implicated in the pathogenesis of gastritis. Patients may not respond to conventional medical treatments, which suggests the involvement of unknown factors in the pathogenesis of gastritis[1,2].
The gastrointestinal system contains sensory neuropeptides, such as vasoactive intestinal peptide (VIP), substance P (SP), and calcitonin gene-related peptide (CGRP)[3]. SP increases the migration of immune cells and cytokine production; therefore, it has suggested that SP may regulate inflammatory processes and wound healing[4-8]. VIP has anti-inflammatory effects through (1) decreasing the secretion of proinflammatory cytokines (TNFα, IL-6, and IL-12); (2) increasing the secretion of anti-inflammatory cytokines (such as IL-10); and (3) reducing Th1 activation[9,10]. CGRP has a vasodilatory effect, and blocking the CGRP pathway causes an insufficient hyperemic response. Disturbances in neuropeptide content may result in mucosal damage, physiological stress, and mild irritation[10,11].
Neutral endopeptidase (NEP, CD10) is a cell-surface membrane-bound enzyme commonly located in the brush border of the mucosa of the small intestine. NEP has been suggested to function in the growth and differentiation of intestinal epithelial cells. NEP hydrolyzes SP and may terminate its biological activity[12]. To date, no studies have investigated changes in SP, VIP, CGRP, and NEP levels in the gastric mucosa of children. Advances in our knowledge of the underlying mechanisms will help in developing new treatment options.
The primary objective of this study was to investigate the roles of neuronal and non-neuronal peptides in the pathogenesis of chronic gastritis in children. The secondary objective was to investigate the changes in NEP expression and activity in normal and inflamed mucosa.
This study was conducted between May 2012 and May 2013 at the Pediatric Gastroenterology Department of the Akdeniz University Faculty of Medicine. The study protocol was approved by the Ethics Committee at the Faculty of Medicine, Akdeniz University, Antalya, Turkey (protocol number: 14.05.2012/110). All patients and/or their families gave written and verbal consent to participate in the study.
Patients under the age of 18 who presented to the outpatient clinic with abdominal pain and dyspepsia (epigastric pain, discomfort, burning, and fullness, with bloating, early satiety, nausea, and vomiting) lasting longer than 3 mo and who underwent upper gastrointestinal system endoscopy were included in the study group. The control group included individuals who presented with complaints other than dyspepsia, such as weight loss, iron deficiency anemia, and suspected celiac disease, and who underwent upper gastrointestinal endoscopy.
At baseline, patients who were determined to have gastritis ± duodenitis via endoscopic examination were included in the study group, and patients who had normal upper endoscopic examination results were included in the control group. After the histological examination (based on the Sydney classification and activity scores) patients were re-grouped[13]. Patients whose histological examination results were normal or who had Sydney I gastritis were included in the control group. Patients with Sydney II or III gastritis were included in the study group. Patients with and without duodenitis (based on a histological assessment) were analyzed separately via CD10 staining.
Pediatric patients who were scheduled to undergo endoscopy for any reason and whose families gave written and verbal consent to be included in the study; Patients who did not have any contraindications for endoscopic biopsy.
Patients who had chronic diseases, such as malignancy, diabetes mellitus, celiac disease, hemolytic uremic syndrome, immune deficiency, and rheumatic diseases; Patients who were receiving immunosuppressive agents, anticoagulants, chemotherapeutic drugs, or any non-steroidal anti-inflammatory drug; Patients who had received a proton pump inhibitor, H2 blocker or any antibiotics, including H. pylori eradication therapy, within the previous three months.
The same pediatric gastroenterologist (Islek A) performed all endoscopic examinations and biopsy procedures using a Fujinon video endoscope. The region between the oropharynx and the second part of the duodenum was examined during the endoscopic examinations, and endoscopic diagnoses were recorded.
Two biopsy samples were taken from the antrum of all patients and used for the detection of H. pylori using the rapid urease test. In patients with gastritis (based on the endoscopic assessment), two biopsy samples, one from where the gastric lesion was severe (S1) and one from where the gastric lesion was less severe (S2), were obtained from the antrum for pathological examination and immunohistochemical staining. The S2 biopsies were taken from tissue approximately 3-5 cm from S1. In addition, two more gastric biopsy samples from S1 and S2 were taken for quantitative measurements (ELISA) of tissue peptide levels and NEP enzyme activity.
In patients with normal appearing gastric mucosa, two biopsy samples from the antrum (C) were obtained for pathological examination and immunohistochemical staining, and two more gastric biopsy samples from the antrum were taken for quantitative measurements (ELISA) of tissue peptide levels and NEP enzyme activity.
In all patients, three biopsy samples from the second part of duodenum were obtained for histological examination and NEP staining.
Paraffin blocks were prepared from all collected tissue samples, and samples were cut into 4-μm-thick sections. Sections were then incubated at 60 °C for 5 min and stained with a CD10 primary antibody (anti-CD10, Clone: 2A1H5E1; Thermo Fisher Scientific Inc., United Kingdom) using a closed-system automated immunohistochemical staining device (Ventana, Roche, United States) at the Department of Pathology. The Sydney classification system was used to classify the degree of gastritis[13]. Biopsy samples for the ELISA and enzyme activity assays were snap-frozen in liquid nitrogen and kept at -80 °C until the analysis.
We previously established a method to differentially measure the SP levels present in nerve endings and in non-neuronal tissue[14,15]. Briefly, biopsy specimens were cut into small pieces and kept in 1 mL of 2% acetic acid at 95 °C for 10 min. Sequential collections of the supernatant were performed in which the first 10-min extraction included predominately neuronal SP, whereas the second extraction, which was re-incubated in 1 mL of 2% acetic acid at 95 °C for 50 min, primarily yielded non-neuronal SP. Supernatants were dried completely and then reconstituted in 150-300 μL of sample buffer from an SP EIA kit (Cayman Chem., Catalog No. 583751). From each sample, 25 and 50 μL were used for immunoassays, which gave results with a 95% confidence interval. Tissue extractions were also used for quantifying VIP (Bachem-Pennisula Laboratories, cat. No. S1183) and CGRP (Phoenix Pharmaceuticals, cat. No. EK-015-02) via immunoassay. The detection limit for SP was 4-500 pg/mL, and we tested multiple dilutions of the samples to avoid very low and high concentrations because the assay is most sensitive within the 20-80 percentile (approximately 10-250 pg/mL). Under these conditions, both intra- and inter-assay variabilities were < 20%. The half maximal inhibitory concentration (IC50) for VIP measurements was 200 pg/mL, and the same precautions were taken to keep intra- and inter-assay variabilities < 20%.
Dansyl-d-Ala-Gly-p-nitro-Phe-Gly, dansyl-d-Ala-Gly, and phosphoramidon, a specific NEP inhibitor, were purchased from Sigma (St. Louis, MO, United States). Measurement of NEP activity was performed as previously described, with some modifications[16]. Briefly, snap-frozen tissues were weighed and sonicated five times on ice for 15 s in ice-cold 50 mmol/L Tris-HCl (pH 7.4) buffer that included 1% Triton X-100. The homogenates were centrifuged at 10000 ×g for 3 min to remove cellular debris and nuclei and then stored at -80 °C until used.
Samples (10 μL) were pre-incubated with enalapril, an angiotensin-converting enzyme (ACE) inhibitor, to prevent the cleavage of the fluorogenic substrate (N-dansyl-d-Ala-Gly-p-nitro-Phe-Gly) by ACE, in the presence or absence of phosphoramidon. Following this preincubation, the fluorogenic substrate was added, and samples were incubated for an additional 2 h at 37 °C. The final concentrations were 16.5 μmol/L for enalapril, 16.5 μmol/L for phosphoramidon, and 200 μmol/L for the substrate in a reaction volume of 160 μL. After 2 h of incubation, the fluorescence absorbance of the sample was recorded using a BIOTEC FX800 Reader. The amount of product was estimated by measuring its fluorescence intensity at 562 nm, with excitation at 342 nm. Arbitrary fluorescence units for each sample were compared against a standard curve prepared using dansyl-d-Ala-Gly (cleavage product) to determine the NEP-specific activity per mg tissue. To verify the method, tissue samples from freshly frozen kidneys were included as a positive control.
Tissue neprilysin (NEP, CD10) levels were also examined via immunohistochemistry. Immunostaining was performed on formalin-fixed paraffin-embedded sections. Sections from each block were deparaffinized and heated in a microwave oven for 10 min for antigen retrieval. Endogenous peroxidase was blocked by incubating sections in 3% hydrogen peroxide in methanol for 10 min. Each sequential incubation was then followed by a thorough washing of the slides in distilled water and phosphate-buffered saline (0.001%, Sigma). After incubation with a monoclonal CD antibody (Thermo Scientific, Fremont, CA, United States) for 60 min, the sections were allowed to react with a biotinylated secondary antibody for 15 min, followed by an incubation with streptavidin for an additional 15 min. Finally, all slides were treated with DAB for color development and were then counterstained with Mayer’s hematoxylin. Negative controls were created using non-reactive TBS-1% BSA at the same concentration as the primary antibody. Serial sections from an abdominal lymph node from a patient diagnosed with diffuse large-cell lymphoma were used as positive controls for CD10 staining. Staining was evaluated separately in foveolar, glandular, and endocrine cells. The staining patterns were classified as focal or diffuse. The intensity of staining was classified as weak, moderate, or strong. The distribution of staining (cytoplasmic or membranous) was also noted.
Histopathological examinations were performed by the same experienced pathologist (OE). The evaluations were performed according to the Sydney classification system[13]. Grouping (control or study group) was performed according to the chronicity and degree of the activity score (based on mononuclear cell infiltration).
H. pylori infection diagnoses were performed with rapid urease tests and histopathological examinations.
Statistical analysis were performed using SPSS software 18.0 and GraphPad InStat 3 software. Paired or unpaired t-tests were used for comparing parametric values in accordance with a normal distribution, and Pearson’s chi-square tests were used for analyzing descriptive statistics. A P value < 0.05 was considered to be statistically significant.
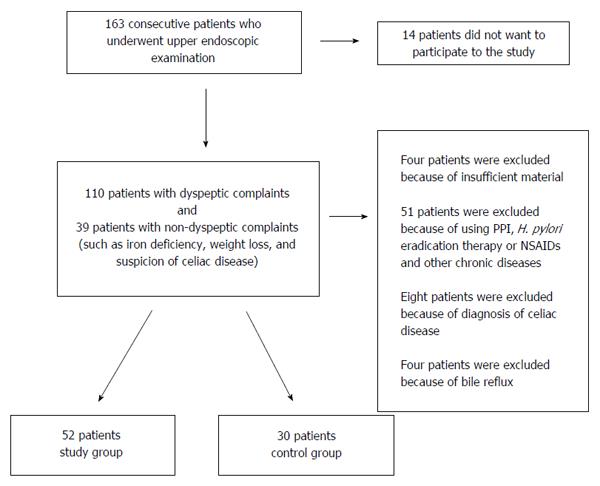
Of the 163 consecutive patients who underwent upper endoscopic examination, 14 were excluded because of their refusal to participate in the study, 8 because of a diagnosis of celiac disease, four because of insufficient samples, and 51 because of the previous use of proton pump inhibitor, H. pylori eradication therapy or NSAIDs and the presence of other chronic diseases. In addition, four patients who had bile reflux during the endoscopic examination were excluded from the study because of the insufficient number of patients for a statistical analysis. After the histological examination, the study group comprised 52 patients who had grade II or III chronic gastritis, and the control group, who had grade I chronic gastritis or histologically normal gastric mucosal samples, comprised 30 patients. Histologically defined gastritis was present in all patients whose endoscopic examinations indicated gastritis. After the histological examination, ten patients with grade I gastritis were moved from the study group to the control group, and ten patients with grade II gastritis were moved from control group to the study group (Figure 1). The demographic characteristics of the patients are shown in Table 1.
| Patient characteristics | Study group | Control group | P value | |
| n | 52 | 30 | ||
| Age (yr) | ||||
| mean | 12.47 ± 4.087 | 11.6 ± 4.065 | ||
| median | 13.5 | 12.6 | 0.847 | |
| range | 6-17 | 6-17 | ||
| Sex (F/M) | 29/23 | 17/13 | 0.981 | |
| Grading of gastritis (Sydney class, activity score) | S1 | S2 | Normal gastric mucosa; 14 patients | - |
| Mild (Grade I) | - | 44 | ||
| Moderate (Grade II) | 24 | 8 | Grade I gastritis; 16 patients | |
| Severe (Grade III) | 28 | - | ||
| H. pylori | 23 | 6 | 0.027 | |
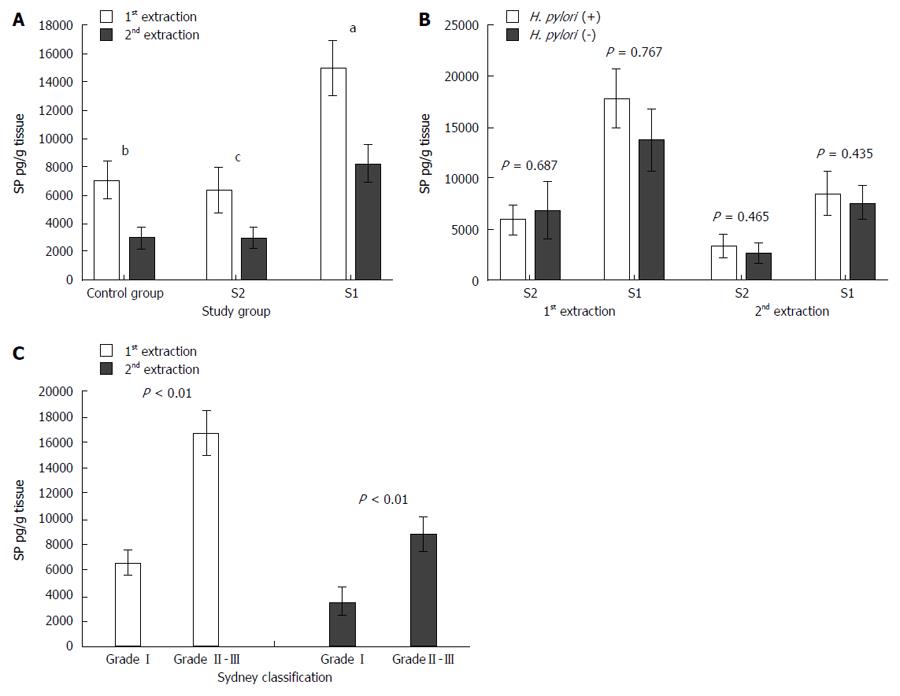
The S1 and S2 biopsy samples underwent a 2-step extraction, and their SP levels were measured via ELISA. Although the first extraction mostly contained SP from neurons, the SP in the second extraction reflected levels outside of neurons[17].
In both the first and second extractions, SP levels were significantly higher in S1 than in C samples (14980.11 ± 1950.82 pg/g vs 6913.20 ± 1360.56 pg/g tissue in the first extraction and 8249.51 ± 1342.45 pg/g vs 2977.23 ± 777.21 pg/g tissue in the second extraction, respectively). Moreover, SP levels were significantly higher in the S1 than in S2 samples in both extractions (14980.11 ± 1950.82 pg/g vs 6370.18 ± 1603.87 pg/g tissue in the first extraction and 8249.51 ± 1342.45 pg/g vs 2980.37 ± 742.35 pg/g tissue in the second extraction, respectively). However, there were no significant differences in SP levels between the S2 and C samples in either the first or second extractions (6370.18 ± 1603.87 pg/g vs 6913.20 ± 1360.56 pg/g tissue in the first extraction and 2980.37 ± 742.35 pg/g vs 2977.23 ± 777.21 pg/g tissue in the second extraction, respectively) (Figure 2A).
H. pylori was detected in 29 of 82 individuals in the study. There were no significant differences in SP levels between H. pylori-positive and H. pylori-negative individuals (Figure 2B).
There were significant differences in SP levels between patients with mild (Sydney I) and moderate/severe (Sydney II or Sydney III) gastritis. SP levels were higher in the patients with Sydney II or Sydney III gastritis than in those with Sydney I gastritis (16734.86 ± 1754.35 pg/g vs 6534.27 ± 986.45 pg/g tissue in the first extraction and 8790.83 ± 1326.92 pg/g vs 3567.23 ± 1065.21 pg/g tissue in the second extraction, respectively) (Figure 2C).
We did not observe any significant difference in SP levels in the first and second extractions in the study group (S1 and S2) and control group (C) between girls and boys.
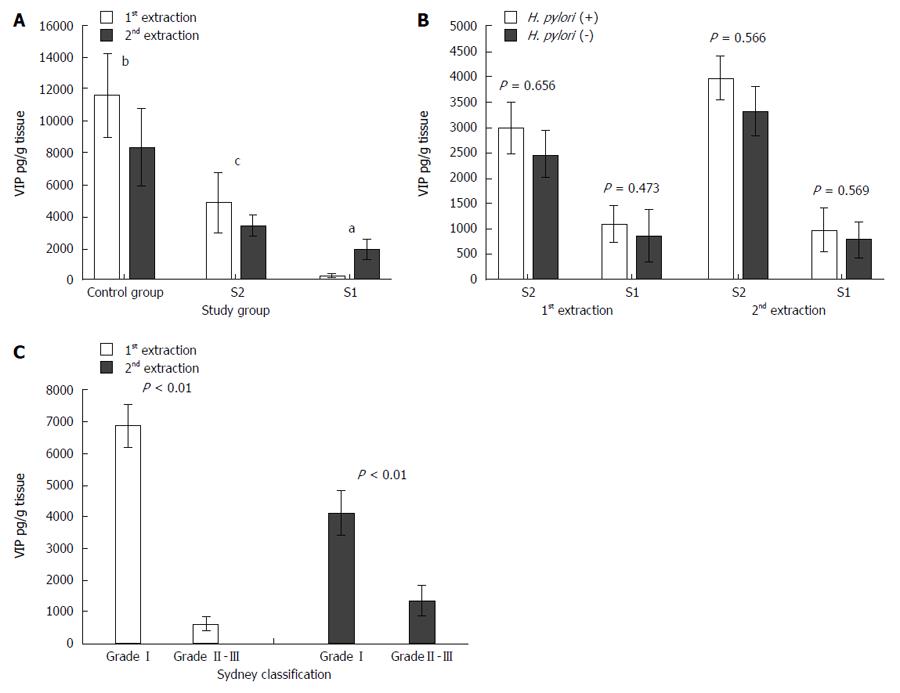
In the first and second extractions, VIP levels were significantly higher in the C samples than in both S2 (11633.70 ± 2596.89 pg/g vs 4867.64 ± 1862.84 pg/g tissue in the first extraction and 8339.03 ± 2437.23 vs 3435.87 ± 707.74 pg/g tissue in the second extraction, respectively) and S1 (11633.70 ± 2596.89 pg/g vs 288.21 ± 116.24 pg/g tissue in the first extraction and 8339.03 ± 2437.23 pg/g vs 1895.53 ± 655.41 pg/g tissue in the second extraction, respectively) samples. Also, VIP levels were significantly lower in S1 than in S2 samples in both the first and second extractions (288.21 ± 116.24 pg/g vs 4867.64 ± 1862.84 pg/g tissue in the first extraction and 1895.53 ± 655.41 pg/g vs 3435.87 ± 707.74 pg/g tissue in the second extraction, respectively) (Figure 3A).
No significant differences in VIP levels was detected between H. pylori (+) and H. pylori (-) individuals (Figure 3B).
There were significant differences in VIP levels between patients with mild (Sydney grade I) and moderate/severe (Sydney grade II or III) gastritis. VIP levels were higher in patients with a mild disease than in patients with a moderate/severe disease (6876.12 ± 896.31 pg/g vs 256.68 ± 46.87 pg/g tissue in the first extraction and 4045.23 ± 876.21 pg/g vs 1042.12 ± 241.12 pg/g tissue in the second extraction, respectively) (Figure 3C).
We did not observe any significant difference in VIP levels in the first and second extractions in the study group (S1 and S2) and control group (C) between girls and boys.
In the first and second extractions, CGRP levels were significantly lower in S1 than in the C samples (229.56 ± 49.63 pg/g vs 1184.18 ± 80.46 pg/g tissue in the first extraction and 397.80 ± 145.84 vs 1184.18 ± 80.46 pg/g tissue in the second extraction, respectively) and in S2 than in C samples (947.42 ± 265.65 pg/g vs 1184.18 ± 80.46 pg/g tissue in the first extraction and 618.02 ± 206.71 vs 1184.18 ± 80.46 pg/g tissue in the second extraction, respectively). Moreover, CGRP levels were lower S1 than in S2 samples (229.56 ± 49.63 pg/g vs 947.42 ± 265.65 pg/g tissue in the first extraction and 397.80 ± 145.84 pg/g vs 618.02 ± 206.71 pg/g tissue in the second extraction, respectively) (Figure 4A).
There was no significant difference in CGRP levels between H. pylori (+) and (-) individuals (Figure 4B).
There were significant differences in CGRP levels between patients with mild (Sydney grade I) and moderate/severe (Sydney grade II or III) gastritis. CGRP levels were higher in patients with grade I gastritis than in those with grade II or III gastritis (1063.87 ± 276.51 pg/g vs 312.34 ± 43.61 pg/g tissue in the first extraction and 862.85 ± 112.29 pg/g vs 328.13 ± 49.12 pg/g tissue in the second extraction, respectively) (Figure 4C).
We did not observe any significant difference in CGRP levels in the first and second extraction in the study group (S1 and S2) and control group (C) between girls and boys.
The gastric mucosa samples of 45 patients from the study group and 29 patients from the control group were stained for CD10, and the staining characteristics of epithelial cells and lymphoplasmacytic cells were recorded. We also measured NEP activity in freshly frozen tissues.
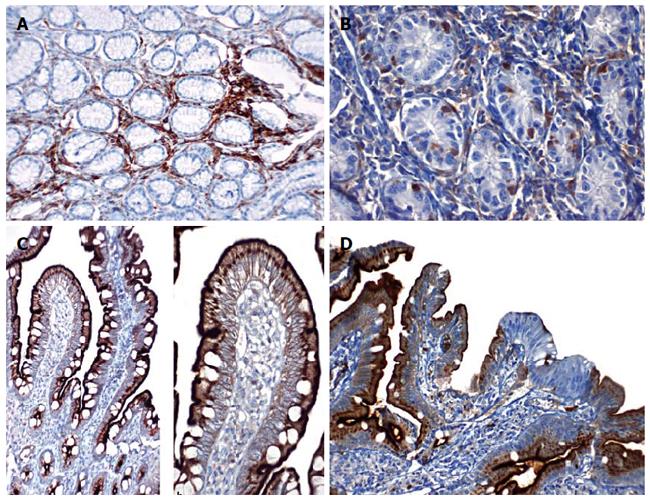
In the study and control groups, no CD10 staining at the gastric epithelium was observed (Figure 5A and B). In accordance with this observation, we did not detect measurable levels of NEP-like activity in these samples.
In 38 of the 45 patients in the study group, CD10 staining was positive (focal or diffuse) in lymphoplasmacytic cells in S1 samples, whereas no staining was observed in seven patients. In 28 S1 biopsy samples from patients, diffuse staining was observed in lymphocytic cells, and focal staining was observed in 10 patient samples. In 15 S2 biopsy samples from patients, CD10 staining was diffuse, and in 23 patient samples, the staining was focal. Based on the statistical analysis of these results, S1 staining was significantly more diffuse than S2 staining (Pearson's chi-square test, P = 0.002). In the H. pylori (+) patients, intense staining was observed in the germinal centers of lymphoid follicles.
Despite the positive staining of some samples, we could not detect measurable levels of NEP-like activity, suggesting that factors extracted during the sample preparation inhibit the enzymatic activity of NEP[17].
In 27 of the 29 patients in the control group, CD10 staining was positive in lymphoplasmacytic cells, whereas no staining was observed in samples from two patients. In eight patients, sample staining was diffuse. However, samples from 19 patients had focal staining. These results indicate that the gastric mucosa (lymphoplasmacytic cells) of the control group had less diffuse staining than did the severe and less severe areas of gastritis of the study group (P = 0.011 and P = 0.040, respectively; Pearson’s χ2 test).
We investigated CD10 staining in the duodenal mucosa, which reflects NEP activity. The patients without duodenitis (n = 30) had more pronounced diffuse and strong CD10 staining than did the patients with duodenitis (n = 52, P = 0.012) (Figure 5C). The loss of CD10 staining was more pronounced in cases of severe (based on the severity of mononuclear cell infiltration) duodenitis (33/52).
Gastritis, which is defined as an inflammation of the gastric mucosa, is a major cause of chronic abdominal pain in children[1]. In the current study, we investigated the roles of neuronal and non-neuronal peptides in the pathogenesis of chronic childhood gastritis. We observed differential effects of the disease on peptide levels. Specifically, SP was increased and CGRP and VIP were decreased in patients with severe gastritis. The changes were more prominent in S1 areas (where the gastritis was severe), but significant changes were also observed in the neighboring tissue (areas with less severe gastritis). In addition, the changes correlated with the pathological grade. The expression of CD10, the enzyme primarily involved in the hydrolysis of SP was also decreased in patients with duodenitis.
SP is an inflammatory peptide that is mainly released from sensory nerve endings. In the gastrointestinal tract, SP and its receptor have been reported to be extensively expressed in myenteric and submucosal nerve plexuses. In addition, SP is found in neutrophils, eosinophils, macrophages, and dendritic cells. SP plays roles in muscle contraction, epithelial ion transport, vascular permeability, and the regulation of immune function[18-23]. The inflammatory activity of SP has often been shown in in vitro studies and in animal experiments. Specifically, the activation of SP-containing nerve endings increases neutrophil, lymphocyte, monocyte, and fibroblast chemotaxis, suggesting a role in neurogenic inflammation[24-26]. Although experimental studies have shown that SP plays an important role in the formation of ulcers, it is not known how the levels of SP change in association with childhood gastritis. Here, we used a quantitative approach and found that SP is increased in both neuronal and non-neuronal diseased-tissue in a grade-dependent manner. Furthermore, the presence of an H. pylori infection did not alter SP levels; hence, SP probably exerts its inflammatory effect independent of H. pylori infections.
These findings correlate well with the inflammatory role of SP but differ from the results reported for adult patients in a study using a very similar approach[17]. Specifically, Erin et al[17] evaluated 57 adult patients with gastritis and reported two-way changes in SP levels. In patients with gastritis, SP levels in gastric mucosa with a lesion were found to be similar to the levels in healthy gastric mucosa, even though the amount of neuronal SP was significantly lower in lesion-free gastric mucosa than in healthy mucosa. The researchers suggested that neuronal SP is suppressed in lesion-free regions in patients with gastritis, which may suggest a degeneration of sensory nerve endings. However, the researchers did not compare their findings based on the Sydney classification system in that study. Also, differences between adult and pediatric patients might be due to lower physiological levels of neuronal SP in children. This possibility should be evaluated further. Our results, together with previously published data on SP, suggest that an SP antagonist may have therapeutic value in treating children with chronic gastritis.
VIP has anti-inflammatory effects by reducing the secretion of proinflammatory cytokines (TNFα, IL-6, and IL-12), increasing the secretion of anti-inflammatory cytokines (such as IL-10), and reducing Th1 activation[27]. VIP analogs have been shown to be useful in inflammatory and autoinflammatory models, such as models of endotoxic shock, Crohn’s disease, ulcerative colitis, rheumatoid arthritis, Parkinson’s disease, and brain injury[28]. Information regarding the change in VIP levels in upper gastrointestinal diseases is very limited. In an experimental cold-induced stress ulcer rat model, the administration of intraperitoneal VIP led to decreased histamine and methylhistamine levels and healing of mucosal ulcers[9], and a few animal studies have demonstrated gastro-protective effects of VIP[29,30]. In a study with a limited number of adult H. pylori (+) gastritis patients, VIP levels were shown to be decreased in the gastric mucosa. Researchers have suggested that this decrease in VIP levels may play a role in the pathogenesis of gastritis[10]. In 1985, 8 adult patients with duodenal ulcers were reported to have increased levels of VIP in their proximal duodenal mucosa[31]. In 2012, Erin et al[17] evaluated 57 adult patients and reported that neuronal VIP levels were significantly higher in healthy gastric mucosa samples than in samples from patients with gastritis and ulcers. In addition, reductions in VIP levels, particularly VIP of neuronal origin, were shown to be more pronounced in patients with peptic ulcers. Therefore, the researchers suggested that reductions in VIP levels may lead to inflammatory gastrointestinal pathologies, such as gastritis and ulcers. In our study, we found that VIP levels were significantly higher in healthy gastric mucosa and in areas with mild gastritis (control group) compared with the levels in areas with moderate-severe gastritis (both S1 and S2). Moreover, we also found that VIP levels in S1 samples were significantly lower than levels in S2 samples in the first and second extractions.
In our study, we did not find any relationship between decreased VIP levels and H. pylori infections. VIP increases the secretion of HCO3- in the mucus, contributing to the protection of the underlying epithelium[32]. The decreased levels of VIP may lead to the degradation of the gastric mucus barrier and damage to gastric epithelial cells, which in turn allows the easy penetration of H. pylori. The results of our study suggest that reductions in VIP rather than H. pylori infections causes gastritis, which is consistent with the findings of the study by Erin et al[17] on adult patients. Our study is the first to evaluate the VIP levels in childhood gastritis and suggests that mimetics of VIP may have a therapeutic value in treating childhood gastritis.
Blocking the CGRP pathway (such as via the ablation of afferent nerves with capsaicin) decreases the protective hyperemic response. In experimental studies, the administration of CGRP has been shown to result in the stimulation of sensory nerves, and in various experimental gastric ulcer models, CGRP has been found to create a protective effect. CGRP also accelerates the healing of gastric mucosal lesions and gastric ulcers created by various ulcerogenic factors[10,33,34]. It has been suggested that CGRP and its receptor in the transient receptor potential vanilloid type-1 pathway might be novel targets for therapeutic agents in treating gastric mucosal injury and visceral sensitivity in functional bowel syndrome[35,36].
Sipos et al[10] evaluated 10 adult patients with gastritis and found that CGRP- containing immunoreactive nerve endings were slightly decreased relative to the number present in the control group. In our study, we found that CGRP levels were lower in S1 and S2 samples in the first and second extractions in children with gastritis compared with levels in the controls. Moreover, the CGRP levels were lower in S1 than in S2 samples. These results indicate that decreased levels of CGRP might be involved in the pathogenesis of gastritis. To our knowledge, this study is the first to evaluate changes in CGRP and their effects on pediatric patients with gastritis. Finally, we observed no differences in SP, CGRP, or VIP levels in the H. pylori (+) vs H. pylori (-) groups, but we found significant differences in patients with grade I vs II-III gastritis. The reason for this is related to the fact that almost half of the patients in the study group, where severe gastritis was more common, were H. pylori (-). Therefore, we suggest that changes in neuropeptides can cause gastric mucosal damage independent of H. pylori infection.
NEP is a membrane-bound cell-surface enzyme that is densely concentrated in the brush border of the small intestinal mucosa. In healthy mucosa, linear (regular) CD10 staining was observed via immunohistochemical methods. Trejdosiewicz et al[37] reported no CD10 staining in healthy gastric mucosa in patients with celiac disease. In our study, we did not observe any NEP enzyme activity in the gastric mucosa or epithelial CD10 staining. In adults with chronic gastritis, intraepithelial neutrophil density indicates the extent of mucosal damage. Neutrophil density has been shown to be far lower in children than in adults, but lymphocyte infiltration is more prominent[38]. In our study, diffuse CD10 staining of gastric mucosal lymphoplasmacytic cells was significantly lower in the control group than in the study group. Moreover, we observed more intense CD10 staining in S1 than in S2 samples. Hematopoietic progenitor cells, called common lymphoid progenitors, have the potential to transform into T, B, and natural killer cells and express NEP. The more frequent detection of CD10-positive lymphoplasmacytic cell-inflamed regions suggests that cells expressing NEP may play a role in the onset or aggravation of inflammation.
NEP hydrolyzes SP at the Gln6-Phe7, Phe7-Phe8 and Gly9-Leu regions and creates SP fragments with different activities[12]. In our study, we observed elevated SP levels and an increased presence of CD10 positive cells in regions with lesions, which can be interpreted as a reactive response. The role and significance of epithelial NEP expression seem to differ from the role and significance of its expression in immune cells. Epithelial NEP expression seems to be important in the protection of the mucosa. NEP may have a role in the growth and differentiation of intestinal epithelial cells, and it has been used in support of diagnoses of microvilli inclusion disease using light microscopy[37,39-41]. In a study of ulcerative colitis patients, a regular staining pattern was detected in healthy ileal mucosa, but no staining was observed in inflamed mucosa[42], and changes in NEP expression were reported in a patient with intestinal metaplasia accompanied by gastric adenocarcinoma[43]. Moreover, we observed a more prominent diffuse CD10 staining pattern in the duodenal mucosa of patients without duodenitis than in those with duodenitis.
Our study is the first to evaluate the level and expression of NEP in inflamed gastric mucosa and the duodenum, and our results suggests that the loss of epithelial NEP activity is involved in duodenal inflammation. The changes observed in inflammatory infiltrates most likely signify the reactive infiltration of immature immune cells associated with gastritis. In conclusion, based on these findings, we propose that decreased levels of VIP and CGRP and increased levels of SP contribute to the pathological changes in the gastric mucosa observed in patients with gastritis. Hence, new treatments targeting these molecules may have therapeutic and preventive actions.
Experiments were performed in part at the Health Sciences Research Center of Akdeniz University (SBAUM). The authors thank the Senior Technicians Özlem Duymus and Nilüfer Ekinci for their technical help.
Chronic gastritis is an important childhood health problem. The disruption of balance between aggressive and protective factors of gastric mucosa has been demonstrated to lead to gastritis. And Helicobacter pylori (H. pylori) infection is the most important factor implicated in the pathogenesis of gastritis. However, neither the gastric mucosa’s protective mechanisms nor the pathogenesis of gastritis have been fully elucidated. Patients may not respond to conventional medical treatments or H. pylori eradication therapy which suggests the involvement of unknown factors in the pathogenesis of gastritis. The gastrointestinal system contains some regulatory sensory neuropeptides that may play a role in gastritis pathogenesis. There are insufficient data regarding in this issue.
It has been suggested that substance P (SP) may regulate inflammatory processes, vasoactive intestinal peptide (VIP) has anti-inflammatory effects, and calcitonin gene-related peptide (CGRP) has a vasodilatory effect. Neutral endopeptidase (NEP) hydrolyzes SP, it may terminate its biological activity and it has been suggested to function in the growth and differentiation of intestinal epithelial cells. To date, no studies have investigated changes in SP, VIP, CGRP, and NEP levels in the gastric mucosa of children. Disturbances in neuropeptide content may result in mucosal damage. Advances in current knowledge of the underlying mechanisms will help in developing new treatment options.
In a recent study with adults authors found that, neuronal SP levels decreased significantly in normally appearing mucosa of patients with gastritis while levels of non-neuronal SP increased in diseased areas of gastritis and ulcer. The content of VIP in both disease-involved and uninvolved mucosa, and expression of NEP, markedly decreased in patients with gastritis or ulcer. Since VIP, as well as SP fragments, formed following hydrolysis with NEP is recognized to have gastroprotective effects, decreased levels of VIP, SP and NEP may predispose to cellular damage. In the present study, the authors aimed to investigate the roles of neuronal and non-neuronal peptides in the pathogenesis of chronic gastritis in children.
Advances in the knowledge of the authors’ underlying mechanisms will help in developing new treatment options. The use of SP antagonists, VIP analogues or targeting the CGRP pathway with novel therapeutic agents can be a good treatment option.
Inflammation of the gastric mucosa is defined as gastritis. The gastrointestinal system contains sensory neuropeptides, such as VIP, SP, and CGRP. Disturbances in neuropeptide content may result in gastritis.
Authors reported that in this study decreased levels of VIP and CGRP and increased levels of SP contribute to pathological changes in gastric mucosa in children. The results suggest that new treatments targeting these molecules may have therapeutic and preventive effects.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Garcia-Olmo D, Patne SCU, Tsukamoto T S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Rowland M, Bourke B, Drumm B. Acid-Peptic Disease. Walker’s Pediatric Gastrointestinal Disease. Hamilton, Ontario, Canada: BC Decker Inc 2008; 153-163. |
| 2. | McColl KE, el-Omar E, Gillen D. Helicobacter pylori gastritis and gastric physiology. Gastroenterol Clin North Am. 2000;29:687-703, viii. [PubMed] |
| 3. | Stead RH. Innervation of mucosal immune cells in the gastrointestinal tract. Reg Immunol. 1992;4:91-99. [PubMed] |
| 4. | Weinstock JV, Blum AM. Release of substance P by granuloma eosinophils in response to secretagogues in murine schistosomiasis mansoni. Cell Immunol. 1990;125:380-385. [PubMed] |
| 5. | Killingsworth CR, Shore SA, Alessandrini F, Dey RD, Paulauskis JD. Rat alveolar macrophages express preprotachykinin gene-I mRNA-encoding tachykinins. Am J Physiol. 1997;273:L1073-L1081. [PubMed] |
| 6. | Joos GF, Germonpré PR, Pauwels RA. Role of tachykinins in asthma. Allergy. 2000;55:321-337. [PubMed] |
| 7. | Bost KL, Breeding SA, Pascual DW. Modulation of the mRNAs encoding substance P and its receptor in rat macrophages by LPS. Reg Immunol. 1992;4:105-112. [PubMed] |
| 8. | Erin N, Ercan F, Yegen BC, Arbak S, Okar I, Oktay S. Role of capsaicin-sensitive nerves in gastric and hepatic injury induced by cold-restraint stress. Dig Dis Sci. 2000;45:1889-1899. [PubMed] |
| 9. | Tunçel N, Tunçel M, Aboul-Enein HY. Effects of the vasoactive intestinal peptide on stress-induced mucosal ulcers and modulation of methylation of histamine in gastric tissue of the rats. Farmaco. 2003;58:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Sipos G, Altdorfer K, Pongor E, Chen LP, Fehér E. Neuroimmune link in the mucosa of chronic gastritis with Helicobacter pylori infection. Dig Dis Sci. 2006;51:1810-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Takeuchi K, Ueshima K, Ohuchi T, Okabe S. The role of capsaicin-sensitive sensory neurons in healing of HCl-induced gastric mucosal lesions in rats. Gastroenterology. 1994;106:1524-1532. [PubMed] |
| 12. | Bunnett NW, Wu V, Sternini C, Klinger J, Shimomaya E, Payan D, Kobayashi R, Walsh JH. Distribution and abundance of neutral endopeptidase (EC 3.4.24.11) in the alimentary tract of the rat. Am J Physiol. 1993;264:G497-G508. [PubMed] |
| 13. | Rugge M, Genta RM. Staging and grading of chronic gastritis. Hum Pathol. 2005;36:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 14. | Erin N, Clawson GA. Parameters affecting substance P measurement in heart, lung, and skin. Biotechniques. 2004;37:232, 234, 236 passim. [PubMed] |
| 15. | Erin N, Ulusoy O. Differentiation of neuronal from non-neuronal Substance P. Regul Pept. 2009;152:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Carter TL, Pedrini S, Ghiso J, Ehrlich ME, Gandy S. Brain neprilysin activity and susceptibility to transgene-induced Alzheimer amyloidosis. Neurosci Lett. 2006;392:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Erin N, Türker S, Elpek O, Yıldırım B. Differential changes in Substance P, VIP as well as neprilysin levels in patients with gastritis or ulcer. Peptides. 2012;35:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Erin N, Zik B, Sarigül M, Tütüncü S. The effects of chronic low-dose capsaicin treatment on substance P levels. Regul Pept. 2009;153:83-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Holzer P, Livingston EH, Saria A, Guth PH. Sensory neurons mediate protective vasodilatation in rat gastric mucosa. Am J Physiol. 1991;260:G363-G370. [PubMed] |
| 20. | Pernow B. Substance P--a putative mediator of antidromic vasodilation. Gen Pharmacol. 1983;14:13-16. [PubMed] |
| 21. | Reinshagen M, Patel A, Sottili M, French S, Sternini C, Eysselein VE. Action of sensory neurons in an experimental at colitis model of injury and repair. Am J Physiol. 1996;270:G79-G86. [PubMed] |
| 22. | Batbayar B, Somogyi J, Zelles T, Fehér E. Immunohistochemical analysis of substance P containing nerve fibres and their contacts with mast cells in the diabetic rat’s tongue. Acta Biol Hung. 2003;54:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Ottaway CA, Stanisz AM. Neural-immune interactions in the intestine: Implications for inflammatory bowel disease. Inflammatory bowel disease. Baltimore: Williams and Wilkins 1995; 281-300. |
| 24. | Kähler CM, Sitte BA, Reinisch N, Wiedermann CJ. Stimulation of the chemotactic migration of human fibroblasts by substance P. Eur J Pharmacol. 1993;249:281-286. [PubMed] |
| 25. | Ansel JC, Brown JR, Payan DG, Brown MA. Substance P selectively activates TNF-alpha gene expression in murine mast cells. J Immunol. 1993;150:4478-4485. [PubMed] |
| 26. | Cocchiara R, Bongiovanni A, Albeggiani G, Azzolina A, Geraci D. Substance P selectively activates TNF-alpha mRNA in rat uterine immune cells: a neuroimmune link. Neuroreport. 1997;8:2961-2964. [PubMed] |
| 27. | Delgado M, Pozo D, Ganea D. The significance of vasoactive intestinal peptide in immunomodulation. Pharmacol Rev. 2004;56:249-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 305] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 28. | Harmar AJ, Arimura A, Gozes I, Journot L, Laburthe M, Pisegna JR, Rawlings SR, Robberecht P, Said SI, Sreedharan SP. International Union of Pharmacology. XVIII. Nomenclature of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide. Pharmacol Rev. 1998;50:265-270. [PubMed] |
| 29. | Palus K, Całka J. The Influence of Prolonged Acetylsalicylic Acid Supplementation-Induced Gastritis on the Neurochemistry of the Sympathetic Neurons Supplying Prepyloric Region of the Porcine Stomach. PLoS One. 2015;10:e0143661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Long L, Wang J, Chen N, Zheng S, Shi L, Xu Y, Luo C, Deng Y. Curcumin Ameliorates Reserpine-Induced Gastrointestinal Mucosal Lesions Through Inhibiting IκB-α/NF-κB Pathway and Regulating Expression of Vasoactive Intestinal Peptide and Gastrin in Rats. J Med Food. 2016;19:528-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Domschke S, Bloom SR, Adrian TE, Lux G, Bryant MG, Domschke W. Gastroduodenal mucosal hormone content in duodenal ulcer disease. Hepatogastroenterology. 1985;32:198-201. [PubMed] |
| 32. | Yao B, Hogan DL, Bukhave K, Koss MA, Isenberg JI. Bicarbonate transport by rabbit duodenum in vitro: effect of vasoactive intestinal polypeptide, prostaglandin E2, and cyclic adenosine monophosphate. Gastroenterology. 1993;104:732-740. [PubMed] |
| 33. | Dömötör A, Kereskay L, Szekeres G, Hunyady B, Szolcsányi J, Mózsik G. Participation of capsaicin-sensitive afferent nerves in the gastric mucosa of patients with Helicobacter pylori-positive or-negative chronic gastritis. Dig Dis Sci. 2007;52:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Clementi G, Amico-Roxas M, Caruso A, Cutuli VM, Maugeri S, Prato A. Protective effects of calcitonin gene-related peptide in different experimental models of gastric ulcers. Eur J Pharmacol. 1993;238:101-104. [PubMed] |
| 35. | Luo XJ, Liu B, Dai Z, Yang ZC, Peng J. Stimulation of calcitonin gene-related peptide release through targeting capsaicin receptor: a potential strategy for gastric mucosal protection. Dig Dis Sci. 2013;58:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Evangelista S. Capsaicin receptor as target of calcitonin gene-related peptide in the gut. Prog Drug Res. 2014;68:259-276. [PubMed] |
| 37. | Trejdosiewicz LK, Malizia G, Oakes J, Losowsky MS, Janossy G. Expression of the common acute lymphoblastic leukaemia antigen (CALLA gp100) in the brush border of normal jejunum and jejunum of patients with coeliac disease. J Clin Pathol. 1985;38:1002-1006. [PubMed] |
| 38. | Crone J, Gold BD. Helicobacter pylori infection in pediatrics. Helicobacter. 2004;9 Suppl 1:49-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Danielsen EM, Vyas JP, Kenny AJ. A neutral endopeptidase in the microvillar membrane of pig intestine. Partial purification and properties. Biochem J. 1980;191:645-648. [PubMed] |
| 40. | Groisman GM, Amar M, Livne E. CD10: a valuable tool for the light microscopic diagnosis of microvillous inclusion disease (familial microvillous atrophy). Am J Surg Pathol. 2002;26:902-907. [PubMed] |
| 41. | Koepsell SA, Talmon G. Light microscopic diagnosis of microvillus inclusion disease on colorectal specimens using CD10. Am J Surg Pathol. 2010;34:970-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 42. | Lloyd JM, Owens SR. CD10 immunohistochemistry stains enteric mucosa, but negative staining is unreliable in the setting of active enteritis. Mod Pathol. 2011;24:1627-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Endoh Y, Tamura G, Motoyama T, Ajioka Y, Watanabe H. Well-differentiated adenocarcinoma mimicking complete-type intestinal metaplasia in the stomach. Hum Pathol. 1999;30:826-832. [PubMed] |