Published online Sep 28, 2016. doi: 10.3748/wjg.v22.i36.8168
Peer-review started: May 4, 2016
First decision: May 27, 2016
Revised: June 9, 2016
Accepted: July 20, 2016
Article in press: July 21, 2016
Published online: September 28, 2016
Processing time: 145 Days and 12.5 Hours
To investigate expression of four alpha-carbonic anhydrases (CAs) in colorectal carcinomas (CRC) and compare the results with patients’ survival.
Colorectal carcinoma samples from 539 CRC patients and control tissues were arranged as tissue microarrays and analyzed with antibodies against CA II, CA VII, CA IX, and CA XII. Intensity and extent of staining were both scored from 0 to 3 in each sample. These enzyme expression levels were then correlated to patients’ survival and clinicopathological parameters, which were tumor differentiation grade and stage, site of tumor, patients’ age, and gender. Kaplan-Meier analysis and Cox regression hazard ratio model were used to analyze survival data.
CA II and CA XII staining intensities correlated with patients’ survival in that higher expression indicated poorer prognosis. In Cox regression analysis one unit increase in the CA II intensity increased the hazard ratio to 1.19 fold (CI: 1.04-1.37, P = 0.009). A significant correlation was also found when comparing CA XII staining intensity with survival of CRC patients (HR = 1.18, 95%CI: 1.01-1.38, P = 0.036). The extent of CA XII immunostaining did not correlate to the patients’ survival (P = 0.242, Kaplan-Meier analysis). A significant interaction between age group and extent of the CA II staining was found. Increased extent of CA II had a significant hazard ratio among patients 65 years and older (1.42, 95%CI: 1.16-1.73, P = 0.0006). No correlations were found between CA VII (intensity P = 0.566, extent P = 0.495, Kaplan-Meier analysis), or CA IX (intensity P = 0.879, extent P = 0.315, Kaplan-Meier analysis) immunostaining results and survival, or the other parameters.
The present findings indicate that CA II and CA XII could be useful in predicting survival in CRC.
Core tip: Our aim was to investigate expression of four alpha-carbonic anhydrases (CAs) in colorectal carcinomas (CRC) and compare the results with patients’ survival. CRC samples were arranged as tissue microarrays and analyzed with antibodies against CA II, CA VII, CA IX, and CA XII. Enzyme expression levels were correlated to patients’ survival and clinicopathological parameters. CA II and CA XII staining intensities correlated with patients′ survival in that higher expression indicated poorer prognosis. The present findings indicate that CA II and CA XII could be useful in predicting survival in CRC.
- Citation: Viikilä P, Kivelä AJ, Mustonen H, Koskensalo S, Waheed A, Sly WS, Pastorek J, Pastorekova S, Parkkila S, Haglund C. Carbonic anhydrase enzymes II, VII, IX and XII in colorectal carcinomas. World J Gastroenterol 2016; 22(36): 8168-8177
- URL: https://www.wjgnet.com/1007-9327/full/v22/i36/8168.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i36.8168
Colorectal cancer (CRC) is the most common malignancy of the gastrointestinal (GI) tract; it is the second most common cancer in women and third in men[1]. Its incidence shows substantial geographical variation, resulting in a 10-fold difference between some countries[1]. The high number of patients with CRC, precancerous lesions or polyps causes significant challenges to national healthcare systems, their chances to recognize tumors at an early stage, as well as possibilities to offer the most effective treatment. According to recent data the incidence of CRC is rising in many traditionally low-incidence countries like Japan, Korea, China, and Eastern Europe, which is thought to be a result of cultural and dietary changes towards a Western lifestyle[1]. Even though the overall incidence is increasing, at the same time some positive development has occurred in many previous high-risk countries due to more effective diagnostics and treatment.
CRC usually develops from precursor lesions, i.e., colonic adenomas[2]. The prevalence of adenomas clearly increases by age. Postmortem studies indicate that 30%-40% of individuals from Western countries have adenomas, most of them asymptomatic[3]. A majority of these are benign and never turn malignant[2]. In familial syndromes, younger patients develop adenomas which proceed to cancer with a much higher frequency[2-4]. Removal of precancerous lesions at colonoscopy prevents them from proceeding to cancer. Thus, early diagnosis and complete removal of precancerous lesions are key factors for successful outcome.
The diagnostic arsenal in CRC includes several clinicopathological parameters, which can be utilized to determine the malignancy and prognosis. They include tumor stage according to the TNM (tumor, nodes, metastasis), Dukes or Astler-Coller classification, as well as tumor grade, microsatellite instability, and molecular markers, recently reviewed by Marzouk and Schofield[5]. The intensively studied molecular markers include such as tumor suppressor gene p53 and its mutations as well as antigen Ki-67[6,7]. However, new biomarkers are urgently needed to predict survival and improve stratification of CRC patients for different treatment options.
Carbonic anhydrases (CAs) constitute a group of zinc-binding enzymes, which catalyze the reversible hydration of CO2 to bicarbonate. This reaction is crucial for maintenance of pH homeostasis of the body. Through this chemical reaction they are involved in several downstream physiological processes, such as bone resorption, vision, and production of saliva, bile, pancreatic juice and gastric juice[8-10]. The mammalian alpha-CA family includes 16 known isoforms of which 15 can be found in humans. These isoforms show marked differences in their kinetics and cellular and subcellular distributions[8,9].
Under hypoxic conditions cells produce acidic metabolic products via anaerobic glycolysis. This pathway is inhibited in the presence of enough oxygen. Notably, tumor cells have a tendency to upregulate glucose intake and increase the rate of anaerobic glycolysis even when the amount of oxygen is sufficient[11]. Tumor cells need CA enzymes and many other proteins, such as ion transporters, to maintain neutral intracellular pH[12]. During this process extracellular pH decreases, which in turn, disturbs physiological processes of the surrounding normal tissue and promotes cancer growth[11,13]. Indeed, increased glucose intake and hypoxia are often linked to more aggressive and invasive tumor growth, signs that correlate with poor prognosis[11]. It has been suggested that partial hypoxia may contribute to cell selection, favoring a shift from a pre-malignant phenotype to more malignant forms, in which the oxygen free metabolism plays a major role in making it possible for cells to survive in challenging hypoxic environments[11].
During the last 20 years, CA proteins have been studied as potential markers for various cancers. Cytosolic CA II is the most widely expressed isoform in normal tissues, such as gastric, pancreatic, biliary, and intestinal epithelia[9,14]. It is often absent or only weakly expressed in malignant tumors. Recently, CA II was shown to be highly overexpressed in gastrointestinal stromal tumors, and it was suggested as a potential biomarker for this mesenchymal tumor type[15]. CA VII, another cytosolic isozyme, shows a more restricted tissue distribution than CA II. It is predominantly expressed in the brain, where it contributes to bicarbonate-driven GABAergic excitation[16]. A recent study showed that CA VII is overexpressed in glioblastomas, suggesting that it may represent another tumor-associated CA isoform[17].
CA IX has attracted lots of attention, because its expression is limited to few normal tissues, such as gastric, intestinal and gall bladder epithelia, but it is highly overexpressed in hypoxic tumors[9,18,19]. CA XII is another isoform, which is overexpressed in several cancers, even though it is also present in various normal tissues. It has been demonstrated to be present in both normal intestinal epithelium and malignant colorectal tumors[9,19,20]. CA IX and XII are known to be regulated via von Hippel Lindau / hypoxia inducible factor pathway[21].
The aim of this study was to investigate the expression of isozymes CA II, CA VII, CA IX, and CA XII in CRC. The immunohistochemical expression levels were correlated to clinicopathological data. Our results show that both CA II and CA XII staining intensities correlate with survival rate of CRC patients, suggesting a potential role of these enzymes as prognostic biomarkers.
In total, 840 patients underwent surgery for CRC at Helsinki University Hospital during years 1983-2001. Tissue specimens and clinical data from 645 patients were available for our study. These tumors were classified with Dukes classification which was a standard classification system during the sample collection period. No information of TNM - classification was available for the analysis. The Ethical Committee of Helsinki University Hospital (Dnro 226/E6/2006) and National Supervisory Authority for Welfare and Health (Dnro 10041/06.01.03.01/2012) granted permission for the use of these samples. Survival data were available for all patients and obtained from patient records, the Finnish Cancer Registry and Statistics Finland. The clinicopathological characteristics of the patients are described in Table 1.
| n (%) | |
| Total | 539 (100) |
| Sex | |
| Male | 294 (54.5) |
| Female | 245 (45.5) |
| Age | |
| < 65 yr | 231 (42.9) |
| ≥ 65 yr | 308 (57.1) |
| Tumor location | |
| Right side of colon | 157 (29.1) |
| Left side of colon | 138 (25.6) |
| Rectum | 244 (45.3) |
| Stage | |
| Dukes A | 82 (15.2) |
| Dukes B | 191 (35.4) |
| Dukes C | 136 (25.2) |
| Dukes D | 130 (24.1) |
| Differentiation (grade) | |
| High | 19 (3.5) |
| Medium high | 352 (65.3) |
| Medium low | 141 (26.2) |
| Low | 27 (5.0) |
Tumor samples were arranged as tissue microarrays. Three parallel series included one tumor sample each taken with a 1 mm needle. Of 645 patient samples we obtained, 106 ended up with no scoring results, because samples were washed out or displaced during the staining process. The remaining 539 samples were considered representative enough to be analyzed and scored. Each microscope slide contained four spots of normal human pancreas as positive controls. Five µm sections of formalin-fixed paraffin embedded tumor specimens were deparaffinised and immunostained with rabbit anti-human CA II, CA VII, and CA XII sera or with monoclonal anti-human CA IX antibody (M75). These antibodies have been previously utilized in numerous studies and are specific for each isozyme[22-25]. Immunoperoxidase staining was performed in an automated Lab Vision Autostainer 480 (LabVision Corporation, Fremont, CA, United States) by Power Vision+ Poly-HRP Immunohistochemistry kit reagents (ImmunoVision Technologies Co) including the following steps: (1) rinsing in wash buffer; (2) treatment in 3% H2O2 in ddH2O for five minutes and rinsing with wash buffer; (3) blocking with cow colostrum diluted 1:2 in Tris-buffered saline containing 0.05% Tween-20 for 30 min and rinsing in wash buffer; (4) incubation with primary antibody (polyclonal antibodies diluted 1:2000 and monoclonal M75 diluted 1:100) for 30 min; (5) rinsing in wash buffer three times for five minutes; (6) incubation in poly-HRP-conjugated anti-rabbit/mouse IgG for 30 min and rinsing in wash buffer three times for five minutes; (7) incubation in DAB (3,3’-diaminobenzidine tetrahydrochloride) solution (one drop of DAB solution A and one drop of DAB solution B in 1 mL of ddH2O) for six minutes and rinsing in ddH2O; (8) CuSO4 treatment for five minutes to enhance the signal and rinsing in ddH2O; (9) treatment with hematoxylin for one minute; and (10) rinsing with ddH2O. All procedures were performed at room temperature. The mounting of the sections was performed using Entellan Neu (Merck; Darmstadt, Germany) and was finally examined and photographed with a Zeiss Axioskop 40 microscope (Carl Zeiss; Göttingen, Germany). The intensity of the staining was scored on a scale of 0 to 3 as follows: 0, no reaction; 1, weak reaction; 2, moderate reaction; 3, strong reaction. The extent of the staining was scored as 1 when 1%-10% of the cells stained, 2 when 11%-50% of the cells stained, and 3 when 51%-100% of the cells stained. A negative score (0) was given to tissue sections that had no evidence of specific immunostaining. In addition to the analysis of tumor cells, CA II immunostaining was also evaluated in endothelial cells and CA VII in tumor stroma, because these enzymes showed significant staining reactions in these specific locations.
All statistical analyses were performed by an experienced biostatistician (H. Mustonen). Results are announced as number of patients and percentage of patients, the Kaplan-Meier mean survival time with 95%CI, and the Cox regression hazard ratios with their 95%CI. P values < 0.05 were considered statistically significant. Two-tailed tests were used.
Kaplan-Meier analysis and Cox regression hazard ratio model were used to analyze survival data. The assumption of constant hazard ratios over time was tested by including a time dependent covariate for each testable variable. Because neither Dukes classification nor histological differentiation grade status followed the Cox model assumption, stratified analyses for Dukes stage and differentiation grade were used. For statistical analyses, Dukes stage A and B, as well as C and D were combined to reduce the number of groups to two. The same was done for tumor differentiation status; low and medium low differentiations were combined as well as medium high and high differentiations. In the Cox proportional hazards model we included variables for age, sex, and site of the tumor. Separate analyses were made for each CA staining. If a significant interaction with CA staining and another parameter were found, a split analysis was performed.
In analysis, male patients were compared to females and 65 years-old or older patients were compared to younger ones. Tumor location was also considered in the analysis: right side of the colon including the caecum, ascending colon and right flexure versus left side of the colon, including the left flexure, descending colon, sigmoid colon and rectum. Mid transverse tumors were excluded from the comparison.
When analyzing the expression of each CA enzyme, level 0 was considered the baseline, and the announced hazard ratio rises exponentially every time the scoring value rises one unit. Statistical analyses were performed by SPSS v 21© (IBM corp, New York).
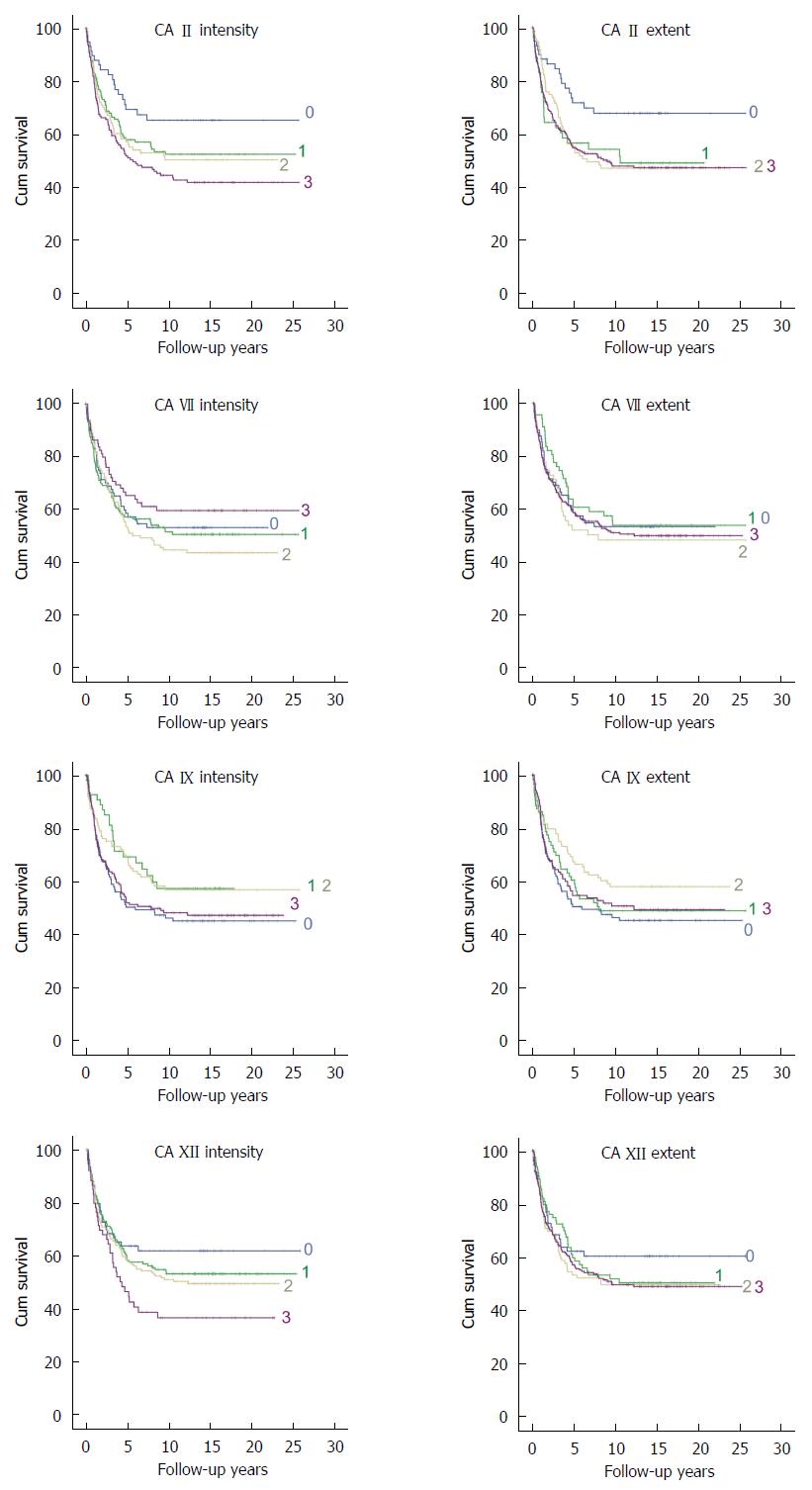
Four CA isozymes, CA II, VII, IX, and XII were selected for the immunohistochemical analysis of colorectal cancer and the summary of these results is presented in Table 2. Figure 1 presents representative images of positive immunostaining of each isozyme in CRC specimens. Normal colon and pancreas specimens are shown as positive controls. Figure 2 shows Kaplan-Meier plots for the staining intensity and extent of each isozyme. In Kaplan-Meier analysis, there was a significant decrease in survival as the intensity of CA II increased. The mean survival time decreased from 17.7 years (95% CI, 14.8-20.6) to 12.3 years (95%CI: 10.5-14.2) when CA II intensity increased from 0 to 3. The extent of CA II staining showed a similar trend (P = 0.022). Correspondingly, the mean survival time decreased from 18.3 years (95%CI: 15.5-21.1) to 13.5 years (95%CI: 12.0-15.0).
| Mean | SD | Median | Mode | 25% | 75% | |
| CA II epithelium intensity | 1.8 | 1.1 | 2 | 3 | 1 | 3 |
| CA II epithelium extent | 2.2 | 1.1 | 3 | 3 | 2 | 3 |
| CA II endothelium | 0.9 | 0.3 | 1 | 1 | 1 | 1 |
| CA VII epithelium intensity | 1.4 | 1 | 1 | 1 | 1 | 2 |
| CA VII epithelium extent | 2 | 1.2 | 3 | 3 | 1 | 3 |
| CA VII stromal intensity | 2.8 | 0.5 | 3 | 3 | 3 | 3 |
| CA VII stromal extent | 2.8 | 0.5 | 3 | 3 | 3 | 3 |
| CA IX epithelium intensity | 1.7 | 1.2 | 2 | 3 | 0 | 3 |
| CA IX epithelium extent | 1.6 | 1.2 | 2 | 3 | 0 | 3 |
| CA XII epithelium intensity | 1.5 | 0.9 | 1 | 2 | 1 | 2 |
| CA XII epithelium extent | 2 | 1.1 | 2 | 3 | 1 | 3 |
In Cox regression analysis, the most significant results were found for CA II staining intensity as shown in Table 3. One unit increase in the CA II intensity increased the age, sex, Dukes, differentiation, and tumor side corrected hazard ratio to 1.19 fold (95%CI: 1.04-1.37, P = 0.009). However, no significant interaction was found between CA II intensity and age, sex, or location of tumor. When extent of the CA II staining was analyzed a significant interaction between age group and extent of the CA II staining was found. Therefore, the analysis was split between the age groups. This revealed that increased extent of CA II had a significant hazard ratio among patients 65 years and older (1.42, 95%CI: 1.16-1.73, P = 0.0006). Notably, in addition to the actual tumor cells, CA II-positive staining was often induced in the endothelium of tumor capillaries. In Kaplan-Meier analysis, vascular endothelial staining of CA II did not show any significant correlation to survival (P = 0.676).
| Hazard ratio | 95%CIlower | 95%CIupper | P value | |
| CA II epithelium intensity analysis | ||||
| CA II epithelium intensity | 1.19 | 1.04 | 1.37 | 0.0092 |
| Sex (men vs females) | 1.20 | 0.92 | 1.58 | 0.1777 |
| Age over 65 yr | 1.78 | 1.35 | 2.35 | P < 0.0001 |
| Side of colon (sin vs dex) | 1.17 | 0.86 | 1.58 | 0.3170 |
| CA II epithelium extent analysis, age ≤ 65 yr | ||||
| CA II epithelium extent | 0.88 | 0.73 | 1.06 | 0.1742 |
| Sex (men vs females) | 0.99 | 0.65 | 1.51 | 0.9564 |
| Side of colon (sin vs dex) | 1.63 | 0.97 | 2.74 | 0.1869 |
| CA II epithelium extent analysis, age > 65 yr | ||||
| CA II epithelium extent | 1.42 | 1.16 | 1.73 | 0.0006 |
| Sex (men vs females) | 1.44 | 1.01 | 2.05 | 0.0435 |
| Side of colon (sin vs dex) | 0.92 | 0.63 | 1.35 | 0.6764 |
| CA XII epithelium intensity analysis | ||||
| CA XII epithelium intensity | 1.18 | 1.01 | 1.38 | 0.0360 |
| Sex (men vs females) | 1.23 | 0.94 | 1.60 | 0.1301 |
| Age over 65 yr | 1.67 | 1.27 | 2.19 | 0.0002 |
| Side of colon (sin vs dex) | 1.09 | 0.81 | 1.47 | 0.5571 |
Epithelial CA VII immunostaining showed no correlation to patients’ survival (Figure 2). In Kaplan-Meier survival analysis, P values were 0.566 for staining intensity and 0.495 for extent. Negative results were also observed for stromal staining (P = 0.816 for intensity; P = 0.591 for extent). There was a significant correlation (P = 0.013) between epithelial staining extent and Dukes classification. These results are shown in Table 4. No significant correlation was found between epithelial or stromal CA VII immunostaining and other parameters.
| CA VII epithelial extent | Dukes A count | % | B count | % | C count | % | D count | % |
| 0 | 15 | 16.1% | 36 | 38.7% | 25 | 26.9% | 17 | 18.3% |
| 1 | 14 | 19.7% | 31 | 43.7% | 14 | 19.7% | 12 | 16.9% |
| 2 | 10 | 15.2% | 23 | 34.8% | 14 | 21.2% | 19 | 28.8% |
| 3 | 28 | 11.4% | 85 | 34.6% | 67 | 27.2% | 66 | 26.8% |
In Kaplan-Meier analysis no significant correlation was found between survival and intensity (P = 0.879) or extent (P = 0.315) of CA IX immunostaining. Additionally, no correlation was found when the CA IX immunostaining was compared with the other tumor or clinical parameters.
In Kaplan-Meier analysis, CA XII was another isozyme showing a significant correlation to survival. The mean survival time decreased from 16.5 years (95%CI: 13.7-19.2) to 9.9 years (95%CI: 7.3-12.4) as the intensity of CA XII increased from 0 to 3. In Cox regression analysis, a significant correlation was found when comparing CA XII staining intensity with survival of CRC patients (HR = 1.18, 95%CI: 1.01-1.38, P = 0.036; Table 3). There was no significant interaction between CA XII intensity and age, gender or location of the tumor. The extent of CA XII immunostaining did not correlate to the patients’ survival (P = 0.242, Kaplan-Meier analysis). CA XII staining extent did not either correlate with clinicopathological parameters.
In general, patients 65 years and older had a significantly increased risk for poorer survival (HR = 1.78, 95%CI: 1.35-2.34, P < 0.0001 in CA II epithelium intensity analysis and 1.67, CI: 1.27-2.19, P = 0.0002 in CA XII epithelium intensity analysis).
In our study, the expression of carbonic anhydrase isozymes CA II and CA XII correlated with patients’ survival. Our results suggest that these proteins might have a value in the assessment of CRC patients, specifically when selecting patients for a more aggressive cancer treatment. Patients with a better prognosis can be treated less aggressively, which diminishes side effects and increases quality of life. The obtained hazard rations were relatively modest, and thus the results need to be interpreted with caution. According to our results, CA IX has no diagnostic or prognostic role in CRC, even though it has been considered a promising biomarker for several other cancers[26]. This finding highlights the unique biology of CRCs in terms of tumor development process.
The role of different CA isozymes in cancer has been under intensive research during the last decade. Studies have been focused on the association between expression of CAs and tumor aggressiveness and patient survival, on their role in tumor metastasis, as well as on their potential role as targets of anticancer drugs[13,27-29]. CA isozymes have shown abnormal expression in various malignant tumors compared to corresponding normal cells and tissues[11,13,17,29-31]. In one study, CA IX was shown to be diffusely expressed in CRC, whereas normal or adenomatous mucosa showed a more limited distribution[18]. Ki-67 immunostainings confirmed that CA IX was expressed in areas with high proliferative activity. Similarly, CA XII showed a more diffuse immunostaining reaction in CRC compared to normal colon or adenomas[20]. Cytosolic CA II isozyme has a different distribution. It is highly expressed in several normal gastrointestinal tract tissues, such as gastric and intestinal mucosa[14], but is clearly downregulated in most colorectal tumors[32]. The same phenomenon may also occur in the case of the other cytosolic CAs[33]. According to Birkenkamp-Demtroder’s study[34], CA VII expression was found to be 4-fold downregulated in sigmoid or rectosigmoid carcinomas compared to the normal tissue. Recently, Niemelä et al[35] reported cDNA microarray expression of all CAs, except for CA XIII, in normal and malignant colorectal specimens. The fold-changes for our target CA isozymes were: CA II [-7.4 (normal mucosa vs sporadic carcinoma)], CA VII (-4.3), CA IX (+2.4) and CA XII (-3.2). In our Figure 1 increased expression is clearly demonstrated for CA IX, whereas the interpretation is more difficult for the other isozymes because of high staining intensities in both normal and tumor tissues.
Even though the pathogenesis of colorectal cancer has been intensively studied during the last decades, there are still molecular mechanisms that clearly warrant more research. For example, it was recently shown that polyps with any advanced neoplastic features are smaller in the right side of the colon than in the left side, and the gene expression is also different[34,36]. In our study, however, the reactivity of CA enzymes showed no correlation to the tumor location.
The main clinicopathological factors affecting colorectal cancer patients’ survival are tumor grade, resection margins, and presence or absence of lymph node metastases[3]. Other factors with prognostic significance include tumor budding, micrometastases, peritoneal carcinomatosis, lymphatic, perineural and venous invasion, and histological properties including level of invasion[5]. In Marzouk and Schofield’s review[5], a number of potential prognostic molecular markers, such as microsatellite instability, BRAF mutation, KRAS mutation, PIK3CA mutations, and PTEN deletion, were evaluated. 18q deletions and thymidylate synthase expression have been associated with unfavorable prognosis and tumor recurrence[5]. Even though many of these molecular markers have been used when choosing patients with metastatic disease for chemotherapy[5], new markers are still needed to distinguish different cancer types or to stratify patients for personalized chemotherapy according to the cancer properties. Further studies are needed to define the clinical value of CA II and CA XII staining for preoperative evaluation and for comparison of these markers in colorectal cancer.
We thank Ms Aulikki Lehmus for skillful technical assistance and Harlan Barker, MSc, for important revisions.
Colorectal cancer (CRC) is the most common malignancy of the gastrointestinal tract. The high number of patients with CRC, precancerous lesions or polyps causes significant challenges to healthcare systems, their chances to recognize tumors at an early stage, as well as possibilities to offer the most effective treatment. Tumor cells need carbonic anhydrases (CAs) and many other proteins, to maintain neutral intracellular pH. During tumor growth extracellular pH decreases and disturbs physiological processes of the surrounding normal tissue and promotes cancer growth. This is why CA proteins have been studied as potential markers for various cancers.
CA II and CA XII staining intensities correlated with colorectal cancer patients’ survival in that higher expression indicated poorer prognosis.
The correlation of both CA II and CA XII staining results with survival of colorectal cancer patients was a novel observation. Surprisingly, CA IX immunostaining results did not show any correlation to patients’ prognosis, even though this enzyme is widely considered a potential predictive marker in several other tumor categories.
These results suggest that CA II and CA XII proteins might be valuable in assessment of CRC patients, specifically when selecting patients for a more aggressive cancer treatment. Patients with a better prognosis can be treated less aggressively, which diminishes side effects and increases quality of life.
CAs are a group of zinc-binding enzymes, which catalyze the reversible hydration of CO2 to bicarbonate.
Biomarkers for colorectal Cancer are still under intense Investigation and novel predictive and prognostic markers are urgently needed. The authors investigate the Protein Expression Levels of several CA in a large series of colorectal cancer and conclude that CA II and CA XII could be useful prognostic markers.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Finland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hokama A, Ierardi E, Ocker M S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1754] [Cited by in RCA: 1882] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 2. | Scholefield JH. ABC of colorectal cancer: screening. BMJ. 2000;321:1004-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Hardy RG, Meltzer SJ, Jankowski JA. ABC of colorectal cancer. Molecular basis for risk factors. BMJ. 2000;321:886-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Cole TR, Sleightholme HV. ABC of colorectal cancer. The role of clinical genetics in management. BMJ. 2000;321:943-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Marzouk O, Schofield J. Review of histopathological and molecular prognostic features in colorectal cancer. Cancers (Basel). 2011;3:2767-2810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Russo A, Bazan V, Iacopetta B, Kerr D, Soussi T, Gebbia N. The TP53 colorectal cancer international collaborative study on the prognostic and predictive significance of p53 mutation: influence of tumor site, type of mutation, and adjuvant treatment. J Clin Oncol. 2005;23:7518-7528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 297] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710-1715. [PubMed] |
| 8. | Supuran CT, Scozzafava A, Casini A. Carbonic anhydrase inhibitors. Med Res Rev. 2003;23:146-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 978] [Cited by in RCA: 976] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 9. | Kivelä AJ, Kivelä J, Saarnio J, Parkkila S. Carbonic anhydrases in normal gastrointestinal tract and gastrointestinal tumours. World J Gastroenterol. 2005;11:155-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 10. | Supuran CT. Carbonic anhydrases as drug targets--an overview. Curr Top Med Chem. 2007;7:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3472] [Cited by in RCA: 3609] [Article Influence: 171.9] [Reference Citation Analysis (0)] |
| 12. | Parks SK, Chiche J, Pouysségur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13:611-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 483] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 13. | Neri D, Supuran CT. Interfering with pH regulation in tumours as a therapeutic strategy. Nat Rev Drug Discov. 2011;10:767-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1260] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 14. | Parkkila S, Parkkila AK, Juvonen T, Rajaniemi H. Distribution of the carbonic anhydrase isoenzymes I, II, and VI in the human alimentary tract. Gut. 1994;35:646-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 102] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Parkkila S, Lasota J, Fletcher JA, Ou WB, Kivelä AJ, Nuorva K, Parkkila AK, Ollikainen J, Sly WS, Waheed A. Carbonic anhydrase II. A novel biomarker for gastrointestinal stromal tumors. Mod Pathol. 2010;23:743-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 16. | Ruusuvuori E, Li H, Huttu K, Palva JM, Smirnov S, Rivera C, Kaila K, Voipio J. Carbonic anhydrase isoform VII acts as a molecular switch in the development of synchronous gamma-frequency firing of hippocampal CA1 pyramidal cells. J Neurosci. 2004;24:2699-2707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Bootorabi F, Haapasalo J, Smith E, Haapasalo H, Parkkila S. Carbonic anhydrase VII - a potential prognostic marker in gliomas. Health. 2011;3:6-12. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Saarnio J, Parkkila S, Parkkila AK, Haukipuro K, Pastoreková S, Pastorek J, Kairaluoma MI, Karttunen TJ. Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol. 1998;153:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 184] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Kivela AJ, Parkkila S, Saarnio J, Karttunen TJ, Kivela J, Parkkila AK, Bartosova M, Mucha V, Novak M, Waheed A. Expression of von Hippel-Lindau tumor suppressor and tumor-associated carbonic anhydrases IX and XII in normal and neoplastic colorectal mucosa. World J Gastroenterol. 2005;11:2616-2625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 20. | Kivelä A, Parkkila S, Saarnio J, Karttunen TJ, Kivelä J, Parkkila AK, Waheed A, Sly WS, Grubb JH, Shah G. Expression of a novel transmembrane carbonic anhydrase isozyme XII in normal human gut and colorectal tumors. Am J Pathol. 2000;156:577-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Ivanov SV, Kuzmin I, Wei MH, Pack S, Geil L, Johnson BE, Stanbridge EJ, Lerman MI. Down-regulation of transmembrane carbonic anhydrases in renal cell carcinoma cell lines by wild-type von Hippel-Lindau transgenes. Proc Natl Acad Sci USA. 1998;95:12596-12601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 281] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 22. | Parkkila AK, Parkkila S, Juvonen T, Rajaniemi H. Carbonic anhydrase isoenzymes II and I are present in the zona glomerulosa cells of the human adrenal gland. Histochemistry. 1993;99:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Bootorabi F, Jänis J, Smith E, Waheed A, Kukkurainen S, Hytönen V, Valjakka J, Supuran CT, Vullo D, Sly WS. Analysis of a shortened form of human carbonic anhydrase VII expressed in vitro compared to the full-length enzyme. Biochimie. 2010;92:1072-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | Pastoreková S, Parkkila S, Parkkila AK, Opavský R, Zelník V, Saarnio J, Pastorek J. Carbonic anhydrase IX, MN/CA IX: analysis of stomach complementary DNA sequence and expression in human and rat alimentary tracts. Gastroenterology. 1997;112:398-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 259] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Karhumaa P, Parkkila S, Türeci O, Waheed A, Grubb JH, Shah G, Parkkila A, Kaunisto K, Tapanainen J, Sly WS. Identification of carbonic anhydrase XII as the membrane isozyme expressed in the normal human endometrial epithelium. Mol Hum Reprod. 2000;6:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Pastorekova S, Parkkila S, Zavada J. Tumor-associated carbonic anhydrases and their clinical significance. Adv Clin Chem. 2006;42:167-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Chia SK, Wykoff CC, Watson PH, Han C, Leek RD, Pastorek J, Gatter KC, Ratcliffe P, Harris AL. Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J Clin Oncol. 2001;19:3660-3668. [PubMed] |
| 28. | Giatromanolaki A, Koukourakis MI, Sivridis E, Pastorek J, Wykoff CC, Gatter KC, Harris AL. Expression of hypoxia-inducible carbonic anhydrase-9 relates to angiogenic pathways and independently to poor outcome in non-small cell lung cancer. Cancer Res. 2001;61:7992-7998. [PubMed] |
| 29. | Haapasalo J, Nordfors K, Järvelä S, Bragge H, Rantala I, Parkkila AK, Haapasalo H, Parkkila S. Carbonic anhydrase II in the endothelium of glial tumors: a potential target for therapy. Neuro Oncol. 2007;9:308-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Robertson N, Potter C, Harris AL. Role of carbonic anhydrase IX in human tumor cell growth, survival, and invasion. Cancer Res. 2004;64:6160-6165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 237] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 31. | Türeci O, Sahin U, Vollmar E, Siemer S, Göttert E, Seitz G, Parkkila AK, Shah GN, Grubb JH, Pfreundschuh M. Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci USA. 1998;95:7608-7613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 279] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Kivela AJ, Saarnio J, Karttunen TJ, Kivelä J, Parkkila AK, Pastorekova S, Pastorek J, Waheed A, Sly WS, Parkkila TS. Differential expression of cytoplasmic carbonic anhydrases, CA I and II, and membrane-associated isozymes, CA IX and XII, in normal mucosa of large intestine and in colorectal tumors. Dig Dis Sci. 2001;46:2179-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Kummola L, Hämäläinen JM, Kivelä J, Kivelä AJ, Saarnio J, Karttunen T, Parkkila S. Expression of a novel carbonic anhydrase, CA XIII, in normal and neoplastic colorectal mucosa. BMC Cancer. 2005;5:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Birkenkamp-Demtroder K, Olesen SH, Sørensen FB, Laurberg S, Laiho P, Aaltonen LA, Orntoft TF. Differential gene expression in colon cancer of the caecum versus the sigmoid and rectosigmoid. Gut. 2005;54:374-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 35. | Niemelä AM, Hynninen P, Mecklin JP, Kuopio T, Kokko A, Aaltonen L, Parkkila AK, Pastorekova S, Pastorek J, Waheed A. Carbonic anhydrase IX is highly expressed in hereditary nonpolyposis colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:1760-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Gupta S, Balasubramanian BA, Fu T, Genta RM, Rockey DC, Lash R. Polyps with advanced neoplasia are smaller in the right than in the left colon: implications for colorectal cancer screening. Clin Gastroenterol Hepatol. 2012;10:1395-1401.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |