Published online Sep 21, 2016. doi: 10.3748/wjg.v22.i35.7926
Peer-review started: April 29, 2016
First decision: July 13, 2016
Revised: August 1, 2016
Accepted: August 10, 2016
Article in press: August 10, 2016
Published online: September 21, 2016
Processing time: 140 Days and 1 Hours
Management of cancers of the digestive system has progressed rapidly into the molecular era. Despite the significant recent achievements in the diagnosis and treatment of these patients, the number of deaths for these tumors has currently plateaued. Many investigations have assessed the role of HER2 in tumors of the digestive system in both prognostic and therapeutic settings, with heterogeneous results. Novel testing and treatment guidelines are emerging, in particular in gastric and colorectal cancers. However, further advances are needed. In this review we provide a comprehensive overview of the current state-of-knowledge of HER2 alterations in the most common tumors of the digestive system and discuss the operational implications of HER2 testing.
Core tip: Numerous studies have broadened our understanding of HER2 as a critical oncogene in many human cancers, including tumors of the digestive system. Due to the increasing importance of HER2 testing in this heterogeneous group of tumors, in this review we seek to outline the current state of knowledge of HER2 alterations in the most common malignancies occurring in the digestive system, to examine the operational implications of HER2 testing as a biomarker and potentially targetable gene, and discuss immediate future perspectives for pathologists and gastroenterologists.
- Citation: Fusco N, Bosari S. HER2 aberrations and heterogeneity in cancers of the digestive system: Implications for pathologists and gastroenterologists. World J Gastroenterol 2016; 22(35): 7926-7937
- URL: https://www.wjgnet.com/1007-9327/full/v22/i35/7926.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i35.7926
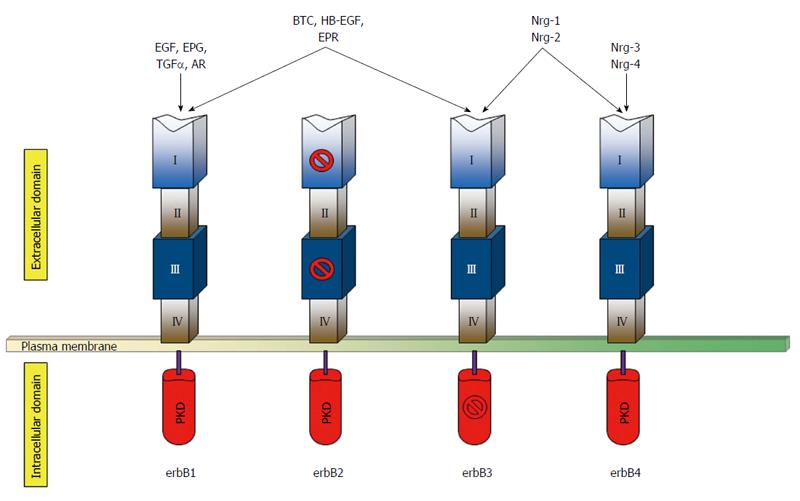
The epidermal growth factor receptor 2 is a proto-oncogene that was first identified in the early 1980s in rodent neural tumor cell lines and therefore named neu[1]. Given the homology between the human gene and that of the rodent, adherence to appropriate nomenclature is pivotal to avoid any confusion. In this review, HER2/neu will refer to the gene across both species, while HER2 and erbB2 will be used specifically to indicate the human gene and its protein product, respectively[2]. HER2/neu belongs to one of the most studied growth factor receptor systems in cancer, the erbB tyrosine kinase family[1-4]. This family consists of four members encoding the homologous epidermal growth factor receptor proteins erbB1, 2, 3, 4 that are ubiquitously expressed in epithelial, mesenchymal, and neuronal normal cells and their cellular progenitors[5,6]. Each of these receptors is composed of an extracellular ligand-binding domain, a transmembrane segment and an intracellular protein kinase domain with a carboxyl terminal segment holding site of phosphorylation or tyrosine residues (Figure 1)[5,7,8]. Among the four erbB proteins, erbB2 is functionally characterized by an extraordinarily strong catalytic kinase activity, representing a key oncoprotein that triggers cornerstone intracellular signaling events for cell growth and survival, ultimately leading to increased signal transduction and activation of the MAPK and PI3K/Akt pathways[4,6,9]. Importantly, erbB2 is not involved in ligand binding of the growth factors unless is overexpressed[10], while the other members of its family represent active receptors in basal conditions also[5,9], as outlined in Figure 1.
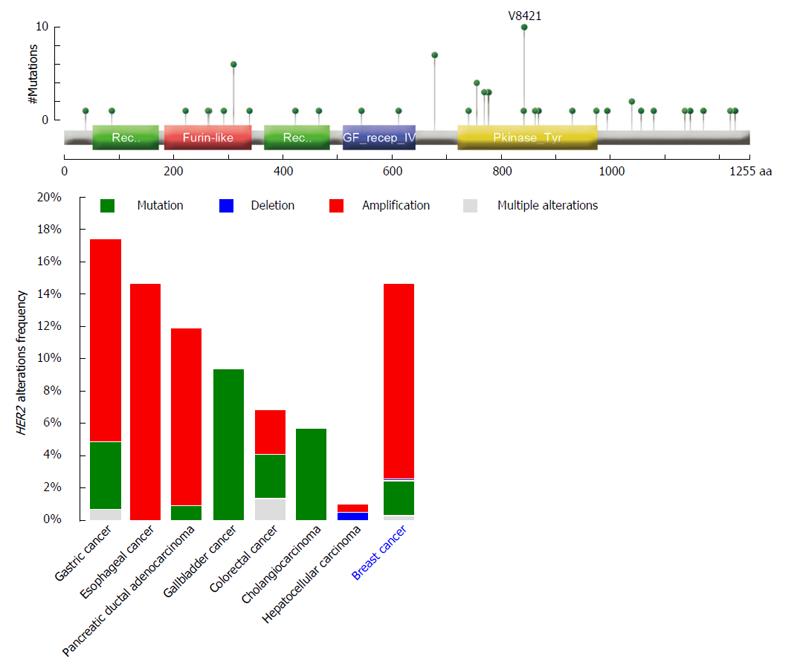
Numerous preclinical and clinical studies, beginning with the intuition of Slamon and collaborators on the role of HER2 in breast cancer, have broadened our understanding of this oncogene in many human cancers, including digestive system cancers (DSC)[8,11]. While HER2 represents a prognostic marker of aggressive behavior in many DSC[8,12,13], the importance of this oncogene remains closely related to its role as a potentially targetable cancer gene[4,14]. To date, anti-HER2 antibodies such as trastuzumab, pertuzumab, the new conjugate ado-trastuzumab emtansine, and HER2-inhibitors (e.g., lapatinib) have received the United States Food and Drug Administration (FDA) approval not only in HER2-positive breast cancers but also in HER2-positive metastatic gastric cancer (GC)[15]. Massively parallel sequencing studies have recently revealed that a substantial proportion of DSC are genetically characterized by HER2 alterations (Figure 2)[16]. However, highly different percentages in the incidence of these molecular aberrations, ranging from 0 to 50%, have been reported even within the same anatomic site, such as the pancreas (Table 1)[16-19]. These partially discordant observations could have been, at least in part, responsible for the nihilistic view of HER2 in the targeted therapeutic regimens for extra-gastric DSC. Many groups are currently establishing the role of HER2 in DSC in both prognostic and therapeutic settings. However, targeting of tumors that overexpress erbB2, albeit representing the reality for advanced GC and gastroesophageal junction (GEJ) cancer, is considered a reasonable future option[8,20]. At present, the role of trastuzumab in DSC is being explored by a variety of translational research molecular pathology studies, as well as clinical trials.
| Primary tumor | erbB2 overexpression frequencies | HER2 amplification frequencies | Ref. |
| Esophageal cancer | 4%-22% | 4%-14% | [33-36,38,39] |
| Gastric cancer | 13%-22% | 10%-18% | [12,41-47] |
| Colorectal cancer | 2%-11% | 3% | [48-57] |
| Biliary tract cancer | 5%-76% | 1%-8% | [27,75,86,88-90] |
| Pancreatic cancer | 0%-50% | 2%-29% | [17-19,64-66,68] |
| Liver cancer | 2%-5% | 0-1% | [72-79] |
Management of DSC has progressed rapidly into the molecular era[21-30]. However, the “trastuzumab-revolution” that we have experienced in the breast has yet to be realized in the digestive system tract and its accessory organs[8,31]. Due to the increasing importance of HER2 testing in cancer and the new exciting challenges that precision medicine is providing, in this review we seek to describe the current state of knowledge of HER2 alterations in the most common DSC, to discuss the operational issues of HER2 testing, and to outline forthcoming clinical perspectives, in particular focusing on the cutting-edge tools available for HER2 characterization and targeting in the digestive system.
Esophageal cancer (EC), excluding GEJ tumors, is among the ten most prevalent tumors worldwide and ranks fifth in cancer mortality in men and eighth in women[32]. Squamous cell carcinoma (SCC) represents the most frequent histological type[20]. The poor prognosis of EC results from the delayed diagnosis and poor efficacy of current treatments, being in most cases limited to a palliative role[23]. In the largest meta-analysis of the prognostic significance of erbB2 overexpression and gene amplification in EC patients, 22% of tumors were HER2-positive, regardless of histotype[33]. However, these data are likely to be overestimated. Indeed, the overexpression of erbB2 has been observed in 12%-17% of adenocarcinomas (ADC) in more recent studies[34], whereas less than 4% of esophageal SCCs are HER2-amplified[35]. Taken together, no significant differences in survival rates have been reported in patients diagnosed with HER2-positive esophageal ADC compared with the HER2-negative cases. However, the great heterogeneity among indexed studies on HER2 prognostic role in these malignancies demands further investigations. Interestingly, the prognostic influence of HER2 amplification as a biomarker is slightly greater in SCC compared to ADC[33,35]. On the other hand, the small number of HER2-positive SCCs, the lack of large-cohort studies, and the absence of standardized methods for HER2 testing limit our knowledge of HER2 significance in SCC of the esophagus[36]. At present, the optimal treatment for EC remains controversial. In this regard, neoadjuvant chemotherapy with subsequent surgery represent the standard approach in the United Kingdom[37,38], whereas in Europe and United States neoadjuvant chemo-radiotherapy followed by surgery is preferred[39]. However, the individualization and optimization of therapy for EC might come across HER2 and its epistatic interactions with other potentially actionable cancer genes. Indeed, it has recently been reported that possible alterations in epidermal growth factor receptor (EGFR), telomerase reverse transcriptase (TERT), and HER2 are bona fide predictor of response to HER2-target therapy in EC, particularly in SCCs[35,40]. At present, RTOG 1010 (Radiation Therapy, Paclitaxel, and Carboplatin With or Without Trastuzumab in Treating Patients With EC) is the only ongoing phase III trial (https://clinicaltrials.gov/ct2/show/study/NCT01196390) randomizing patients with HER2-positive esophageal ADC to chemoradiation with or without trastuzumab.
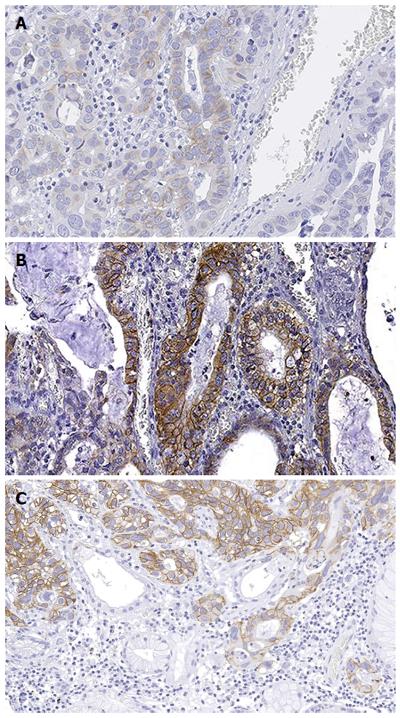
GC, including GEJ cancer, is closely related to environmental factors, reflecting its characteristic geographical distribution[32]. Although GC rates have gradually decreased during the past decades, this tumor still represents the third leading cause of cancer-related death globally[32]. The vast majority of GCs can be divided into three distinct subtypes based on Lauren’s histopathologic classification: intestinal-type, showing glandular architecture, diffuse-type, with poorly cohesive cells arranged in an infiltrative pattern, and mixed-type, bearing hybrid characteristics[20]. This morphologic heterogeneity replicates an intrinsic molecular complexity. Recently, The Cancer Genome Atlas (TCGA) network proposed a novel molecular classification of GC, dividing these tumors into four major molecular subtypes, namely tumors positive for Epstein-Barr, microsatellite unstable tumors, genomically stable tumors, and tumors with chromosomal instability[41]. Among these molecular subgroups, microsatellite unstable tumors preferentially occur in the body and antrum, and are characterized by an extraordinarily high number of mutations with the lack of targetable amplifications, including HER2 amplification. Chromosomal instability subtype encompasses the majority of GC, has a predilection for the GEJ, is associated with intestinal-type histology, and exhibit the highest rates of HER2 amplification among all molecular subtypes[41]. Overall, GCs overexpressing erbB2 and/or showing HER2 amplification, range from 13% to 22% of cases[12,42]. Meta-analysis data suggest that GC harboring HER2 amplification fares worse[43]; however, the prognostic value of HER2 remains controversial in the stomach[44-46]. This is probably due to the heterogeneous HER2 status patterns in tumors arising in the stomach that basically mirror the intra-tumor morphologic and molecular heterogeneity of GC (Figure 3)[12]. Indeed, in contrast to breast carcinoma, up to 90% of erbB2-positive GCs are reported to harbor erbB2 overexpression in less than 5% of tumor cells[12,46]. From a therapeutic perspective, it is currently recommended to administer trastuzumab in combination with cytotoxic therapies in HER2-positive GC patients[42]. In this setting, the addition of trastuzumab to chemotherapy increased the objective response rate from 35% to 47%, improving progression-free survival from 5.5 mo to 6.7 mo and overall survival from 11.1 mo to 13.8 mo[5,42,47]. Clinical trials aiming to examine the efficacy of lapatinib in combination with paclitaxel compared with paclitaxel alone in the treatment of HER2-positive GC are on-going (https://clinicaltrials.gov/ct2/show/study/NCT01705340). Other clinical trials are currently exploring the effect of adding pertuzumab to chemotherapy (https://clinicaltrials.gov/ct2/show/study/NCT01461057) and comparing trastuzumab to paclitaxel or docetaxel as second-line treatment (https://clinicaltrials.gov/ct2/show/study/NCT01641939).
Colorectal cancer (CRC) is a major contributor to cancer morbidity and mortality, with 1.36 million new cases and more than half million deaths per year worldwide[32]. Several studies assessed HER2 status in CRC, with some authors reporting membranous erbB2 overexpression in 2%-11% and others reporting cytoplasmic overexpression in 47%-68% of cases[48-52]. However, it is currently acknowledged that the amplification of HER2 occurs in only 3% of CRC[53]. In accordance to this notion, a recent comprehensive genomic characterization of CRC revealed a recurrent amplicon at 17q21.1 in 4% of these tumors[54]. This locus contains seven genes, including HER2[55]. The operational implications of HER2 amplification in CRC, however, remain elusive, with a number of studies reporting contradictory links to prognosis[53,56,57]. How to assess HER2 status in CRC cancer remains a matter of debate among pathologists; however, a panel of HER2 experts recently provided a reproducible and rigorous testing algorithm[57]. Intriguingly, no intra-tumor heterogeneity is described in CRC except for anecdotic reports[57]. Albeit HER2 seems not to represent a reliable prognostic marker in CRC, this molecular alteration is strongly associated with wild-type status of Kirsten rat sarcoma viral oncogene homolog (KRAS) and amplification DNA topoisomerase 2-alpha (TOP2A)[16,58,59]. This observation is not trivial, raising the hypothesis that HER2 amplification might be a bona fide alternative driver of Ras-Raf-MEK-ERK pathway activation in CRC. In contrast to HER2, intratumor heterogeneity of KRAS mutation status is reported and crucial in selecting patients for anti-EGFR therapy in CRC[12]. For these reasons, and given the homogeneity in erbB2 expression, it has recently been proposed that HER2 status should be assessed as a putative biomarker of resistance to anti-EGFR therapy in KRAS wild-type patients and, if further studies confirm that TOP2A amplifications are associated with anthracycline sensitivity, as a predictor to response[57,59,60]. At present, results from phase II and III trials suggest that HER2-positive CRC should be treated with trastuzumab[61].
Pancreatic cancer (PC) accounts for approximately 2% of new cancers, and is responsible for 7% of cancer-related death yearly worldwide[32]. The vast majority of PCs is represented by invasive ductal ADC arising in the head of the gland; 20% of cases involve the body or the tail[20]. The poor prognosis for these patients is attributed to delayed diagnosis, early metastasis, and the limited efficacy of available systemic treatments[62]. Systemic therapies are only modestly effective; however, there is emerging evidence that small groups of patients may respond well to specific treatments. Amplification of HER2 gene and/or overexpression of its product have been implicated in the development of PC[63]. However, the reported rates of erbB2 overexpression in these neoplasms are extremely variable, ranging from 0 to 50% of cases[17-19,64]. Furthermore, the prognostic role of HER2 amplification in PC has been investigated in numerous studies, again, with heterogeneous results[63]. As a consequence, the diagnostic criteria and prevalence of HER2 amplification in pancreatic ductal ADC remain unclear. Preclinical studies support the potential efficacy of trastuzumab in PC[65,66], although clinical trials have been disadvantaged by small cohorts[67,68]. In one phase II trial, 17 patients showing HER2 amplification were treated with capecitabine combined with trastuzumab[68]. Although the therapy was well tolerated, progression-free and overall survivals were not favorable compared to standard chemotherapy. At present, there is no consensus on the treatment modalities of HER2-positive pancreatic ductal ADC.
Hepatocellular carcinoma (HCC), is the fifth most common cancer in men and the seventh most common cancer in women, resulting in approximately 700000 deaths yearly[32]. In recent years, these tumors showed increasing incidence, albeit variable throughout the world[32,69]. The asymptomatic nature of early disease and the limited use of screening protocols in high-risk individuals often lead to diagnosis in advanced stages, with subsequent requirement of systemic therapy[70,71]. To date, sorafenib (a kinase inhibitor) is the only approved drug in patients with advanced HCC, with modest effectiveness at prolonging patients’ overall survival[72,73]. Given the scarce therapeutic armamentarium currently available, capturing the complexity underpinning HCC biology represents a high-priority goal[70]. In this setting, the role of HER2 in HCC has been explored in several studies yielding, however, to extremely discrepant results due to the diverse methods used for HER2 testing, even including cytoplasmic expression[74-76]. It is currently recognized that there is a low frequency of erbB2 strong membranous IHC overexpression in HCC[77-79]. Among these few cases, only a minority of tumors is reported to harbor HER2 amplification[80,81]. Therefore, it is unlikely that patients with HCC would benefit from treatment with trastuzumab. In addition, recent studies suggest that there is also little indication for using HER2 as a prognostic biomarker in these tumors[79].
Biliary tract cancer (BTC) encompasses a heterogeneous group of rare tumors originating in either the intra- or extrahepatic ducts[20]. This collection of neoplasms includes cholangiocarcinoma, gallbladder cancer (GBC), and ampulla of Vater cancer[20]. Among them, GBC is the most frequent type with an annual incidence of 2.5 cases per 100000 individuals[32]. Taken together, BTC mortality varies between geographic areas, accounting for higher mortality rates in South America and South-East Asia[69]. The current state-of-knowledge on BTC, regrettably, can be summarized briefly: complete tumor resection is the best chance of survival[82]. However, most cases are detected at an advanced stage, when surgical approaches are no longer feasible, and recurrences and distant metastases are common, with subsequent poor survival rates[82]. In addition, adjuvant chemotherapy has not shown sufficient benefit for BTC, while the efficacy of molecular targeted agents is still extremely disappointing[83,84]. To improve the prognosis of BTC, identification of prognostic markers and effective therapeutic targets is essential[85]. BTCs showing erbB2 overexpression and/or HER2 amplification range between 5% and 76%[86], including an estimated 13% of erbB2-positive GBC[87]. This wide range is largely dependent upon the lack of standardized methods used among different studies[27,88]. Intratumor erbB2-expression heterogeneity has not been investigated thoroughly in BTC[75,86,88-90]. However, 51% of cases in a small cohort study showed erbB2 positivity using 50% of positive tumor cells as a threshold, suggesting the presence of heterogeneous protein expression[27]. Furthermore, in this subset of tumors, 83% of cases with heterogeneous erbB2 immunohistochemical (IHC) overexpression displayed HER2 amplification by fluorescent in situ hybridization (FISH)[27]. Nevertheless, no data regarding HER2 amplification heterogeneity have been reported in literature. The highest concordance between erbB2 expression and gene amplification was demonstrated for advanced BTC with high scores of erbB2 expression in the vast majority of tumors cells, present only in a minority of cases[68,88]. Taking into account overall and disease-free survival, HER2 amplification seems not to have a prognostic role in BTC[27]. On the other hand, form a therapeutic standpoint, a subset of HER2-positive GBC responded well to trastuzumab treatment, both in monotherapy and in combination with taxane[85,90-92]. Despite these encouraging observations, an early phase clinical trial of trastuzumab for HER2-positive locally advanced or metastatic GBC was terminated in the United States because of the lack of participants (https://clinicaltrials.gov/ct2/show/study/NCT00478140). In this respect, it would be extremely beneficial to involve countries with a higher incidence of BTC in large-cohort clinical trials.
These data highlight that erbB2 overexpression and/or HER2 amplification occur more frequently in ADC of the upper gastrointestinal tract compared to the rest of the DSC. In routine diagnostic practice and in research settings, erbB2 IHC scoring, along with the assessment of other prognostic and predictive factors, remains a cornerstone in the DSC pathology[44,57,93,94]. At present, it is taking place a consensus in extending the scoring system currently adopted in gastric ADC for all other DSC[57]. Compared to the breast, erbB2 IHC in DSC has a few substantial differences not only from intra- and inter-tumor heterogeneity standpoints but also in terms of cellular staining patterns. Indeed, erbB2 expression is mainly restricted to intestinal-type, gland-forming GC, and incomplete, often basolateral or even only lateral membranous IHC staining is the rule rather than an exception for HER2-positive GC[93,95]. Hence, circularity of IHC staining is no longer a criterion for erbB2 IHC scoring in the digestive tract[95]. A cornerstone work on esophageal ADC aimed to compare the erbB2 scoring system routinely employed in the breast to that used in GC[96,97]. However, no similar studies have been performed on other DSCs, therefore further analyses are warranted. In this era of precision medicine, there are increasing evidences that digital image analysis tools are able to capture the whole spectrum of erbB2 IHC expression patterns and therefore represent useful tools for determining HER2 status and its heterogeneity in DSC[12,98,99]. Both the membranous and nuclear features of the cells should be identified and scored, while the settings for cell count and differentiation between stroma and neoplasia should take into account the morphologic features of the cells (e.g., curvature, color intensity, size, roundness, compactness, elongation) as well as each histologic pattern (e.g., glandular, solid). Digital image analysis technologies grant a rapid and quantitative record of the percentage of stained tumor cells and their membrane staining distribution, allowing a precise and reproducible patients’ stratification also capturing intra-tumor heterogeneity[12]. To verify equivocal IHC results, FISH, silver in situ hybridization (SISH), chromogenic in situ hybridization (CISH) assays are widely performed[8,12,57,93,96]. In particular, FISH identifies the number of HER2 gene copies in conjunction with the number of chromosome 17 centromere (CEP17) copies[94]. This scoring is considered more objective and quantitative than IHC, however FISH reproducibility is strictly dependent on technical issues (e.g., thickness of tissue sections)[8,100-103]. On the other hand, CISH is re-emerging as a more cost-effective assay, using conventional enzymatic reactions and being applicable to standard formalin-fixed paraffin embedded (FFPE) tissues[104]. This method shows high levels of quality and reproducibility, particularly in GC[105]. In a way akin to CISH, SISH is a rapid automated assay that can be interpreted using conventional microscopies, allowing pathologists to evaluate HER2 status within the context of tissue morphology[103,106].
Resistance to trastuzumab and other anti-erbB2 therapies is an event that may occur during the course of therapy or de novo (Table 2)[107]. Drug resistance has been widely studied in breast cancer but not in the DSC, with subsequent lack of a detailed molecular characterization of this phenomenon. Intra-tumor and tumor-to-metastasis heterogeneity are among the most important characteristics that determine resistance to anti-erbB2 therapy in DSC and should always be taken into account when selecting patients eligible for these treatments and clinical trials[12,19,52,96,108]. Indeed, there are several molecular evidences that genetic heterogeneity is not restricted to passenger genes but that also bona fide driver genetic alterations such as HER2 gene amplification can be heterogeneously distributed within a given tumor[109]. The therapeutic implications of this concept are yet to be ascertained, although it is intuitive that only the HER2-positive neoplastic population would be sensitive to anti-erbB2 drugs[110]. Furthermore, it is not clear whether HER2 amplification is an early event and subsequently lost in the HER2-negative components, or whether HER2 amplification might be subclonally acquired at a relatively late stage of tumorigenesis[12,27]. In addition, somatic mutations in HER2 have been described in a small subset of DSC and there are functional evidences that at least a subset of mutations targeting HER2 might be responsible for the development of resistance to trastuzumab therapy, in a way akin to breast cancer[8,109]. For example, alterations leading to increased heterodimerization of HER2 with EGFR or HER3 are thought to induce resistance to trastuzumab therapy[107]. Furthermore, cleavage of the full-length erbB2 protein produces a truncated membrane-associated fragment called p95HER2 with increased kinase activity in GC cell lines[111,112]. Up to 30% of breast cancers may harbor this alteration, showing poor prognosis and lower rates of response to trastuzumab therapy compared to patients with full-length HER2[111]. Furthermore, 11% of HCC but none of BTC have been found to harbor HER2 (H878Y) somatic mutation occurring in the tyrosine kinase domain, resulting in c.2632C>T[81,113,114]. This specific mutation has been proposed as a predictor of response to HER2- and/or EGFR-targeted therapy in HCC. Interestingly, a phase II trial observed that the dual EGFR/HER2 tyrosine kinase inhibitor lapatinib was active in HCC but not in BTC, suggesting that mutations in the tyrosine kinase domain of HER2 in HCC may underlie responsiveness to agents that target HER2 and/or EGFR[115]. Generally, erbB2-directed therapy appears to be beneficial in erbB2-positive gallbladder cancers; however, tumors harboring HER2 mutation (V777L), in the kinase domain, followed into the non-responders category[91]. In addition to the alterations in erbB2 receptors, mutations in genes involved in the signaling pathways activated by these receptors are also correlated with failure of therapeutic response to erbB2 inhibitors[116]. A preclinical trial is testing the hypothesis that HER2 amplification might be used, under certain conditions, as a “molecular bait” for trastuzumab-emtansine precision chemotherapy to overcome anti-erbB2 resistance in HER2-positive metastatic CRC[117]. Moreover, PIK3CA mutations and PTEN inactivation could affect the effectiveness of erbB2-targeting therapy. Thus, it might be advantageous to clarify not only HER2 alterations but also the PI3K-Akt pathway status to optimize HER2-targeting therapy[118]. In this regard, massively parallel sequencing and bioinformatic analyses are likely to represent the next frontier in the identification of complex mechanisms of trastuzumab resistance in this broad group of tumors[8,118-120]. A better knowledge of the biology underpinning HER2 status in the digestive system should be regarded as a priority for the development of effective strategies to overcome resistance.
| Type | Agent | Target | Mechanisms of resistance | Factors involved |
| Monoclonal antibodies | Trastuzumab | erbB2 | Alterations in tyrosine kinase domain; overexpression of alternative erbB isoforms and dimerization receptors; loss of downstream checkpoints; dimerization and interaction with other receptors | p95HER2, MUC4 EGFR, erbB ligands (TGFα, EGF, HB), PTEN IGF1R, MET |
| Pertuzumab | erbB2 | |||
| T-DM1 | erbB2 | |||
| Tyrosine kinase inhibitors | Lapatinib | erbB1, erbB2 | Alterations in tyrosine kinase domain; acquisition of HER2 mutations; activation of further downstream signaling pathways | KIT and PDGFRA receptor signaling pathway; PI3K-AKT, mTOR |
| Neratinib | erbB2, erbB4 | |||
| Afatinib | erbB1, erbB2, erbB4 | |||
| Canertinib | erbB1, erbB2, erbB4 | |||
| Inhibitors of the downstream targets | Everolimus | mTOR | Activation of further downstream signaling pathways | PI3K-AKT, mTOR, MEK, MAPK |
| BKM120 | PI3K/AKT | |||
| BEZ-235 | PI3K/AKT/mTOR | |||
| GS-1101 | PI3K | |||
| NVP-BKM120 | PI3K | |||
| GDC-0941 | PI3K | |||
| GSK458 | PI3K/mTOR | |||
| GDC-0980 | PI3K/mTOR | |||
| PI-103 | PI3K/mTOR | |||
| hsp90 inhibitors | Tanespimycin | hsp90 | Up-regulation of alternative pathways | NF-κB, MAPK |
| Retaspimycin | hsp90 | |||
| AUY922 | hsp90 |
Substantial progress has been made in the management of patients with advanced-stage DSC in recent years, with the realization of tangible improvements in terms of outcome and life quality. In particular, trastuzumab has greatly improved the therapeutic approach to patients with advanced GC but not yet in other DSC. However, no validated HER2 testing strategies are available for non-gastric DSC and subsequently tailored treatments are yet to be implemented in this broad group of malignancies. Several drugs targeting erbB2 or its downstream signals are under development in CRC, GBC, and EC, including ongoing phase 3 clinical trials in CRC. Nowadays, the focus on HER2 expression/amplification status alone is not able to capture the underlying mechanisms of disease progression and resistance. In this setting, PIK3CA mutation or PTEN loss has been evaluated as a possible predictive biomarker and has also been used as one of the inclusion criteria. Understanding the interplay between HER2 and the PI3K-Akt pathway alterations would be pivotal in the development of new therapeutic strategies. Further studies focused on the epistasis between molecular alterations and associations between molecular alterations and tumor microenvironment are warranted to accurately and robustly predict anti-erbB2 treatment outcome in DSC. Thus, a comprehensive clinical and pathogenomic approach is fundamental in appropriately characterizing HER2 status in DSC at an individualized level for both precision therapy and accurate prognostication.
The authors would like to thank Dr. Francesca Boggio for providing significant contribution in the recording of the audio core tip.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Genta FA S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 2. | Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469-6487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 906] [Cited by in RCA: 840] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 3. | Thompson DM, Gill GN. The EGF receptor: structure, regulation and potential role in malignancy. Cancer Surv. 1985;4:767-788. [PubMed] |
| 4. | Wong DJ, Hurvitz SA. Recent advances in the development of anti-HER2 antibodies and antibody-drug conjugates. Ann Transl Med. 2014;2:122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 5. | Roskoski R. The ErbB/HER family of protein-tyrosine kinases and cancer. Pharmacol Res. 2014;79:34-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 978] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 6. | Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83-86. [PubMed] |
| 7. | Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, Lee J, Yarden Y, Libermann TA, Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418-425. [PubMed] |
| 8. | Yan M, Parker BA, Schwab R, Kurzrock R. HER2 aberrations in cancer: implications for therapy. Cancer Treat Rev. 2014;40:770-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 9. | Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4920] [Cited by in RCA: 5113] [Article Influence: 213.0] [Reference Citation Analysis (1)] |
| 10. | Ghosh R, Narasanna A, Wang SE, Liu S, Chakrabarty A, Balko JM, González-Angulo AM, Mills GB, Penuel E, Winslow J. Trastuzumab has preferential activity against breast cancers driven by HER2 homodimers. Cancer Res. 2011;71:1871-1882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 172] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 11. | Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177-182. [PubMed] |
| 12. | Fusco N, Rocco EG, Del Conte C, Pellegrini C, Bulfamante G, Di Nuovo F, Romagnoli S, Bosari S. HER2 in gastric cancer: a digital image analysis in pre-neoplastic, primary and metastatic lesions. Mod Pathol. 2013;26:816-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Rakha EA, Reis-Filho JS, Ellis IO. Combinatorial biomarker expression in breast cancer. Breast Cancer Res Treat. 2010;120:293-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8204] [Cited by in RCA: 8139] [Article Influence: 339.1] [Reference Citation Analysis (0)] |
| 15. | Dowsett M, Cooke T, Ellis I, Gullick WJ, Gusterson B, Mallon E, Walker R. Assessment of HER2 status in breast cancer: why, when and how? Eur J Cancer. 2000;36:170-176. [PubMed] |
| 16. | cBioPortal for Cancer Genomics. The Cancer Genome Atlas. Available from: http://www.cbioportal.org/. |
| 17. | Komoto M, Nakata B, Amano R, Yamada N, Yashiro M, Ohira M, Wakasa K, Hirakawa K. HER2 overexpression correlates with survival after curative resection of pancreatic cancer. Cancer Sci. 2009;100:1243-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Stoecklein NH, Luebke AM, Erbersdobler A, Knoefel WT, Schraut W, Verde PE, Stern F, Scheunemann P, Peiper M, Eisenberger CF. Copy number of chromosome 17 but not HER2 amplification predicts clinical outcome of patients with pancreatic ductal adenocarcinoma. J Clin Oncol. 2004;22:4737-4745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Chou A, Waddell N, Cowley MJ, Gill AJ, Chang DK, Patch AM, Nones K, Wu J, Pinese M, Johns AL. Clinical and molecular characterization of HER2 amplified-pancreatic cancer. Genome Med. 2013;5:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 20. | Bosman FT, World Health Organization, International Agency for Research on Cancer. WHO classification of tumours of the digestive system. Lyon: International Agency for Research on Cancer 2010; . |
| 21. | Xu W, Yang Z, Lu N. Molecular targeted therapy for the treatment of gastric cancer. J Exp Clin Cancer Res. 2016;35:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 22. | Woo J, Cohen SA, Grim JE. Targeted therapy in gastroesophageal cancers: past, present and future. Gastroenterol Rep (Oxf). 2015;3:316-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Blum Murphy MA, Elimova E, Ajani JA. Current concepts and future potential in neoadjuvant chemotherapy for esophageal cancer. Expert Rev Gastroenterol Hepatol. 2016;10:383-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Sartore-Bianchi A, Ardini E, Bosotti R, Amatu A, Valtorta E, Somaschini A, Raddrizzani L, Palmeri L, Banfi P, Bonazzina E. Sensitivity to Entrectinib Associated With a Novel LMNA-NTRK1 Gene Fusion in Metastatic Colorectal Cancer. J Natl Cancer Inst. 2016;108:pii djv306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Sahin IH, Iacobuzio-Donahue CA, O’Reilly EM. Molecular signature of pancreatic adenocarcinoma: an insight from genotype to phenotype and challenges for targeted therapy. Expert Opin Ther Targets. 2016;20:341-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Kim HY, Park JW. Clinical trials of combined molecular targeted therapy and locoregional therapy in hepatocellular carcinoma: past, present, and future. Liver Cancer. 2014;3:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Yoshida H, Shimada K, Kosuge T, Hiraoka N. A significant subgroup of resectable gallbladder cancer patients has an HER2 positive status. Virchows Arch. 2016;468:431-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Sicklick JK, Fanta PT, Shimabukuro K, Kurzrock R. Genomics of gallbladder cancer: the case for biomarker-driven clinical trial design. Cancer Metastasis Rev. 2016;35:263-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Marks EI, Yee NS. Molecular genetics and targeted therapeutics in biliary tract carcinoma. World J Gastroenterol. 2016;22:1335-1347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 30. | Stotz M, Gerger A, Haybaeck J, Kiesslich T, Bullock MD, Pichler M. Molecular Targeted Therapies in Hepatocellular Carcinoma: Past, Present and Future. Anticancer Res. 2015;35:5737-5744. [PubMed] |
| 31. | Hortobagyi GN. Trastuzumab in the treatment of breast cancer. N Engl J Med. 2005;353:1734-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 209] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 32. | Global Cancer Facts & Figures 3rd Edition. Atlanta: American Cancer Society 2015; . |
| 33. | Chan DS, Twine CP, Lewis WG. Systematic review and meta-analysis of the influence of HER2 expression and amplification in operable oesophageal cancer. J Gastrointest Surg. 2012;16:1821-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Yoon HH, Shi Q, Sukov WR, Wiktor AE, Khan M, Sattler CA, Grothey A, Wu TT, Diasio RB, Jenkins RB. Association of HER2/ErbB2 expression and gene amplification with pathologic features and prognosis in esophageal adenocarcinomas. Clin Cancer Res. 2012;18:546-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 35. | Gonzaga IM, Soares-Lima SC, de Santos PT, Blanco TC, de Reis BS, Quintella DC, de Oliveira IM, de Faria PA, Kruel CD, Andreollo NA. Alterations in epidermal growth factor receptors 1 and 2 in esophageal squamous cell carcinomas. BMC Cancer. 2012;12:569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Langer R, Rauser S, Feith M, Nährig JM, Feuchtinger A, Friess H, Höfler H, Walch A. Assessment of ErbB2 (Her2) in oesophageal adenocarcinomas: summary of a revised immunohistochemical evaluation system, bright field double in situ hybridisation and fluorescence in situ hybridisation. Mod Pathol. 2011;24:908-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Walker RA, Bartlett JM, Dowsett M, Ellis IO, Hanby AM, Jasani B, Miller K, Pinder SE. HER2 testing in the UK: further update to recommendations. J Clin Pathol. 2008;61:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 38. | Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet. 2002;359:1727-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1121] [Cited by in RCA: 1084] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 39. | Ilson DH. Esophageal cancer chemotherapy: recent advances. Gastrointest Cancer Res. 2008;2:85-92. [PubMed] |
| 40. | Galsky MD, Von Hoff DD, Neubauer M, Anderson T, Fleming M, Nagarwala Y, Mahoney JM, Midwinter D, Vocila L, Zaks TZ. Target-specific, histology-independent, randomized discontinuation study of lapatinib in patients with HER2-amplified solid tumors. Invest New Drugs. 2012;30:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4858] [Article Influence: 441.6] [Reference Citation Analysis (2)] |
| 42. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5328] [Article Influence: 355.2] [Reference Citation Analysis (3)] |
| 43. | Chen C, Yang JM, Hu TT, Xu TJ, Yan G, Hu SL, Wei W, Xu WP. Prognostic role of human epidermal growth factor receptor in gastric cancer: a systematic review and meta-analysis. Arch Med Res. 2013;44:380-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 44. | Gu J, Zheng L, Wang Y, Zhu M, Wang Q, Li X. Prognostic significance of HER2 expression based on trastuzumab for gastric cancer (ToGA) criteria in gastric cancer: an updated meta-analysis. Tumour Biol. 2014;35:5315-5321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Li J, Jing J. Comment on Gu et al. entitled “Prognostic significance of HER2 expression based on trastuzumab for gastric cancer (ToGA) criteria in gastric cancer: an updated meta-analysis”. Tumour Biol. 2014;35:6177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | Grabsch H, Sivakumar S, Gray S, Gabbert HE, Müller W. HER2 expression in gastric cancer: Rare, heterogeneous and of no prognostic value - conclusions from 924 cases of two independent series. Cell Oncol. 2010;32:57-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 130] [Reference Citation Analysis (0)] |
| 47. | Jácome AA, Coutinho AK, Lima EM, Andrade AC, Dos Santos JS. Personalized medicine in gastric cancer: Where are we and where are we going? World J Gastroenterol. 2016;22:1160-1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 48. | Kavanagh DO, Chambers G, O’Grady L, Barry KM, Waldron RP, Bennani F, Eustace PW, Tobbia I. Is overexpression of HER-2 a predictor of prognosis in colorectal cancer? BMC Cancer. 2009;9:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 49. | Wu SW, Ma CC, Yang Y. The prognostic value of HER-2/neu overexpression in colorectal cancer: evidence from 16 studies. Tumour Biol. 2014;35:10799-10804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Pappas A, Lagoudianakis E, Seretis C, Tsiambas E, Koronakis N, Toutouzas K, Katergiannakis V, Manouras A. Clinical role of HER-2/neu expression in colorectal cancer. J BUON. 2013;18:98-104. [PubMed] |
| 51. | Schuell B, Gruenberger T, Scheithauer W, Zielinski Ch, Wrba F. HER 2/neu protein expression in colorectal cancer. BMC Cancer. 2006;6:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Li Q, Wang D, Li J, Chen P. Clinicopathological and prognostic significance of HER-2/neu and VEGF expression in colon carcinomas. BMC Cancer. 2011;11:277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 53. | Wu SW, Ma CC, Li WH. Does overexpression of HER-2 correlate with clinicopathological characteristics and prognosis in colorectal cancer? Evidence from a meta-analysis. Diagn Pathol. 2015;10:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6773] [Cited by in RCA: 6673] [Article Influence: 513.3] [Reference Citation Analysis (0)] |
| 55. | Available from: http://www.ensembl.org. |
| 56. | Song Z, Deng Y, Zhuang K, Li A, Liu S. Immunohistochemical results of HER2/neu protein expression assessed by rabbit monoclonal antibodies SP3 and 4B5 in colorectal carcinomas. Int J Clin Exp Pathol. 2014;7:4454-4460. [PubMed] |
| 57. | Valtorta E, Martino C, Sartore-Bianchi A, Penaullt-Llorca F, Viale G, Risio M, Rugge M, Grigioni W, Bencardino K, Lonardi S. Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod Pathol. 2015;28:1481-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 222] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 58. | Richman SD, Chambers P, Seymour MT, Daly C, Grant S, Hemmings G, Quirke P. Intra-tumoral heterogeneity of KRAS and BRAF mutation status in patients with advanced colorectal cancer (aCRC) and cost-effectiveness of multiple sample testing. Anal Cell Pathol (Amst). 2011;34:61-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 59. | Richman SD, Southward K, Chambers P, Cross D, Barrett J, Hemmings G, Taylor M, Wood H, Hutchins G, Foster JM. HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials. J Pathol. 2016;238:562-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 190] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 60. | Tu J, Yu Y, Liu W, Chen S. Significance of human epidermal growth factor receptor 2 expression in colorectal cancer. Exp Ther Med. 2015;9:17-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Siena S, Sartore-Bianchi A, Lonardi S, Trusolino L, Martino C, Bencardino K, Leone F, Zagonel V, Valtorta E, Torri V. Trastuzumab and lapatinib in HER2-amplified metastatic colorectal cancer patients (mCRC): The HERACLES trial. J Clin Oncol. 2015;33 Suppl:3508. |
| 62. | Vaccaro V, Melisi D, Bria E, Cuppone F, Ciuffreda L, Pino MS, Gelibter A, Tortora G, Cognetti F, Milella M. Emerging pathways and future targets for the molecular therapy of pancreatic cancer. Expert Opin Ther Targets. 2011;15:1183-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 63. | Li X, Zhao H, Gu J, Zheng L. Prognostic role of HER2 amplification based on fluorescence in situ hybridization (FISH) in pancreatic ductal adenocarcinoma (PDAC): a meta-analysis. World J Surg Oncol. 2016;14:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Ueda S, Ogata S, Tsuda H, Kawarabayashi N, Kimura M, Sugiura Y, Tamai S, Matsubara O, Hatsuse K, Mochizuki H. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004;29:e1-e8. [PubMed] |
| 65. | Büchler P, Reber HA, Büchler MC, Roth MA, Büchler MW, Friess H, Isacoff WH, Hines OJ. Therapy for pancreatic cancer with a recombinant humanized anti-HER2 antibody (herceptin). J Gastrointest Surg. 2001;5:139-146. [PubMed] |
| 66. | Kimura K, Sawada T, Komatsu M, Inoue M, Muguruma K, Nishihara T, Yamashita Y, Yamada N, Ohira M, Hirakawa K. Antitumor effect of trastuzumab for pancreatic cancer with high HER-2 expression and enhancement of effect by combined therapy with gemcitabine. Clin Cancer Res. 2006;12:4925-4932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 67. | Safran H, Iannitti D, Ramanathan R, Schwartz JD, Steinhoff M, Nauman C, Hesketh P, Rathore R, Wolff R, Tantravahi U. Herceptin and gemcitabine for metastatic pancreatic cancers that overexpress HER-2/neu. Cancer Invest. 2004;22:706-712. [PubMed] |
| 68. | Harder J, Ihorst G, Heinemann V, Hofheinz R, Moehler M, Buechler P, Kloeppel G, Röcken C, Bitzer M, Boeck S. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br J Cancer. 2012;106:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 69. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25543] [Article Influence: 1824.5] [Reference Citation Analysis (7)] |
| 70. | Chen J, Gao J. Advances in the study of molecularly targeted agents to treat hepatocellular carcinoma. Drug Discov Ther. 2014;8:154-164. [PubMed] |
| 71. | Villanueva A, Hernandez-Gea V, Llovet JM. Medical therapies for hepatocellular carcinoma: a critical view of the evidence. Nat Rev Gastroenterol Hepatol. 2013;10:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 72. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10271] [Article Influence: 604.2] [Reference Citation Analysis (2)] |
| 73. | Llovet JM, Decaens T, Raoul JL, Boucher E, Kudo M, Chang C, Kang YK, Assenat E, Lim HY, Boige V. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol. 2013;31:3509-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 486] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 74. | Ito Y, Takeda T, Sakon M, Tsujimoto M, Higashiyama S, Noda K, Miyoshi E, Monden M, Matsuura N. Expression and clinical significance of erb-B receptor family in hepatocellular carcinoma. Br J Cancer. 2001;84:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 237] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 75. | Collier JD, Guo K, Mathew J, May FE, Bennett MK, Corbett IP, Bassendine MF, Burt AD. c-erbB-2 oncogene expression in hepatocellular carcinoma and cholangiocarcinoma. J Hepatol. 1992;14:377-380. [PubMed] |
| 76. | Nakopoulou L, Stefanaki K, Filaktopoulos D, Giannopoulou I. C-erb-B-2 oncoprotein and epidermal growth factor receptor in human hepatocellular carcinoma: an immunohistochemical study. Histol Histopathol. 1994;9:677-682. [PubMed] |
| 77. | Heinze T, Jonas S, Kärsten A, Neuhaus P. Determination of the oncogenes p53 and C-erb B2 in the tumour cytosols of advanced hepatocellular carcinoma (HCC) and correlation to survival time. Anticancer Res. 1999;19:2501-2503. [PubMed] |
| 78. | Vlasoff DM, Baschinsky DY, De Young BR, Morrison CD, Nuovo GJ, Frankel WL. C-erb B2 (Her2/neu) is neither overexpressed nor amplified in hepatic neoplasms. Appl Immunohistochem Mol Morphol. 2002;10:237-241. [PubMed] |
| 79. | Hsu C, Huang CL, Hsu HC, Lee PH, Wang SJ, Cheng AL. HER-2/neu overexpression is rare in hepatocellular carcinoma and not predictive of anti-HER-2/neu regulation of cell growth and chemosensitivity. Cancer. 2002;94:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Xian ZH, Zhang SH, Cong WM, Wu WQ, Wu MC. Overexpression/amplification of HER-2/neu is uncommon in hepatocellular carcinoma. J Clin Pathol. 2005;58:500-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Bekaii-Saab T, Williams N, Plass C, Calero MV, Eng C. A novel mutation in the tyrosine kinase domain of ERBB2 in hepatocellular carcinoma. BMC Cancer. 2006;6:278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 82. | Chan E, Berlin J. Biliary tract cancers: understudied and poorly understood. J Clin Oncol. 2015;33:1845-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 83. | Zhu GQ, Shi KQ, You J, Zou H, Lin YQ, Wang LR, Braddock M, Chen YP, Zheng MH. Systematic review with network meta-analysis: adjuvant therapy for resected biliary tract cancer. Aliment Pharmacol Ther. 2014;40:759-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Horgan AM, Amir E, Walter T, Knox JJ. Adjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysis. J Clin Oncol. 2012;30:1934-1940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 515] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 85. | Roa I, de Toro G, Schalper K, de Aretxabala X, Churi C, Javle M. Overexpression of the HER2/neu Gene: A New Therapeutic Possibility for Patients With Advanced Gallbladder Cancer. Gastrointest Cancer Res. 2014;7:42-48. [PubMed] |
| 86. | Harder J, Waiz O, Otto F, Geissler M, Olschewski M, Weinhold B, Blum HE, Schmitt-Graeff A, Opitz OG. EGFR and HER2 expression in advanced biliary tract cancer. World J Gastroenterol. 2009;15:4511-4517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 87. | Javle M, Rashid A, Churi C, Kar S, Zuo M, Eterovic AK, Nogueras-Gonzalez GM, Janku F, Shroff RT, Aloia TA. Molecular characterization of gallbladder cancer using somatic mutation profiling. Hum Pathol. 2014;45:701-708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 88. | Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, Hirohashi S, Shibata T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418-425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 318] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 89. | Pignochino Y, Sarotto I, Peraldo-Neia C, Penachioni JY, Cavalloni G, Migliardi G, Casorzo L, Chiorino G, Risio M, Bardelli A. Targeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomas. BMC Cancer. 2010;10:631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 90. | Law LY. Dramatic response to trastuzumab and paclitaxel in a patient with human epidermal growth factor receptor 2-positive metastatic cholangiocarcinoma. J Clin Oncol. 2012;30:e271-e273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Javle M, Churi C, Kang HC, Shroff R, Janku F, Surapaneni R, Zuo M, Barrera C, Alshamsi H, Krishnan S. HER2/neu-directed therapy for biliary tract cancer. J Hematol Oncol. 2015;8:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 178] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 92. | Sorscher S. Marked radiographic response of a HER-2-overexpressing biliary cancer to trastuzumab. Cancer Manag Res. 2013;9:1-3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 93. | Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, Ochiai A, Rüschoff J, Henkel T. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology. 2008;52:797-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 868] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 94. | Sapino A, Goia M, Recupero D, Marchiò C. Current Challenges for HER2 Testing in Diagnostic Pathology: State of the Art and Controversial Issues. Front Oncol. 2013;3:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 95. | Mrklic I, Bendic A, Kunac N, Bezic J, Forempoher G, Durdov MG, Karaman I, Prusac IK, Pisac VP, Vilovic K. Her-2/neu assessment for gastric carcinoma: validation of scoring system. Hepatogastroenterology. 2012;59:300-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Sato-Kuwabara Y, Neves JI, Fregnani JH, Sallum RA, Soares FA. Evaluation of gene amplification and protein expression of HER-2/neu in esophageal squamous cell carcinoma using Fluorescence in situ Hybridization (FISH) and immunohistochemistry. BMC Cancer. 2009;9:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 97. | Mimura K, Kono K, Hanawa M, Mitsui F, Sugai H, Miyagawa N, Ooi A, Fujii H. Frequencies of HER-2/neu expression and gene amplification in patients with oesophageal squamous cell carcinoma. Br J Cancer. 2005;92:1253-1260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 98. | Feuchtinger A, Stiehler T, Jütting U, Marjanovic G, Luber B, Langer R, Walch A. Image analysis of immunohistochemistry is superior to visual scoring as shown for patient outcome of esophageal adenocarcinoma. Histochem Cell Biol. 2015;143:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 99. | Laurinaviciene A, Dasevicius D, Ostapenko V, Jarmalaite S, Lazutka J, Laurinavicius A. Membrane connectivity estimated by digital image analysis of HER2 immunohistochemistry is concordant with visual scoring and fluorescence in situ hybridization results: algorithm evaluation on breast cancer tissue microarrays. Diagn Pathol. 2011;6:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 100. | Yan M, Schwaederle M, Arguello D, Millis SZ, Gatalica Z, Kurzrock R. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev. 2015;34:157-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 101. | Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997-4013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2910] [Cited by in RCA: 3055] [Article Influence: 254.6] [Reference Citation Analysis (0)] |
| 102. | English DP, Roque DM, Santin AD. HER2 expression beyond breast cancer: therapeutic implications for gynecologic malignancies. Mol Diagn Ther. 2013;17:85-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 103. | Penault-Llorca F, Bilous M, Dowsett M, Hanna W, Osamura RY, Rüschoff J, van de Vijver M. Emerging technologies for assessing HER2 amplification. Am J Clin Pathol. 2009;132:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 104. | Min L, Shou C. In Situ Hybridization of Breast Cancer Markers. Methods Mol Biol. 2016;1406:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 105. | Pyo JS, Sohn JH, Kim WH. Concordance rate between HER2 immunohistochemistry and in situ hybridization in gastric carcinoma: systematic review and meta-analysis. Int J Biol Markers. 2016;31:e1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Papouchado BG, Myles J, Lloyd RV, Stoler M, Oliveira AM, Downs-Kelly E, Morey A, Bilous M, Nagle R, Prescott N. Silver in situ hybridization (SISH) for determination of HER2 gene status in breast carcinoma: comparison with FISH and assessment of interobserver reproducibility. Am J Surg Pathol. 2010;34:767-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 107. | Gagliato DM, Jardim DL, Marchesi MS, Hortobagyi GN. Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 108. | Marx AH, Tharun L, Muth J, Dancau AM, Simon R, Yekebas E, Kaifi JT, Mirlacher M, Brümmendorf TH, Bokemeyer C. HER-2 amplification is highly homogenous in gastric cancer. Hum Pathol. 2009;40:769-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 109. | Ng CK, Martelotto LG, Gauthier A, Wen HC, Piscuoglio S, Lim RS, Cowell CF, Wilkerson PM, Wai P, Rodrigues DN. Intra-tumor genetic heterogeneity and alternative driver genetic alterations in breast cancers with heterogeneous HER2 gene amplification. Genome Biol. 2015;16:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 110. | Lee S, de Boer WB, Fermoyle S, Platten M, Kumarasinghe MP. Human epidermal growth factor receptor 2 testing in gastric carcinoma: issues related to heterogeneity in biopsies and resections. Histopathology. 2011;59:832-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 111. | Arribas J, Baselga J, Pedersen K, Parra-Palau JL. p95HER2 and breast cancer. Cancer Res. 2011;71:1515-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 112. | Oshima Y, Tanaka H, Murakami H, Ito Y, Furuya T, Kondo E, Kodera Y, Nakanishi H. Lapatinib sensitivities of two novel trastuzumab-resistant HER2 gene-amplified gastric cancer cell lines. Gastric Cancer. 2014;17:450-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 113. | Mazzanti R, Arena U, Tassi R. Hepatocellular carcinoma: Where are we? World J Exp Med. 2016;6:21-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 94] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 114. | Li L, Wang H. Heterogeneity of liver cancer and personalized therapy. Cancer Lett. 2016;379:191-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 220] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 115. | Whang YE, Armstrong AJ, Rathmell WK, Godley PA, Kim WY, Pruthi RS, Wallen EM, Crane JM, Moore DT, Grigson G. A phase II study of lapatinib, a dual EGFR and HER-2 tyrosine kinase inhibitor, in patients with castration-resistant prostate cancer. Urol Oncol. 2013;31:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 116. | Arienti C, Zanoni M, Pignatta S, Del Rio A, Carloni S, Tebaldi M, Tedaldi G, Tesei A. Preclinical evidence of multiple mechanisms underlying trastuzumab resistance in gastric cancer. Oncotarget. 2016;7:18424-18439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 117. | Siena S, Bardelli A, Sartore-Bianchi A, Martino C, Siravegna G, Magrì A, Leone F, Zagonel V, Lonardi S, Amatu A. HER2 amplification as a ‘molecular bait’ for trastuzumab-emtansine (T-DM1) precision chemotherapy to overcome anti-HER2 resistance in HER2 positive metastatic colorectal cancer: The HERACLES-RESCUE trial. 2016;Gastrointestinal Cancers Symposium, 2016. |
| 118. | Marchiò C, De Filippo MR, Ng CK, Piscuoglio S, Soslow RA, Reis-Filho JS, Weigelt B. PIKing the type and pattern of PI3K pathway mutations in endometrioid endometrial carcinomas. Gynecol Oncol. 2015;137:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 119. | Fusco N, Sciarra A, Guerini-Rocco E, Marchiò C, Vignani F, Colombo P, Ferrero S. Rediscovering Secondary Tumors of the Prostate in the Molecular Era. Adv Anat Pathol. 2016;23:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 120. | Yamamoto H, Watanabe Y, Maehata T, Morita R, Yoshida Y, Oikawa R, Ishigooka S, Ozawa S, Matsuo Y, Hosoya K. An updated review of gastric cancer in the next-generation sequencing era: insights from bench to bedside and vice versa. World J Gastroenterol. 2014;20:3927-3937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 56] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |