Published online Sep 14, 2016. doi: 10.3748/wjg.v22.i34.7787
Peer-review started: April 21, 2016
First decision: May 12, 2016
Revised: May 20, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: September 14, 2016
Processing time: 142 Days and 0.2 Hours
To compare 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) features in gastric lymphoma and gastric carcinoma.
Patients with newly diagnosed gastric lymphoma or gastric carcinoma who underwent 18F-FDG PET/CT prior to treatment were included in this study. We reviewed and analyzed the PET/CT features of gastric wall lesions, including FDG avidity, pattern (focal/diffuse), and intensity [maximal standard uptake value: (SUVmax)]. The correlation of SUVmax with gastric clinicopathological variables was investigated by χ2 test, and receiver-operating characteristic (ROC) curve analysis was performed to determine the differential diagnostic value of SUVmax-associated parameters in gastric lymphoma and gastric carcinoma.
Fifty-two patients with gastric lymphoma and 73 with gastric carcinoma were included in this study. Abnormal gastric FDG accumulation was found in 49 patients (94.23%) with gastric lymphoma and 65 patients (89.04%) with gastric carcinoma. Gastric lymphoma patients predominantly presented with type I and type II lesions, whereas gastric carcinoma patients mainly had type III lesions. The SUVmax (13.39 ± 9.24 vs 8.35 ± 5.80, P < 0.001) and SUVmax/THKmax (maximal thickness) (7.96 ± 4.02 vs 4.88 ± 3.32, P < 0.001) were both higher in patients with gastric lymphoma compared with gastric carcinoma. ROC curve analysis suggested a better performance of SUVmax/THKmax in the evaluation of gastric lesions between gastric lymphoma and gastric carcinoma in comparison with that of SUVmax alone.
PET/CT features differ between gastric lymphoma and carcinoma, which can improve PET/CT evaluation of gastric wall lesions and help differentiate gastric lymphoma from gastric carcinoma.
Core tip:18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) feature in gastric lymphomas compared to that in gastric carcinomas were investigated. Gastric lymphoma patients predominantly presented with type I and type II lesions, whereas gastric carcinoma patients mainly with type III lesions. The SUVmax and SUVmax/THKmax were both higher in patients with gastric lymphomas compared to that in patients with gastric carcinomas. A ROC curve analysis suggested a better performance of SUVmax/THKmax in the evaluation of gastric lesions in comparison with that of SUVmax alone. The differences existed in the PET/CT feature could improve the PET/CT evaluation of gastric lesions and contribute to the identification of gastric lymphomas from gastric carcinomas.
- Citation: Li XF, Fu Q, Dong YW, Liu JJ, Song XY, Dai D, Zuo C, Xu WG. 18F-fluorodeoxyglucose positron emission tomography/computed tomography comparison of gastric lymphoma and gastric carcinoma. World J Gastroenterol 2016; 22(34): 7787-7796
- URL: https://www.wjgnet.com/1007-9327/full/v22/i34/7787.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i34.7787
Lymphoma, mainly non-Hodgkin’s lymphoma (NHL), may be extranodal in origin in 25%-40% of patients, depending on geography[1-3]. The gastrointestinal tract is the most common site of primary extranodal NHL, occurring in 4%-20% of patients[4], accounting for 20%-30% of extranodal cases at diagnosis[5]. Second only to gastric carcinoma, gastric lymphoma is an important malignant tumor of the stomach. Histopathology of gastric lymphoma is predominantly high-grade diffuse large B-cell lymphoma (DLBCL) and low-grade mucosa-associated lymphoid tissue (MALT) lymphoma[3].
Imaging plays an important role in noninvasive evaluation of patients with extranodal lymphoma before treatment[6,7]. Hybrid positron emission tomography (PET)/computed tomography (CT) integrates 18F-fluorodeoxyglucose (FDG) PET and CT scanning, thus simultaneously providing functional, metabolic information based on PET and structural, anatomical information based on CT[8-10]. A growing number of studies have supported the application of 18F-FDG PET/CT in initial staging, treatment response assessment, and follow-up of patients with gastric lymphoma of various histological subtypes[11-14]. However, differences were also present in some previous studies, due in part to lower FDG accumulation in low-grade lymphoma than in aggressive lymphoma, and to the presence of physiological tracer uptake in the stomach[12,15].
Endoscopic examination and direct biopsy are well-established methods for differential diagnosis between gastric lymphoma and gastric carcinoma[16-23]. However, 18F-FDG PET/CT has the advantages of detecting gastric lymphoma that is limited to the submucosal stage, which may be missed by gastroscopy, and in finding unanticipated lesions outside the stomach[23,24]. In addition, 18F-FDG PET/CT is significant for the diagnosis of gastric lesions in patients for whom endoscopic examination is not acceptable.
Clinical manifestations and radiological features of gastric lymphoma and gastric carcinoma are in general nonspecific, such as abdominal pain, dyspepsia, gastric ulcers, and irregular thickness of the gastric wall. Besides, the marked differences between gastric lymphoma and gastric carcinoma with regard to therapeutic options and prognosis further highlight the significance of accurate detection and differentiation of the two tumors. To the best of our knowledge, few studies have focused on the imaging differences between gastric lymphoma and gastric carcinoma using 18F-FDG PET/CT[18,19]. The purpose of the present investigation was to characterize the PET/CT performance in evaluation of gastric lymphoma in comparison with that in gastric carcinoma.
Consecutive patients with newly diagnosed gastric lymphomas and with newly diagnosed gastric carcinomas by 18F-FDG PET/CT performed about one week prior to any treatment, and underwent operation between July 2014 and January 2006 in our institution were included in this study. All diagnoses were confirmed by endoscopic biopsy or postoperative pathological findings. This study was reviewed and approved by the Tianjin Medical University Cancer Institute and Hospital Institutional Review Board, and written informed consent was obtained from all the patients. Patients were staged according to the Lugano classification and the Tumor Node Metastasis (TNM) classification, respectively[8]. The demographic and clinicopathological characteristics of the included patients are presented in Table 1. It is worth mentioning that gastric carcinoma patients were divided into subgroups of mucinous adenocarcinoma and non-mucinous adenocarcinoma to facilitate the interpretation of the 18F-FDG PET/CT results, and mucinous adenocarcinoma subgroup consisted of gastric mucinous adenocarcinoma and signet ring cell carcinoma.
| Variables | Gastric lymphomas | Gastric carcinomas | P value |
| Total number of patients | 52 | 73 | |
| Age, yr, median (range) | 56 (8-90) | 62 (31-84) | 0.0261 |
| Gender (male/female) | 29/23 | 48/25 | 0.2582 |
| Histopathological subtype, n | |||
| DLBCL: 33 | Mucinous: 13 | ||
| MALT: 19 | Non-mucinous: 60 | ||
| Stage, n | Lugano (I/II1/II2/IV) | TNM (I/II/III/IV) | 0.3263 |
| 19/7/2/24 | 12/18/12/31 | ||
| Involved regions | |||
| Cardia | 7 (13.5) | 30 (41.1) | 0.0122 |
| Fundus | 16 (30.8) | 14 (19.2) | 0.2452 |
| Body | 40 (76.9) | 32 (43.8) | 0.0592 |
| Antrum | 28 (53.8) | 26 (35.6) | 0.2052 |
| ≥ 2 regions | 34 (65.38) | 20 (27.40) | 0.0092 |
| THKmax, cm, mean (range) | 1.97 (0.3-6.6) | 2.00 (0.3-9.2) | 0.9134 |
| Splenomegalia | 12 (23.1) | 6 (8.2) | 0.0462 |
| Involved lymph nodes in retroperitoneal space below renal hilus | 15 (28.8) | 8 (11.0) | 0.0372 |
| Mucosal ulceration | 18 (34.62) | 53 (72.60) | 0.0232 |
The patients were required to fast at least 6 h prior to 18F-FDG PET/CT examination, with injection of approximately 3.70-4.81 MBq/kg. Blood glucose was measured to ensure the level was below 6.8 mmol/L. After injection, patients were kept lying comfortably for an uptake period of 45-60 min. Before the examination, patients were asked to drink 500-800 mL water to distend the stomach and to accelerate renal tracer elimination. Scanning from head to thigh was performed using a PET/CT system (Discovery ST4, General Electric Healthcare, Waukesha, WI, United States). The protocol included an initial CT scan followed by PET data acquisition. The initial CT was performed with 120 kV, 100 mA and a slice thickness of 5 mm. PET data were obtained in a three-dimensional mode with an acquisition time of 2 min for each bed position (for a total of 6-8 bed positions). The CT-based attenuation-corrected PET images were reconstructed using an iterative algorithm. After completion of data acquisition, separate PET images, CT images and fused PET/CT data were available for review in coronal, sagittal and axial planes using the manufacturer’s review station (Xeleris, General Electric Healthcare).
The 18F-FDG PET/CT images were visually interpreted by a consensus of at least two experienced nuclear medicine physicians who were aware of the clinical manifestation, but blinded to the specific histological diagnosis of the patients.
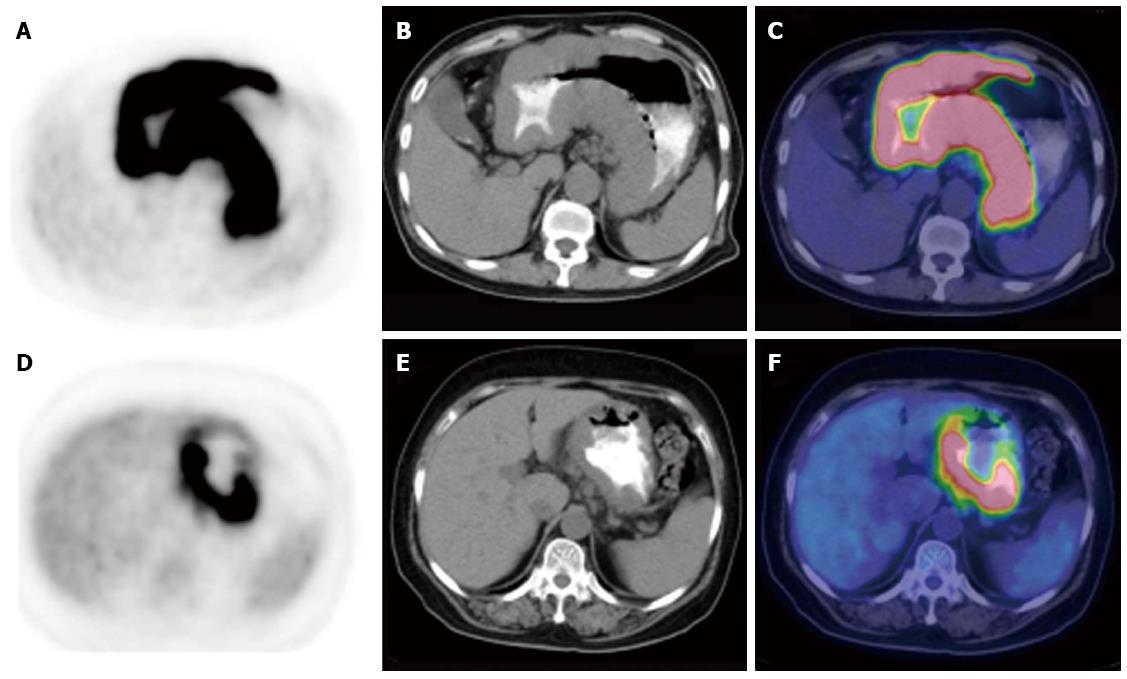
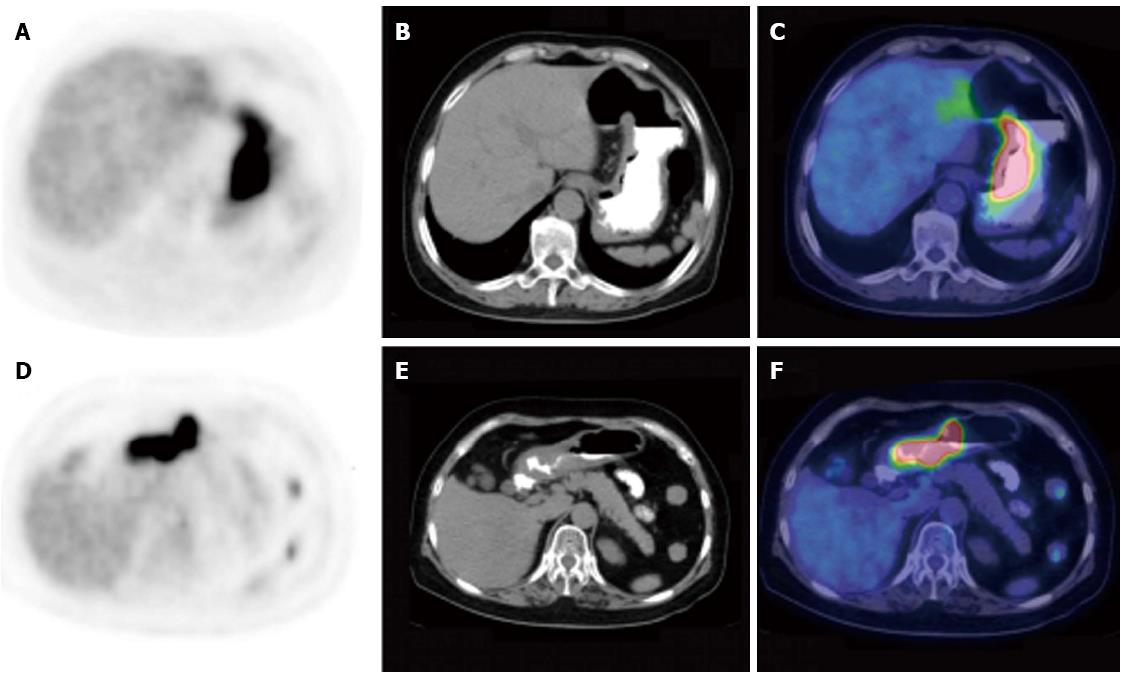
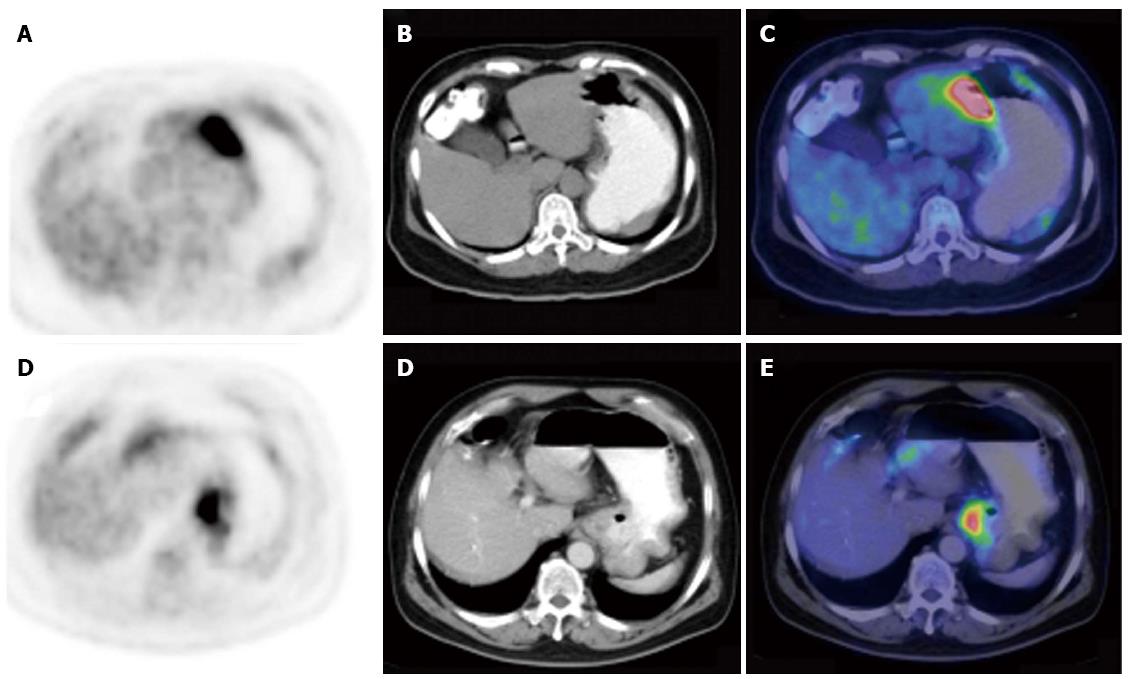
The maximal thickening measurement of the gastric wall based on CT component was recorded to define the size of the lesion. To determine the intensity of gastric FDG uptake semi-quantitatively, the maximal standard uptake value (SUVmax) was measured. The patterns of PET/CT scan were classified into three subtypes according to the infiltrative and thickening extent of the lesion in the stomach: type I, infiltrating more than one-third of the total gastric wall and with diffuse thickening; type II, infiltrating less than one-third and with segmental thickening; and type III, with local uptake and local thickening.
The data were expressed as mean ± SD. Student’s t test, Wilcoxon’s rank-sum test, analysis of variance and χ2 test were used to determine the statistical difference in demographic and clinical characteristics, SUVmax and categorical data between patients with various histological subtypes. The relationship between SUVmax and gastric clinicopathological variables was investigated by χ2 test. A receiver-operating characteristic (ROC) curve analysis was performed to identify the values of SUVmax or SUVmax/maximal thickness (THKmax) in the differential diagnosis of gastric lymphomas and gastric carcinomas, and the total area under the curve (AUC), 95%CI and a best cutoff threshold of SUVmax or SUVmax/THKmax were calculated to quantify the differential diagnostic value of these indicators. The statistical methods of this study were reviewed by Yu-Bei Huang from the Department of Epidemiology and Biostatistics in our hospital.
All calculation and statistical analyses were performed using the statistical package for social science 21.0 version (SPSS, iNC, Chicago, IL, United States). P < 0.05 was considered statistically significant.
Fifty-two patients with gastric lymphoma and 73 with gastric carcinoma were included in this study. As expected, the histopathological subtype of gastric lymphoma was predominantly high-grade DLBCL (n = 33) or low-grade MALT lymphoma (n = 19). Gastric carcinoma patients were divided into mucinous adenocarcinoma (n = 13) or non-mucinous adenocarcinoma (n = 60) subgroups to facilitate comparison with gastric lymphoma patients. The incidence of the involved regions of the stomach (the cardia, fundus, body and the antrum) was 13.5% (7/52), 30.8% (16/52), 76.9% (40/52) and 53.8% (28/52) for gastric lymphoma and 41.1% (30/73), 19.2% (14/73), 43.8% (32/73) and 35.6% (26/73) for gastric carcinoma, respectively. The THKmax of the gastric wall lesions in patients with gastric lymphoma and gastric carcinoma is compared in Table 1. There was no significant difference with regard to THKmax in patients with gastric lymphoma in comparison with gastric carcinoma (P = 0.913). Twelve cases (23.1%) with splenomegaly and 15 (28.8%) with retroperitoneal lymph node involvement below the renal hilus were observed in the gastric lymphoma group, while the corresponding numbers in the gastric carcinoma group were lower (P = 0.046, P = 0.037). Unlike gastric carcinoma, which is an epithelium-derived malignancy, gastric lymphoma is derived from the submucous layer and mostly infiltrates beneath the mucinous membrane, so mucosal ulceration was less common than in gastric carcinoma (34.62% vs 72.6%, P = 0.023) (Table 1).
The presence of gastric 18F-FDG uptake and SUVmax are summarized in Table 2. Gastric FDG uptake was demonstrated in all 52 patients with gastric lymphoma and in 72 of the 73 patients (98.63%) with gastric carcinoma (P = 0.957). However, abnormal gastric FDG accumulation was deemed present if the intensity of gastric 18F-FDG uptake was higher than the hepatic uptake. Forty-nine (94.23%) gastric lymphoma patents and 65 (89.04%) gastric carcinoma patients had increased gastric FDG uptake. The SUVmax was higher in patients with gastric lymphoma compared with gastric carcinoma (13.39 ± 9.24 vs 8.35 ± 5.80, P < 0.001). With regard to the 18F-FDG PET/CT pattern of gastric wall lesions, the incidence of type I lesions (Figure 1) (P = 0.002) and type II lesions (Figure 2) (P = 0.038) was significantly higher, but the incidence of type III lesions (Figure 3) (P < 0.001) was significantly lower in gastric lymphoma than gastric carcinoma patients. Fu et al[19] suggested SUVmax/THKmax as a valid and practical biomarker in discriminating primary gastric lymphoma from advanced gastric carcinoma. As illustrated in Table 2, SUVmax/THKmax was significantly higher in patients with gastric lymphoma in comparison with gastric carcinoma (7.96 ± 4.02 vs 4.88 ± 3.32, P < 0.001).
| Gastric lymphoma | Gastric carcinoma | P value | |
| Presence of gastric FDG uptake | 52 (100) | 72 (98.63) | 0.9571 |
| Gastric FDG uptake > liver | 49 (94.23) | 65 (89.04) | 0.8291 |
| PET/CT pattern | |||
| Type I | 23 (44.23) | 9 (12.33) | 0.0021 |
| Type II | 22 (42.31) | 14 (19.18) | 0.0381 |
| Type III | 7 (13.46) | 50 (68.49) | < 0.0011 |
| SUVmax (mean ± SD) | 13.39 ± 9.24 | 8.35 ± 5.80 | < 0.0012 |
| SUVmax/THKmax (mean ± SD) | 7.96 ± 4.02 | 4.88 ± 3.32 | < 0.0012 |
SUVmax was higher in gastric lymphoma patients with DLBCL than in those with MALT (18.41 ± 7.78 vs 4.66 ± 2.72, P < 0.001) and higher in patients with advanced Lugano stage (II1/II2/IV) than in those with stage I (15.53 ± 8.87 vs 9.97 ± 8.88, P = 0.026) (Table 3). In gastric carcinoma patients, SUVmax was higher in the non-mucinous adenocarcinoma subgroup than in mucinous adenocarcinoma subgroup (9.02 ± 6.14 vs 5.28 ± 2.06, P = 0.032) and higher in advanced TNM stage (III/IV) than in stage I/II gastric carcinoma patients (10.57 ± 6.27 vs 5.17 ± 2.96, P < 0.001) (Table 4). With regard to THKmax, there were no significant differences between patients with gastric lymphoma and gastric carcinoma according to cell types and TNM/Lugano stage. We divided patients into low SUVmax (< mean value) and high SUVmax (≥ mean value) subgroups. Association of SUVmax with clinicopathological features was evaluated by χ2 test among patients with gastric lymphoma and gastric carcinoma. As shown in Tables 3 and 4, Pearson correlation analysis suggested a significant association of high-grade gastric lymphoma (DLBCL) (P < 0.001), non-mucinous adenocarcinoma (P = 0.046) and advanced stage gastric carcinoma (P < 0.001) with high SUVmax. However, investigation of the relationship of SUVmax to advanced stage gastric lymphoma did not identify a strong positive correlation.
| Characteristics | n | SUVmax and THKmax | SUVmax | |||
| mean ± SD | P value | High (n) | Low (n) | P value | ||
| Sex | ||||||
| Male | 29 | 14.31 ± 9.58 | 0.4231 | 14 | 15 | 0.5102 |
| Female | 23 | 12.22 ± 8.85 | 9 | 14 | ||
| Age (yr) | ||||||
| < mean | 22 | 12.23 ± 8.25 | 0.4461 | 10 | 12 | 0.8792 |
| ≥ mean | 30 | 14.23 ± 9.95 | 13 | 17 | ||
| Histopathological subtype | ||||||
| DLBCL | 33 | 18.41 ± 7.78 | < 0.0013 | 22 | 11 | < 0.0015 |
| 2.33 ± 1.434 | ||||||
| MALT | 19 | 4.66 ± 2.72 | 0.5674 | 1 | 18 | |
| 1.36 ± 1.254 | ||||||
| Lugano stage | ||||||
| I | 19 | 9.97 ± 8.88 | 0.0261 | 6 | 13 | 0.2702 |
| 1.52 ± 1.194 | ||||||
| II1/II2/IV | 33 | 15.53 ± 8.87 | 0.2444 | 17 | 16 | |
| 2.23 ± 1.514 | ||||||
| Characteristics | n | SUVmax and THKmax | SUVmax | |||
| mean ± SD | P value1 | High (n) | Low (n) | P value2 | ||
| Sex | ||||||
| Male | 48 | 7.55 ± 4.53 | 0.4572 | 15 | 33 | 0.2803 |
| Female | 25 | 9.89 ± 7.54 | 11 | 14 | ||
| Age (yr) | ||||||
| < mean | 35 | 7.14 ± 4.56 | 0.0881 | 9 | 26 | 0.0903 |
| ≥ mean | 38 | 9.46 ± 6.61 | 17 | 21 | ||
| Histopathological subtype | ||||||
| Mucinous | 13 | 5.28 ± 2.06 | 0.0322 | 1 | 12 | 0.0465 |
| 1.75 ± 0.934 | ||||||
| Non-mucinous | 60 | 9.02 ± 6.14 | 0.7814 | 25 | 35 | |
| 2.07 ± 1.374 | ||||||
| TNM stage | ||||||
| I/II | 30 | 5.17 ± 2.96 | < 0.0012 | 2 | 28 | < 0.0015 |
| 1.57 ± 0.804 | ||||||
| III/IV | 43 | 10.57 ± 6.27 | 0.2074 | 24 | 19 | |
| 2.32 ± 1.504 | ||||||
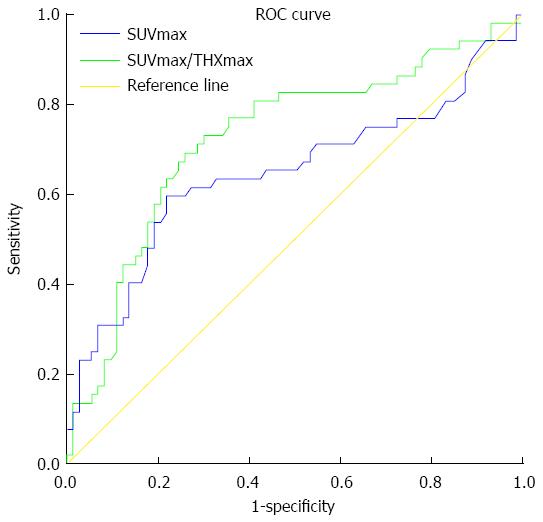
Comparative receiver-operating characteristic (ROC) curves were generated to obtain the best cutoff threshold for SUVmax or SUVmax/THKmax for differential diagnosis between gastric lymphoma and gastric carcinoma (Figure 4). The AUC was 0.645 (95%CI: 0.540-0.750) and 0.725 (95%CI: 0.631-819). This suggests a more acceptable discrimination of SUVmax/THKmax in comparison with that of SUVmax alone, with a best cutoff threshold at 10.4 and 5.9 for SUVmax and SUVmax/THKmax, respectively. There was no profound difference in specificity (0.781 vs 0.740) for SUVmax and SUVmax/THKmax. The corresponding sensitivity significantly increased from 0.596 to 0.692 when SUVmax alone was replaced by SUVmax/THKmax to evaluate the diagnostic performance of 18F-FDG PET/CT in the differential diagnosis of gastric lymphoma and gastric carcinoma.
Gastric carcinoma and gastric lymphoma are the two most commonly encountered malignancies in the stomach. 18F-FDG PET/CT examination is a well-recognized noninvasive imaging modality in staging and treatment response evaluation for gastric wall lesions, including in gastric lymphoma and gastric carcinoma[12,20]. The advantages of 18F-FDG PET/CT over conventional imaging techniques have been well characterized in numerous studies[21,22]. However, few studies have investigated the application of 18F-FDG PET/CT in the initial differential diagnosis of gastric lymphoma and gastric carcinoma. We aimed to distinguish gastric lymphoma from gastric carcinoma through assessing 18F-FDG uptake pattern and intensity in gastric wall lesions.
Regarding the 18F-FDG PET/CT pattern, gastric lymphoma patients predominantly presented with diffuse/segmental tracer uptake (type I and type II), whereas gastric carcinoma patients showed mainly local tracer uptake (type III). 18F-FDG uptake intensity, measured by SUVmax or SUVmax/THKmax, was significantly higher in patients with gastric lymphoma than gastric carcinoma. Consequently, higher SUVmax and larger SUVmax/THKmax suggest that gastric lymphoma is more likely. Furthermore, the presence of splenomegaly or involvement of lymph nodes in the retroperitoneal space below the renal hilus may provide additional clues in diagnosing gastric lymphoma. More importantly, SUVmax/THKmax was a more reliable indicator to distinguish gastric lymphoma from gastric carcinoma compared to SUVmax alone.
As expected, DLBCL and MALT lymphomas accounted for the majority of gastric lymphoma subtypes, and the gastric carcinoma patients were divided into mucinous adenocarcinoma and non-mucinous adenocarcinoma subgroups to facilitate comparison with gastric lymphoma patients. MALT lymphoma is indolent, and usually develops as local lesions. Gastric MALT lymphoma detected by 18F-FDG PET/CT has been widely studied, as the stomach is the most commonly involved organ[11,16,17]. However, there are controversial results with regard to the usefulness of PET/CT scan in the diagnosis of gastric MALT lymphoma. Some studies have suggested that 18F-FDG PET/CT is not a useful imaging method in patients with low-grade lymphoma, such as MALT lymphoma, as 18F-FDG uptake in MALT lymphoma is lower than in aggressive lymphoma, such as DLBCL[12,15]. Especially for gastric MALT lymphoma, the sensitivity of 18F-FDG PET/CT was low because of the physiological or inflammatory FDG accumulation in the stomach, which usually resulted in false-negative diagnosis. Subsequently, 18F-FDG PET/CT was shown to be useful for evaluating the protrusion type of gastric MALT lymphoma in which mass lesions are formed[16]. In addition, diffuse or local uptake can occur in chronic-gastritis-like type or depressed type of gastric MALT lymphoma, so endoscopic biopsy is recommend even if the gastroscopy findings suggest chronic gastritis[16-19,23]. Explanations for this discrepancy have been proposed, including the presence of heterogeneous cell populations in gastric MALT lymphoma[11]; the partial volume effect of mucosal or small lesions of gastric MALT lymphoma detected by endoscopy[16,17]; and gastric MALT lymphoma existing in combination with DLBCL, or transformation into DLBCL during follow-up[25,26]. So, MALT lymphoma with emerging foci of intense 18F-FDG uptake are susceptible to conversion to DLBCL.
MALT lymphoma is generally considered to be a non-18F-FDG-avid type of lymphoma due to its small volume and indolent behavior[27]. Plasmacytic differentiation (PCD) has been recently suggested as an important factor influencing the detection rate of MALT lymphoma by 18F-FDG PET/CT[28]. In contrast, Tsukamoto et al[29] demonstrated no significant effect of PCD in MALT lymphoma detection. The differences among the studies could be explained by the different stages of the recruited cases, even among those with the same pathological subtype. Unfortunately, we could not confirm the critical role of PCD in 18F-FDG PET/CT evaluation of gastric MALT lymphoma due to the small population size and predominance of PCD in extragastric MALT lymphoma[29].
To facilitate comparison with gastric lymphoma, we did not further classify subgroups of gastric carcinoma (mucinous vs non-mucinous). As indicated by several previous studies, gastric mucinous and signet ring cell adenocarcinoma frequently present with significantly low 18F-FDG intensity due to low expression of glucose transporter-1 and low glucose metabolism. On the contrary, the non-mucinous adenocarcinoma subgroup of gastric carcinoma patients exhibited markedly higher FDG uptake compared with the mucinous adenocarcinoma subgroup because of higher metabolic activity[30].
SUV is a semi-quantitative measure of the normalized concentration of radioactivity in a lesion, and SUVmax is one of the most widely used parameters as an indicator of lesions with a high metabolic rate[31]. However, SUVmax is a single voxel value that shows the highest intensity of 18F-FDG uptake within the region of interest and may not represent total tumor metabolism[32]. Instead of SUVmax, volumetric parameters of metabolism such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) derived from 18F-FDG PET have been recently used for differential analysis, stage stratification survival analysis, and oncogenomic alteration for a variety of malignancies, such as pancreatic cancer[33], non-small-cell lung cancer[34] and head and neck cancer[35]. In view of the potential clinical value of MTV and TLG in the diagnostic performance of PET/CT, we will focus on differentiating gastric lymphoma from gastric carcinoma based on these volumetric parameters of metabolism, and perform a correlation analysis between prognosis and MTV or TLG.
On the basis of studies from Wu et al[18] and Fu et al[19], we further characterized the differences in 18F-FDG PET/CT findings between gastric lymphoma and gastric carcinoma and added significant information to the previous studies. First, a larger sample size in our study was an advantage. Second, in the analysis of associated clinicopathological features with SUVmax, we performed subgroup analysis based on sex, age, cell type and staging in gastric lymphoma and gastric carcinoma. In contrast, Wu et al[18] simply analyzed the difference in THKmax and SUVmax of the gastric wall lesions between patients with gastric lymphoma and gastric cancer, with and without extragastric involvement. Third, unlike Fu et al[19], who focused on the FDG intensity (SUVmax) of primary lesions and abnormalities detected by CT, including THKmax and mucosal ulcerations, our study was more comprehensive. We reviewed and analyzed the PET/CT features of gastric wall lesions including CT-detected abnormalities (THKmax and ulcerations), FDG avidity and involved region, pattern (focal/diffuse), and intensity (SUVmax). In addition, the correlation of SUVmax with gastric clinicopathological variables was investigated by χ2 test in our study. Finally, a ROC curve analysis was performed to determine the differential diagnostic value of SUVmax/THKmax in gastric lymphoma and gastric carcinoma.
Our study had some limitations. First, the small number of patients, in the gastric lymphoma group. Furthermore, we excluded one case of Burkitt’s lymphoma and one case of NK/T cell lymphoma to facilitate obtaining categorical data for the gastric lymphoma patients with different histological subtypes. Second, the retrospective nature of the present study could not completely rule out bias in the patient selection. In addition, the differences between our and a previous study[18] are explained by referring to the comparison of maximal thickness between gastric lymphoma and gastric carcinoma. Therefore, the results and conclusions of our study need to be verified by more prospective studies with a large population.
In conclusion, there were differences in 18F-FDG PET/CT features of gastric lymphoma compared with gastric carcinoma. The former predominantly presented with diffuse/segmental tracer uptake (type I and type II), whereas the latter showed mainly local tracer uptake (type III). Regarding 18F-FDG uptake intensity, measured by SUVmax or SUVmax/THKmax, a higher SUVmax and a larger SUVmax/THKmax suggest that gastric lymphoma is more likely. In addition, SUVmax/THKmax was a more reliable indicator for differentiation of gastric lymphoma from gastric carcinoma in comparison with SUVmax alone.
We would like to thank Xiao-Zhou Yu, Lei Zhu, Xiang Zhu, Wen-Chao Ma and Hui Huang in our department for their support and assistance in this study.
The usefulness of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) in a variety of malignancies is well established. However, the role of 18F-FDG PET/CT in gastric lymphomas or gastric carcinomas is challenging due to the physiologic FDG uptake in the stomach and difference in the level of tracer activity in various pathological subtypes.
To the best of our knowledge, there have been few studies focusing on the imaging differences between gastric lymphomas and gastric carcinomas using 18F-FDG PET/CT, and divergence also existed in some previous studies. The purpose of the present investigation was to distinguish gastric lymphomas from gastric carcinomas based on the characteristics of PET/CT evaluation for gastric wall lesions.
Gastric lymphomas predominantly presented with diffuse/segmental 18F-FDG uptake, whereas gastric carcinomas mainly with local uptake, and a higher SUVmax and a larger SUVmax/THKmax. Compared to SUVmax alone, SUVmax/THKmax showed a better performance in the differential diagnosis between gastric lymphomas and gastric carcinomas.
This study indicated significant differences in the 18F-FDG PET/CT features between gastric lymphomas and gastric carcinomas. The findings can help differentiate gastric lymphomas from gastric carcinomas based on the PET/CT evaluation.
18F-FDG PET/CT imaging and evaluation with SUVmax/THKmax parameters in gastric wall lesions were considered to be valuable in the differentiation of gastric lymphomas from gastric carcinomas.
This is an interesting study that compared differences in the PET/CT findings between the gastric lymphoma and gastric carcinoma.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lee SY, Yoshida H S- Editor: Ma YJ L- Editor: Ma JY E- Editor: Ma S
| 1. | Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252-260. [PubMed] |
| 2. | Chua SC, Rozalli FI, O’Connor SR. Imaging features of primary extranodal lymphomas. Clin Radiol. 2009;64:574-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Zucca E, Conconi A, Cavalli F. Treatment of extranodal lymphomas. Best Pract Res Clin Haematol. 2002;15:533-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Al-Akwaa AM, Siddiqui N, Al-Mofleh IA. Primary gastric lymphoma. World J Gastroenterol. 2004;10:5-11. [PubMed] |
| 5. | Psyrri A, Papageorgiou S, Economopoulos T. Primary extranodal lymphomas of stomach: clinical presentation, diagnostic pitfalls and management. Ann Oncol. 2008;19:1992-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Lu P. Staging and classification of lymphoma. Semin Nucl Med. 2005;35:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Kwee TC, Kwee RM, Nievelstein RA. Imaging in staging of malignant lymphoma: a systematic review. Blood. 2008;111:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 8. | Rodríguez-Vigil B, Gómez-León N, Pinilla I, Hernández-Maraver D, Coya J, Martín-Curto L. Positron emission tomography/computed tomography in the management of Hodgkin’s disease and non-Hodgkin’s lymphoma. Curr Probl Diagn Radiol. 2006;35:151-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Metser U, Goor O, Lerman H, Naparstek E, Even-Sapir E. PET-CT of extranodal lymphoma. AJR Am J Roentgenol. 2004;182:1579-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Ilica AT, Kocacelebi K, Savas R, Ayan A. Imaging of extranodal lymphoma with PET/CT. Clin Nucl Med. 2011;36:e127-e138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Radan L, Fischer D, Bar-Shalom R, Dann EJ, Epelbaum R, Haim N, Gaitini D, Israel O. FDG avidity and PET/CT patterns in primary gastric lymphoma. Eur J Nucl Med Mol Imaging. 2008;35:1424-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Phongkitkarun S, Varavithya V, Kazama T, Faria SC, Mar MV, Podoloff DA, Macapinlac HA. Lymphomatous involvement of gastrointestinal tract: evaluation by positron emission tomography with (18)F-fluorodeoxyglucose. World J Gastroenterol. 2005;11:7284-7289. [PubMed] |
| 13. | Yi JH, Kim SJ, Choi JY, Ko YH, Kim BT, Kim WS. 18F-FDG uptake and its clinical relevance in primary gastric lymphoma. Hematol Oncol. 2010;28:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Beal KP, Yeung HW, Yahalom J. FDG-PET scanning for detection and staging of extranodal marginal zone lymphomas of the MALT type: a report of 42 cases. Ann Oncol. 2005;16:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Hoffmann M, Kletter K, Becherer A, Jäger U, Chott A, Raderer M. 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) for staging and follow-up of marginal zone B-cell lymphoma. Oncology. 2003;64:336-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Hirose Y, Kaida H, Ishibashi M, Uozumi J, Arikawa S, Kurata S, Hayabuchi N, Nakahara K, Ohshima K. Comparison between endoscopic macroscopic classification and F-18 FDG PET findings in gastric mucosa-associated lymphoid tissue lymphoma patients. Clin Nucl Med. 2012;37:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Enomoto K, Hamada K, Inohara H, Higuchi I, Tomita Y, Kubo T, Hatazawa J. Mucosa-associated lymphoid tissue lymphoma studied with FDG-PET: a comparison with CT and endoscopic findings. Ann Nucl Med. 2008;22:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Wu J, Zhu H, Li K, Wang XG, Gui Y, Lu GM. (18)F-fluorodeoxyglucose positron emission tomography/computed tomography findings of gastric lymphoma: Comparisons with gastric cancer. Oncol Lett. 2014;8:1757-1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Fu L, Li H, Wang H, Xu B, Fan Y, Tian J. SUVmax/THKmax as a biomarker for distinguishing advanced gastric carcinoma from primary gastric lymphoma. PLoS One. 2012;7:e50914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Mochiki E, Kuwano H, Katoh H, Asao T, Oriuchi N, Endo K. Evaluation of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography for gastric cancer. World J Surg. 2004;28:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Moog F, Bangerter M, Diederichs CG, Guhlmann A, Merkle E, Frickhofen N, Reske SN. Extranodal malignant lymphoma: detection with FDG PET versus CT. Radiology. 1998;206:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 181] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 22. | Raanani P, Shasha Y, Perry C, Metser U, Naparstek E, Apter S, Nagler A, Polliack A, Ben-Bassat I, Even-Sapir E. Is CT scan still necessary for staging in Hodgkin and non-Hodgkin lymphoma patients in the PET/CT era? Ann Oncol. 2006;17:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 23. | Andriani A, Zullo A, Di Raimondo F, Patti C, Tedeschi L, Recine U, Caruso L, Bonanno G, Chiarenza A, Lizzani G. Clinical and endoscopic presentation of primary gastric lymphoma: a multicentre study. Aliment Pharmacol Ther. 2006;23:721-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Sharma P, Suman SK, Singh H, Sharma A, Bal C, Malhotra A, Kumar R. Primary gastric lymphoma: utility of 18F-fluorodeoxyglucose positron emission tomography-computed tomography for detecting relapse after treatment. Leuk Lymphoma. 2013;54:951-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Song KH, Yun M, Kim JH, Yang WI, Kang DR, Chung JB, Lee YC. Role of F-FDG PET Scans in Patients with Helicobacter pylori-Infected Gastric Low-Grade MALT Lymphoma. Gut Liver. 2011;5:308-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Tian J, Chen L, Wei B, Shao M, Ding Y, Yin D, Yao S. The value of vesicant 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) in gastric malignancies. Nucl Med Commun. 2004;25:825-831. [PubMed] |
| 27. | Elstrom R, Guan L, Baker G, Nakhoda K, Vergilio JA, Zhuang H, Pitsilos S, Bagg A, Downs L, Mehrotra A. Utility of FDG-PET scanning in lymphoma by WHO classification. Blood. 2003;101:3875-3876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 301] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 28. | Hoffmann M, Wöhrer S, Becherer A, Chott A, Streubel B, Kletter K, Raderer M. 18F-Fluoro-deoxy-glucose positron emission tomography in lymphoma of mucosa-associated lymphoid tissue: histology makes the difference. Ann Oncol. 2006;17:1761-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 29. | Tsukamoto N, Kojima M, Hasegawa M, Oriuchi N, Matsushima T, Yokohama A, Saitoh T, Handa H, Endo K, Murakami H. The usefulness of (18)F-fluorodeoxyglucose positron emission tomography ((18)F-FDG-PET) and a comparison of (18)F-FDG-pet with (67)gallium scintigraphy in the evaluation of lymphoma: relation to histologic subtypes based on the World Health Organization classification. Cancer. 2007;110:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Kawamura T, Kusakabe T, Sugino T, Watanabe K, Fukuda T, Nashimoto A, Honma K, Suzuki T. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92:634-641. [PubMed] |
| 31. | Paidpally V, Chirindel A, Lam S, Agrawal N, Quon H, Subramaniam RM. FDG-PET/CT imaging biomarkers in head and neck squamous cell carcinoma. Imaging Med. 2012;4:633-647. [PubMed] |
| 32. | Moon SH, Hyun SH, Choi JY. Prognostic significance of volume-based PET parameters in cancer patients. Korean J Radiol. 2013;14:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Shi S, Ji S, Qin Y, Xu J, Zhang B, Xu W, Liu J, Long J, Liu C, Liu L. Metabolic tumor burden is associated with major oncogenomic alterations and serum tumor markers in patients with resected pancreatic cancer. Cancer Lett. 2015;360:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Im HJ, Pak K, Cheon GJ, Kang KW, Kim SJ, Kim IJ, Chung JK, Kim EE, Lee DS. Prognostic value of volumetric parameters of (18)F-FDG PET in non-small-cell lung cancer: a meta-analysis. Eur J Nucl Med Mol Imaging. 2015;42:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 35. | Pak K, Cheon GJ, Nam HY, Kim SJ, Kang KW, Chung JK, Kim EE, Lee DS. Prognostic value of metabolic tumor volume and total lesion glycolysis in head and neck cancer: a systematic review and meta-analysis. J Nucl Med. 2014;55:884-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |