Published online Sep 28, 2016. doi: 10.3748/wjg.v22.i36.8203
Peer-review started: March 18, 2016
First decision: July 12, 2016
Revised: July 25, 2016
Accepted: August 8, 2016
Article in press: August 8, 2016
Published online: September 28, 2016
Processing time: 194 Days and 15.4 Hours
To investigate clinicopathological features of early stage gastric cancer with enteroblastic differentiation (GCED).
We retrospectively investigated data on 6 cases of early stage GCED and 186 cases of early stage conventional gastric cancer (CGC: well or moderately differentiated adenocarcinoma) who underwent endoscopic submucosal dissection or endoscopic mucosal resection from September 2011 to February 2015 in our hospital. GCED was defined as a tumor having a primitive intestine-like structure composed of cuboidal or columnar cells with clear cytoplasm and immunohistochemical positivity for either alpha-fetoprotein, Glypican 3 or SALL4. The following were compared between GCED and CGC: age, gender, location and size of tumor, macroscopic type, ulceration, depth of invasion, lymphatic and venous invasion, positive horizontal and vertical margin, curative resection rate.
Six cases (5 males, 1 female; mean age 75.7 years; 6 lesions) of early gastric cancer with a GCED component and 186 cases (139 males, 47 females; mean age 72.7 years; 209 lesions) of early stage CGC were investigated. Mean tumor diameters were similar but rates of submucosal invasion, lymphatic invasion, venous invasion, and non-curative resection were higher in GCED than CGC (66.6% vs 11.4%, 33.3% vs 2.3%, 66.6% vs 0.4%, 83.3% vs 11% respectively, P < 0.01). Deep submucosal invasion was not revealed endoscopically or by preoperative biopsy. Histologically, in GCED the superficial mucosal layer was covered with a CGC component. The GCED component tended to exist in the deeper part of the mucosa to the submucosa by lymphatic and/or venous invasion, without severe stromal reaction. In addition, Glypican 3 was the most sensitive marker for GCED (positivity, 83.3%), immunohistochemically.
Even in the early stage GCED has high malignant potential, and preoperative diagnosis is considered difficult. Endoscopists and pathologists should know the clinicopathological features of this highly malignant type of cancer.
Core tip: We evaluated the comparison of clinicopathological features between 6 cases of early stage gastric cancer with enteroblastic differentiation (GCED) and 186 cases of early stage conventional gastric cancer (CGC: well/ moderately differentiated adenocarcinoma). Lymphatic, venous, and submucosal invasion rates were higher in GCED than CGC (33.3% vs 2.3%, 66.6% vs 0.4%, 66.6% vs 11.4% respectively, P < 0.01). In addition, Glypican 3 was the most sensitive marker for GCED (positivity, 83.3%), immunohistochemically. GCED has high malignant potential even at an early stage, and preoperative diagnosis is considered difficult. Further investigations are needed to establish optimal treatment approaches for GCED.
- Citation: Matsumoto K, Ueyama H, Matsumoto K, Akazawa Y, Komori H, Takeda T, Murakami T, Asaoka D, Hojo M, Tomita N, Nagahara A, Kajiyama Y, Yao T, Watanabe S. Clinicopathological features of alpha-fetoprotein producing early gastric cancer with enteroblastic differentiation. World J Gastroenterol 2016; 22(36): 8203-8210
- URL: https://www.wjgnet.com/1007-9327/full/v22/i36/8203.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i36.8203
Gastric cancer with enteroblastic differentiation (GCED) was proposed as a very rare variant of alpha-fetoprotein-producing gastric cancer (AFPGC) and its clinicopathological features have not been well elucidated. There are few reports about GCED, and most are case reports. In these earlier reported cases, GCED was histologically characterized as having a primitive intestine-like structure composed of cuboidal or columnar cells with clear cytoplasm[1-3]. Murakami et al[4] reported 29 cases of GCED in which stages ranged from an early to an advanced stage based on clinicopathologic and immunohistochemical characteristics. They proposed that GCED showed aggressive behavior such as lymphatic and venous invasion, lymph node metastasis, and liver metastasis, and its clinicopathologic features were similar to those of AFPGC.
Recently, with the advent and widespread use of endoscopic submucosal dissection (ESD), indications for endoscopic treatment of early stage gastric cancer have been expanding rapidly. Therefore, it is necessary for endoscopists to know the clinicopathological features of various histological types of early gastric cancer. However, no report has focused on the early stage of GCED. This study aimed to clarify the clinicopathological features of early stage GCED by comparisons with the early stage of conventional gastric cancer (CGC), including well or moderately differentiated carcinoma.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Juntendo University School of Medicine Institutional Review Board.
All procedures were recorded in endoscopy databases on cases that underwent endoscopic resection in our hospital. The databases were examined to identify all cases of early stage GCED and CGC that underwent ESD or endoscopic mucosal resection from September 2011 to February 2015. We evaluated the comparison between early gastric cancer with a GCED component and early stage CGC clinicopathologically.
GCED was defined as a tumor having a primitive intestine-like structure composed of cuboidal or columnar cells with clear cytoplasm and immunohistochemical positivity for either alpha-fetoprotein (AFP) (rabbit polyclonal, 1:1000; Dako, Glostrup, Denmark), Glypican 3 (clone 1G12, 1:200; BioMosaics, Burlington, VT, United States) or SALL4 (clone 6E3, 1:100; Abnova, Taipei, Taiwan). Results of histology and immunohistochemical staining were evaluated by two pathologists specialized in the gastrointestinal tract. We determined curative resection criteria according to Gastric Cancer Treatment Guidelines 2010 or 2014 provided by the Japanese Gastric Cancer Association[5,6].
Statistical analyses were conducted using SPSS (version 15.0 for Windows; SPPS Inc., Chicago, IL, United States) software. The comparison of clinicopathological features between GCEDs and CGCs were examined by the χ2 test and the Mann-Whitney U test. The level of significance was set at P < 0.05.
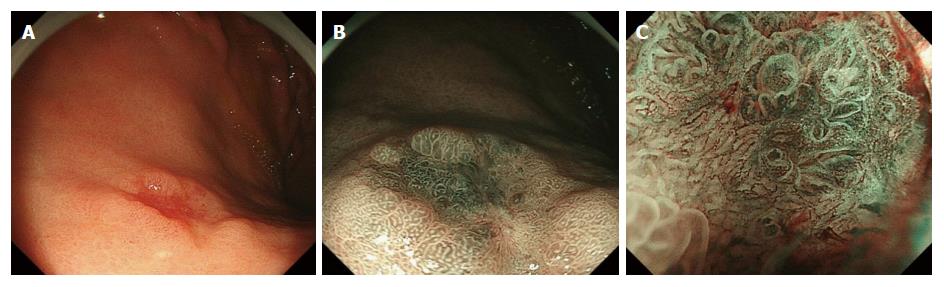
This study included 192 cases (144 males, 48 females; mean age 72.8 ± 7 years; 215 lesions) of early stage GCED and CGC. Among 192 cases, there were 6 GCED cases (5 males, 1 female; mean age 75.7 years; 6 lesions) and 186 CGC cases (139 males, 47 females; mean age 72.7 years; 209 lesions). Table 1 shows the clinicopathological findings of the patients with GCED and Table 2 shows results of the comparison between patients with GCED and CGC. Four cases who were checked for Helicobacter pylori (H. pylori) infection by H. pylori-immunoglobulin G were positive. No significant differences were observed in tumor location (U/M/L = 2/2/2 and 23/91/95 in GCED and CGC, respectively), mean tumor size (15.0 and 15.2 mm in GCED and CGC, respectively), and macroscopic types (flat or depressed type/ elevated type = 4/2 and 92/117 in GCED and CGC, respectively). Endoscopically, we could not find specific features of GCED or anticipate deep submucosal invasion (Figure 1). No lesion was diagnosed as GCED by examination of biopsy specimens. Regarding the depth of tumor invasion, the total submucosal invasion rate was significantly higher in GCED than CGC (66.6% vs 11.4%, P < 0.01). Positive rates for lymphatic and venous invasion were significantly higher in GCED than CGC (33.3% vs 2.3% and 66.6% vs 0.4%, P < 0.01). Therefore, the curative resection rate was significantly lower in GCED than CGC (16.7% vs 89.0%, P < 0.01). Moreover, when comparing only SM invasive cancer, the positive rate for venous invasion was significantly higher in GCED than CGC (100% vs 4.2%, P < 0.01) and the curative resection rate was significantly lower in GCED than CGC (0% vs 50%, P < 0.01). In 2 of the 6 GCED cases, additional surgery was performed (case 3: T1N0M0, stage IA; case 6: T1N1M0, stage IIA). Recurrence or metastasis was not seen in any of the 4 GCED cases that were followed from 28 to 51 mo (median 40 mo).
| Case | 1 | 2 | 3 | 4 | 5 | 6 |
| Age, yr | 61 | 77 | 78 | 75 | 80 | 83 |
| Male/female | Male | Male | Male | Male | Female | Male |
| Tumor location: U/M/L | L | M | U | U | L | M |
| Tumor size, mm | 8 | 11 | 14 | 18 | 6 | 36 |
| Macroscopic type | 0-IIc | 0-IIc | 0-IIc | 0-IIa | 0-IIc | 0-IIa + I |
| H. pylori infection | HPIgG+ | HPIgG+ | HPIgG- | NA | HPIgG+ | HPIgG+ |
| Procedure method | ESD | ESD | ESD with additional surgery | ESD | ESD | ESD with additional surgery |
| Depth of invasion (μm) | M | SM (1500) | SM (1000) | SM (200) | M | SM (2000) |
| Lymphatic invasion | (-) | (-) | (-) | (-) | (+) | (+) |
| Venous invasion | (-) | (+) | (+) | (+) | (-) | (+) |
| Observation period (mo) | 51, ANED | 42, ANED | N/A | 38, ANED | N/A | 28, ANED |
| Immunohistochemical analysis | ||||||
| AFP | (+) | (-) | (-) | (-) | (-) | (-) |
| Glypican3 | (+) | (+) | (+) | (+) | (-) | (+) |
| SALL4 | (+) | (-) | (-) | (-) | (+) | (-) |
| MUC2 | (-) | (-) | (-) | (+) | (-) | (-) |
| MUC5AC | (-) | (-) | (-) | (+) | (-) | (-) |
| MUC6 | (-) | (+) | (+) | (+) | (-) | (+) |
| CD10 | (+) | (+) | (+) | (+) | (+) | (+) |
| P53 | (-) | (-) | (+) | (+) | (+) | (-) |
| GCED | CGC | P value | |
| Number of lesions, n | 6 (2.7) | 209 (97.3) | N/A |
| Age, yr; median (range) | 75.7 (61-83) | 72.7 (40-92) | 0.39 |
| Male/female | 5/1 | 139/47 | 0.63 |
| Tumor location: U/M/L | 2/2/2 | 23/91/95 | 0.24 |
| Tumor size, mm, mean (range) | 15.0 (6-36) | 15.2 (2-60) | 0.96 |
| Macroscopic type: elevated type/flat or depressed type | 2/4 | 92/117 | 0.60 |
| Ulceration | 0 | 19 (9.0) | 0.43 |
| Depth of invasion: M/SM | 2/4 | 185/24 | < 0.01 |
| Rate of submucosal invasive cancer | 66.60% | 11.40% | |
| Median SM invasive depth, μm (range) | 1500(200-2000) | 795.8(100-5000) | 0.40 |
| Lymphatic invasion | 2 (33.3) | 5 (2.3) | < 0.01 |
| Venous invasion | 4 (66.6) | 1 (0.4) | < 0.01 |
| Positive horizontal margin | 0 | 9 (4.3) | 0.60 |
| Positive vertical margin | 1 (16.7) | 5 (2.3) | < 0.05 |
| Curative resection1 | 1 (16.7) | 186 (89.0) | < 0.01 |
| The following is a comparison only for SM invasive cancer | |||
| Number of SM invasive lesions, n | 4 | 24 | N/A |
| Lymphatic invasion | 1 (25) | 5 (20.8) | 0.88 |
| Venous invasion | 4 (100) | 1 (4.2) | < 0.01 |
| Positive horizontal margin | 0 | 1 (4.2) | 0.33 |
| Positive vertical margin | 1 (16.7) | 2 (8.3) | 0.55 |
| Curative resection1 | 0 | 12 (50) | < 0.01 |
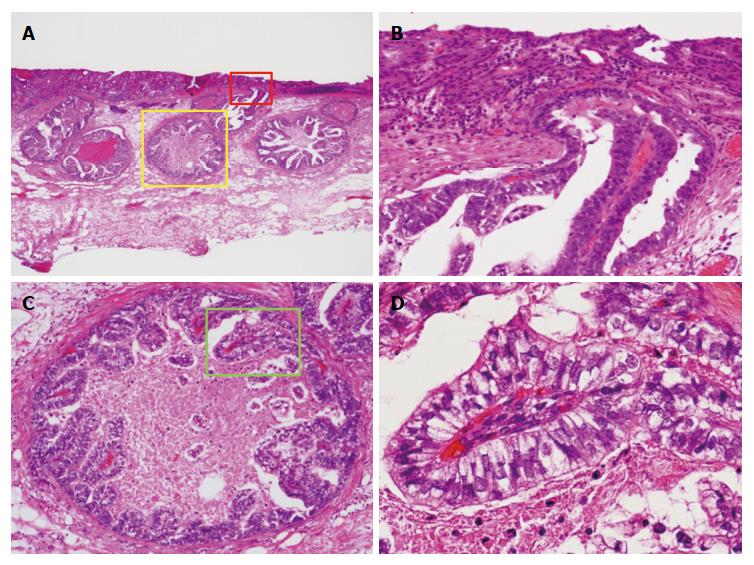
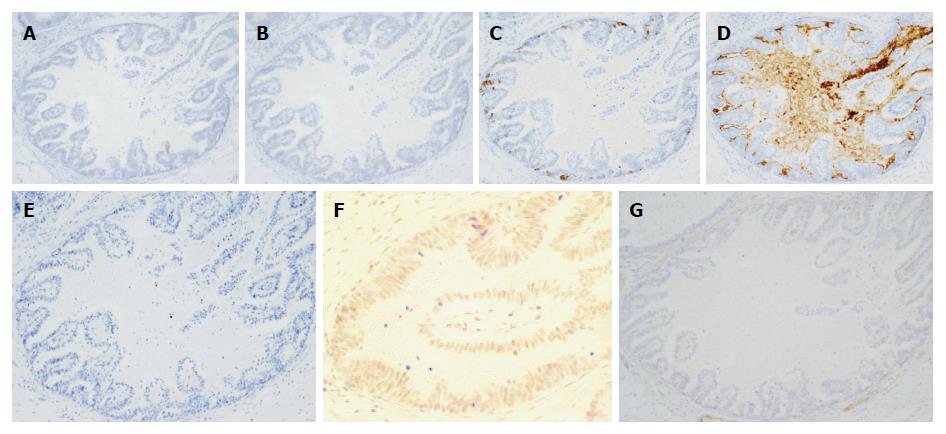
Histological examination of GCED demonstrated the presence of tubulopapillary carcinoma with clear cytoplasm at the deeper part of the mucosa and submucosa by lymphatic and/or venous invasion, and there was no severe stromal reaction. In all GCED cases, the superficial mucosal layer was covered with a conventional tubular adenocarcinoma (Figure 2). All GCED patients were positive for at least one of three enteroblastic lineage markers (AFP, Glypican 3, SALL 4). Glypican 3 was the most sensitive marker (positivity, 83.3%). Four cases were classified as having the gastrointestinal phenotype (4/6; 66.7%), and two as the intestinal type (2/6; 33.3%) according to combinations of the expression of CD10, MUC2, MUC5AC, and MUC6 (Figure 3).
AFP was initially found in 1956 and is a fetal serum protein produced mainly in the fetal liver and yolk sac. After birth, AFP rapidly disappears and is not detected in the serum of healthy adults[7]. The serum level of AFP is increased in some patients with hepatocellular carcinoma (HCC) or yolk sac tumor. In clinical practice, AFP has been considered to be a useful tumor marker in screening or monitoring patients with HCCs or yolk sac tumors. However, some diseases other than HCCs and yolk sac tumor were also associated with high serum levels of AFP, and gastric cancer is one of the most common[2,8-15]. Since the first report of AFPGC by Bourreille et al[16] in 1970, many cases have been reported, and the specifics of this disease have been gradually clarified.
Histologically, typical AFPGC has a liver-like structure and has been frequently reported as hepatoid adenocarcinoma[17-20]. However, there are also histologic types of carcinoma other than hepatoid adenocarcinoma. In 1994, Matsunou et al[1] reported two cases of AFP-producing gastric carcinoma with enteroblastic differentiation (AFP-producing clear cell gastric carcinoma), and the tumor characteristics were as follows: (1) columnar carcinoma cells growing primarily in tubulopapillary and glandular patterns; (2) abundant glycogen, but no mucin production in the clear cytoplasm; (3) gut hormone-containing cells scattered among clear carcinoma cells; (4) carcinoma cells producing oncofetal glycoproteins such as AFP and CEA; and (5) ultrastructurally, carcinoma cells showing well-developed microvilli with core filaments, whose rootlets formed occasional terminal webs, consistent with absorptive epithelium of fetal intestine or enteroblastic differentiation. Motoyama et al[21] proposed that AFPGCs should be divided into three subtypes: (1) hepatoid type; (2) yolk sac type; and (3) fetal gastrointestinal type. The fetal gastrointestinal type is thought to be equivalent to GCED[1,3]. It is said that most AFPGCs have various mixtures of these histologic types and CGC. Kinjo et al[2] also reported that in a histologic type of submucosal invasive cancer of AFPGC, the positivity rates for CGC and GCED in the mucosal component were significantly higher than in the invasive component. On the other hand, hepatoid gastric carcinoma (HGC) was significantly positive in the invasive component. In this study, pathological findings in all GCED cases showed that the mucosal layer was composed of conventional tubular adenocarcinoma and GCED. In addition, the superficial mucosal layer was covered with a conventional tubular adenocarcinoma, and there were no findings of GCED components exposed to the superficial layer. Therefore, since we could only collect superficial mucosal layer tissue for the biopsy specimens, we felt that GCED could not be diagnosed by examination of biopsy specimens. The GCED component exists only in the deeper part of the mucosa to the submucosa by lymphatic and/or venous invasion but not in any other histological type of AFPGC (HGC and yolk sac type). As in previous reports[2,4] these findings suggested that GCED could be thought to develop from CGC and then gradually develop hepatoid features during the process of tumor invasion and proliferation. We considered that there is a possibility that early stage GCED represents the early developmental stage of AFPGC, which has various mixtures of histological types.
Endoscopically, we diagnose the depth of invasion according to endoscopic features of gastric cancers with deep submucosal invasion (≥ 500 μm in depth), such as remarkable redness, uneven surface, margin elevation, ulceration, and enlarged folds[22]. However, we could not anticipate the presence of deep submucosal invasion because none of these findings were revealed in our 6 GCED cases. The reason may be that there were few macroscopic changes in endoscopic morphology because the main submucosal invasive form of GCED depends on lymphatic and/or venous invasion and there were no severe stromal reactions.
In previous reports, AFPGC was usually associated with a poor prognosis with a high incidence of lymphatic invasion, venous invasion, and synchronous and metachronous liver metastasis[23-25]. Hirasaki et al[26] studied 24 cases of AFP-producing early gastric cancer and reported that liver metastasis was seen in 2 cases (8.3%) and lymph node metastasis was seen in 18 of 24 cases (75.0%). From these results, they suggested that lymph node metastasis should be generally taken into consideration in AFP-producing early gastric cancer even if lymph node swelling is not seen on diagnostic imaging and that such patients should be under close periodic observation. Furthermore, Adachi et al[23] studied 270 cases of AFPGC and reported that there were 61 patients who underwent curative gastrectomy for stage I or II AFPGC, and that 39 of those patients (64%) were alive during a median follow-up period of 27 mo. From these results, it is difficult to say that the prognosis was good. Although in this study the mean diameters of GCED and CGC were about the same, comparisons of M and SM invasive cancer, positive rates of lymphatic and venous invasion, and non-curative resection rates were significantly higher in GCED than CGC. Furthermore, when comparing only SM invasive cancer, the positive rate of venous invasion was significantly higher in GCED than CGC (100% vs 4.2%, P < 0.01). Although lymph node swelling cannot be seen on diagnostic imaging, lymph node metastasis occurred in 1 of 2 GCED cases who underwent additional surgery. In addition, although all of the 4 GCED cases for whom we could follow their complete progress were alive during the median follow-up period of 40 mo, 5 of 6 GCED cases (83.3%; 4 of 4 SM invasive cancer, 100%) had endoscopic non-curative resection because of deep submucosal invasion, lymphatic and/or venous invasion, and a positive margin. Therefore, we think that GCED, as well as AFPGC, may have a high incidence of liver and lymph node metastasis because of the high level of affinity to vessels and is associated with a poor prognosis in comparison with CGC. In this study, 2 of 5 cases who had endoscopic non-curative resection were followed up without additional surgery. One of the 5 cases had additional surgery and the remaining 2 were followed elsewhere. Although the 2 cases that we are following are alive without a recurrence (case 2: 42 mo, case 5: 38 mo), it is thought that close periodic observation is strictly required in such cases.
The present study had some limitations. Since GCED is a disease with a very low incidence rate, the number of patients was small. Furthermore, the data were collected retrospectively in a single medical center, which may produce selection bias.
In conclusion, GCED is rare, but has distinct clinicopathological features, especially in terms of high malignant potential and difficulty in preoperative diagnosis by biopsy specimens and prediction of submucosal invasion by endoscopic findings. It is necessary for endoscopists and pathologists to be aware of the clinicopathological features of such a rare and highly malignant type of cancer. In addition, when we detect specific histological findings for GCED with hematoxylin and eosin stain, immunohistochemical examination should be performed to make a precise diagnosis. However, there has been no report that evaluated a large number of cases. Further investigations are needed to elucidate the natural history of GCED by assessing the long-term outcome and establishing optimal treatment approaches for GCED.
Gastric cancer with enteroblastic differentiation (GCED) was proposed as a very rare variant of alpha-fetoprotein-producing gastric cancer (AFPGC). In previous reports, AFPGC was usually associated with a poor prognosis with a high incidence of lymphatic invasion, venous invasion, and synchronous and metachronous liver metastasis. Recently, with the advent and widespread use of endoscopic submucosal dissection (ESD), indications for endoscopic treatment of early stage gastric cancer have been expanding rapidly. Therefore, it is necessary for endoscopists to know the clinicopathological features of various histological types of early gastric cancer. However, the clinicopathological features and the optimal treatment approaches of GCED have not been well elucidated. In this study, we investigated clinicopathological features of early stage of GCED.
There are few reports about GCED. Most of these are case reports, and there is no report that focused on the early stage of GCED. In these earlier reported cases, GCED was histologically characterized as having a primitive intestine-like structure composed of cuboidal or columnar cells with clear cytoplasm, and GCED showed aggressive behavior such as lymphatic and venous invasion, lymph node metastasis, and liver metastasis.
The results of this study contribute to clarifying the clinicopathological features of early stage GCED by comparisons with the early stage of conventional gastric cancer (CGC), including well or moderately differentiated carcinoma. In this study, even if it is early stage cancer, GCED has distinct clinicopathological features, especially in terms of high malignant potential with a high incidence of lymphatic and venous invasion, difficulty in preoperative diagnosis by biopsy specimens and prediction of submucosal invasion by endoscopic findings. In addition, Glypican 3 was the most sensitive marker for GCED (positivity, 83.3%), immunohistochemically.
If the authors detect specific histological findings for GCED with hematoxylin and eosin stain, immunohistochemical examination, especially Glypican 3 should be performed to make a precise diagnosis. However, there has been no report that evaluated a large number of cases. Further investigations are needed to elucidate the natural history of GCED by assessing the long-term outcome and establishing optimal treatment approaches for GCED.
ESD is a well established technique of endoscopic resection that allows for en bloc removal of GI epithelial lesions. Glypican 3, it is an oncofetal protein that is expressed in the human fetal liver and placenta. It was discovered as a potential serological and histochemical marker whose expression is specific for hepatocellular carcinoma. SALL 4, it is an oncofetal protein that is expressed in the human fetal liver and silenced in the adult liver, but it is reexpressed in a subgroup of patients who have hepatocellular carcinoma and an unfavorable prognosis.
This most remarkable point of this manuscript is the analysis of the influence of this rare type of tumor and its bad prognosis in comparison to the conventional one that justifies the research in this subtype.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Varona MA S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Matsunou H, Konishi F, Jalal RE, Yamamichi N, Mukawa A. Alpha-fetoprotein-producing gastric carcinoma with enteroblastic differentiation. Cancer. 1994;73:534-540. [PubMed] |
| 2. | Kinjo T, Taniguchi H, Kushima R, Sekine S, Oda I, Saka M, Gotoda T, Kinjo F, Fujita J, Shimoda T. Histologic and immunohistochemical analyses of α-fetoprotein--producing cancer of the stomach. Am J Surg Pathol. 2012;36:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Yamabuki T, Omi M, Yonemori A, Misu K, Inomata H, Abiko Y, Mori M, Nihei K. Gastrointestinal Obstruction due to Solitary Lymph Node Recurrence of Alpha-Fetoprotein-Producing Gastric Carcinoma with Enteroblastic Differentiation. Case Rep Gastroenterol. 2014;8:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Murakami T, Yao T, Mitomi H, Morimoto T, Ueyama H, Matsumoto K, Saito T, Osada T, Nagahara A, Watanabe S. Clinicopathologic and immunohistochemical characteristics of gastric adenocarcinoma with enteroblastic differentiation: a study of 29 cases. Gastric Cancer. 2016;19:498-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1895] [Article Influence: 135.4] [Reference Citation Analysis (0)] |
| 6. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1913] [Article Influence: 239.1] [Reference Citation Analysis (1)] |
| 7. | Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest. 1956;8:174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 319] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Hamanaka W, Yoneda S, Shirakusa T, Shirahama H, Tashiro Y, Iwasaki A, Shiraishi T, Tsuru H. Alpha-fetoprotein (AFP)-producing adrenocortical carcinoma--long survival with various therapeutic strategies including a lung resection: report of a case. Surg Today. 2008;38:275-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Isonishi S, Ogura A, Kiyokawa T, Suzuki M, Kunito S, Hirama M, Tachibana T, Ochiai K, Tanaka T. Alpha-fetoprotein (AFP)-producing ovarian tumor in an elderly woman. Int J Clin Oncol. 2009;14:70-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Yamagata T, Yamagata Y, Nakanishi M, Matsunaga K, Minakata Y, Ichinose M. A case of primary lung cancer producing alpha-fetoprotein. Can Respir J. 2004;11:504-506. [PubMed] |
| 11. | Hocking GR, Shembrey M, Hay D, Ostör AG. Alpha-fetoprotein-producing adenocarcinoma of the sigmoid colon with possible hepatoid differentiation. Pathology. 1995;27:277-279. [PubMed] |
| 12. | Kodama T, Kameya T, Hirota T, Shimosato Y, Ohkura H, Mukojima T, Kitaoka H. Production of alpha-fetoprotein, normal serum proteins, and human chorionic gonadotropin in stomach cancer: histologic and immunohistochemical analyses of 35 cases. Cancer. 1981;48:1647-1655. [PubMed] |
| 13. | Koyama S, Ebihara T, Osuga T. Histologic and immunohistochemical studies of alpha-fetoprotein (AFP)-producing gastric carcinoma. Gastroenterol Jpn. 1987;22:419-427. [PubMed] |
| 14. | Masuzawa M, Lee PK, Kamada T, Akeyama T, Abe H, Shimano T, Mori T, Morino H, Ishiguro S. Carcinoembryonic antigen, alpha-fetoprotein and carcinoplacental alkaline phosphatase in gastric carcinoma metastatic to the liver. Cancer. 1977;39:1175-1180. [PubMed] |
| 15. | Nishimura H, Okamoto Y, Takahashi M, Fujita T. Occurrence of alpha-fetoprotein, Regan isoenzyme, and variant alkaline phosphatase in the serum of a patient with gastric cancer. Gastroenterology. 1976;71:497-499. [PubMed] |
| 16. | Bourreille J, Metayer P, Sauger F, Matray F, Fondimare A. [Existence of alpha feto protein during gastric-origin secondary cancer of the liver]. Presse Med. 1970;78:1277-1278. [PubMed] |
| 17. | Ishikura H, Kirimoto K, Shamoto M, Miyamoto Y, Yamagiwa H, Itoh T, Aizawa M. Hepatoid adenocarcinomas of the stomach. An analysis of seven cases. Cancer. 1986;58:119-126. [PubMed] |
| 18. | Ye MF, Tao F, Liu F, Sun AJ. Hepatoid adenocarcinoma of the stomach: a report of three cases. World J Gastroenterol. 2013;19:4437-4442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Yang J, Wang R, Zhang W, Zhuang W, Wang M, Tang C. Clinicopathological and prognostic characteristics of hepatoid adenocarcinoma of the stomach. Gastroenterol Res Pract. 2014;2014:140587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Lin CY, Yeh HC, Hsu CM, Lin WR, Chiu CT. Clinicopathologial features of gastric hepatoid adenocarcinoma. Biomed J. 2015;38:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Motoyama T, Aizawa K, Watanabe H, Fukase M, Saito K. alpha-Fetoprotein producing gastric carcinomas: a comparative study of three different subtypes. Acta Pathol Jpn. 1993;43:654-661. [PubMed] |
| 22. | Abe S, Oda I, Shimazu T, Kinjo T, Tada K, Sakamoto T, Kusano C, Gotoda T. Depth-predicting score for differentiated early gastric cancer. Gastric Cancer. 2011;14:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Adachi Y, Tsuchihashi J, Shiraishi N, Yasuda K, Etoh T, Kitano S. AFP-producing gastric carcinoma: multivariate analysis of prognostic factors in 270 patients. Oncology. 2003;65:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Li XD, Wu CP, Ji M, Wu J, Lu B, Shi HB, Jiang JT. Characteristic analysis of α-fetoprotein-producing gastric carcinoma in China. World J Surg Oncol. 2013;11:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long Z, Zhu H, Wang Y. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol. 2010;102:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Hirasaki S, Tanimizu M, Tsuzuki T, Tsubouchi E, Hidaka S, Hyodo I, Tajiri H. Seronegative alpha-fetoprotein-producing early gastric cancer treated with endoscopic mucosal resection and additional surgery. Intern Med. 2004;43:926-930. [PubMed] |