Published online Oct 7, 2016. doi: 10.3748/wjg.v22.i37.8322
Peer-review started: April 13, 2016
First decision: June 20, 2016
Revised: July 7, 2016
Accepted: August 8, 2016
Article in press: August 8, 2016
Published online: October 7, 2016
Processing time: 172 Days and 7.9 Hours
To investigate the effects of orally gavaged aqueous rhubarb extract (RE) on 5-fluorouracil (5-FU)-induced intestinal mucositis in rats.
Female Dark Agouti rats (n = 8/group) were gavaged daily (1 mL) with water, high-dose RE (HDR; 200 mg/kg) or low-dose RE (LDR; 20mg/kg) for eight days. Intestinal mucositis was induced (day 5) with 5-FU (150 mg/kg) via intraperitoneal injection. Intestinal tissue samples were collected for myeloperoxidase (MPO) activity and histological examination. Xenopus oocytes expressing aquaporin 4 water channels were prepared to examine the effect of aqueous RE on cell volume, indicating a potential mechanism responsible for modulating net fluid absorption and secretion in the gastrointestinal tract. Statistical significance was assumed at P < 0.05 by one-way ANOVA.
Bodyweight was significantly reduced in rats administered 5-FU compared to healthy controls (P < 0.01). Rats administered 5-FU significantly increased intestinal MPO levels (≥ 307%; P < 0.001), compared to healthy controls. However, LDR attenuated this effect in 5-FU treated rats, significantly decreasing ileal MPO activity (by 45%; P < 0.05), as compared to 5-FU controls. 5-FU significantly reduced intestinal mucosal thickness (by ≥ 29% P < 0.001) as compared to healthy controls. LDR significantly increased ileal mucosal thickness in 5-FU treated rats (19%; P < 0.05) relative to 5-FU controls. In xenopus oocytes expressing AQP4 water channels, RE selectively blocked water influx into the cell, induced by a decrease in external osmotic pressure. As water efflux was unaltered by the presence of extracellular RE, the directional flow of water across the epithelial barrier, in the presence of extracellular RE, indicated that RE may alleviate water loss across the epithelial barrier and promote intestinal health in chemotherapy-induced intestinal mucositis.
In summary, low dose RE improves selected parameters of mucosal integrity and reduces ileal inflammation, manifesting from 5-FU-induced intestinal mucositis.
Core tip: Aqueous rhubarb extract partially improved selected parameters of 5-fluorouracil (5-FU)-induced intestinal mucositis in rats. Exposure to 5-FU decreased bodyweight, yet high-dose rhubarb extract (RE) and low-dose RE (LDR) showed no changes. Myeloperoxidase activity was significantly decreased in rats treated with LDR and 5-FU when compared to the intestinal mucositis control group. Ileal mucosal thickness was significantly improved (19%) in animals with intestinal mucositis and treated with LDR. In xenopus oocytes expressing AQP4 water channels, RE blocked swelling induced by a decrease in external osmotic pressure which indicated that water influx across the epithelial barrier was selectively blocked by RE.
- Citation: Bajic JE, Eden GL, Lampton LS, Cheah KY, Lymn KA, Pei JV, Yool AJ, Howarth GS. Rhubarb extract partially improves mucosal integrity in chemotherapy-induced intestinal mucositis. World J Gastroenterol 2016; 22(37): 8322-8333
- URL: https://www.wjgnet.com/1007-9327/full/v22/i37/8322.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i37.8322
Traditional herbal medicines have been used for centuries in the maintenance and improvement of health or the treatment of illnesses. Globally, ancient herbal remedies have been created based on theories, beliefs and experiences representing various cultures at different times throughout history[1]. Consequently, traditional herbal medicines are being investigated increasingly for their potential to treat and reduce the symptoms of a wide variety of diseases and disorders, specifically cancer and its treatment-related side-effects. Many cancer patients seek alternative medicines that will complement their standard-care treatments with the hope that they will improve symptoms associated with either the cancer or their anti-cancer treatments[2].
Cancer is a life-threatening illness affecting millions of individuals world-wide. In westernized countries approximately 50% of the population will develop cancer before the age of 85[3]. Chemotherapy forms one of the most common strategies for cancer treatment. Cytotoxic chemotherapy drugs, such as 5-fluorouracil (5-FU), act by inhibiting DNA synthesis of not only malignant cells, but also rapidly dividing cells lining the intestinal mucosa[4]. An increase in cell apoptosis stimulates the production of reactive oxygen species (ROS) and pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and IL-4 resulting in further tissue and blood vessel damage[5,6]. This cascade of events results in a range of debilitating clinical side-effects, from nausea and vomiting to inflammation and ulceration of the gastrointestinal tract; and sepsis may occur if untreated[7,8]. These painful and life-threatening side-effects collectively form a disorder known as intestinal mucositis which affects approximately 60% of patients undergoing chemotherapy[9]. Current therapies for intestinal mucositis seek to reduce the severity of symptoms rather than acting as a curative or preventative measure[10,11]. Thus, treatments are required with the potential to eliminate or reduce the adverse side-effects of cancer chemotherapy.
Recently, in experimental systems, plant extracts such as grape seed extract (GSE) and Iberogast® have been investigated as potential treatments for intestinal mucositis on the basis of their anti-inflammatory and antioxidant constituents[12-14]. Indeed, plant-sourced molecules and compounds are commonly perceived to be safer therapeutics compared to synthetic compounds[15]. There are limited studies on the pharmacology of herbal medicines, yet such extracts may offer protection against intestinal mucositis in an experimental setting. The scientific study of further plant-based extracts is therefore warranted.
Rhubarb, Rheum spp., is a herbaceous perennial plant with a long, fleshy stalk, commonly used for cooking and medicine. Dried rhubarb rhizomes were traditionally used in Chinese medicine as a natural remedy for gastrointestinal complications including diarrhoea, constipation and inflammation[16]. The pharmacological effects have been attributed to the stalk of the plant[17,18]. Two main active constituents (ethanol-soluble and water soluble) have been classified in rhubarb stalks. Anthraquinones form the main ethanol-soluble active constituent of rhubarb stalks[14]. These constituents have exhibited a diarrhoeal effect in mice providing a possible purgative mechanism of action[18]. In contrast, the aqueous extract of rhubarb has recently demonstrated anti-diarrhoeal properties, believed to be mediated by tannins through regulation of intestinal water secretion and absorption[18]. Importantly, chemotherapy recipients experiencing intestinal mucositis have altered membrane integrity and impaired water absorption and secretion[7,19].
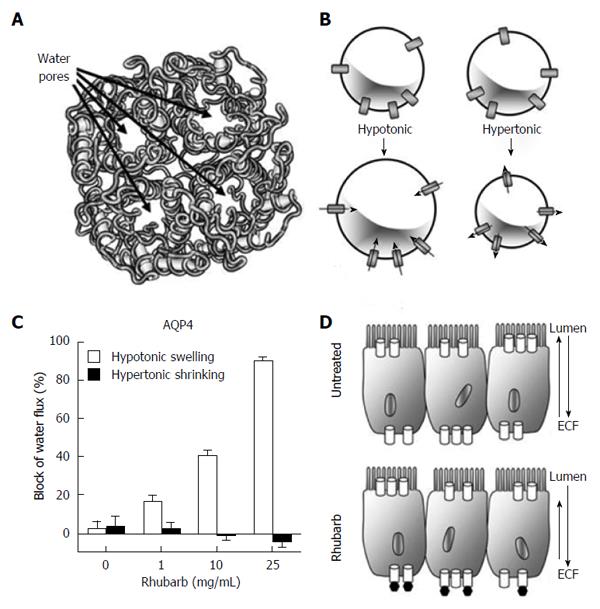
Aquaporins (AQPs) are integral membrane proteins responsible for the regulation of water transport across a membrane via an osmotic gradient[20,21]. Aquaporin channels are tetramers with a water pore located in each subunit of the channel (Figure 1A). Water molecules move in single file through aquaporin pores, down osmotic and hydrostatic gradients. As one molecule enters via the extracellular region of the channel, another molecule is displaced into the cytoplasm and vice versa[22]. Currently, 13 mammalian AQPs have been identified (AQP 0-12). AQPs are abundant in tissues reliant on high water permeability to maintain correct function[21,23] and are involved in metabolic processes such as kidney, lung, brain and gastrointestinal function[24-26]. In the human gastrointestinal tract, AQPs 3, 7 and 8 are expressed throughout the mucosal epithelia, and AQP1 is present in endothelial cells of the vasculature. In early stage inflammatory bowel disease, tight junctions and transport systems are impaired, leading to a leaky epithelium. Clinical human biopsies showed that levels of expression of AQPs1 and 3 are reduced in Crohn’s Disease and AQPs 7 and 8 are decreased in ulcerative colitis, based on quantitative PCR and immunolabelling assays[27]. As well, the typical apical localisation of AQP8 in bowel was lost, and the appearance of a faint basolateral signal suggested intestinal epithelial cell polarity was disrupted.
Aquaporin-4 (AQP 4) is believed to provide the principal mechanism for bidirectional water transport across the basolateral membrane of small intestinal enterocytes[28]. These water channels ensure that efficient water absorption and secretion is maintained, thus allowing for adequate hydration and optimal stool consistency[29]. Liu et al[17] demonstrated that the anti-diarrhoeal effect of rhubarb tannins extract occurred via the inhibition of AQP 2 and 3 expression in vitro and in a mouse model of magnesium sulphate-induced diarrhoea. In addition, the water-soluble polysaccharides of rhubarb have protected the gastrointestinal tract against inflammation resulting from 2,4,6-trinitrobenzene sulfonic acid-induced colitis[17]. The anti-inflammatory mechanism of action underlying rhubarb extract (RE) remains unclear; however, it is thought that tannins may reduce the production of pro-inflammatory cytokines such as IL-4 and IFN-γ[17]. Consequently, RE was explored for its anti-inflammatory potential in intestinal mucositis and its potential to influence water transport across the intestinal mucosa[17,18].
In the current study, an aqueous fraction of rhubarb was investigated for its potential to reduce intestinal damage induced by the antimetabolite chemotherapy drug, 5-FU in rats. It was hypothesised that RE would decrease the severity of intestinal mucositis by improving histopathological parameters and potentially regulate faecal output via water secretion into the intestinal lumen.
Rhubarb stems (2.5 kg) were sectioned (1 cm) and boiled with absolute ethanol to remove alcohol-soluble components. Once cooled, the liquid was discarded and the residues were further boiled with water. The aqueous rhubarb components were retained for dehydration to obtain a concentrated powder[17]. Dehydration was conducted by freeze-drying at the South Australian Research and Development Institute, West Beach, South Australia. Four grams of powder were obtained for every 500 g of fresh rhubarb. Based on fractionation of the extract, the active agent appears to be a water-soluble ethanol-insoluble glycopeptide. Lectin array profiling has indicated that mannose and N-acetylglucosamine are predominant components of the carbohydrate structure. The precise chemical structure and possible presence of more than one isoform with biological activity remains to be determined.
Six week old female Dark Agouti rats (n = 32; 110-150 g) were sourced from the Animal Resources Centre (Western Australia) and Laboratory Animal Services (The University of Adelaide, South Australia). All animal experimentation was approved by the Animal Ethics Committee of the University of Adelaide (S-2010-111). The animal protocol described in this study was designed to minimise pain or discomfort to the animals and complied with the National Health and Medical Research Council Code of Practice for Animal Care in Research and Teaching. Prior to the experimentation period, rats were individually housed in Tecniplast™ (PA, United States) metabolism cages for 48 hours to acclimatise. Rats received ad libitum water and 18% Casein diet[30] and were exposed to a 12 h light-dark cycle in a temperature controlled room (22 °C). After the acclimatisation phase, rats were randomly allocated to four treatment groups (n= 8/group): Water + Saline, Water + 5-FU, Low-Dose Rhubarb (LDR; 20 mg/kg BW) + 5-FU and High-Dose Rhubarb (HDR; 200 mg/kg BW) + 5-FU. Water, HDR and LDR (1 mL) were administered daily via orogastric gavage on days 0 to 7. LDR dose for gavage was based on the estimated dose required to block aquaporin water channel activity in the oocyte expression system, and the dose HDR was selected as a 10 fold higher concentration for comparison.
Daily recordings of body weight, feed and water intake and faecal and urine output were conducted. Faecal pellets were collected daily, weighed and placed in a drying oven at 70 °C for 72 h. The percentage weight loss was used as an indication of moisture content in the faecal samples. On day 5, rats were injected with 5-FU (150 mg/kg BW; Hospira Australia Pty Ltd, Melbourne, Victoria) to induce intestinal mucositis. The single high dose of 5-FU used in the current study was determined from previous studies in our laboratory[31]. Following 5-FU administration, daily disease activity index (DAI) scoring was performed by a blinded researcher based on overall condition, weight loss and stool consistency. Each parameter was scored based on a scale of 0 (normal) to 3 (maximal severity) giving a maximum daily total of 9 for severely affected rats[32,33].
Rats were humanely euthanized on day 8 via carbon dioxide asphyxiation. Day 8 of the experimental period represented 3 d post 5-FU exposure and due to the acute nature of 5-FU-induced intestinal mucositis, this was determined to be the optimal day when histological damage in the intestine was most evident. The gastrointestinal tract was removed and emptied, then the lengths of each section [duodenum, jejunum, jejuno-ileum junction (JI), ileum and colon] were recorded and weighed.
Segments (2 cm and 4 cm) of the small intestine tract were collected at approximately 10% (jejunum) and 90% (ileum) of the total small intestine length for histological and biochemical analysis, respectively. Samples for histological analysis were fixed in 10% buffered formalin for 24 h and transferred to 70% ethanol for preservation. Segments for biochemical analysis were weighed and snap-frozen in liquid nitrogen prior to storage at -80 °C. The remaining thoracic and abdominal organs (thymus, lungs, heart, spleen, kidneys, liver, stomach and caecum) were weighed and discarded.
Myeloperoxidase (MPO) is an enzyme present in the intracellular granules of neutrophils and provides a quantitative analysis of acute inflammation. The assay was performed with slight modification from Beyer et al[34]. Segments of the small intestinal tract (jejunum, JI and ileum; 4 cm) were thawed and prepared for MPO assay via homogenization in 10 mmol/L phosphate buffer (pH 6.1). Homogenised samples were centrifuged at 13000 rpm for 12 min and the supernatant was discarded. The remaining pellet was resuspended with 0.5% hexadecyltrimethyl ammonium bromide buffer and vortexed prior to a final centrifuge (13000 rpm for 2 min). Supernatant from each sample (50 μL aliquot) was dispensed into a 96-well plate and the MPO reaction was initiated with an O-dianisidine dihydrochloride solution (200 μL/well; 4.2 mg O-dianisidine dihydrochloride, 12.5 μL hydrogen peroxide (30%) in 2.5 mL potassium phosphate buffer (50 mmol/L, pH 6.1) and 22.5 mL distilled H2O). A spectrometer (Victor X4 Multilabel Reader, Perkin Elmer, Singapore) measured absorbance (450 nm) at one minute intervals over a 15 min period. The change in absorbance was used to calculate MPO activity within a tissue sample (MPO units/g of intestinal tissue).
Intestinal samples stored in 70% ethanol were embedded with paraffin wax and cross-sectioned at 4 μm. Histological slides were stained with haematoxylin and eosin for qualitative and quantitative analysis. Qualitative measurements of 40 villus and crypts per intestinal section (jejunum, JI and ileum) were performed blinded using Image ProPlus software for Windows (version 5.1.1; Media Cybernetics, Silver Spring MD, United States) connected to a Nikon Eclipse 50i light microscope (Nikon Cooperation, Japan) and a ProGres C5 digital camera (Jenoptik, Germany). Intestinal sections were also analysed quantitatively using disease severity scores based on 11 criteria described by Howarth et al[32]. Each criterion was scored on a scale of 0 (normal) to 3 (severely damaged) for five cross-sections of each intestinal region. The median score for each criterion was calculated and the scores of all criteria were summed to give an overall disease severity score; with a score of 33 indicating maximal tissue damage[32,33].
Unfertilized oocytes from Xenopus laevis were prepared as described previously[35,36] and maintained in ND96 saline (96 mmol/L NaCl, 2 mmol/L KCl, 1 mmol/L MgCl2, 1.8 mmol/L CaCl2, and 5 mmol/L HEPES, pH 7.55) supplemented with 100 μg/mL penicillin,100 U/mL streptomycin, and 10% horse serum. Oocytes were injected with 50 nL of water containing 1 ng of rat AQP4 wild-type cRNA and were incubated for 2 or more days at 16-18 °C prior to osmotic swelling and shrinking assays in saline without antibiotics or serum. Hypotonic saline (50%) was prepared by diluting isotonic saline with an equal volume of water, whilst 200% hypertonic saline was prepared by doubling the NaCl concentration of the saline. Volume change rates were measured by videomicroscopy at 0.5 frames/s over 30 s using NIH ImageJ software (http://rsbweb.nih.gov/ij/), as described previously[35,36].
Statistical analyses were conducted using IBM SPSS Statistics version 19 for Windows (SPSS Inc., Chicago, IL, United States) and GraphPad Prism 6.02 for Windows (GraphPad Software Inc., San Diego, CA, United States). Normality tests were performed on all data sets to determine parametric and non-parametric data. All parametric data (metabolic data, MPO activity and villus height/crypt depth measurements) was analysed using one-way ANOVA with Tukey post hoc test. Non-parametric data (DSS and DAI) was analysed using Kruskal-Wallis with Mann Whitney U post hoc test. All data were expressed as mean ± SEM with the exception of disease severity scores which were expressed as medians and range. Values of P < 0.05 were considered significant.
Cloned rat AQP 4 water channels expressed in Xenopus oocytes were analysed quantitatively for osmotically-driven changes in cell volume in the presence and absence of dried reconstituted aqueous RE. Decreased external osmotic pressure (50% hypotonic saline) induced a volume increase (swelling) that was blocked by RE (Figure 1B and C). In contrast, the volume decrease (shrinking) induced by 200% hypertonic saline was not significantly altered by RE (Figure 1B and C), indicating that the blocking effect of RE was directional. In the presence of extracellular RE, water influx into the cell mediated by AQP4 was selectively blocked, whereas water efflux was not altered, providing a potentially useful tool for differentially modulating net fluid absorption and secretion in the gastrointestinal tract. The current ex vivo study predicted that RE would act on basolateral AQP 4 channels and alleviate water loss across the barrier epithelium (Figure 1D), thereby promoting intestinal health in the experimental setting of chemotherapy-induced intestinal mucositis.
Low dose rhubarb (LDR) and high dose rhubarb (HDR) had no significant effect on metabolic parameters (bodyweight, feed and water intake and faecal and urine output) when compared to controls prior to administration of 5-FU (Table 1). After 5-FU administration, feed intake was significantly decreased (by 60%; P < 0.001) in comparison to healthy controls (Table 2). Furthermore, in 5-FU treated rats administered HDR, feed intake was further reduced by 55% when compared to 5-FU controls (P < 0.01). However, normal feed intake was maintained in 5-FU treated rats administered LDR. Although feed intake was significantly reduced in 5-FU controls, there was no reduction in wet faecal output compared to healthy controls. However, in 5-FU treated rats administered HDR, faecal output was reduced by 41% in comparison to 5-FU controls. There were no significant effects on water intake and urine output between control and RE treatment groups (Table 2). Similarly, no significant effects on faecal moisture content were evident among all treatment groups, before or after 5-FU administration (data not shown).
| Water | LDR | HDR | |
| Food intake (g) | 51.0 ± 0.7 | 52.3 ± 2.0 | 53.8 ± 1.0 |
| Water Intake (mL) | 122.5 ± 7.3 | 129.4 ± 12.0 | 115.0 ± 7.1 |
| Wet faecal output (g) | 6.2 ± 0.3 | 6.8 ± 0.3 | 6.6 ± 0.4 |
| Urine output (mL) | 79.3 ± 5.6 | 79.8 ± 6.1 | 85.8 ± 5.0 |
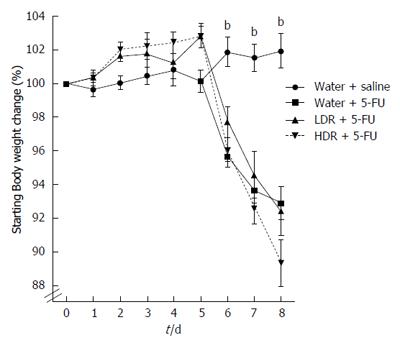
A reduction in feed intake was consistent with decreased bodyweight after 5-FU administration (Figure 2). Prior to inducing intestinal mucositis with 5-FU, RE had no significant effect on bodyweight. Treatment with 5-FU resulted in a significant reduction in bodyweight compared to normal controls (P < 0.01). However, compared to 5-FU controls, HDR and LDR had no effect on mean bodyweight following 5-FU administration.
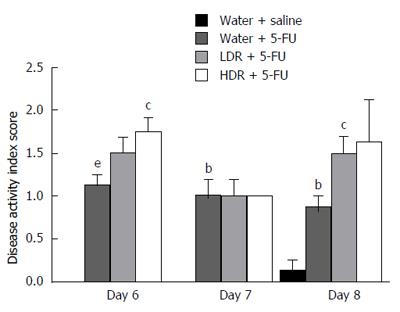
Administration of 5-FU significantly increased DAI scores in comparison to healthy controls (P < 0.01; Figure 3). Days 6 and 8 produced significantly greater DAI scores in 5-FU treated rats administered HDR and LDR, respectively, compared to 5-FU controls; otherwise, RE treatments had no significant effect on symptomatic disease activity.
Visceral and gastrointestinal organ weights were expressed as a proportion of bodyweight (Tables 3 and 4). Reductions in relative thymus (by ≥ 35%; P < 0.001) and relative spleen weight (by ≥ 23%; P < 0.001) were apparent in all rats treated with 5-FU when compared to healthy controls (Table 3). In 5-FU treated rats, HDR and LDR had no significant effect on visceral organ weights compared to 5-FU controls.
| Water + saline | Water + 5-FU | LDR + 5-FU | HDR + 5-FU | |
| Thymus | 14.6 ± 1.3 | 6.6 ± 0.5e | 9.4 ± 0.6 | 9.5 ± 0.9 |
| Heart | 37.5 ± 0.8 | 39.0 ± 1.0 | 39.4 ± 0.8 | 39.1 ± 0.7 |
| Lung | 60.0 ± 2.2 | 63.0 ± 2.5 | 67.3 ± 4.7 | 71.9 ± 3.0 |
| Liver | 362.9 ± 6.5 | 362.7 ± 11.0 | 358.8 ± 7.2 | 339.7 ± 7.2 |
| Spleen | 20.3 ± 0.5 | 15.6 ± 0.3e | 15.2 ± 0.4 | 14.6 ± 0.5 |
| Kidneys | 75.6 ± 5.3 | 86.5 ± 1.7 | 88.7 ± 1.1 | 89.4 ± 2.7 |
| Caecum | 39.7 ± 1.1 | 43.7 ± 2.4 | 49.2 ± 2.5 | 47.0 ± 2.1 |
| Stomach | 57.3 ± 2.6 | 55.3 ± 1.1 | 58.8 ± 0.9 | 61.9 ± 1.2 |
A significant decrease in the combined jejunum and ileum relative weight (by ≥ 10%; P < 0.01) was evident in all 5-FU treated rats (Table 4). However, this effect was not present in the duodenum. There was also no effect of HDR or LDR on relative duodenum weight and the combined relative weights of jejunum and ileum in 5-FU treated rats, compared to 5-FU controls. Administration of 5-FU had no effect on relative colon weight in comparison to healthy controls. However, when compared to 5-FU controls, administration of LDR to 5-FU treated rats significantly increased colon weight (29%; P < 0.01). Additionally, 5-FU significantly reduced the combined jejunum and ileum length in comparison to healthy controls (Table 5). However, this effect was not evident in the duodenum and colon. The administration of HDR and LDR to 5-FU treated rats had no effect on gastrointestinal organ lengths in comparison to 5-FU controls.
| Water + saline | Water + 5-FU | LDR + 5-FU | HDR+ 5-FU | |
| Duodenum | 5.5 ± 0.2 | 4.8 ± 0.1 | 5.1 ± 0.2 | 4.8 ± 0.2 |
| Jejunum and ileum | 71.6 ± 2.3 | 64.8 ± 0.9a | 62.9 ± 1.8 | 63.5 ± 1.7 |
| Colon | 11.1 ± 0.3 | 10.6 ± 0.4 | 11.2 ± 0.2 | 10.8 ± 0.4 |
Healthy small intestinal sections achieved median disease severity scores of ≤ 2. Administration of 5-FU caused significant damage to intestinal structure in the jejunum, JI and ileum; achieving median (range) scores of 21 (18-30), 21 (14-27) and 22 (17-25), respectively, when assessed by semi-quantitative histological scores based on 11 parameters (Figure 4). However, RE had no significant effect on intestinal structure, relative to 5-FU controls.
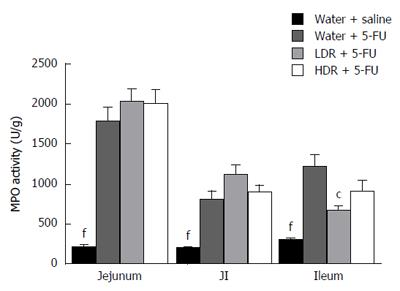
Increased intestinal MPO activity is a common feature of chemotherapy-induced intestinal mucositis[31]. When compared to healthy controls, 5-FU resulted in increased MPO activity by 780% in the jejunum and 310% in the JI and ileum (Figure 5). RE had no significant effect on MPO activity within the jejunum and the JI in 5-FU treated rats. However, administration of LDR to 5-FU treated rats resulted in reduced MPO activity by 45% (P < 0.05) in the ileum, compared to 5-FU controls.
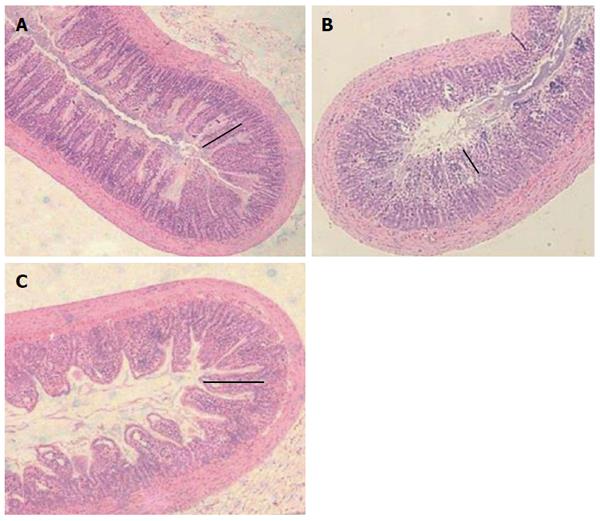
The combined measurements of villus height and crypt depth provided an overall indication of mucosal thickness and thus, damage (Figure 6). Administration of 5-FU significantly decreased mucosal thickness by 29% in the jejunum, and 34% in both the JI and ileum when compared to healthy controls. RE had no significant effect on villus height and crypt depth in the jejunum, compared to 5-FU controls. This effect was mirrored in the JI, with the exception of crypt depth which was significantly greater (P < 0.05) in 5-FU treated rats receiving HDR. More importantly, administration of LDR to 5-FU treated rats resulted in significantly greater ileal villus heights and crypt depths relative to 5-FU controls; significantly increasing overall ileal mucosal thickness by 19% (Figure 7).
Intestinal mucositis remains a debilitating side-effect of chemotherapy treatment. The current study utilised a rat model of intestinal mucositis to investigate the potential for aqueous RE to protect against damage to the intestinal mucosa and regulate water transport in the intestine. The water-soluble components of rhubarb appeared to target more distal regions of the alimentary tract, partially improving selected parameters of the ileum, such as mucosal thickness and MPO activity associated with the clinical manifestations of 5-FU-induced intestinal mucositis.
Administration of 5-FU significantly decreased feed intake and bodyweight as previously described[12,31,37]. A reduction in feed intake and bodyweight is observed in cancer patients due to nausea and pain associated with chemotherapy treatment[38,39]. Interestingly, in the current study, daily administration of HDR to 5-FU treated rats further reduced appetite but maintained bodyweight. It is therefore plausible that the caloric index of HDR may have been contributing to the reduced appetite, yet maintenance of bodyweight in the rats receiving high dose RE.
In the current study, intraperitoneal administration of 5-FU caused significant damage to small intestinal structure, further impacting on intestinal weight and length. Previous studies of experimental intestinal mucositis have noted a correlation between small intestinal weight and mucosal integrity which was also demonstrated in the current study[13,31]. Jejunum and ileum weights were significantly decreased in 5-FU treated rats, accompanied by increased villus and crypt damage when compared to healthy controls. Enterocyte apoptosis in 5-FU treated rats was likely responsible for the reduced small intestinal weight. However, RE administered to 5-FU treated rats had no significant effect on intestinal weight, compared to 5-FU controls, which suggested that RE did not enhance cell regeneration after 5-FU toxicity.
Administration of 5-FU may result in exposure of the submucosa to harsh luminal conditions[6]. As a compensatory mechanism, the muscularis externa contracts to reduce submucosal contact with the luminal environment in an attempt to prevent bacterial translocation. In the current study, the length of the total jejunum and ileum was reduced by 5-FU treatment, as described previously by Mashtoub et al[31]. However, consistent with previous studies, this effect was not present in the duodenum and colon as 5-FU damage was less severe in these regions of the intestine[12,13,31].
In the current study, LDR treatment resulted in a significant increase in ileal villus height and crypt depth; possibly representing LDR promoted crypt cell regeneration and hence, increased migration of rejuvenated cells to the villus. Alternatively, LDR may have exerted an anti-oxidative effect, mediated by the water soluble polysaccharides of rhubarb which may have protected the intestinal mucosa against cell apoptosis; maintaining villus and crypt structure. A reduction in ileal MPO activity by LDR in 5-FU treated rats indicated a decrease in neutrophil activity which further supports the anti-oxidative and anti-inflammatory properties of RE. These results are consistent with previous studies which have exploited plant polysaccharides for their anti-inflammatory and antioxidant properties[12,40,41]. Cheah et al[12,14] examined grape seed extract (GSE), a tannin rich by-product of the wine and grape juice industries, in the setting of chemotherapy-induced intestinal mucositis. It was discovered that GSE could partially ameliorate small intestinal inflammation and mucosal damage caused by 5-FU cytotoxicity. Tannins, an active constituent of GSE and possibly RE, possess the ability to prevent the overproduction of ROS or decrease the production of pro-inflammatory cytokines such as IL-4 and IFN-γ[12,17]. Further investigations are therefore required to understand the protective and anti-inflammatory mechanism of action of RE in improving acute intestinal inflammation and damage to the mucosa.
A significant improvement in ileal mucosal integrity and inflammation was observed in 5-FU rats treated with LDR, but not HDR. Limited RE studies have been conducted, therefore the low and high dose range of 20 mg/kg and 200 mg/kg were selected in the current study to determine the effects of RE across a broad dose range. The efficacy of RE in the current study may therefore have been dose-dependent. Prior to this study, the effects of RE on 5-FU-induced mucosal damage and inflammation were unknown and accordingly, the RE optimal concentration remains undefined. The present study suggested that the effectiveness of RE at varying concentrations may follow a normally distributed relationship. Potentially, at high concentrations (≥ 200 mg/kg BW), no significant effects may have been observed due to steric involution of bioactive binding sites. Further studies are therefore required to determine the optimal concentration to attain maximal mucosal protection.
Chemotherapy recipients experiencing intestinal mucositis have altered membrane integrity and impaired water absorption and secretion[7]. Any molecule of a similar size or shape possesses the capability to attach to the pore vestibule and block the transport of water through AQP channels. Pharmacological blockers of aquaporin fluid fluxes are thought to occlude the pore vestibule and impede the bi-directional transport of water through the channel[42-44]. In the current study, RE present in the circulatory system may have targeted AQP 4 channels within enterocytes, resulting in a unidirectional blockade, and thereby decreased water secretion into the lumen of the small intestine. This hypothesised theory is further explained in Figure 1D. Wang et al[29] determined that AQP 4 knockout mice had significantly higher stool moisture content in comparison to wild-type (P < 0.05). This suggested that stool consistency was dependent on the functionality of AQP 4 channels. This study also established that AQP 4 channels are scarce within the large intestine. Furthermore, within the large intestine, AQP 4 channels are only present on the initial section of the proximal colon[29]. Therefore, it is probable that fluid absorption and secretion across AQP 4 channels in the small intestine may have been partly responsible for the moisture content of the faeces in the current study. Further in vivo studies should identify the expression levels of AQP 4 and other aquaporins to determine morphological and potential functional changes after 5-FU exposure. Qin et al[18] demonstrated that aqueous RE improved stool consistency in mice with castor oil and magnesium sulphate-induced diarrhoea. Furthermore, aqueous RE caused constipation when administered to normal mice suggesting that RE may have been acting on AQP 4 channels to alter water absorption in the intestine. Consequently, further studies are required to determine the moisture content of caecal fluid to confirm or refute the hypothesis that RE affects stool consistency. This would allow for comparison of water absorption and secretion in the small intestine, independent of the colon. A reduction in caecal moisture content would suggest that RE was preventing fluid secretion across small intestinal AQP 4 channels.
In summary, the present study demonstrated that the ancient herbal remedy RE in its aqueous form, at relatively low dose, offers partial protection to the distal intestinal mucosa against tissue damage and inflammation associated with 5-FU-induced intestinal mucositis. Further studies are warranted to identify the anti-inflammatory and antioxidant properties of RE via examination of inflammatory cytokines in blood and tissue. This provides preliminary information regarding the potential use of RE as an adjunct to chemotherapy to improve particular histological manifestations of intestinal mucositis. Moreover, the reduced ileal inflammation and improved mucosal thickness suggests further therapeutic potential for other gastrointestinal inflammatory disorders that ultimately affect the more distal regions of the alimentary tract. However, the potential drug-drug interactions of RE and chemotherapy drugs, such as 5-FU should be thoroughly investigated as recent studies have highlighted concern over such interactions[45]. Future research should also focus on analysing moisture content of caecal fluid to determine whether RE acts as a unidirectional blocker of AQP 4 channels in the small intestine. Finally, further investigation into the active constituents of RE would be beneficial to improve our understanding of its potential utility in bowel disease and its associated mechanism of action.
The authors would like to thank Elizabeth Brown and Joseph Fabian for their assistance with pilot studies. Additionally, the authors would like to thank Shuguan Bi at the University of California Santa Barbara for assistance with lectin array profiling.
The need to discover effective treatment approaches for chemotherapy-induced intestinal mucositis is growing as cancer incidence continues to increase and thus, the incidence of treatment-related side-effects increases. Traditional medicines are continually being examined for their therapeutic potential in cancer and chemotherapy settings. Accordingly, the aqueous extract of rhubarb (Rheum Spp.) was investigated for its potential to improve intestinal integrity and acute inflammation in experimentally-induced intestinal mucositis in rats.
To our knowledge, this is the first study of its kind to identify the therapeutic effect of aqueous rhubarb extract (RE) in experimentally-induced intestinal mucositis.
This is the first study examining the potential for aqueous RE to improve intestinal integrity and acute inflammation in a rat model of 5-FU-induced intestinal mucositis.
The promising findings presented in the current study indicate that a low dose of aqueous RE improves selected parameters of 5-fluorouracil (5-FU)-induced intestinal mucositis. Future studies should determine the active factor of the compound so that it can be extracted and further examined for clinical efficacy.
5-FU is a widely utilised chemotherapy drug used to treat a range of cancer types from colon to breast cancer. It may be used independently however, is most commonly used in combination with other chemotherapy drugs, such as Methotrexate. RE was obtained from the stalks of the traditional herbal medicine Rheum spp. The low dose of RE (LDR) was based on the estimated dose required to block aquaporin water channel activity in the oocyte expression system, and the high dose (HDR) was selected as a 10 fold higher concentration for comparison. Aquaporins (AQPs) are integral membrane proteins responsible for the regulation of water transport across a membrane via an osmotic gradient. Currently, 13 mammalian AQPs have been identified (AQP 0-12). AQPs are abundant in tissues reliant on high water permeability to maintain correct function and are involved in metabolic processes such as kidney, lung, brain and gastrointestinal function.
This manuscript is well written. The scientific hypothesis and the appropriate tests are well explained and conducted. Results are fairly discussed, notably the question of the need for further experiments investigating an optimal dose.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liew FY, Touchefeu Y S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Wachtel-Galor S, Benzie IF. Herbal Medicine: An Introduction to Its History, Usage, Regulation, Current Trends, and Research Needs. Herbal Medicine: Biomolecular and Clinical Aspects. Boca Raton FL: Llc 2011; . |
| 2. | Ashikaga T, Bosompra K, O’Brien P, Nelson L. Use of complimentary and alternative medicine by breast cancer patients: prevalence, patterns and communication with physicians. Support Care Cancer. 2002;10:542-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Australian Institute of Health and Welfare & Australasian Association of Cancer Registries. Cancer in Australia: An overview of 2012. : Canberra 2012; . |
| 4. | Sonis ST. Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol. 1998;34:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 345] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Soares PM, Mota JM, Souza EP, Justino PF, Franco AX, Cunha FQ, Ribeiro RA, Souza MH. Inflammatory intestinal damage induced by 5-fluorouracil requires IL-4. Cytokine. 2013;61:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Sonis ST, Elting LS, Keefe D, Peterson DE, Schubert M, Hauer-Jensen M, Bekele BN, Raber-Durlacher J, Donnelly JP, Rubenstein EB. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100:1995-2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 985] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 7. | Gibson RJ, Keefe DM. Cancer chemotherapy-induced diarrhoea and constipation: mechanisms of damage and prevention strategies. Support Care Cancer. 2006;14:890-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Sakai H, Sagara A, Matsumoto K, Hasegawa S, Sato K, Nishizaki M, Shoji T, Horie S, Nakagawa T, Tokuyama S. 5-Fluorouracil induces diarrhea with changes in the expression of inflammatory cytokines and aquaporins in mouse intestines. PLoS One. 2013;8:e54788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Lalla RV, Peterson DE. Treatment of mucositis, including new medications. Cancer J. 2006;12:348-354. [PubMed] |
| 10. | Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J, Elting LS, Fox PC, Cooksley C, Sonis ST. Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer. 2004;100:2026-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 492] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 11. | Yazbeck R, Howarth GS. Complementary medicines: emerging therapies for intestinal mucositis. Cancer Biol Ther. 2009;8:1629-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Cheah KY, Howarth GS, Yazbeck R, Wright TH, Whitford EJ, Payne C, Butler RN, Bastian SE. Grape seed extract protects IEC-6 cells from chemotherapy-induced cytotoxicity and improves parameters of small intestinal mucositis in rats with experimentally-induced mucositis. Cancer Biol Ther. 2009;8:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Wright TH, Yazbeck R, Lymn KA, Whitford EJ, Cheah KY, Butler RN, Feinle-Bisset C, Pilichiewicz AN, Mashtoub S, Howarth GS. The herbal extract, Iberogast, improves jejunal integrity in rats with 5-Fluorouracil (5-FU)-induced mucositis. Cancer Biol Ther. 2009;8:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Cheah KY, Howarth GS, Bastian SE. Grape seed extract dose-responsively decreases disease severity in a rat model of mucositis; concomitantly enhancing chemotherapeutic effectiveness in colon cancer cells. PLoS One. 2014;9:e85184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Schepetkin IA, Quinn MT. Botanical polysaccharides: macrophage immunomodulation and therapeutic potential. Int Immunopharmacol. 2006;6:317-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 871] [Cited by in RCA: 887] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 16. | Peigen X, Liyi H, Liwei W. Ethnopharmacologic study of Chinese rhubarb. J Ethnopharmacol. 1984;10:275-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Liu L, Guo Z, Lv Z, Sun Y, Cao W, Zhang R, Liu Z, Li C, Cao S, Mei Q. The beneficial effect of Rheum tanguticum polysaccharide on protecting against diarrhea, colonic inflammation and ulceration in rats with TNBS-induced colitis: the role of macrophage mannose receptor in inflammation and immune response. Int Immunopharmacol. 2008;8:1481-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Qin Y, Wang JB, Kong WJ, Zhao YL, Yang HY, Dai CM, Fang F, Zhang L, Li BC, Jin C. The diarrhoeogenic and antidiarrhoeal bidirectional effects of rhubarb and its potential mechanism. J Ethnopharmacol. 2011;133:1096-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Carneiro-Filho BA, Lima IP, Araujo DH, Cavalcante MC, Carvalho GH, Brito GA, Lima V, Monteiro SM, Santos FN, Ribeiro RA. Intestinal barrier function and secretion in methotrexate-induced rat intestinal mucositis. Dig Dis Sci. 2004;49:65-72. [PubMed] |
| 20. | Agre P, King LS, Yasui M, Guggino WB, Ottersen OP, Fujiyoshi Y, Engel A, Nielsen S. Aquaporin water channels--from atomic structure to clinical medicine. J Physiol. 2002;542:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 809] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 21. | King LS, Kozono D, Agre P. From structure to disease: the evolving tale of aquaporin biology. Nat Rev Mol Cell Biol. 2004;5:687-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 693] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 22. | Cui Y, Bastien DA. Water transport in human aquaporin-4: molecular dynamics (MD) simulations. Biochem Biophys Res Commun. 2011;412:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Ishibashi K. New members of mammalian aquaporins: AQP10-AQP12. Handb Exp Pharmacol. 2009;251-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Nicchia GP, Nico B, Camassa LM, Mola MG, Loh N, Dermietzel R, Spray DC, Svelto M, Frigeri A. The role of aquaporin-4 in the blood-brain barrier development and integrity: studies in animal and cell culture models. Neuroscience. 2004;129:935-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 25. | Frigeri A, Gropper MA, Turck CW, Verkman AS. Immunolocalization of the mercurial-insensitive water channel and glycerol intrinsic protein in epithelial cell plasma membranes. Proc Natl Acad Sci USA. 1995;92:4328-4331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 306] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 26. | Mobasheri A, Marples D, Young IS, Floyd RV, Moskaluk CA, Frigeri A. Distribution of the AQP4 water channel in normal human tissues: protein and tissue microarrays reveal expression in several new anatomical locations, including the prostate gland and seminal vesicles. Channels (Austin). 2007;1:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Ricanek P, Lunde LK, Frye SA, Støen M, Nygård S, Morth JP, Rydning A, Vatn MH, Amiry-Moghaddam M, Tønjum T. Reduced expression of aquaporins in human intestinal mucosa in early stage inflammatory bowel disease. Clin Exp Gastroenterol. 2015;8:49-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Koyama Y, Yamamoto T, Tani T, Nihei K, Kondo D, Funaki H, Yaoita E, Kawasaki K, Sato N, Hatakeyama K. Expression and localization of aquaporins in rat gastrointestinal tract. Am J Physiol. 1999;276:C621-C627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 206] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 29. | Wang KS, Ma T, Filiz F, Verkman AS, Bastidas JA. Colon water transport in transgenic mice lacking aquaporin-4 water channels. Am J Physiol Gastrointest Liver Physiol. 2000;279:G463-G470. [PubMed] |
| 30. | Tomas FM, Murray AJ, Jones LM. Modification of glucocorticoid-induced changes in myofibrillar protein turnover in rats by protein and energy deficiency as assessed by urinary excretion of Ntau-methylhistidine. Br J Nutr. 1984;51:323-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Mashtoub S, Tran CD, Howarth GS. Emu oil expedites small intestinal repair following 5-fluorouracil-induced mucositis in rats. Exp Biol Med (Maywood). 2013;238:1305-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Howarth GS, Francis GL, Cool JC, Xu X, Byard RW, Read LC. Milk growth factors enriched from cheese whey ameliorate intestinal damage by methotrexate when administered orally to rats. J Nutr. 1996;126:2519-2530. [PubMed] |
| 33. | Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 442] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 34. | Beyer AJ, Smalley DM, Shyr YM, Wood JG, Cheung LY. PAF and CD18 mediate neutrophil infiltration in upper gastrointestinal tract during intra-abdominal sepsis. Am J Physiol. 1998;275:G467-G472. [PubMed] |
| 35. | Campbell EM, Birdsell DN, Yool AJ. The activity of human aquaporin 1 as a cGMP-gated cation channel is regulated by tyrosine phosphorylation in the carboxyl-terminal domain. Mol Pharmacol. 2012;81:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Yool AJ, Morelle J, Cnops Y, Verbavatz JM, Campbell EM, Beckett EA, Booker GW, Flynn G, Devuyst O. AqF026 is a pharmacologic agonist of the water channel aquaporin-1. J Am Soc Nephrol. 2013;24:1045-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Torres DM, Tooley KL, Butler RN, Smith CL, Geier MS, Howarth GS. Lyprinol only partially improves indicators of small intestinal integrity in a rat model of 5-fluorouracil-induced mucositis. Cancer Biol Ther. 2008;7:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 38. | Green R, Horn H, Erickson JM. Eating experiences of children and adolescents with chemotherapy-related nausea and mucositis. J Pediatr Oncol Nurs. 2010;27:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 39. | Smith JL, Malinauskas BM, Garner KJ, Barber-Heidal K. Factors contributing to weight loss, nutrition-related concerns and advice received by adults undergoing cancer treatment. Adv Med Sci. 2008;53:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Cheng CL, Koo MW. Effects of Centella asiatica on ethanol induced gastric mucosal lesions in rats. Life Sci. 2000;67:2647-2653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 41. | Garrido G, González D, Lemus Y, García D, Lodeiro L, Quintero G, Delporte C, Núñez-Sellés AJ, Delgado R. In vivo and in vitro anti-inflammatory activity of Mangifera indica L. extract (VIMANG). Pharmacol Res. 2004;50:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Seeliger D, Zapater C, Krenc D, Haddoub R, Flitsch S, Beitz E, Cerdà J, de Groot BL. Discovery of novel human aquaporin-1 blockers. ACS Chem Biol. 2013;8:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Wacker SJ, Aponte-Santamaría C, Kjellbom P, Nielsen S, de Groot BL, Rützler M. The identification of novel, high affinity AQP9 inhibitors in an intracellular binding site. Mol Membr Biol. 2013;30:246-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Migliati E, Meurice N, DuBois P, Fang JS, Somasekharan S, Beckett E, Flynn G, Yool AJ. Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol Pharmacol. 2009;76:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 45. | Ma L, Zhao L, Hu H, Qin Y, Bian Y, Jiang H, Zhou H, Yu L, Zeng S. Interaction of five anthraquinones from rhubarb with human organic anion transporter 1 (SLC22A6) and 3 (SLC22A8) and drug-drug interaction in rats. J Ethnopharmacol. 2014;153:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |