Published online Sep 7, 2016. doi: 10.3748/wjg.v22.i33.7500
Peer-review started: April 4, 2016
First decision: May 12, 2016
Revised: May 30, 2016
Accepted: June 28, 2016
Article in press: June 28, 2016
Published online: September 7, 2016
Processing time: 159 Days and 2.1 Hours
Split liver transplantation (SLT), while widely accepted in pediatrics, remains underutilized in adults. Advancements in surgical techniques and donor-recipient matching, however, have allowed expansion of SLT from utilization of the right trisegment graft to now include use of the hemiliver graft as well. Despite less favorable outcomes in the early experience, better outcomes have been reported by experienced centers and have further validated the feasibility of SLT. Importantly, more than two decades of experience have identified key requirements for successful SLT in adults. When these requirements are met, SLT can achieve outcomes equivalent to those achieved with other types of liver transplantation for adults. However, substantial challenges, such as surgical techniques, logistics, and ethics, persist as ongoing barriers to further expansion of this highly complex procedure. This review outlines the current state of SLT in adults, focusing on donor and recipient selection based on physiology, surgical techniques, surgical outcomes, and ethical issues.
Core tip: Split liver transplantation (SLT) in adults is usually performed with the right trisegment graft or less frequently with the hemiliver graft. Both graft types require highly complex surgical techniques. Compared with the right trisegment graft, hemiliver SLT requires stricter donor and recipient selection to prevent graft dysfunction associated with size-mismatch. To achieve ideal graft-recipient paring, a clear understanding of surgical anatomy and recipient physiology is needed. With favorable circumstances, outcomes of adult SLT can be comparable to whole liver transplantation. The routine use of SLT, however, remains controversial due to various challenges, particularly under the current “sickest first” liver allocation policy.
- Citation: Hashimoto K, Fujiki M, Quintini C, Aucejo FN, Uso TD, Kelly DM, Eghtesad B, Fung JJ, Miller CM. Split liver transplantation in adults. World J Gastroenterol 2016; 22(33): 7500-7506
- URL: https://www.wjgnet.com/1007-9327/full/v22/i33/7500.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i33.7500
Liver transplantation using partial grafts was born in the late 1980’s as a rescue modality for a severe pediatric donor shortage. In 1984, Bismuth et al[1] described a new technique to decrease the size of an adult liver to fit a pediatric recipient. After successful experiences with this procedure, a new technique of “splitting” a whole liver graft was successfully introduced, allowing the simultaneous transplant of two recipients from one deceased donor liver[2-4]. Unlike reduced-size grafts, split liver transplantation (SLT) was initially characterized by higher morbidity and mortality[4,5]. Over time, however, technical advancements and better donor-recipient selection have led to more frequent use of SLT and better outcomes.
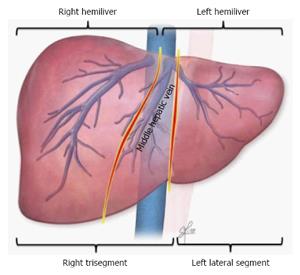
In SLT, deceased donor livers most commonly are split into a smaller left lateral segment (segment II and III) for children and a larger right trisegment (segment I, IV-VIII) for adults (Figure 1). This combination has contributed tremendously to the reduction of pediatric waiting list mortality[6]. Gains in knowledge have introduced the use of 2 hemiliver grafts, a left lobe (segment I-IV) and a right lobe (segment V-VIII), for transplant in 2 adults or adult-sized recipients (Figure 1). Although hemiliver SLT theoretically doubles the number of liver grafts for adults, this technique has been underutilized due to technical, logistical, and ethical challenges[7]. Further advancement of SLT for adults requires a full understanding of the current state of SLT, focusing on the unique aspects of partial grafting from deceased donors. This review outlines existing practice in adult SLT, including donor and recipient matching, surgical techniques, and outcomes. Finally, ethical issues of adult SLT will be discussed, including how to justify SLT vs whole liver graft transplant and in what situations SLT provides the best benefits under the current liver allocation system guided by the Model for End-Stage Liver Disease (MELD) score.
Careful donor selection and thorough consideration of split graft quality are essential in adult SLT. The upper donor age limit for SLT generally is considered to be between 40 and 50 years of age[8-10]. Prolonged ICU stay before organ recovery is unfavorable, but not a contraindication. Donor liver enzymes should be normal or mildly elevated[11,12]. If other risk factors are absent, split grafts with higher values of liver enzymes can be used[9,10]. While the impact of donor hypernatremia remains unknown, it can be unfavorable. The presence of obesity, history of heavy alcohol use, and low platelet counts upon donor admission could be a surrogate for hidden negative pathophysiology such as graft steatosis and fibrosis. The use of vasopressor to maintain donor hemodynamics can increase the risk of poor graft quality. Despite the lack of scientific evidence, these factors seem to be important to determine whether the liver is suitable for SLT.
During organ recovery, visual and manual evaluation by the donor team is of utmost importance. In the presence of abnormal visualization, a liver biopsy should be performed to rule out any pathology including macrosteatosis, inflammation, fibrosis, and cholestasis. When other donor and recipient factors are ideal, the presence of mild steatosis or inflammation is acceptable. Once the decision is made to proceed with splitting, coordination between donor and recipient teams is crucial in order to minimize cold ischemia time, which is the only modifiable donor factor.
Once a donor liver is deemed to be splittable, choosing and matching an appropriate recipient is extremely important. Hemiliver SLT for adult recipients carries the potential risk of graft failure due to size mismatch, but with a right trisegment graft, graft size does not usually influence surgical outcomes. Recipient selection, therefore, can be more liberal with SLT utilizing a right trisegment graft than when hemiliver grafts are used[7]. Equally important, a right trisegment graft provides venous outflow similar to a whole liver graft and will generally tolerate portal hypertension in recipients[7]. On the other hand, recipient selection for hemiliver SLT requires more comprehensive assessment. Generally, teenagers or small adults with minimal portal hypertension are ideal recipients for hemiliver grafts. The use of hemiliver grafts for high-risk recipients, such as those with high MELD scores or severe portal hypertension, remains controversial[13]. Larger grafts should be used for recipients with severe portal hypertension in order to avoid small-for-size syndrome[10].
For living donor liver transplantation (LDLT), in order to meet a recipient’s metabolic demand, the minimal graft size has been reported to be as small as a graft-to-recipient weight ratio (GRWR) of 0.6%-0.8%[14,15]. In contrast, the acceptable minimal graft size in adult SLT is unknown. Because split grafts have often experienced prolonged cold ischemia and brain death-related hemodynamic instability, recipients receiving split grafts appear to require a higher GRWR[16]. Lee et al[9] reported that a GRWR of 1.0% was the minimal requirement in hemiliver SLT to avoid early graft dysfunction. To achieve such graft-recipient matching, split grafts should be taken from larger donors and transplanted into smaller recipients[10].
Graft size estimation is crucial in hemiliver SLT. Since liver imaging is rarely available in deceased donors, graft size estimation usually relies on standard calculation formulas using donor body surface area or body weight: whole liver volume (mL) = 1072.8 × body surface area (m2) - 345.7 for Caucasians[17] and 706.2 × body surface area (m2) + 2.4 for Asians[18]. More simply, whole liver weight can be estimated as 2% of donor body weight[19]. Lobe size can be determined based on standard lobar distribution, approximately 35% for the left lobe and 65% for the right lobe. It should be noted that since these estimations are not always accurate, graft weight can be underestimated to increase the risk of small-for-size related graft failure. Therefore, direct assessment by donor surgeons is quite important.
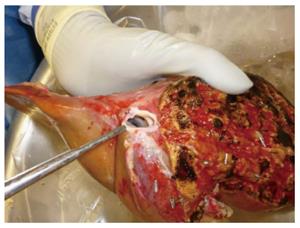
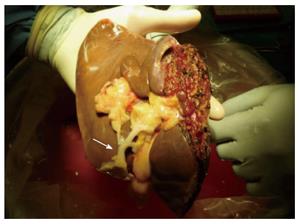
Lack of consensus regarding sharing patterns of major vessels and bile ducts between 2 split grafts, particularly when a liver is shared by 2 different centers, is one of the most important technical challenges facing SLT. The ideal and most favorable sharing pattern was originally described by Bismuth et al[3] in 1989. The principle concept of this sharing pattern is its avoidance of multiple small branches that would need to be reconstructed in recipients. Impeccable knowledge of surgical liver anatomy is crucial to understand why this sharing pattern is ideal in SLT. The left lobe frequently has a single branch of the portal vein, hepatic duct, and venous outflow that is a common channel of the left and middle hepatic veins (Figure 2), but multiple branches of small hepatic arteries often exist. On the other hand, the right lobe often has a single right hepatic artery, but multiple branches are commonly seen in the venous drainage, hepatic duct, and portal vein. According to the original sharing pattern by Bismuth, the left-sided graft retains the celiac trunk leaving a single right hepatic artery in the right-sided graft in order to avoid multiple small branches of hepatic artery in the left-sided graft (Figure 3). Then, the right-sided graft retains the remaining main branches, including the common hepatic duct, main portal vein, and vena cava[3]. Such a sharing pattern can lower the risk of surgical complications by avoiding multiple complex anastomoses. In current clinical practice, however, the primary transplant team often prefers to keep all main branches without consideration of actual donor anatomy or recipient needs, even leaving small multiple branches in the contralateral graft. While the primary team has the priority to keep main branches, the final decision should be made with flexibility based on donor anatomy and recipient need[10]. Such comprehensive sharing by 2 teams facilitates increased use of split grafts and improves recipient outcomes.
SLT is a unique operation that requires establishing 2 complete sets of vascular inflow and outflow as well as biliary drainage from one liver graft. SLT organ recovery requires highly complex surgical techniques. Detailed techniques of in situ splitting are described previously[20]. The first and most important step for successful SLT is the capability of donor team to make a timely and reliable decision about whether to proceed with splitting. In order to achieve this, the donor team needs to fully understand the recipient situation, including body size, medical urgency, severity of portal hypertension, and surgical anatomy. With visualization of the donor liver, careful assessment of suitability for SLT should be conducted in terms of size, quality, and anatomy. Intraoperative cholangiogram is mandatory to determine splittability. Second, donor operation time should be minimized because of frequent hemodynamic instability in brain dead donors and to avoid compromising graft quality of other organs to be recovered. Hepatic hilar dissection also should be minimized, except for anatomical assessment, because this step can be performed safely on the back table. Liver hanging maneuver is effective for in situ parenchymal transection[20,21]. It is important to have a low threshold to cross clamp in case the donor becomes unstable during in situ splitting, necessitating a switch to the ex vivo technique. Finally, complex back table procedures include the division of vessels and bile duct and venous reconstruction to facilitate venous drainage of the anterior segment in the right lobe graft[20].
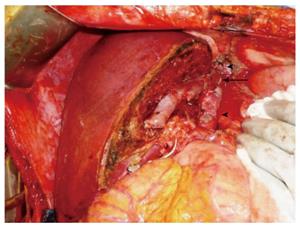
Excellent venous outflow is essential for successful SLT. Since the right trisegment graft usually retains the entire vena cava, caval anastomosis can be done with either the piggyback or the standard technique, as is done in whole liver transplantation. Such anatomical advantage promises excellent venous outflow. In SLT using the left hemiliver, our standard technique at Cleveland Clinic uses the common channel of the left and middle hepatic veins anastomosed to the recipient venous cuff created with all 3 hepatic veins as the piggyback technique in whole liver transplantation (Hashimoto, unpublished data). This technique promises excellent venous outflow. When the vena cava is retained with the right hemiliver graft, excellent venous outflow can be achieved with a new middle hepatic vein draining into the donor vena cava (Figure 4). When the vena cava is not retained with the right hemiliver graft, a complex venous reconstruction is necessary, as is done with LDLT. Portal inflow should be modified in split grafts of marginal size[22]. Splenic artery ligation, splenectomy, and hemi-portocaval shunt are well known techniques for portal inflow modification. Of these, the use of hemi-portocaval shunt is controversial because of increased risk of portal steal phenomenon[23]. In biliary reconstruction, unnecessary tissue dissection disrupts blood supply to the recipient bile duct and increases the risk of biliary ischemia, bile leak and stricture. Thus, the minimal dissection technique utilized in LDLT should be used for SLT to optimize blood supply to the recipient bile duct, particularly when choledococholedocostomy is performed[24].
SLT using the right trisegment graft initially had an increased risk of morbidity and mortality in adult recipients[4,5]. While surgical outcomes have improved with experience, outcomes for right trisegment graft transplantation are still controversial[25]. Due to the procedure’s technical complexity, the incidence of biliary and vascular complications can be as high as 40% and 25%, respectively. However, when multiple risk factors are avoided (short ischemia time, non-urgent recipient status, young donor age, etc.), the right trisegment graft can achieve excellent outcomes and is no longer considered to be marginal by experienced centers[26,27].
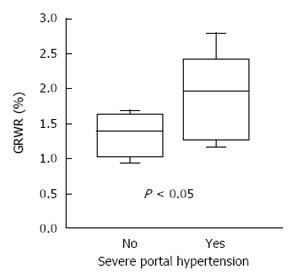
Data of hemiliver SLT for 2 adults are limited. Aseni et al[28] reported a recent Italian multicenter experience of hemiliver SLT, showing inferior 5-year survival compared to whole liver transplantation (63% vs 83%). However, under certain circumstances, long-term survival after hemiliver SLT is equivalent to whole liver transplantation or LDLT[9,10,12,29]. Importantly, the impact of graft size on survival seems to be more prominent in hemiliver SLT compared to the right trisegment graft. Accordingly, appropriate graft-recipient selection is critical to avoid small-for-size grafting and to promote optimal outcomes. As mentioned in Recipient selection, when a GRWR is greater than 1.0%, hemiliver graft survival appears to be favorable[9]. Our experience at Cleveland Clinic also demonstrates that avoiding smaller grafts for recipients with severe portal hypertension facilitates desirable outcomes (Figure 5)[10]. This strategy increases safety and effectiveness of hemiliver grafts and could result in wider application of hemiliver SLT.
The small-for-size grafts that can result from SLT, particularly hemiliver grafts, often receive excessive portal flow, which causes hepatic arterial spasm via hepatic arterial buffer response[30,31]. Importantly, this may increase the risk of hepatic artery thrombosis[31]. Such arterial spasm can cause poor blood supply to the graft biliary system, resulting in an increased risk of biliary complications[32]. Another important surgical risk is early graft failure due to graft-recipient size mismatch. When a small-for-size graft is used for a recipient with severe portal hypertension, modification of the portal inflow may be necessary to prevent graft failure. If this occurs, early retransplantation should be considered before the onset of renal failure or sepsis.
Creating two extended criteria split grafts from a standard criteria whole liver raises a variety of ethical issues[33,34]. Since partial grafting per se is a risk factor for graft failure[35], one ethical issue is whether it is best to proceed with SLT or wait for a smaller whole liver graft. To justify the use of split grafts, SLT needs to show similar or better outcomes compared to whole liver transplantation, as LDLT has been able to demonstrate[36]. Unfortunately, SLT is not yet considered the standard of care for adult recipients, but it does potentially give recipients greater opportunity for a life-saving transplant. Given unanswered ethical questions, however, recipients should have the unequivocal right to refuse a split graft with complete and accurate national and center-specific information. Thorough discussion of the risks and benefits of SLT with transplant candidates should take place at the time of evaluation, listing, and organ offer[37].
The use of split grafts for high MELD recipients is controversial[10,13]. Under the philosophy of the “sickest first” liver allocation, splittable donors are often allocated to those with a high MELD score who are generally unsuitable for SLT. When a donor liver is splittable, the best reason to proceed with SLT is when a primary recipient is too small to receive a large whole donor liver. Since small adult candidates are often bypassed on the waiting list when a large donor becomes available, SLT can overcome the large-for-size mismatch and increase opportunity for transplantation for these candidates. For small recipients, split grafts can provide enough liver volume to tolerate portal hyperperfusion, which is considered to be one of the major factors resulting in small-for-size related graft failure. According to our experience, after the primary recipient is transplanted, the leftover split graft can be used safely and effectively for the secondary recipient with similar outcomes[10]. While this graft-recipient combination helps achieve excellent survival after SLT, such ideal matching rarely happens under the MELD allocation. Even with ideal matching, various challenges and higher complication rates result in the underutilization of split grafts, particularly when hemiliver SLT is indicated.
SLT is an important technique to increase the availability of livers for adults in need of life-saving liver transplantation. As experience has grown worldwide, resulting in technical advancements and better donor-recipient matching, this highly complex surgical technique has become more feasible and has achieved excellent outcomes. However, the routine application of adult SLT will only be possible when certain challenges are addressed and resolved. While ideal donor-recipient matching is hindered under the current “sickest first” liver allocation, patients can still benefit from SLT under certain circumstances. Continued experience and advancement of SLT will better define the role of SLT in addressing the current severe donor shortage and reducing wait list mortality in adults.
The authors would like to acknowledge Ms. Sally Garrett Karyo for her assistance.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ozsoy M, Sonzogni A S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Bismuth H, Houssin D. Reduced-sized orthotopic liver graft in hepatic transplantation in children. Surgery. 1984;95:367-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 2. | Pichlmayr R, Ringe B, Gubernatis G, Hauss J, Bunzendahl H. [Transplantation of a donor liver to 2 recipients (splitting transplantation)--a new method in the further development of segmental liver transplantation]. Langenbecks Arch Chir. 1988;373:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 347] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Bismuth H, Morino M, Castaing D, Gillon MC, Descorps Declere A, Saliba F, Samuel D. Emergency orthotopic liver transplantation in two patients using one donor liver. Br J Surg. 1989;76:722-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 182] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Emond JC, Whitington PF, Thistlethwaite JR, Cherqui D, Alonso EA, Woodle IS, Vogelbach P, Busse-Henry SM, Zucker AR, Broelsch CE. Transplantation of two patients with one liver. Analysis of a preliminary experience with ‘split-liver’ grafting. Ann Surg. 1990;212:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Broelsch CE, Emond JC, Whitington PF, Thistlethwaite JR, Baker AL, Lichtor JL. Application of reduced-size liver transplants as split grafts, auxiliary orthotopic grafts, and living related segmental transplants. Ann Surg. 1990;212:368-375; discussion 375-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 426] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 6. | Busuttil RW, Goss JA. Split liver transplantation. Ann Surg. 1999;229:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 227] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Hashimoto K, Eghtesad B. Split liver transplantation. Contemporary liver transplantation. Switzerland: Springer 2016; . |
| 8. | Emre S, Umman V. Split liver transplantation: an overview. Transplant Proc. 2011;43:884-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Lee WC, Chan KM, Chou HS, Wu TJ, Lee CF, Soong RS, Wu TH, Lee CS. Feasibility of split liver transplantation for 2 adults in the model of end-stage liver disease era. Ann Surg. 2013;258:306-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Hashimoto K, Quintini C, Aucejo FN, Fujiki M, Diago T, Watson MJ, Kelly DM, Winans CG, Eghtesad B, Fung JJ. Split liver transplantation using Hemiliver graft in the MELD era: a single center experience in the United States. Am J Transplant. 2014;14:2072-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Humar A, Ramcharan T, Sielaff TD, Kandaswamy R, Gruessner RW, Lake JR, Payne WD. Split liver transplantation for two adult recipients: an initial experience. Am J Transplant. 2001;1:366-372. [PubMed] |
| 12. | Broering DC, Wilms C, Lenk C, Schulte am Esch J, Schönherr S, Mueller L, Kim JS, Helmke K, Burdelski M, Rogiers X. Technical refinements and results in full-right full-left splitting of the deceased donor liver. Ann Surg. 2005;242:802-812, discussion 812-813. [PubMed] |
| 13. | Nadalin S, Schaffer R, Fruehauf N. Split-liver transplantation in the high-MELD adult patient: are we being too cautious? Transpl Int. 2009;22:702-706. [PubMed] |
| 14. | Kaido T, Mori A, Ogura Y, Hata K, Yoshizawa A, Iida T, Yagi S, Uemoto S. Lower limit of the graft-to-recipient weight ratio can be safely reduced to 0.6% in adult-to-adult living donor liver transplantation in combination with portal pressure control. Transplant Proc. 2011;43:2391-2393. [PubMed] |
| 15. | Selzner M, Kashfi A, Cattral MS, Selzner N, Greig PD, Lilly L, McGilvray ID, Therapondos G, Adcock LE, Ghanekar A. A graft to body weight ratio less than 0.8 does not exclude adult-to-adult right-lobe living donor liver transplantation. Liver Transpl. 2009;15:1776-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Pratschke J, Wilhelm MJ, Kusaka M, Basker M, Cooper DK, Hancock WW, Tilney NL. Brain death and its influence on donor organ quality and outcome after transplantation. Transplantation. 1999;67:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 255] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Heinemann A, Wischhusen F, Püschel K, Rogiers X. Standard liver volume in the Caucasian population. Liver Transpl Surg. 1999;5:366-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 194] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, Momose Y, Komiyama A, Makuuchi M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 703] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 19. | Lee SG. Living-donor liver transplantation in adults. Br Med Bull. 2010;94:33-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Hashimoto K, Fung J. In situ liver splitting. Abdominal organ retrieval and transplantation bench surgery. Oxford: Wiley-Blackwell 2013; 101-115. |
| 21. | Liddo G, Buc E, Nagarajan G, Hidaka M, Dokmak S, Belghiti J. The liver hanging manoeuvre. HPB (Oxford). 2009;11:296-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Boillot O, Delafosse B, Méchet I, Boucaud C, Pouyet M. Small-for-size partial liver graft in an adult recipient; a new transplant technique. Lancet. 2002;359:406-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 23. | Lee SG. A complete treatment of adult living donor liver transplantation: a review of surgical technique and current challenges to expand indication of patients. Am J Transplant. 2015;15:17-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 268] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 24. | Soejima Y, Fukuhara T, Morita K, Yoshizumi T, Ikegami T, Yamashita Y, Sugimachi K, Taketomi A, Maehara Y. A simple hilar dissection technique preserving maximum blood supply to the bile duct in living donor liver transplantation. Transplantation. 2008;86:1468-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 25. | Mallik M, Callaghan CJ, Hope M, Gibbs P, Davies S, Gimson AE, Griffiths WJ, Pettigrew GJ. Comparison of liver transplantation outcomes from adult split liver and circulatory death donors. Br J Surg. 2012;99:839-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 26. | Corno V, Colledan M, Dezza MC, Guizzetti M, Lucianetti A, Maldini G, Pinelli D, Giovanelli M, Zambelli M, Torre G. Extended right split liver graft for primary transplantation in children and adults. Transpl Int. 2006;19:492-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Maggi U, De Feo TM, Andorno E, Cillo U, De Carlis L, Colledan M, Burra P, De Fazio N, Rossi G. Fifteen years and 382 extended right grafts from in situ split livers in a multicenter study: Are these still extended criteria liver grafts? Liver Transpl. 2015;21:500-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Aseni P, De Feo TM, De Carlis L, Valente U, Colledan M, Cillo U, Rossi G, Mazzaferro V, Donataccio M, De Fazio N. A prospective policy development to increase split-liver transplantation for 2 adult recipients: results of a 12-year multicenter collaborative study. Ann Surg. 2014;259:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Zambelli M, Andorno E, De Carlis L, Rossi G, Cillo U, De Feo T, Carobbio A, Giacomoni A, Bottino G, Colledan M. Full-right-full-left split liver transplantation: the retrospective analysis of an early multicenter experience including graft sharing. Am J Transplant. 2012;12:2198-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Demetris AJ, Kelly DM, Eghtesad B, Fontes P, Wallis Marsh J, Tom K, Tan HP, Shaw-Stiffel T, Boig L, Novelli P. Pathophysiologic observations and histopathologic recognition of the portal hyperperfusion or small-for-size syndrome. Am J Surg Pathol. 2006;30:986-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 31. | Quintini C, Hirose K, Hashimoto K, Diago T, Aucejo F, Eghtesad B, Vogt D, Pierce G, Baker M, Kelly D. “Splenic artery steal syndrome” is a misnomer: the cause is portal hyperperfusion, not arterial siphon. Liver Transpl. 2008;14:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Hashimoto K, Miller CM, Quintini C, Aucejo FN, Hirose K, Uso TD, Trenti L, Kelly DM, Winans CG, Vogt DP. Is impaired hepatic arterial buffer response a risk factor for biliary anastomotic stricture in liver transplant recipients? Surgery. 2010;148:582-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Vulchev A, Roberts JP, Stock PG. Ethical issues in split versus whole liver transplantation. Am J Transplant. 2004;4:1737-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Collett D, O’Neill J, Neuberger J. Splitting livers - balancing the gain and the pain. Transpl Int. 2008;21:218-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1486] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 36. | Freise CE, Gillespie BW, Koffron AJ, Lok AS, Pruett TL, Emond JC, Fair JH, Fisher RA, Olthoff KM, Trotter JF. Recipient morbidity after living and deceased donor liver transplantation: findings from the A2ALL Retrospective Cohort Study. Am J Transplant. 2008;8:2569-2579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 236] [Cited by in RCA: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 37. | Organ Procurement and Transplantation Network. Ethics - Split versus whole liver transplantation. Available from: https://optn.transplant.hrsa.gov/resources/ethics/split-versus-whole-liver-transplantation/. |