Published online Sep 28, 2016. doi: 10.3748/wjg.v22.i36.8211
Peer-review started: May 13, 2016
First decision: June 20, 2016
Revised: July 24, 2016
Accepted: August 23, 2016
Article in press: August 23, 2016
Published online: September 28, 2016
Processing time: 137 Days and 18.7 Hours
To evaluate the perspective of gastroenterologists regarding the impact of fecal calprotectin (FC) on the management of patients with inflammatory bowel disease (IBD).
Patients with known IBD or symptoms suggestive of IBD for whom the physician identified that FC would be clinically useful were recruited. Physicians completed an online “pre survey” outlining their rationale for the test. After receipt of the test results, the physicians completed an online “post survey” to portray their perceived impact of the test result on patient management. Clinical outcomes for a subset of patients with follow-up data available beyond the completion of the “post survey” were collected and analyzed.
Of 373 test kits distributed, 290 were returned, resulting in 279 fully completed surveys. One hundred and ninety patients were known to have IBD; 147 (77%) with Crohn’s Disease, 43 (21%) Ulcerative Colitis and 5 (2%) IBD unclassified. Indications for FC testing included: 90 (32.2%) to differentiate a new diagnosis of IBD from Irritable Bowel Syndrome (IBS), 85 (30.5%) to distinguish symptoms of IBS from IBD in those known to have IBD and 104 (37.2%) as an objective measure of inflammation. FC levels resulted in a change in management 51.3% (143/279) of the time which included a significant reduction in the number of colonoscopies (118) performed (P < 0.001). Overall, 97.5% (272/279) of the time, the physicians found the test sufficiently useful that they would order it again in similar situations. Follow-up data was available for 172 patients with further support for the clinical utility of FC provided.
The FC test effected a change in management 51.3% of the time and receipt of the result was associated with a reduction in the number of colonoscopies performed.
Core tip: Fecal calprotectin (FC) is a biomarker that provides a method of non-invasive assessment for intestinal inflammation. We evaluated the perspective of a group of gastroenterologists regarding the clinical use of FC, in particular the impact it has on the management of patients with inflammatory bowel disease (IBD). Patients with suspected or known IBD were recruited for study participation. A “pre survey” and “post survey” were completed by the physician prior to and after receipt of the FC result respectively. Of the 279 FC and surveys completed, FC levels resulted in a change in management 51.3% of the time and resulted in a significant reduction in colonoscopies performed.
- Citation: Rosenfeld G, Greenup AJ, Round A, Takach O, Halparin L, Saadeddin A, Ho JK, Lee T, Enns R, Bressler B. FOCUS: Future of fecal calprotectin utility study in inflammatory bowel disease. World J Gastroenterol 2016; 22(36): 8211-8218
- URL: https://www.wjgnet.com/1007-9327/full/v22/i36/8211.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i36.8211
Calprotectin is a cytosolic protein of mucosal neutrophils of the colon and small bowel that are extruded into the gastrointestinal (GI) tract when neutrophils undergo apoptosis[1]. Fecal calprotectin (FC) levels can be measured quantitatively in the stool from patients with mucosal inflammation in the bowel wall as seen in active inflammatory bowel disease (IBD)[2]. In fact, FC levels have been shown to be closely correlated with endoscopic evidence of mucosal inflammation in the intestine[3]. FC is a simple, non-invasive test that can be measured with an ELISA or a rapid detection device.
Calprotectin has previously been shown to be a reliable marker of mucosal inflammation and several studies have explored the use of FC to distinguish the symptoms of irritable bowel syndrome (IBS) from IBD[4,5], to monitor response to therapy in patients with known IBD[6] and to assess for mucosal healing[7]. Furthermore, recent studies have evaluated the ability of FC to predict disease flares in patients with IBD in remission[8-10] as well as in the surveillance for post-operative Crohn’s disease (CD) recurrence[11].
While the use of FC in clinical practice is increasing, there are limited studies that have looked at the physician experience and perspective of the use FC for the differentiation of GI symptoms as IBS or IBD, as well as for the assessment of IBD activity. This study evaluated the impact of FC test results in the setting of the aforementioned indications on the medical management of patients and the need for colonoscopy in an outpatient clinical cohort, as well as patient outcomes stratified to FC test results.
This was a multicenter, prospective cohort study including adult community and academic gastroenterology practices in British Columbia (BC), Canada. Gastroenterologists who had an affiliation with the University of BC (UBC) were invited to participate. Clinicians identified patients for whom FC was considered to be an appropriate next step in diagnosis and/or management of symptoms. Patients with known ischemic colitis, infectious enterocolitis or colorectal cancer or who were pregnant were excluded. Other exclusions included a history of extensive bowel resection (subtotal colectomy or more than three bowel resections), an ostomy, an ileoanal pouch, concurrent daily use of non-steroidal anti-inflammatory drugs or the inability to collect a stool sample and return it within three days.
Eligible patients were invited to enroll by the attending gastroenterologist during clinical care from February 2012 to August 2013. Written informed consent was obtained and patients were provided with the Easy Sampler™ stool collection kit and written instructions regarding collection of a first morning stool sample. Five to 15 g of stool were collected and processed within five days of collection.
The stool samples were prepared and analysed according to the manufacturer’s instructions using the BÜHLMANN Quantum Blue™ Calprotectin kit (BÜHLMANN Laboratories AG, Schönenbuch, Switzerland). All samples were processed by a trained research assistant at the Gastrointestinal Research Institute in Vancouver, BC. FC levels were expressed as micrograms per gram of feces with a range of 100 to > 1800 μg/g. Physicians were informed that a FC level of > 250 μg/g was considered indicative of inflammatory activity, however it was at the discretion of the individual treating physician as to how to incorporate the FC result into patient management.
After enrollment, but prior to receiving the FC result, the attending physician completed an online “pre-survey” using an internet based survey tool (Survey Monkey Inc., Palo Alto, CA, United States) (Supplementary Material 1). The baseline survey captured demographic data as well as any history of IBD, the indication for the test, concurrent investigations being ordered and current management of the symptoms.
During completion of the survey, the physician indicated the clinical scenario for FC testing, with the use of the following categories: (1) differentiation of GI symptoms between IBS and IBD in the absence of a known diagnosis of IBD; (2) differentiation of symptoms in patients known to have IBD; (3) assessment of response to therapy in a patient with IBD; and (4) objective marker of disease activity in a patient with IBD. Categories 3 and 4 were separated in the survey to clearly identify the indication for the test, however were subsequently combined as the indication of inflammatory disease activity assessment to aid with analysis. The FC result was then sent to the physician and the physician completed a “post-survey” using the same internet based survey tool (Supplementary Material 2). This survey was designed to assess the impact of the FC result on the diagnosis and management of the patient’s symptoms. Outcomes according to survey responses are presented within Results under the sub-heading survey analysis.
The two surveys had not been used previously but were piloted by the primary investigator to ensure ease of use and that the surveys represented the actual management decisions of the physician. During the study, 20 patient charts were audited to ensure that the information in the survey accurately reflected the patient management.
Validity of the post-survey was further ensured by follow up of a subset of patients to determine if there was a deviation from the management proposed in the post-survey response. In particular, colonoscopy occurrence within six months following FC collection was considered relevant with respect to reliability of colonoscopy deferral indicated by post-survey responses. For this subset of patients, medical records were retrospectively reviewed in November 2015, enabling follow-up extending to 26 mo after the final post-survey submission. Findings are presented in Results under the sub-heading of follow-up analysis.
To gain insight into the predictability of FC for future IBD outcomes, within the aforementioned patient subset, clinical events in the twelve months following obtainment of FC were also considered, in particular the need for steroids, immunomodulators, biologics, hospitalization or surgery.
The primary outcome was the frequency with which the FC result resulted in a change in the management of the patient as indicated by the pre- and post- surveys. Secondary outcomes included the deferral of colonoscopies as a result of the FC level, the proportion of events for which the physician found the FC level to be useful in the care of the patient and the association of the FC result to subsequent clinical, endoscopic and/or radiological outcomes.
The primary outcome of change in management was analysed according to a positive result being > 250 μg/g in addition to > 100 μg/g, given the heterogeneity in the indication for FC in the study cohort as well as the uncertainty in the current literature relating to what is considered a “positive” and “negative” FC. Within the follow up subset, a FC result of > 100 μg/g was considered positive.
As this pilot study was predominantly descriptive in nature and not designed to test a specific hypothesis, no sample size calculation was performed. Using the Fisher’s exact and McNemar χ2 tests respectively, there was comparison of the impact of a positive and negative FC test on a decision to change patient management and on the number of colonoscopies performed. Statistical calculations were performed using GraphPad Prism (GraphPad Software Inc., United States) and Statistical Analysis (Version 9.4, United States) software. The statistical methods were overseen by Dr. Terry Lee. (Centre for Health Evaluation and Outcome Sciences, St Paul’s Hospital).
Ethics approval was obtained from the Providence Health Care and the UBC research ethics board and the trial was registered at ClinicalTrials.gov (NCT01676324). All of the authors had access to the study data and reviewed and approved the final manuscript.
Seventeen gastroenterologists from eight different community and academic centers throughout BC distributed a total of 373 test kits. Seven of the 17 gastroenterologists were from academic centres. Two hundred and ninety (77.7%) kits were returned for processing resulting in 279 fully completed surveys. Of the 279 patients who returned the kits for processing, 177 (63.4%) were from academic centres.
The patients ranged between 19 and 79 years of age. One hundred and ninety (70%) were previously known to have IBD; 147 (77%) with CD, 43 (23%) with ulcerative colitis and 5 (3%) with IBD unclassified. The number of FC tests for each indication group were: 1.90 (32.2%) to differentiate a new diagnosis of IBS from IBD; 2.85 (30.5%) to distinguish GI symptoms due to active inflammation from IBS in those known to have IBD and 3.104 (37.2%) as an objective measure of disease activity in IBD. Of the 83 samples that were not returned, the indication for the test was to distinguish a new diagnosis of IBS from IBD in 31.3% (26/83), to assess abdominal symptoms in patients known to have IBD in 28.9% (24/83) and as a measure of inflammatory disease activity in 39.8% (33/83).
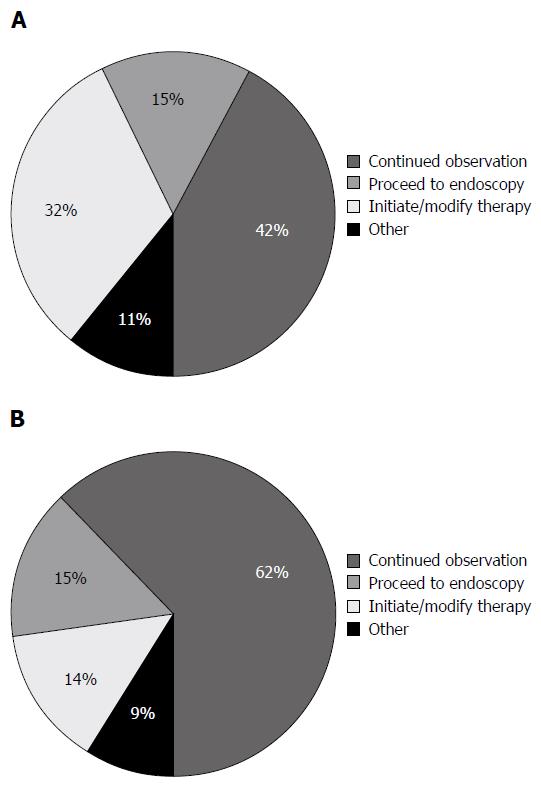
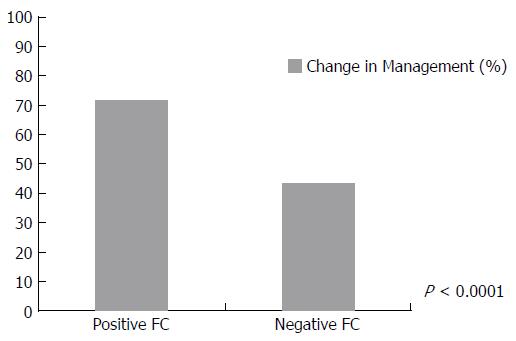
Overall, FC test resulted in a change in management 51.3% of the time (143/279) according to the physician response to the post-survey. Table 1 shows the breakdown of the impact of FC on patient management based on the indication for performing the test, the disease type, the disease location and current treatment. Specific management decisions following receipt of FC test result are shown in Figure 1. When using either a cut-off of 250 or 100 μg/g, patients were significantly more likely to have a change in management when the FC result was positive (250 μg/g, P < 0.0001 (Figure 2)) (100 μg/g, P = 0.0009).
| Change management | Total | Yes | No | Unsure | Pos FC (> 250 μg/g) | Neg FC (< 250 μg/g) |
| n | 279 | 143 | 121 | 15 | 81 | 198 |
| Age | 39.4 ± 13.5 | 38.9 | 40 | 40.5 | ||
| Disease location, n (%) | ||||||
| Crohn's disease | 147 | 56 (38.1) | 88 (59.9) | 3 (2.0) | 55 (37.4) | 92 (62.6) |
| UC | 43 | 17 (39.5) | 20 (46.5) | 6 (14.0) | 20 (46.5) | 23 (53.5) |
| Current Rx | ||||||
| 5-ASA | 44 | 16 | 24 | 4 | 18 | 26 |
| Steroids | 23 | 17 | 4 | 2 | 11 | 12 |
| Biologics | 58 | 32 | 24 | 2 | 24 | 34 |
| IMM | 63 | 35 | 24 | 4 | 28 | 35 |
| None | 136 | 67 | 61 | 8 | 24 | 112 |
| Indication for FC test | ||||||
| To diagnose IBD | 90 | 40 | 45 | 5 | 7 | 83 |
| To distinguish IBS from IBD | 85 | 46 | 37 | 2 | 33 | 52 |
| As an objective measure of disease activity | 104 | 57 | 39 | 8 | 41 | 63 |
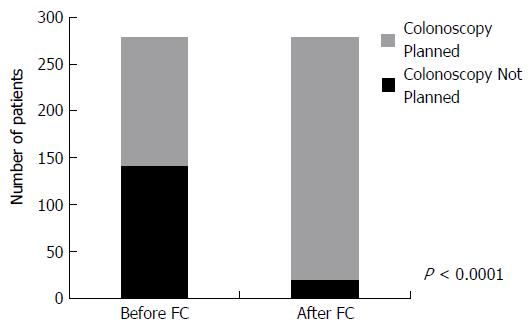
For 142 patients, the physician reported that if the FC test was not available, they would have performed a colonoscopy. After receipt of the FC result, a colonoscopy was planned according to the post-survey responses for 20 patients resulting in 122 colonoscopies (86%) that were not performed in favour of performing a FC test. Conversely, for 137 patients, a colonoscopy was not planned, however, after receipt of the FC result, 4 patients underwent a colonoscopy as per the post-survey responses. Combining these two groups, there was a significant reduction in the total number of colonoscopies performed (118) due to the availability of the FC test (P < 0.001) (Figure 3).
A change in medical therapy was planned for 36 patients, however, only six patients had a change in medical therapy after the physician received the FC result. Of the 243 patients for whom no change in therapy was planned, 41 underwent a change in their therapy after the FC test. For 60% (28/47) of the patients who underwent a change in therapy after the FC result, this change involved the initiation of a biologic (Infliximab or Adalimumab).
Physicians reported the test to be sufficiently useful 97.5% of the time and to the extent that they would order it again for a similar patient in a similar clinical situation. For the seven cases when the test was not felt to be useful, three FC results were greater than 250 μg/g (454, > 1800, > 1800) and four less than 250 μg/g (< 100, < 100, 150, 233). Whether or not the FC level was positive (with either a cut off of > 100 or 250) did not significantly impact whether or not the physician found the test to be useful (P = 0.4281 for > 250 μg/g; P = 0.1405 for > 100 μg/g).
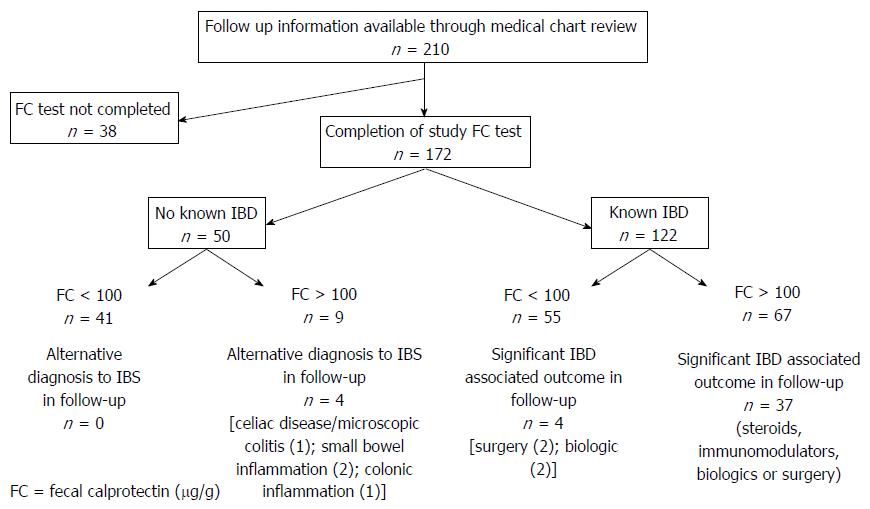
Medical record review was possible for 210 of the initial 373 patients recruited to the study. All of these patients had been recruited from an academic referral centre. For 172 of these 210 patients, FC testing and surveys were completed. (Figure 4) Of the remaining 38 patients who did not complete study FC testing, 31 patients were known to have IBD. Four of the 38 patients had endoscopic investigations instead at the discretion of the treating physician, while seven patients did not return for follow up after the initial recommendation for FC testing.
Review of available medical records for the 172 patients within the follow up subset indicated that for 33 patients there was deviation from the intended management that had been stated in the post-survey response. Accordingly, when considering the impact of FC on occurrence of colonoscopy, data pertaining to the number of colonoscopies that occurred in the six months following performance of FC within this follow-up subgroup was analyzed. As indicated by the pre-survey, the physicians reported that for 95 patients if the FC test was not available, they would have performed a colonoscopy. After receipt of the FC result, colonoscopy occurred in 24 of these patients over the subsequent six months and hence 72 colonoscopies (75%) were not performed as a result of the use of FC. Conversely, for 77 patients, a colonoscopy was not intended, however, after receipt of FC result, 11 patients underwent a colonoscopy. Combining these two groups, similarly to the original cohort analysis, there remained a significant reduction in the total number of colonoscopies performed (P < 0.001).
A degree of deviation between post-survey response and actual therapeutic management was detected during the follow-up analysis. Despite the post-survey responses indicating initiation or modification of medical therapy for 36 patients, this did not end up occurring for ten [therapy not initiated (8); not ceased (1); different immunomodulator started (1)].
Within the follow up cohort, 50 patients had undergone FC in the absence of an established diagnosis of IBD. Forty one patients had a FC result < 100 μg/g of whom eight subsequently had normal endoscopic assessment. Of the remaining 33 patients with a FC < 100 μg/g, there have not been subsequent presentations and/or assessments to suggest an alternative diagnosis to IBS since the FC testing. While for a proportion of patients there has been no further follow up at the academic centre since FC testing, this was considered likely to be reflective of no further clinical presentations given the nature of patients to be referred back to the same academic centre within the region.
Of the remaining nine patients with a FC result > 100 μg/g in whom a diagnosis of IBS or IBD was yet to be established, four had abnormalities on subsequent investigations, while five have not had further investigations or presentations.
Within the IBD Cohort, 55 patients had a FC result < 100 μg/g. Of these patients, colonoscopy had been deferred in 24 following receipt the FC result. In 22 of these 24 patients there were no significant clinical outcomes recorded in the following 12 mo. One patient required surgery due to to small bowel obstruction attributable to adhesions, and another patient had a CT enterography demonstrating active ileal disease for which an an anti-TNF agent was commenced. Of the remaining 31 patients with a FC < 100 μg/g, two patients had discordant findings endoscopically. One patient was found to have active ileocolonic disease within six months, however there had been recent anti-TNF cessation and another patient had endoscopic detection of active ileal disease in the setting of post-operative recurrence surveillance, and was recommenced on anti-TNF therapy.
Of the 67 patients with a FC result > 100 μg/g, when reviewing the available medical records, there were clinically relevant outcomes over the following six months in 37 patients with either a need for steroids, immunomodulators, biologics, surgery or a combination of such management. Such changes had been captured in 23 of the post-survey responses.
The role of FC in GI disease assessment is continuing to evolve since it was first described in 1980, with such evolution including the indications for which FC is considered useful, as well as what defines the appropriate cut-off level. Accompanying such progression is ongoing debate as to the reliability and clinical significance of this biomarker.
Given persistent contention regarding the applicability of FC, this study aimed to systematically assess physician opinion of the utility of FC in the clinical management of patients with either suspected IBD or IBS or established IBD. Our results resonate the previously described utility of FC in this area of gastroenterology, with responding physicians indicating 97% of the time the test was sufficiently useful[5,12]. Furthermore, the FC test impacted patient management more than half of the time with the greatest impact being an 86% reduction in the number of colonoscopies performed. Seventeen percent of patients had a change in their medical therapy as a result of the FC test and for 28 (60%) of these patients that change involved the initiation of a biologic. Such findings exemplify the high degree of utility of FC that was expressed by the study gastroenterologists.
There is mounting evidence that treatment of patients with IBD should be to a target of mucosal healing[6,10,13] and not simply improvement in symptoms. This is due to a lack of correlation of symptoms with IBD activity, particularly for patients with CD. In the current study, 35 of 41 patients underwent a change in medical therapy for IBD after the physician received an elevated FC, implying that such patients were likely clinically quiescent and hence initially no change in management was planned. However, receipt of the positive FC result resulted in a modification of therapy inferring that the goal of therapy was a reduction in mucosal inflammation and not just an improvement in symptoms, while also is likely reflective of the physician trust in FC as a marker of disease activity. As physicians gain more experience and confidence with FC results, one can anticipate even higher rates of change in medical therapy based on FC results.
Follow up of patient outcomes for a subgroup of the study population indicated that receipt of a FC result continued to minimize the need for colonoscopy for at least six months following completion of the post-survey, with 74% of the initially intended colonoscopies remaining deferred. Furthermore, follow up data also provided reassurance for the sensitivity of FC value of < 100 μg/g in the differentiation of IBS from IBD, with no suggestion of alternative diagnoses to IBS during follow up. However, a threshold of a FC result of 100 μg/g was at the expense of sensitivity, with five patients with a FC result greater than 100 μg/g continuing to have the most likely diagnosis of IBS during follow up. Previous studies have considered the diagnostic accuracy of FC levels to differentiate IBD from IBS, including Kennedy et al[5] who reported a sensitivity and specificity of 96% and 87% respectively when a FC threshold of 100 μg/g was used in an outpatient cohort of 895 patients. Within our follow up subgroup, a clinically significant change occurred in 55% of patients with IBD who had an elevated FC above 100 μg/g, which is consistent with several studies demonstrating the utility of an elevated FC for predicting clinical flare in IBD[8-10]. Finally, the finding that a proportion of patients with a normal FC were subsequently found to have active small bowel disease is reflective of the known limitation of FC in this setting.
Targeting treatment to objective measures of disease activity in our current cost conscious environment is a very challenging task. An accurate and inexpensive marker of luminal inflammation such as FC may play a critical role in achieving targeted therapy. Overall, 118 colonoscopies were deferred as a result of the FC test in the current study. Previous studies looking at the cost of colonoscopy have estimated the costs to range between a low of $352 in Canada to a high of $2146 in the United States[14,15]. The Canadian data is now approaching ten years old and taking the average cost reported in this study and adjusting for cost increases and inflation, one could conservatively estimate the cost to be at least $500 in Canada. This represents nearly $50000 saved on colonoscopy alone with the use of FC testing and does not consider any savings from optimization of medical management for patients with IBD (reduction of surgeries or hospitalization). Another study has also shown FC to be cost effective as a screening tool for IBD in both adults and children when the pre test probability was ≤ 75% and ≤ 65% respectively[4].
Limitations to this study include potential bias created by patient inclusion being solely dependent on the discretion of the involved physician. This accordingly has contributed to a heterogeneous patient population and is reflective of the limited guidance regarding the most appropriate use of FC at the time of study design. Given this was a real-life study, there was also unavoidable potential for inclusion of patients with co-existing undiagnosed conditions that could influence the FC level such as colonic polyps or additional immunologically driven digestive disorders. Another potential source of bias is that more than 20% of patients did not return FC samples. However, such patients were similarly distributed across the indications for the test suggesting that this did not influence the patient’s decision to carry out the test. It is acknowledged that questionnaires were not previously validated although a degree of quality control was performed during the study to endeavor to ensure that the surveys accurately reflected the clinical practice of the doctors, with initial perusal of 20 surveys, and then a further follow up of 210 patients. The considerable duration of follow-up possible for the latter patients did enable adjustment for variation between what had been indicated by the physician on the post-survey response and what actually occurred.
Finally, at the time of initial study design, a FC level of 250 μg/g was considered appropriate for analysis of outcomes, however at the time of follow up analysis in 2015, a level of 100 μg/g was considered more appropriate. The “grey zone” between 100 and 250 μg/g and the accompanying need for additional investigations, particularly in IBD was highlighted in the recently published clinician guide to using FC to identify and monitor disease activity in IBD[12].
In conclusions, FC is a simple, non-invasive test that is gaining widespread use in the diagnosis and management of IBD. This is the first Canadian data evaluating the role of FC in clinical practice, with demonstration that physicians find FC testing to be very useful not only for managing patients known to have IBD, but also to diagnose IBD in those with GI symptoms. This study also demonstrated the potential of FC to generate substantial cost savings to patients and the healthcare system in general. While the study was not designed to statistically assess clinical outcomes according to FC result, the descriptive findings are in concurrence with recommendations that FC can be used to differentiate between IBS and IBD, as well as be a guide to optimizing IBD therapy. It is hoped that studies such as this in addition to ongoing FC research pursuits will provide ongoing impetus for the availability and affordability of FC to clinicians and patients to be considered a priority by health service administrators.
The importance of objective, non-invasive assessment of luminal gastrointestinal (GI) symptoms in the diagnosis and management of inflammatory bowel disease (IBD) is gaining increasing recognition. One strategy to meet such a need is the use of the biomarker, fecal calprotectin (FC).
There is expanding literature regarding the use of FC, including differentiating symptoms of irritable bowel syndrome (IBS) from IBD, to monitor response to therapy in patients with known IBD and in the surveillance for post-operative Crohn’s disease recurrence. Nonetheless, there is limited knowledge regarding the perspective of physicians using FC in the diagnostic and management algorithms for IBD with respect to potential impact on patient management.
The authors present the findings of this study which considered the perceived utility of FC within an academic group of gastroenterologists. Physician perspective was obtained through the use of on-line surveys, while medical record review occurred in addition to this to determine clinical outcomes following FC testing. Faecal calprotectin was considered to have an impact on patient management in at least 50% of clinical circumstances in which it was used, including a reduction in the number of colonoscopies performed. Furthermore FC results and subsequent clinical outcomes provided support for the use of such a test in the differentiation of IBS from IBD as well as guiding therapeutic decisions in IBD.
This study supports the use of FC in clinical gastroenterology practice for the assessment of luminal GI symptoms to enable the differentiation of IBS and IBD and in the management of established IBD. This study also prompts further consideration of the potential cost-saving benefit of FC.
FC is a cytosolic neutrophilic protein in the mucosa of the colon and small bowel which is released as a result of apoptosis. It can be measured quantitatively in the stool from patients with mucosal inflammation in the bowel wall as seen in active IBD.
The implementation of FC in clinical practice is a topic of importance and interest to the gastroenterology and wider primary care community. This manuscript is well written and the methodology, whilst imperfect, has characterised the role of calprotectin well in real world practice, showing a potential benefit in terms of cost reduction by reducing number of colonoscopies in this group.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Day AS, de Silva AP, Lakatos PL, Serban DE, van Langenberg DR S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Røseth AG, Aadland E, Jahnsen J, Raknerud N. Assessment of disease activity in ulcerative colitis by faecal calprotectin, a novel granulocyte marker protein. Digestion. 1997;58:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 258] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Costa F, Mumolo MG, Ceccarelli L, Bellini M, Romano MR, Sterpi C, Ricchiuti A, Marchi S, Bottai M. Calprotectin is a stronger predictive marker of relapse in ulcerative colitis than in Crohn’s disease. Gut. 2005;54:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 424] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 3. | Røseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999;34:50-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 307] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 4. | Yang Z, Clark N, Park KT. Effectiveness and cost-effectiveness of measuring fecal calprotectin in diagnosis of inflammatory bowel disease in adults and children. Clin Gastroenterol Hepatol. 2014;12:253-262.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Kennedy NA, Clark A, Walkden A, Chang JC, Fascí-Spurio F, Muscat M, Gordon BW, Kingstone K, Satsangi J, Arnott ID. Clinical utility and diagnostic accuracy of faecal calprotectin for IBD at first presentation to gastroenterology services in adults aged 16-50 years. J Crohns Colitis. 2015;9:41-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Kopylov U, Rosenfeld G, Bressler B, Seidman E. Clinical utility of fecal biomarkers for the diagnosis and management of inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:742-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 131] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 7. | Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, Seibold F. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol. 2010;105:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 421] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 8. | García-Sánchez V, Iglesias-Flores E, González R, Gisbert JP, Gallardo-Valverde JM, González-Galilea A, Naranjo-Rodríguez A, de Dios-Vega JF, Muntané J, Gómez-Camacho F. Does fecal calprotectin predict relapse in patients with Crohn’s disease and ulcerative colitis? J Crohns Colitis. 2010;4:144-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 9. | Mao R, Xiao YL, Gao X, Chen BL, He Y, Yang L, Hu PJ, Chen MH. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm Bowel Dis. 2012;18:1894-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 232] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 10. | Molander P, af Björkesten CG, Mustonen H, Haapamäki J, Vauhkonen M, Kolho KL, Färkkilä M, Sipponen T. Fecal calprotectin concentration predicts outcome in inflammatory bowel disease after induction therapy with TNFα blocking agents. Inflamm Bowel Dis. 2012;18:2011-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 11. | Wright EK, Kamm MA, De Cruz P, Hamilton AL, Ritchie KJ, Krejany EO, Leach S, Gorelik A, Liew D, Prideaux L. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology. 2015;148:938-947.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 224] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 12. | Bressler B, Panaccione R, Fedorak RN, Seidman EG. Clinicians’ guide to the use of fecal calprotectin to identify and monitor disease activity in inflammatory bowel disease. Can J Gastroenterol Hepatol. 2015;29:369-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Smith LA, Gaya DR. Utility of faecal calprotectin analysis in adult inflammatory bowel disease. World J Gastroenterol. 2012;18:6782-6789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (2)] |
| 14. | Sharara N, Adam V, Crott R, Barkun AN. The costs of colonoscopy in a Canadian hospital using a microcosting approach. Can J Gastroenterol. 2008;22:565-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Pyenson B, Scammell C, Broulette J. Costs and repeat rates associated with colonoscopy observed in medical claims for commercial and Medicare populations. BMC Health Serv Res. 2014;14:92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |