Published online Sep 14, 2016. doi: 10.3748/wjg.v22.i34.7851
Peer-review started: April 7, 2016
First decision: May 30, 2016
Revised: June 11, 2016
Accepted: July 6, 2016
Article in press: July 6, 2016
Published online: September 14, 2016
Processing time: 158 Days and 21 Hours
Utilizing the opened round ligament as venous grafts during liver transplantation is useful but controversial, and there are no pathological analyses of this procedure. Herein, we describe the first reported case of a pathological analysis of an opened round ligament used as a venous patch graft in a living donor liver transplantation (LDLT). A 13-year-old female patient with biliary atresia underwent LDLT using a posterior segment graft from her mother. The graft had two hepatic veins (HVs), which included the right HV (RHV; 15 mm) and the inferior RHV (IRHV; 20 mm). The graft RHV and IRHV were formed into a single orifice using the donor’s opened round ligament (60 mm × 20 mm) as a patch graft during bench surgery; it was then anastomosed end-to-side with the recipient inferior vena cava. The recipient had no post-transplant complications involving the HVs, but she died of septic shock with persistent cholangitis and jaundice 86 d after LDLT. The HV anastomotic site had no stenosis or thrombus on autopsy. On pathology, there was adequate patency and continuity between the recipient’s HV and the donor’s opened round ligament. In addition, the stains for CD31 and CD34 on the inner membrane of the opened round ligament were positive. Hepatic venous reconstruction using the opened round ligament as a venous patch graft is effective in LDLT, as observed on pathology.
Core tip: Utilizing the opened round ligament as venous grafts during liver transplantation is useful but controversial, and there are no pathological analyses of this procedure. Herein, we describe the first reported case of pathological analysis of an opened round ligament used as a venous patch graft in living donor liver transplantation. The hepatic venous (HV) anastomotic site had no stenosis or thrombus on autopsy. On pathology, there was adequate patency and continuity between the recipient’s HV and the donor’s opened round ligament. In addition, the stains for CD31 and CD34 on the inner membrane of the opened round ligament were positive.
- Citation: Sanada Y, Sakuma Y, Sasanuma H, Miki A, Katano T, Hirata Y, Okada N, Yamada N, Ihara Y, Urahashi T, Sata N, Yasuda Y, Mizuta K. Immunohistochemical evaluation for outflow reconstruction using opened round ligament in living donor right posterior sector graft liver transplantation: A case report. World J Gastroenterol 2016; 22(34): 7851-7856
- URL: https://www.wjgnet.com/1007-9327/full/v22/i34/7851.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i34.7851
The use of the opened round ligament as a venous patch graft has become accepted in hepatopancreatobiliary surgery because of its easy availability[1-3]. Recent studies have demonstrated the use of the opened round ligament as a venous patch graft in hepatic venous reconstruction during living donor liver transplantation (LDLT)[4-7]. However, there is no consensus regarding the efficacy of using the opened round ligament as a venous patch graft. Although there are reports on the radiological patency of the opened round ligament after LDLT and reports on the pathological assessment of its epithelium at the time of transplant[8,9], pathological analyses of long-term patency and continuity after LDLT have not been reported.
Herein, we describe the first reported case of a pathological analysis on an autopsy specimen of an opened round ligament used as a venous patch graft in hepatic venous reconstruction during LDLT. Approval to conduct this study was obtained from the Ethics Committees of Jichi Medical University.
A 13-year-old female patient with biliary atresia was considered for LDLT because of decompensated liver cirrhosis with jaundice. Her body height and weight were 159.4 cm and 54.0 kg, respectively, and her standard liver volume was 1095 mL. The blood test results were as follows: white blood cells 9600/μL; hemoglobin 8.7 g/dL; hematocrit 26.0%; platelets 149000 /μL; albumin 2.9 g/dL; creatinine 0.35 mg/dL; total bilirubin 31.6 mg/dL; aspartate aminotransferase 93 mU/mL; alanine aminotransferase 50 mU/mL; prothrombin time-international normalized ratio 1.40; and activated partial thromboplastin 58.3 s. The model for end-stage liver disease score was 23.
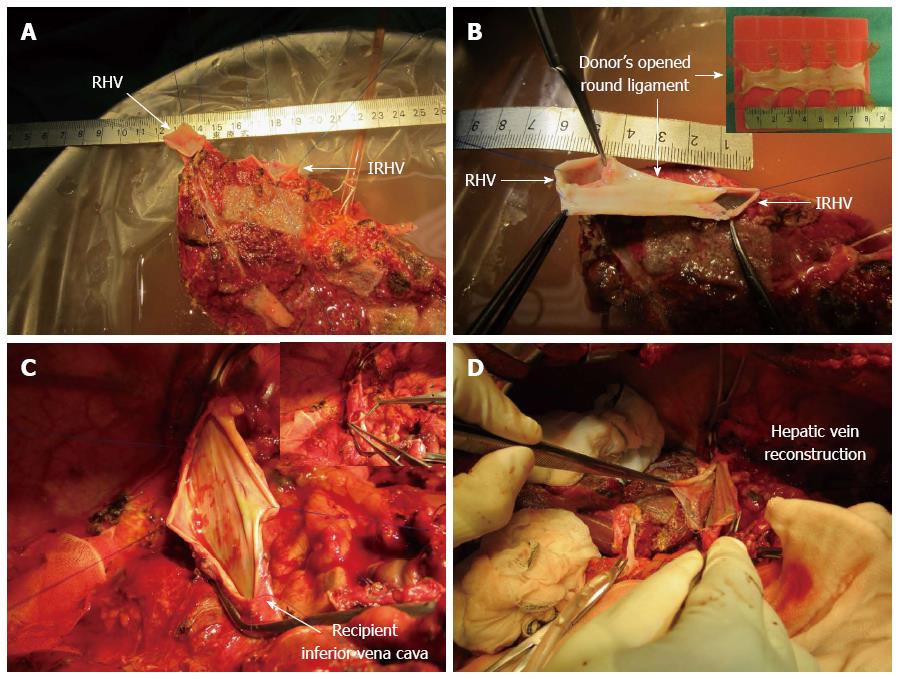
The patient underwent an ABO-compatible LDLT using her mother’s posterior segment graft (390 g; graft volume to standard liver volume ratio: 35.6%). The graft had two hepatic veins (HVs), including the right hepatic vein (RHV; 15 mm) and the inferior RHV (IRHV; 20 mm) (Figure 1A). The graft RHV and IRHV were formed into a single orifice using the donor’s opened round ligament (60 mm × 20 mm) as a venous patch graft during bench surgery (Figure 1B); it was then anastomosed end-to-side with the recipient inferior vena cava (Figure 1C and D). Portal vein reconstruction using an interposition vein graft from the right saphenous vein of the donor was performed between the recipient portal vein and the graft posterior portal vein. Hepatic artery reconstruction using microsurgical technique was performed between the recipient right hepatic artery and the graft posterior hepatic artery. For biliary reconstruction, a Roux-en-Y hepaticojejunostomy was performed. Intraoperative color Doppler ultrasonography was performed to assess the blood flow velocity and pattern after vascular reconstruction, and the flow velocity and pattern were satisfactory. The length of the operation was 21 h and 8 min, and the bleeding volume was 7438 mL. Tacrolimus and methylprednisolone were used for standard post-operative immunosuppressive therapy.
On post-operative day (POD) 8, portal vein thrombosis was detected by color Doppler ultrasonography, and the patient was given intravenous urokinase. On POD 26, acute cellular rejection was diagnosed by a graft liver biopsy, and she was treated with pulse steroids. On POD 40, cytomegalovirus viremia was detected by the C7-HRP test, and she underwent preemptive anti-cytomegalovirus therapy. On POD 48, portal vein balloon dilatation was performed by interventional radiology after the progressive narrowing of the portal vein anastomotic stricture to less than 2 mm was detected by color Doppler ultrasonography. During percutaneous transhepatic portography of the intrahepatic portal vein, bilhemia was detected, and thereafter, she suffered from persistent jaundice (total bilirubin 6.4 mg/dL). On POD 86, she developed a fever with liver dysfunction, and antibiotic treatment for acute cholangitis was administered. However, she died of septic shock with persistent cholangitis and bacteremia (with Serratia marcescens) on POD 88.
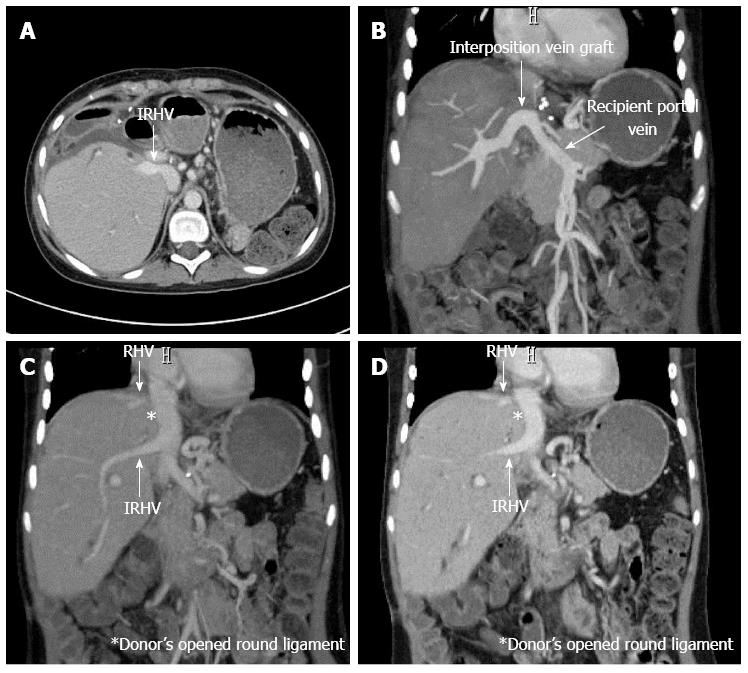
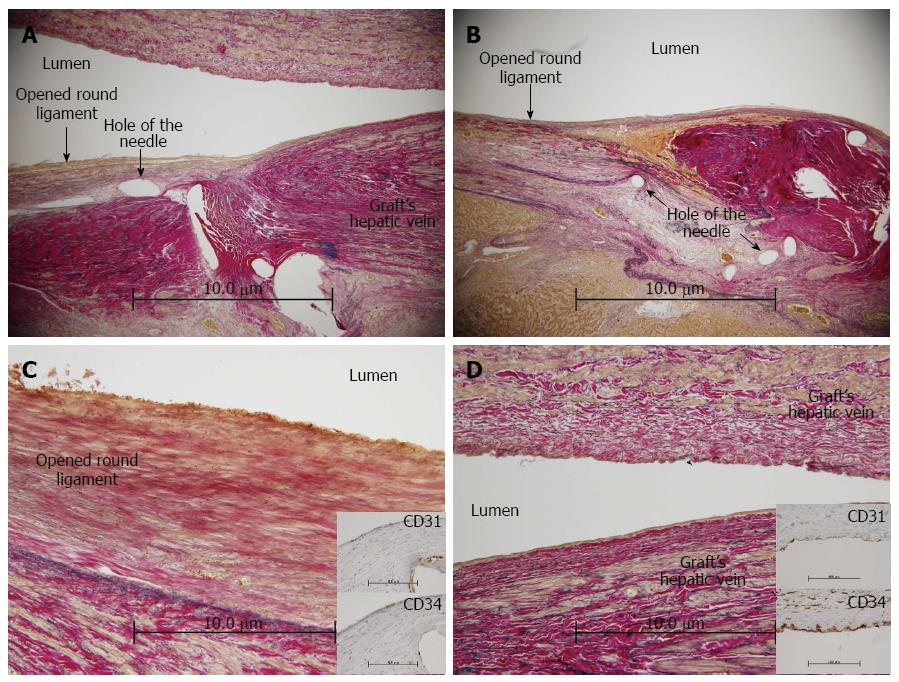
There were no post-transplant complications involving the HVs, and the radiological patency between the opened round ligament and the HV was confirmed on POD 82 (Figure 2). The HV anastomotic site had no stenosis or thrombus on an autopsy (Figure 3A and B). The patency and continuity between the donor’s opened round ligament and the HV were adequate on pathological examination. In addition, the stains for CD31 and CD34 on the inner membrane of the opened round ligament were positive, a finding also observed in the graft HV (Figure 3C and D).
Outflow block in LDLT can potentially result in liver congestion, graft failure, and death[10]. When a right liver graft is used for LDLT and the graft HV is anastomosed end-to-end with the recipient HV or end-to-side with the recipient inferior vena cava, the liver graft expands in all directions in the limited right subphrenic space during liver graft regeneration. Consequently, the HV anastomosis can be compressed and twisted in some circumstances[11]. In addition, multiple HV reconstructions are sometimes required in a right liver graft LDLT. Therefore, reports have indicated that it is important to simplify HV reconstruction and enlarge the graft HV orifice by an all-in-one sleeve patch graft venoplasty, which has shown excellent outcomes[11-14].
The efficacy of various venous grafts used in HV reconstruction has been reported. These grafts include auto-venous grafts (from the inferior mesenteric vein, gonadal vein, external iliac vein, internal jugular vein, and saphenous vein)[13,15,16], venous grafts from a living donor, native portal veins[17], opened round ligaments[4-9,12], cryopreserved homografts[18-22], and polytetrafluoroethylene grafts[23,24]. However, the harvest of a venous graft from a living donor or the recipient himself is not easy. In addition, the availability of cryopreserved homografts is limited, and the adverse effects of polytetrafluoroethylene grafts are unclear. Alternatively, the opened round ligament is easier to use, and less invasive than other venous grafts, but it is controversial in regard to its long-term patency. Recently, use of the opened round ligament as a venous patch graft in hepatic venous reconstruction during LDLT has been reported[4-7]. Although there are reports on the radiological patency of the opened round ligament after LDLT and reports on the pathological assessment of its epithelium at the time of transplant[8,9], there are no pathological analyses of patency and continuity. In this case, we conducted a pathological autopsy evaluation of a donor’s opened round ligament used as a venous patch graft in LDLT. This is the first report that demonstrates the pathological efficacy of the opened round ligament after surgery. The HV anastomotic site had no stenosis or thrombus (Figure 3A and B). On pathology, there was adequate patency and continuity between the recipient HV and the donor’s opened round ligament. In addition, the stains for CD31 and CD34 on the inner membrane of the opened round ligament were positive, a finding also observed in the graft HV (Figure 3C and D). Therefore, we believe that the pathological patency and the existence of venous endothelial cells support the efficacy of using the opened round ligament as a venous patch graft.
In conclusion, using the opened round ligament as a venous patch graft is easy, less invasive, and had been shown to be pathologically effective. The accumulation of further cases and the long-term observation of this case are needed to confirm our findings.
We thank Professor Akira Tanaka (Department of Pathology, Jichi Medical University) for helpful advices regarding pathological evaluations.
A 13-year-old female patient with biliary atresia underwent living donor liver transplantation using a posterior segment graft from her mother, and she died of septic shock with persistent cholangitis and jaundice 86 d after living donor liver transplantation.
The patient was diagnosed septic shock with persistent cholangitis and jaundice 86 d after living donor liver transplantation.
No-obstructive afferent loop syndrome.
Biliary stasis.
The radiological patency between the opened round ligament and the hepatic vein was confirmed on POD 82.
On pathology, there was adequate patency and continuity between the recipient’s hepatic vein and the donor’s opened round ligament. In addition, the stains for CD31 and CD34 on the inner membrane of the opened round ligament were positive, a finding also observed in the graft hepatic vein.
The antibiotic treatment for acute cholangitis was administered.
The efficacy of various venous grafts used in hepatic vein reconstruction has been reported. These grafts include auto-venous grafts (from the inferior mesenteric vein, gonadal vein, external iliac vein, internal jugular vein, and saphenous vein), venous grafts from a living donor, native portal veins, opened round ligaments, cryopreserved homografts, and polytetrafluoroethylene grafts.
Opened round ligament and all-in-one venoplasty.
Hepatic venous reconstruction using the opened round ligament as a venous patch graft is effective in living donor liver transplantation, as observed on pathology.
The manuscript provides anecdotal support for the use of opened round ligament in hepatic vein reconstruction.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Boucek CD S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Yamanaka N, Yasui C, Yamanaka J, Tanaka T, Ando T, Kuroda N, Okamoto E. Recycled use of reopened umbilical vein for venous reconstruction in hepatopancreatobiliary surgery. J Am Coll Surg. 2000;190:497-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Watanabe M, Yamazaki K, Tsuchiya M, Otsuka Y, Tamura A, Shimokawa K, Kaneko H, Teramoto T. Use of an opened umbilical vein patch for the reconstruction of the injured biliary tract. J Hepatobiliary Pancreat Surg. 2007;14:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Gunasekaran G, Mosna LC, Savino JA. Portomesenteric reconstruction using an umbilical vein patch during pancreaticoduodenectomy (Whipple procedure). J Am Coll Surg. 2013;217:e9-e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Ikegami T, Shimada M, Imura S, Morine Y, Kanemura H, Mori H, Arakawa Y, Hanaoka J. Beneficial use of the round ligament as a patch graft for vena cava reconstruction. J Hepatobiliary Pancreat Surg. 2008;15:581-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Sakamoto S, Kasahara M, Shigeta T, Fukuda A, Tanaka H, Matsuno N. Feasibility of using the graft’s umbilical vein as a patch graft for hepatic vein reconstruction in pediatric living donor liver transplantation. Transpl Int. 2010;23:436-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Kamei H, Onishi Y, Ogawa K, Uemoto S, Ogura Y. Living donor liver transplantation using a right liver graft with additional vein reconstructions for patient with situs inversus. Am J Transplant. 2014;14:1453-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Toshima T, Ikegami T, Matsumoto Y, Yoshiya S, Harimoto N, Yamashita Y, Yoshizumi T, Ikeda T, Shirabe K, Maehara Y. One-step venous reconstruction using the donor’s round ligament in right-lobe living-donor liver transplantation. Surg Today. 2015;45:522-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Mergental H, Gouw AS, Slooff MJ, de Jong KP. Venous outflow reconstruction with surgically reopened obliterated umbilical vein in domino liver transplantation. Liver Transpl. 2007;13:769-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Ikegami T, Wang H, Imai D, Bekki Y, Yoshizumi T, Yamashita Y, Toshima T, Soejima Y, Shirabe K, Maehara Y. Pathological analysis of opened round ligaments as venous patch grafts in living donor liver transplantation. Liver Transpl. 2013;19:1245-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Lee S, Park K, Hwang S, Lee Y, Choi D, Kim K, Koh K, Han S, Choi K, Hwang K. Congestion of right liver graft in living donor liver transplantation. Transplantation. 2001;71:812-814. [PubMed] |
| 11. | Lee SG. Techniques of reconstruction of hepatic veins in living-donor liver transplantation, especially for right hepatic vein and major short hepatic veins of right-lobe graft. J Hepatobiliary Pancreat Surg. 2006;13:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Chen CL, Yap AQ, Concejero AM, Liu CY. All-in-one sleeve patch graft venoplasty for multiple hepatic vein reconstruction in living donor liver transplantation. HPB (Oxford). 2012;14:274-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Soejima Y, Ueda N, Fukuhara T, Yoshizumi T, Ikegami T, Yamashita Y, Sugimachi K, Taketomi A, Maehara Y. One-step venous reconstruction for a right lobe graft with multiple venous orifices in living donor liver transplantation. Liver Transpl. 2008;14:706-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Sugawara Y, Makuuchi M, Sano K, Imamura H, Kaneko J, Ohkubo T, Matsui Y, Kokudo N. Vein reconstruction in modified right liver graft for living donor liver transplantation. Ann Surg. 2003;237:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Hwang S, Lee SG, Park KM, Kim KH, Ahn CS, Moon DB, Ha TY. Quilt venoplasty using recipient saphenous vein graft for reconstruction of multiple short hepatic veins in right liver grafts. Liver Transpl. 2005;11:104-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Ikegami T, Shirabe K, Yoshiya S, Soejima Y, Yoshizumi T, Uchiyama H, Toshima T, Motomura T, Maehara Y. One-step reconstruction of the right inferior hepatic veins using auto-venous grafts in living-donor liver transplantation. Surg Today. 2013;43:769-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Mori A, Kaido T, Ogura Y, Ogawa K, Hata K, Yagi S, Yoshizawa A, Isoda H, Shibata T, Uemoto S. Standard hepatic vein reconstruction with patch plasty using the native portal vein in adult living donor liver transplantation. Liver Transpl. 2012;18:602-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Sugawara Y, Makuuchi M, Akamatsu N, Kishi Y, Niiya T, Kaneko J, Imamura H, Kokudo N. Refinement of venous reconstruction using cryopreserved veins in right liver grafts. Liver Transpl. 2004;10:541-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Hwang S, Lee SG, Ahn CS, Park KM, Kim KH, Moon DB, Ha TY. Cryopreserved iliac artery is indispensable interposition graft material for middle hepatic vein reconstruction of right liver grafts. Liver Transpl. 2005;11:644-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Kilic M, Aydin U, Sozbilen M, Ozer I, Tamsel S, Demirpolat G, Atay Y, Alper M, Zeytunlu M. Comparison between allogenic and autologous vascular conduits in the drainage of anterior sector in right living donor liver transplantation. Transpl Int. 2007;20:697-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Yaprak O, Balci NC, Dayangac M, Demirbas T, Guler N, Ulusoy L, Tokat Y, Yuzer Y. Cryopreserved aortic quilt plasty for one-step reconstruction of multiple hepatic venous drainage in right lobe living donor liver transplantation. Transplant Proc. 2011;43:2817-2819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Wang CC, Lopez-Valdes S, Lin TL, Yap A, Yong CC, Li WF, Wang SH, Lin CC, Liu YW, Lin TS. Outcomes of long storage times for cryopreserved vascular grafts in outflow reconstruction in living donor liver transplantation. Liver Transpl. 2014;20:173-181. [PubMed] |
| 23. | Hwang S, Jung DH, Ha TY, Ahn CS, Moon DB, Kim KH, Song GW, Park GC, Jung SW, Yoon SY. Usability of ringed polytetrafluoroethylene grafts for middle hepatic vein reconstruction during living donor liver transplantation. Liver Transpl. 2012;18:955-965. [PubMed] |
| 24. | Thorat A, Jeng LB, Yang HR, Li PC, Li ML, Yeh CC, Chen TH, Hsu SC, Poon KS. Outflow reconstruction for right liver allograft with multiple hepatic veins: “V-plasty” of hepatic veins to form a common outflow channel versus 2 or more hepatic vein-to-inferior vena cava anastomoses in limited retrohepatic space. Liver Transpl. 2016;22:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |