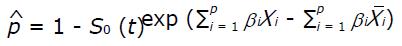Published online Sep 28, 2016. doi: 10.3748/wjg.v22.i36.8194
Peer-review started: May 30, 2016
First decision: July 13, 2016
Revised: August 16, 2016
Accepted: August 30, 2016
Article in press: August 30, 2016
Published online: September 28, 2016
Processing time: 121 Days and 9.6 Hours
To develop a prognostic scoring system for overall survival (OS) of patients undergoing liver resection (LR) for hepatocellular carcinoma (HCC).
Consecutive patients who underwent curative LR for HCC between 2000 and 2013 were identified. The series was randomly divided into a training and a validation set. A multivariable Cox model for OS was fitted to the training set. The beta coefficients derived from the Cox model were used to define a prognostic scoring system for OS. The survival stratification was then tested, and the prognostic scoring system was compared with the European Association for the Study of the Liver (EASL)/American Association for the Study of Liver Diseases (AASLD) surgical criteria by means of Harrell’s C statistics.
A total of 917 patients were considered. Five variables independently correlated with post-LR survival: Model for End-stage Liver Disease score, hepatitis C virus infection, number of nodules, largest diameter and vascular invasion. Three risk classes were identified, and OS for the three risk classes was significantly different both in the training (P < 0.0001) and the validation set (P = 0.0002). Overall, 69.4% of patients were in the low-risk class, whereas only 37.8% were eligible to surgery according to EASL/AASLD. Survival of patients in the low-risk class was not significantly different compared with surgical indication for EASL/AASLD guidelines (77.2 mo vs 82.5 mo respectively, P = 0.22). Comparison of Harrell’s C statistics revealed no significant difference in predictive power between the two systems (-0.00999, P = 0.667).
This study established a new prognostic scoring system that may stratify HCC patients suitable for surgery, expanding surgical eligibility with respect to EASL/AASLD criteria with no harm on survival.
Core tip: European Association for the Study of the Liver (EASL)/American Association for the Study of Liver Diseases (AASLD) guidelines recommend liver resection (LR) for hepatocellular carcinoma (HCC) only for single nodules of any size in patients without tumor related symptoms, no clinically significant portal hypertension and normal bilirubin. In this study we investigated the prognostic factors for survival of patients who underwent LR for HCC. We built a prognostic scoring system to stratify post-resection prognosis, and we identified a larger subset of patients with an expected survival that equates that of patients undergoing LR according to guidelines. Thus, the current EASL/AASLD indications for LR can be safely expanded, with no detrimental effect on patients’ prognosis.
- Citation: Sposito C, Di Sandro S, Brunero F, Buscemi V, Battiston C, Lauterio A, Bongini M, De Carlis L, Mazzaferro V. Development of a prognostic scoring system for resectable hepatocellular carcinoma. World J Gastroenterol 2016; 22(36): 8194-8202
- URL: https://www.wjgnet.com/1007-9327/full/v22/i36/8194.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i36.8194
Hepatocellular carcinoma (HCC) is the most frequent primary tumor of the liver, and it is the third cause of cancer death worldwide[1]. Most HCC cases (from 65% to 90%) occur in the context of chronic hepatitis and cirrhosis[2], which are attributable mainly to chronic hepatitis B virus or hepatitis C virus (HCV) infections, followed by chronic alcohol abuse, obesity and diabetes[3]. The estimated rate of each of these risk factors varies depending on the different regions of the world.
The prognosis of patients with HCC and the choice among the available therapeutic options, largely depends on tumor extension and underlying liver function. According to the European Association for the Study of the Liver (EASL)[4] and the American Association for the Study of Liver Diseases (AASLD)[5] guidelines, treatment allocation is routed by the Barcelona Clinic Liver Cancer staging system (BCLC)[6]. In particular liver resection (LR) is considered as the first-line treatment only for patients at an early stage of the disease, namely those with an optimal liver function (Child-Pugh A, normal bilirubin and absence of clinically relevant portal hypertension), a preserved physical condition (ECOG Performance Status of 0), and a single tumor nodule with no evidence of extra-hepatic spread nor involvement of major vascular structures. In this subset of optimal patients, a 5-year overall survival (OS) of approximately 70% may be expected, similar to that of liver transplantation[7]. Several field practice studies have ascertained that LR is often offered outside these conventional indications, and various authors reported acceptable survival rates for patients with HCC resected at a more advanced stage because of macrovascular invasion[8,9], multiple nodules or impaired liver function[10,11]. In addition, more recent studies demonstrate a survival benefit of radical surgery with respect to the available treatment alternatives across the different BCLC stages[12-14].
The objective of this study is to investigate the prognostic factors for survival of patients who underwent LR for HCC at two referral centers - in which the surgical indication was not restricted to the current guidelines - and to build a prognostic scoring system to stratify post-treatment prognosis and possibly to expand the actual western indications to LR without harmful adverse outcomes.
From January 2000 to March 2013 data from all patients who underwent a curative LR for HCC at the Departments of Gastrointestinal Surgery and Liver Transplantation of the Istituto Nazionale dei Tumori and the Ospedale Niguarda Ca’ Granda of Milan were prospectively collected and entered in a master database. Patients with extrahepatic disease at diagnosis and patients who were censored within two months were excluded from the present study. The master database contained 138 variables, including demographic, clinical, laboratory, treatment and survival data of each patient. The data were retrieved from the database for the purpose of this study after approval from the local institutional review boards.
Criteria for HCC diagnosis were in accordance with EASL/AASLD guidelines evolution[4,5,15]. The diagnosis of HCC was made on sequential contrast-enhanced imaging studies [chest computed tomography (CT), magnetic resonance imaging (MRI), or ultrasound] unless one study conclusively demonstrated a tumor with arterial enhancement and venous washout. In cases lacking conclusive radiological diagnosis, ultrasound-guided biopsy was used in both centers.
LR was performed within conventional guidelines but also beyond EASL/AASLD recommendations in all patients in whom surgical tumor removal was possible with a risk/benefit ratio in favor of surgical indication when compared with other available options such as liver transplantation, loco-regional therapies (ablation, transarterial chemoembolization or radioembolization) or systemic therapies (Sorafenib). Indication for surgery was always discussed in a multidisciplinary HCC board with hepatologists, oncologists, radiologists and surgeons.
No neoadjuvant locoregional/systemic treatments were indicated before surgery. Chest CT scan and contrast-enhanced abdominal CT scan or MRI were used for preoperative staging and volume assessment. The day before surgery, a thorough physical examination was accomplished, together with a complete biochemistry panel including serum alpha-fetoprotein (AFP) levels, and an indocyanine green retention test at 15 min. Liver function and reserve were determined according to the Child-Turcotte-Pugh (CTP) and Model for End-stage Liver Disease (MELD) scores. Presence of clinically relevant portal hypertension was defined as the presence of esophageal or gastric varices detectable at endoscopy or splenomegaly (major diameter > 12 cm) with a platelet count < 100000/mm3[5]. Minor hepatectomy was defined as the resection of ≤ 2 adjacent liver segments[16].
All patients received low-molecular weight heparin the day before surgery and 2 g of cefazolin 30 min before skin incision. After accessing the peritoneal cavity, patients underwent complete abdominal exploration. Intra-operative ultrasound was used to assess tumor characteristics, exclude the presence of adjunctive focal lesions in the liver, ascertain intrahepatic vascular and biliary anatomy, individualize the resection plane with a tumor-free margin of at least 1 cm and eventually decide on resectability. Anatomical resection was always attempted although the final decision on it was strictly dependent on the patient’s tumor and liver conditions. Surgery was always performed within a fluid minimization protocol, particularly during hepatic dissection; a central venous pressure lower than 5 mm/Hg was targeted.
After hospital discharge, patient follow-up was performed in a dedicated liver cancer clinic with hepatological and surgical competences in place to treat the underlying liver diseases and detect possible recurrence of HCC. Physical examination, biochemistry with AFP level measurement, chest CT scan and contrast-enhanced abdominal CT scan or MRI were performed every 4 mo for the first two years and every 6 mo thereafter. Anti-cancer treatment was not applied until recurrence. When recurrence was noted, each patient was treated according to disease presentation.
Categorical variables were reported as the number of cases and percentage; continuous variables were expressed as median and interquartile range (IQR). OS was estimated by the Kaplan-Meier method and calculated from the time from the date of hepatic resection to the earliest of death or last follow-up evaluation. For patients who underwent liver transplant (LT) either for HCC recurrence or end-stage liver disease, survival was censored the day before LT.
All eligible patients were randomly allocated into a training set or a test set in an approximately 1:1 ratio with seed set (16438) to make the procedure reproducible. For all subjects an independent uniform variable was generated and rounded to the closest integer: Two groups were identified according to the 0/1 result. The characteristics of patients in the training and test sets were compared using the Pearson chi-square test (or Fisher exact test, if necessary) for categorical variables, the Student t-test for continuous variables and the log-rank test for time-to-event data.
In the training set a Cox proportional hazards regression model was used to identify the baseline preoperative characteristics predicting OS, and those variables identified as significant in the univariate analysis at the level of P < 0.05 were tested in the multivariable setting. The proportionality assumption was verified by Schoenfeld residual analysis. A prognostic score was then derived using the independent variables weighed according to the estimated β regression coefficient of the final Cox model. The risk estimate associated with each point was then calculated using the Cox proportional hazards model according to the formula:
Math 1
Three prognostic stages (low risk, medium risk and high risk of death at 5 years from surgery) were identified according to the changes in the risk estimates for each point increase of the score. OS curves in the training and test sets for the three prognostic stages were obtained with the Kaplan-Meier method and compared by means of log-rank test.
Patients in the “low-risk” category were considered as “optimal candidates for surgery” according to the model’s predicted survival; the dichotomization of the model (optimal vs non-optimal candidate for surgery) was then compared with the EASL/AASLD indications through calculation of Akaike Information Criteria[17], comparison of Harrell’s C statistics[18] and comparison of survival rates at 5 years.
All analyses were 2-tailed and the threshold of significance was assessed at P < 0.05. The statistical analysis was performed using STATA®, version 13 (StataCorp LP, United States). The statistical methods of this study were reviewed by Dr. Federica Brunero, Clinical Trial Office and Biomedical Statistic, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy.
A total of 917 eligible adult HCC patients were included. Overall, the median age at presentation was 67 years (IQR: 61-73 years). The majority of patients were men (705 subjects, 76.9%) and were predominantly classified as “fully active” (ECOG PS 0, 93.5%). Eight-hundred fifty-three patients (93.1%) had CTP grade A liver function, and 616 patients (72.1%) had MELD score less than or equal to 9[19]. In the majority of cases (46.2%), HCV was the etiology of liver disease and 320 patients (35%) had clinically relevant portal hypertension. Two-hundred sixty-seven patients (29.1%) had a tumor size greater than 5 cm and 897 subjects (97.8%) had up to three tumor nodules. Portal invasion was detected in 36 patients (3.9%). Thirty- and ninety-day mortality rates were 1.1% and 3.5%. Median follow-up of the entire series was 58.1 mo (95%CI: 52.3-63.9). During follow-up 442 deaths were registered. Survival at 5 and 10 years and median survival were 49.3%, 26.2% and 58.7 mo (95%CI: 51.5-65.9) respectively. Recurrence-free survival (RFS) at 3 and 5 years and median RFS were 43.7%, 31.8% and 28.8 mo (95%CI: 25.0-35.6) respectively.
Among the 917 patients, 480 (52.34%) and 437 (47.66%) were assigned randomly to the training set and the validation set, respectively. The demographic, clinical and laboratory characteristics of patients assigned in the two sets are presented in Table 1. Overall, the patients in the training and the validation sets shared similar characteristics, including survival and censoring pattern. Greater than 50% of patients presented with an active HCV infection. Because HCV infection was the prevalent aetiology of cirrhosis, we chose to compare it with all the other aetiologies grouped.
| Training set (n = 480) | Validation set (n = 437) | P value | |
| Age (yr) | 67 (61-73) | 68 (61-73) | 0.684 |
| Gender, male | 374 (77.9) | 331 (75.7) | 0.436 |
| ECOG PS | 0.362 | ||
| 0 | 452 (94.2) | 405 (92.7) | |
| 1-2 | 28 (5.8) | 32 (7.3) | |
| Child-Pugh | 0.118 | ||
| A | 453 (94.4) | 401 (91.8) | |
| B | 27 (5.6) | 36 (8.2) | |
| MELD score | 8 (7-10) | 8 (7-10) | 0.791 |
| Etiology | 0.516 | ||
| Cryptogenic | 104 (21.7) | 88 (20.1) | |
| HBV only | 94 (19.6) | 71 (16.2) | |
| HCV only | 222 (46.25) | 217 (49.7) | |
| Alcohol | 48 (10.0) | 45 (10.3) | |
| HBV + HCV | 12 (2.5) | 16 (3.7) | |
| HCV infection | 247 (51.5) | 246 (56.3) | 0.142 |
| Portal hypertension | 165 (34.4) | 155 (35.5) | 0.728 |
| Platelet count (103/μL) | 157 (25-505) | 154 (26-914) | 0.779 |
| AFP, ng/mL (n = 663) | 14.3 (4.7-121.5) | 11.4 (4.3-71) | 0.160 |
| ICG-R15 (n = 400) | 15 (6.1-25) | 16 (7.7-22.3) | 0.424 |
| Total bilirubin (≥ 1.2 mg/dL) | 148 (30.8) | 126 (28.8) | 0.509 |
| Number of lesions (> 3) | 10 (2.1) | 10 (2.3) | 0.832 |
| Largest diameter (> 5 cm) | 143 (29.8) | 124 (28.4) | 0.637 |
| Portal invasion | 22 (4.6) | 14 (3.2) | 0.283 |
| Extent of hepatectomy (major) | 85 (17.7) | 78 (17.8) | 0.956 |
| Follow-up status (dead) | 240 (50.0) | 202 (46.2) | 0.253 |
| Follow-up time (mo) | 35.9 (16.3-61.0) | 32.5 (16.7-55.8) | 0.254 |
| Overall survival | 59.3 (50.2-66.6) | 56.4 (47.0-75.8) | 0.833 |
Results of the univariate analysis on preoperative characteristics are presented in Supplementary Table 1. Those preoperative variables identified as significant in the univariate analysis at the level of P < 0.05 were fitted in a multivariable Cox proportional hazards regression model within the training set. The proportionality of hazard ratios for all levels of all prognostic factors was verified. The beta coefficients were transformed into relative points and a point system was constructed according to the method described by Sullivan et al[20] (Table 2).
| Variable | HR | 95%CI | P value | β | Points |
| MELD score ≤ 9 | 0 | ||||
| MELD score > 9 | 1.444 | (1.080-1.931) | 0.013 | 0.3674 | 1 |
| HCV infection absent | 0 | ||||
| HCV infection present | 1.468 | (1.112-1.937) | 0.007 | 0.3839 | 1 |
| Number of lesions ≤ 3 | 0 | ||||
| Number of lesions > 3 | 3.253 | (1.434-7.380) | 0.005 | 1.1795 | 3 |
| Largest diameter ≤ 5 cm | 0 | ||||
| Largest diameter > 5 cm | 1.459 | (1.085-1.963) | 0.012 | 0.3779 | 1 |
| Portal invasion absent | 0 | ||||
| Portal invasion present | 3.500 | (2.016-6.073) | < 0.001 | 1.2526 | 3 |
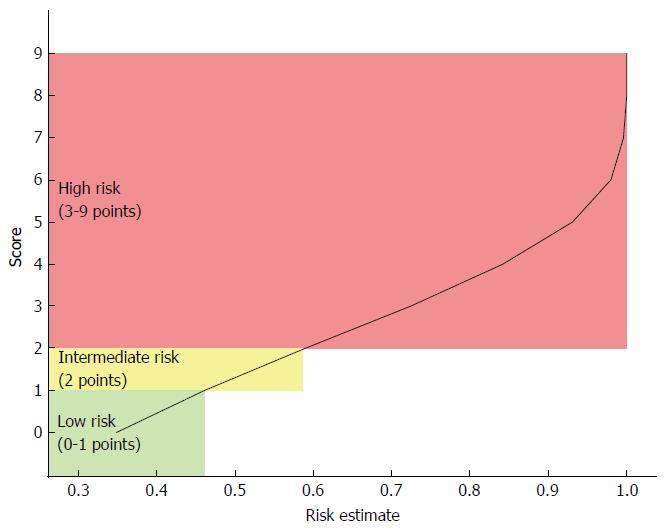
The total score ranged between 0 and 9. The risk estimates were calculated for each score using the Cox proportional hazards model, and three risk stages were defined according to changes in the risk estimates for each point increase (Figure 1): Low risk: 0 to 1 points Intermediate risk: 2 points; High risk: 3 to 9 points.
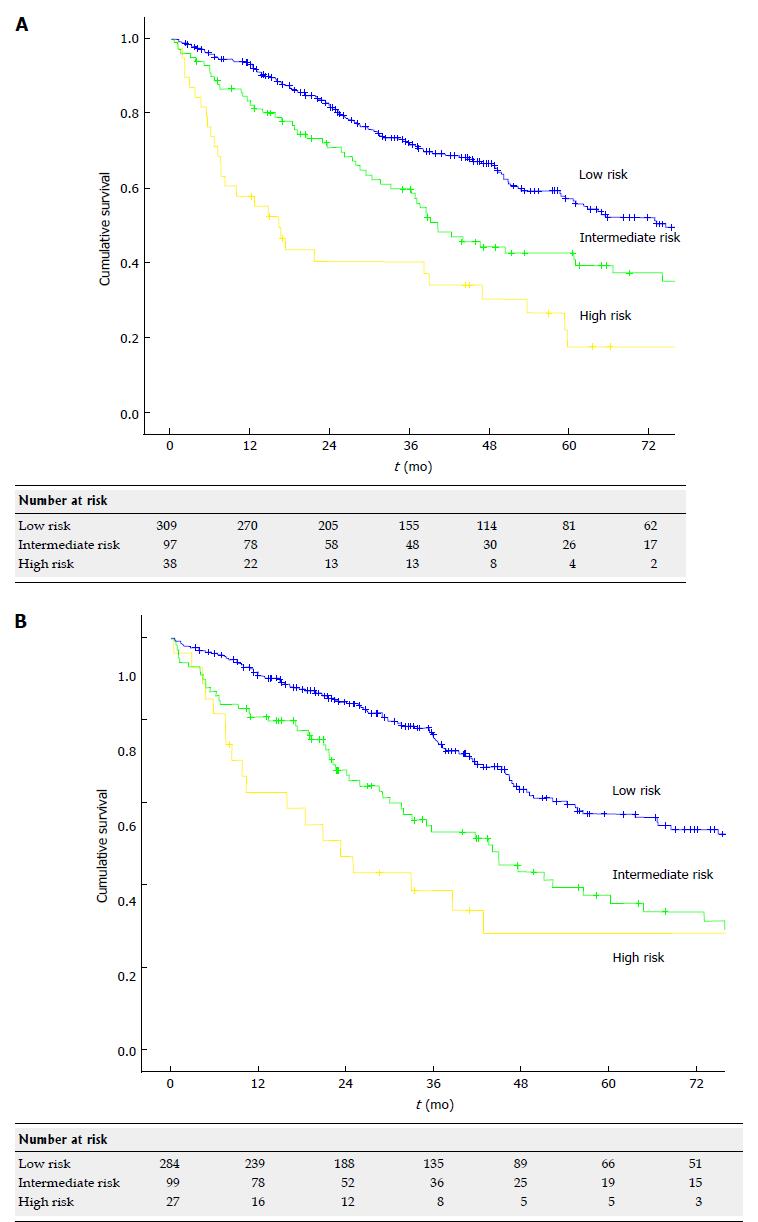
OS curves for the three prognostic stages are presented in Figure 2. In the training set, a significant difference in survival between the three stages was demonstrated (χ2 = 33.56 and P < 0.000), and this finding was confirmed in the validation set (χ2 = 23.67 and P = 0.0002). When considering the case series as a whole, 5-year, 10-year and median survival were 57.2%, 31.2% and 77.2 mo (95%CI: 67.4-87.0) respectively in the low risk category, 40.3% 22.6% and 41.7 mo (95%CI: 34.7-48.7) respectively in the intermediate category and 22.3% 13.4% and 17.4 mo (95%CI: 10.1-24.6) respectively in the high risk category (P < 0.000). Three-year, 5-year and median RFS were 46.4%, 33.8% and 31.5 mo (95%CI: 25.3-35.7) respectively in the low risk category, 40.1% 28.1% and 29.9 mo (95%CI: 25.6-34.2) respectively in the intermediate category and 34.5% 25.9% and 12.5 mo (95%CI: 2.8-22.2) respectively in the high risk category (P = 0.020). Details on sites of HCC recurrence and treatments for recurrence are shown in supplementary Table 2.
Patients in the low-risk category were considered as ideal candidates for LR according to the predicted survival. This criterion allowed the inclusion of 314 patients considered non-ideal candidates according to EASL/AASLD guidelines.
Overall, 593 patients (69.4% of the total of 854 evaluable patients according to both classifications) were ideal candidates for LR according to the proposed Milan score, whereas only 323 patients were ideal candidates for LR according to the EASL/AASLD guidelines (37.8% of the total). This finding resulted in a net increase of 31.6% of patients with ideal indication for LR.
Comparison with the EASL/AASLD surgical guidelines was performed by means of AIC, Harrell’s C statistics and 5-year survival rates. AIC for EASL/AASLD surgical guidelines was 5323.259 and AIC for the Milan score was 4683.745. Harrell’s C was 0.5971 and 0.5849 for the EASL/AASLD surgical guidelines and the proposed criteria respectively (P = 0.617), showing that there is no evidence that the two systems have different predictive power.
The 5-year survival rates for patients who are ideal candidates for surgery according to the two systems were not significantly different (z = -1.6022, P = 0.06), and median survivals did not differ (z = -0.789, P = 0.22) (Table 3).
| No. Of patients (%) | Median OS (95%CI) | 5-yr OS | 10-yr OS | ||
| EASL/AASLD | Ideal | 323 (37.8) | 83 (73-108) | 64.4 | 37.0 |
| Non-ideal | 531 (62.2) | 46 (41-52) | 42.0 | 21.2 | |
| Current study | Ideal | 593 (69.4) | 77 (64-44) | 57.2 | 31.2 |
| Non-ideal | 261 (30.6) | 38 (30-44) | 35.8 | 20.0 | |
LR still represents the cornerstone for any curability attempt in patients with HCC. A large burden of surgical literature has challenged the current Western guidelines. However, LR for HCC is recommended only for single nodules of any size in patients without tumor related symptoms, no clinically significant portal hypertension and normal bilirubin[4,5]. If this profile is not fulfilled, postoperative morbidity may increase, and long-term survival may be significantly reduced. In contrast, when patients meet these criteria, long-term survival may equate that of LT. Thus, LR under restricted conditions maintains its role as a first-line therapeutic option in patients with early HCC[7]. The restrictive approach indicated by Western guidelines was established more than 15 years ago[21], and its conservative recommendations for surgical indications have not evolved over time despite the significant improvement in surgical techniques and technologies and their reflections on patient outcomes. An extension of the recommendations has been repeatedly suggested given that resection can be attempted with high rates of technical success and acceptable survival rates in patients with clinically significant portal hypertension[22], multiple nodules[10] or intrahepatic vascular invasion[9]. In this context, it is not surprising to observe that experienced surgical centres both in the East and in the West adopt a more liberal approach to LR in HCC that does not strictly follow the guidelines. In a recent large multicentre series of patients resected for HCC, less than 30% of cases were considered as ideal candidates for resection according to the current guidelines[12].
In this study, we retrospectively analyzed a large series of approximately 1000 HCC patients who underwent LR at two hospitals in Milan (Italy) with large volumes of activity. Both centres offered LR even outside the current EASL/AASLD guidelines, and indeed greater than 60% of patients were considered as non-ideal candidates for LR. At baseline, patients presented with clinically relevant portal hypertension and/or abnormal bilirubin in greater than 30% of cases and had multifocal tumors in greater than 20% of cases. In addition, the maximum tumor size was larger than 5 cm in greater than 30% of cases. The low perioperative mortality of 3.5% observed at 3 mo and the long follow-up of nearly 60 mo allowed a thorough analysis of those preoperative factors that independently influenced the long-term survival of these patients. To reduce the bias deriving from the absence of an external validation cohort, the case series was randomly divided into a training set and a validation set. We then performed the uni- and multivariable analysis on the training set. As expected, the independent variables related to survival were liver related (MELD score > 9, presence of active HCV infection) and tumor related (number of nodules > 3, the largest diameter of nodules > 5 cm and presence of portal invasion). Interestingly, as previously observed[22], clinically relevant portal hypertension did not independently affect survival. The same occurred for bilirubin above normal levels, which was not independently associated with survival when a composite score, such as MELD, was introduced in the multivariable analysis.
According to the weight of each factor independently related with survival and the corresponding risk estimates, an easy-to-determine prognostic scoring system was built. Then, according to changes in the risk estimates for each point increase, a stratification in three prognostic strata was computed: low (0-1 points), intermediate (2 points) and high (3-9 points) risk population. The corresponding median survivals were 77.2 mo (95%CI: 67.4-87.0), 41.7 mo (95%CI: 34.7-48.7) and 17.4 mo (95%CI: 10.1-24.6), respectively (P < 0.0001). The significant difference in survival, overall and between strata, was confirmed also in the validation set and the entire cohort. This scoring system allows prospecting the post-surgical outcomes by assessing five easily accessible characteristics and thus may help clinicians when selecting between different treatment options for HCC patients.
Patients in the low-risk category were considered as ideal candidates for LR according to the observed survival of approximately 60% at 5 years, which approximates that of patients undergoing LT for HCC. This finding allowed to consider 593 patients (69.4% of the total of 854 evaluable patients) as “ideal candidates” for resection with respect to patients who would have been considered as “ideal candidates” according to EASL/AASLD guidelines (less than 40% patients) and resulted in a net increase of 31.6% of patients with indication for LR. The predictive power of the proposed criteria in the identification of the ideal candidate for resection was similar to that of the current guidelines in terms of AIC and Harrel’s C statistics. Most importantly, inclusion of a significantly increased number of patients in the definition of “ideal candidates” did not result in a significant decrease in terms of survival. After all, the proposed score broadens and enhances the concept of “surgical HCC” that is often discarded in the hepato-oncology community due to an insufficient definition and poor evidence.
There are some limitations of this study. Firstly, despite prospective data collection, this is a retrospective study performed in only two high-volume centres. An external validation in a different population is required to strengthen the study results. Secondly, a different method of defining the training set could have been chosen, e.g., the bootstrapping method, although the presented sample size was sufficiently large to meet generalizability criteria. Thirdly, active HCV infection was identified as an independent prognostic factor in this series, and this result may not totally apply in other settings where other aetiologies of cirrhosis are prevalent. In this respect, the recent introduction of direct antiviral agents to treat HCV infection may reveal other factors with a significant weight on patients’ prognosis in the future. Finally, this study included only patients who underwent open LRs. Some factors, particularly those related to liver function, may have less significant impacts on long-term outcomes for patients undergoing laparoscopic LR[23].
In conclusion, this study provides an easily accessible tool to stratify the prognosis of patients undergoing LR for HCC. The identified subset of patients at low risk could enter the group of ideal candidates for LR given that their prognosis approaches that of patients undergoing LT for HCC. The proposed criteria may expand safely the current EASL/AASLD indications for LR with no detrimental effect on patient prognosis.
The prognosis of patients with hepatocellular carcinoma (HCC) largely depends on tumor extension and underlying liver function. According to Western Guidelines, liver resection (LR) is considered as the first-line treatment only for a restricted subset of patients with an optimal liver function, a preserved physical condition and a single tumor nodule with no evidence of extra-hepatic spread or involvement of major vascular structures. If this profile is fulfilled, postoperative morbidity is low and long-term survival may equate that of liver transplantation.
Several field practice studies have ascertained that LR is often offered outside Western guidelines, and various authors reported acceptable survival rates for patients with HCC resected at a more advanced stage because of macrovascular invasion, multiple nodules or impaired liver function. In addition, more recent studies demonstrate a survival benefit of radical surgery with respect to the available treatment alternatives across the different Barcelona Clinic Liver Cancer staging system stages.
The authors analyzed a large consecutive series of patients who underwent LR for HCC at two Italian centres, of whom greater than 60% of cases were outside Western guidelines. Five variables were identified as independently related to survival: Model for End-stage Liver Disease score > 9, presence of active hepatitis C virus infection, number of nodules > 3, largest diameter of nodules > 5 cm and presence of portal invasion. According to the weight of each variable, an easy-to-determine prognostic scoring system was built that allowed the identification of three risk strata with significantly different survival rates. Overall survival of patients in the low-risk strata was similar to that of patients who underwent LR according to Western guidelines. Considering LR patients in the low-risk strata as “ideal candidates” allowed a net increase of 31.6% of patients with indication for LR with respect to Western guidelines.
This scoring system allows assessment of the post-surgical outcomes by assessing five easily accessible characteristics and thus may help clinicians when selecting between different treatment options for HCC patients. Inclusion of a significantly higher number of patients in the definition of “ideal candidates” did not result in a significant decrease in terms of survival. Thus, the proposed score may broaden and enhance the concept of “surgical HCC” that is often discarded in the hepato-oncology community due to an insufficient definition and poor evidence.
Ideal candidates for LR according to Western guidelines are defined by an optimal liver function (Child-Pugh A, normal bilirubin and absence of clinically relevant portal hypertension), a preserved physical condition (ECOG Performance Status of 0), and a single tumor nodule with no evidence of extra-hepatic spread or involvement of major vascular structures.
The study is interesting and trough a sophisticated statistical analysis of a large group of patients, provides a demonstration of the possibility to expand the obsolete European Association for the Study of the Liver/American Association for the Study of Liver Diseases guidelines. The topic is important and this is a well-organized study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lo Tesoriere R, Toriguchi K S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20515] [Article Influence: 2051.5] [Reference Citation Analysis (20)] |
| 2. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1793] [Article Influence: 85.4] [Reference Citation Analysis (2)] |
| 3. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 4. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4521] [Article Influence: 347.8] [Reference Citation Analysis (2)] |
| 5. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 6. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3596] [Article Influence: 276.6] [Reference Citation Analysis (4)] |
| 7. | Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1331] [Cited by in RCA: 1271] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 8. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Roayaie S, Jibara G, Taouli B, Schwartz M. Resection of hepatocellular carcinoma with macroscopic vascular invasion. Ann Surg Oncol. 2013;20:3754-3760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 10. | Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E, Donadon M. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 417] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 11. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 583] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 12. | Roayaie S, Jibara G, Tabrizian P, Park JW, Yang J, Yan L, Schwartz M, Han G, Izzo F, Chen M. The role of hepatic resection in the treatment of hepatocellular cancer. Hepatology. 2015;62:440-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 322] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 13. | Yin L, Li H, Li AJ, Lau WY, Pan ZY, Lai EC, Wu MC, Zhou WP. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan Criteria: a RCT. J Hepatol. 2014;61:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 14. | Vitale A, Burra P, Frigo AC, Trevisani F, Farinati F, Spolverato G, Volk M, Giannini EG, Ciccarese F, Piscaglia F. Survival benefit of liver resection for patients with hepatocellular carcinoma across different Barcelona Clinic Liver Cancer stages: a multicentre study. J Hepatol. 2015;62:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 189] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 15. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3243] [Article Influence: 135.1] [Reference Citation Analysis (0)] |
| 16. | Bismuth H. Surgical anatomy and anatomical surgery of the liver. World J Surg. 1982;6:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 465] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716-723. [DOI] [Full Text] |
| 18. | Newson R. Comparing the predictive powers of survival models using Harrell’s C or Somers’ D. The STATA Journal. 2010;10:339-358. [DOI] [Full Text] |
| 19. | Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, La Barba G, Zanello M, Grazi GL, Pinna AD. Impact of model for end-stage liver disease (MELD) score on prognosis after hepatectomy for hepatocellular carcinoma on cirrhosis. Liver Transpl. 2006;12:966-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 195] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 20. | Sullivan LM, Massaro JM, D’Agostino RB. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23:1631-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 997] [Cited by in RCA: 1286] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 21. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2876] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 22. | Cucchetti A, Ercolani G, Vivarelli M, Cescon M, Ravaioli M, Ramacciato G, Grazi GL, Pinna AD. Is portal hypertension a contraindication to hepatic resection? Ann Surg. 2009;250:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Sposito C, Battiston C, Facciorusso A, Mazzola M, Muscarà C, Scotti M, Romito R, Mariani L, Mazzaferro V. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. Br J Surg. 2016;103:871-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |