Published online Sep 7, 2016. doi: 10.3748/wjg.v22.i33.7463
Peer-review started: April 10, 2016
First decision: May 12, 2016
Revised: June 30, 2016
Accepted: July 20, 2016
Article in press: July 20, 2016
Published online: September 7, 2016
Processing time: 148 Days and 3.6 Hours
The role of bile acids in colorectal cancer has been well documented, but their role in pancreatic cancer remains unclear. In this review, we examined the risk factors of pancreatic cancer. We found that bile acids are associated with most of these factors. Alcohol intake, smoking, and a high-fat diet all lead to high secretion of bile acids, and bile acid metabolic dysfunction is a causal factor of gallstones. An increase in secretion of bile acids, in addition to a long common channel, may result in bile acid reflux into the pancreatic duct and to the epithelial cells or acinar cells, from which pancreatic adenocarcinoma is derived. The final pathophysiological process is pancreatitis, which promotes dedifferentiation of acinar cells into progenitor duct-like cells. Interestingly, bile acids act as regulatory molecules in metabolism, affecting adipose tissue distribution, insulin sensitivity and triglyceride metabolism. As a result, bile acids are associated with three risk factors of pancreatic cancer: obesity, diabetes and hypertriglyceridemia. In the second part of this review, we summarize several studies showing that bile acids act as cancer promoters in gastrointestinal cancer. However, more question are raised than have been solved, and further oncological and physiological experiments are needed to confirm the role of bile acids in pancreatic cancer carcinogenesis.
Core tip: Bile acids bridge the gap between risk factors and pancreatic cancer, providing a new horizon in pancreatic cancer carcinogenesis.
- Citation: Feng HY, Chen YC. Role of bile acids in carcinogenesis of pancreatic cancer: An old topic with new perspective. World J Gastroenterol 2016; 22(33): 7463-7477
- URL: https://www.wjgnet.com/1007-9327/full/v22/i33/7463.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i33.7463
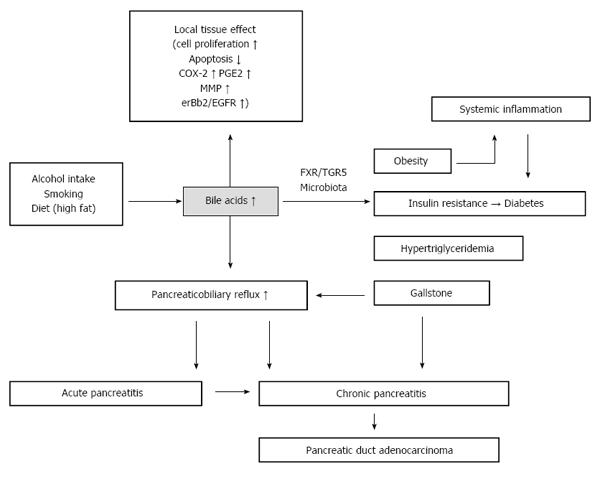
Pancreatic cancer mortality is the fourth leading cause of cancer deaths in males and females and accounts for 7% of all deaths in cancer patients[1]. Therapeutic strategies for these cancers are well developed, but the death rates of pancreatic cancer have remained stable from 1930 to 2011 due to delayed diagnosis and elusive mechanisms of cancer initiation and progression. Pancreas is a retroperitoneal organ, located behind the stomach and in front of the spine. Because of the relatively large space around this organ, pancreatic tumors do not generally cause obstructive symptoms or pain. Moreover, the pancreas contains two types of cells, exocrine and endocrine cells. Most pancreatic tumors are pancreatic duct adenocarcinomas, which originate in exocrine cells, with no changes in hormone secretion. Therefore, early diagnosis of pancreatic cancer is difficult due to a lack of symptoms. Most pancreatic cancer is diagnosed at a late, inoperable, and incurable stage. Scientists have sought to identify early diagnostic markers and to elucidate the underlying mechanisms of pancreatic cancer initiation and progression. Etiological studies have identified a number of risks for developing pancreatic cancer, including (1) alcohol intake; (2) smoking; (3) diet (high-fat and red meat); (4) obesity; (5) diabetes; (6) gallstones; (7) long common channel of the biliary duct and the pancreatic duct; (8) chronic pancreatitis; (9) hypertriglyceridemia; and (10) other risks, including age and sex, race (black population), non-O blood type, autoimmune disease, hereditary pancreatitis, and infectious disease[2]. Notably, 60% of pancreatic cancers occur in the head of the pancreas[3], which is close to the bile tracts, suggesting that bile acids may play a role in pancreatic cancer formation[4,5]. Bile acids were first proposed as a carcinogen in the 1940s[4]. Since then, increasing evidence has shown that bile acids, particularly secondary bile acids, play important roles in the carcinogenesis in gastrointestinal cancers[4] and breast cancer[6]. We review the systemic and local effects of bile acids in pancreatic cancer initiation and progression and propose that bile acids have key roles in different metabolic and oncogenic pathways (Figure 1).
A large body of evidence has shown that alcohol intake significantly increases blood and intestinal bile acids levels[7,8]. Alcohol induces bile acid secretion via two pathways[9]. First, alcohol increases cholesterol 7α-hydroxylase synthesis, rather than directly activating the enzyme[10]. Second, alcohol has an inhibitory effect on gallbladder contraction, leading to a decrease in the amount of bile acid moving into the duodenum. Subsequently, enterohepatic circulation of bile acids is interrupted, resulting in reduced feedback inhibition of bile acid synthesis. Long-term alcohol intake results in prolonged low-dose exposure of the pancreatic epithelial cells to bile acids, which activate intracellular signaling pathways. Equilibrium of the alcohol-bile acids-microbiome axis must be taken into account in the relationship between bile acids and alcohol intake. After consumption of alcohol, fecal deoxycholic acid (DCA), one type of secondary bile acid, increased 3-4 times that of the control groups[7]. Secondary bile acids play an important role in shaping the gut microbiome[11], which is critical for the gut barrier. Additionally, the acute effects of alcohol administration directly impair the duodenum and jejunum barrier[12]. Gut barrier injury leads to changes in gut permeability, resulting in an increase in serum DCA levels and systemic inflammation[13].
Epidemiological and clinical studies have indicated that smoking is a risk factor for pancreatic cancer. Recent reports have shown that nicotine stimulates mutated K-ras activation, as well as other mutations associated with pancreatic cancer, including those in p53, COX-2, SMAD4 and p16INK4A[14,15]. However, little is known about the mechanisms of how smoking causes gene mutation and pancreatic cancer formation. Bile acid concentration in the stomach of smokers is significantly higher than that in non-smokers, and this trend is found even when not actually smoking[16]. Additionally, nicotine induces gastric acid secretion, leading to a significant drop in the pH of the stomach[17]. Gastric acid is a strong regulator of the secretion of bile acids. However, bile acid reflux into the pancreatic duct is associated with intraductal papillary carcinoma in the pancreas[18]. Further investigations are still needed to determine whether smoking also leads to bile acid reflux to the pancreatic duct. Overall, the above findings are not convincing evidence of the association between smoking and pancreatic cancer initiation. The local effects of bile acids, which are induced by smoking, on pancreatic cancer formation may be overestimated, but nicotine may act on pancreatic cells via blood circulation delivery.
Little is known about how diet is associated with cancer formation, partly because there is high variation in diets. The basic function of bile acids is to promote the absorption of dietary fat and help absorb fat-soluble vitamins, as well as to regulate cholesterol metabolism. Dietary fat, which is the strongest regulator, induces secretion of bile acids into the duodenum, resulting in an elevated fecal bile acid concentration. Vegetables and carbohydrates, which do not induce secretion of bile acids, are not associated with pancreatic cancer[19]. Approximately 95% of bile acids are reabsorbed into the intestine and transported to the liver. During this process, bile acids also escape into blood circulation. Studies have shown that the plasma bile acid concentration is correlated with the fecal concentration[20] due to intestinal epithelial cell exposure to bile acids. Accumulating evidence has shown that excess bile acids are associated with colon cancer initiation. However, the pancreas does not directly contact bile acids. How diet-induced bile acids promote pancreatic cancer formation, through local effects (by bile reflux) or though systemic effects (by circulation), remains unknown.
Metabolic syndrome includes the following disorders: abdominal obesity, hypertension, hyperglycemia, hypertriglyceridemia, and low serum high-density protein. Metabolic syndrome and prediabetes share the same disorders. Thus, we here discuss obesity, diabetes and hypertriglyceridemia at the same time. Possible mechanisms linking obesity and cancer include: (1) Insulin or insulin-related growth factors (IGF); (2) microbiome; (3) chronic inflammation; (4) sex hormones; (5) circulating adipokines; and (6) white adipose tissue-derived progenitor cells[21]. Type 2 diabetes is cause by insulin resistance, with hyperinsulinemia. Approximately half of individuals with diabetes are obese[22], and up to 60% of diabetes cases are caused by obesity[23]. A recent study revealed that type 2 diabetes results from chronic inflammation caused by obesity[24]. Above all, when deeply studying the mechanism of these two diseases, it is difficult to identify which is the original metabolic defect, hyperinsulinemia or insulin resistance, and which is secondary. We hypothesize that hyperinsulinemia is the original defect[25]. In parallel, obesity is a complex and multifactorial metabolic disease. Here, we only discuss diet-induced obesity and review several bile acid-related factors.
In addition to the important role of bile acids in nutrient absorption, accumulating evidence indicates that bile acids play key roles in glucose and lipid metabolism. The concentration of deoxycholic acid, a secondary bile acid, is elevated in type 2 diabetes, along with elevation of the hydrophobic 12a-hydroxylated bile acids[26]. In vivo, ob/ob mice also had elevated plasma bile acids[27]. Bariatric surgery[28] and bile acid binding resins improve insulin resistance[29] and ameliorate obesity and type 2 diabetes, indicating that changes in bile acid flow or compositions promote remission of metabolic disorders. The dominant type of bariatric surgery is Roux-en-Y gastric bypass (RYGB)[28], which alters bile acid flow and re-absorption by changing the anatomy of the intestine. Plasma primary bile acids, including chenodeoxycholic acid (CDCA), and cholic acid (CA), increased after surgery, along with increased taurine-conjugated and glycine-conjugated bile acids[30], which indicated that re-absorption increases in the upper intestine. Consequently, fewer bile acids reached the distal intestine, resulting in decreased secondary bile acid pools. Bile acid binding resins predominantly function by decreasing bile acids in the intestine and by blocking re-absorption of bile acids, which limits the total bile acid pool. In other words, both bariatric surgery and bile acid binding resins promote primary bile acid synthesis and re-absorption and limit secondary bile acid synthesis and their concentration in plasma.
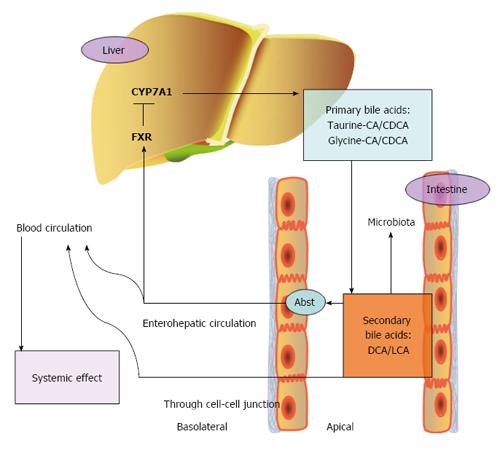
Farnesoid X nuclear receptor (FXR) is a nuclear receptor, and its major ligands are bile acids[31]. A primary bile acid, CDCA, is the strongest agonist of FXR. Secondary bile acids, such as lithocholic acid (LCA) and deoxycholic acids (DCA), are also activators of FXR but have a lower affinity. In contrast, hydrophilic bile acids do not activate FXR[31]. In addition to FXR, bile acids activate other nuclear receptors, such as pregnane-X-receptor, constitutive androstane receptor and vitamin D receptor, inducing different signaling pathways[32]. FXR is predominantly expressed in the liver, intestine, kidney, adrenal gland, pancreas, and reproductive tissues[33]. In the liver, primary bile acids bind to FXR in hepatocytes after re-absorption, leading to increased expression of small heterodimer partner 1 (SHP-1), which is a DNA-binding domain. SHP-1 inhibits expression of cholesterol 7 α-hydroxylase (CYP7A1) via liver receptor homologue 1 (LRH-1) and liver X receptor α (LXRα), resulting in decreased synthesis of bile acids[34]. This is the major mechanism of bile acid re-absorption feedback inhibition of bile acid synthesis. Furthermore, SHP-1 can also inhibit expression of CYP8B1 (cytochrome P450, family 8, subfamily B, polypeptide 1) via hepatocyte nuclear factor 4 (HNF4). CYP8B1 regulates the synthesis of cholic acid (CA), which is hydrophilic. Thus, composition and hydrophobicity of the primary bile acids is determined by CYP8B1[35]. In the intestine, activation of FXR induces the secretion of fibroblast growth factor-19 (FGF-19), FGF-15 in mouse, which binds to fibroblast growth factor receptor 4, to decrease the expression of CYP7A. This is a SHP-1-independent pathway in the regulation of bile acid synthesis[36]. Enterohepatic circulation of bile acids leads to feedback inhibition of bile acid synthesis. Any problems in the steps in this cycle will lead to metabolic diseases, including cholestatic liver disease, gallstones, fatty liver, diabetes and obesity.
Bile acids and FXR are regulators of glucose homeostasis and insulin resistance. Gene encoding phosphoenolpyruvate carboxykinase, glucose-6-phosphatase, and fructose-1,6-biphosphatase (FBP1) are target genes of FXR[37]. All of these are rate-limiting enzymes in glucose metabolism. Activation of FXR or overexpression FXR in the liver reduces the plasma glucose level. FXR deficiency, in the liver not in the intestine, leads to glucose metabolism disruption and results in insulin resistance[37]. However, expression of FXR in the intestine has a negative effect on human disease development. In FXR-/- mice, enhanced glucose clearance and insulin sensitivity were observed, but hepatic insulin sensitivity was not altered[38], indicating that the effect of intestine FXR overcomes the effect of the liver in regulating glucose metabolism. A recent study was also consistent with these findings. In high-fat-induced nonalcoholic fatty liver disease mouse models, changing the composition of bile acids by administration of antibiotics, which results in gut microbiota alternation, led to nonalcoholic fatty liver disease development. This study demonstrated that bile acids or gut microbiota (which will be discussed in a later section) regulate nutrient metabolism in a FXR-dependent manner in the intestine but not in the liver[39]. A intestine-selective, high-affinity FXR inhibitor, glycine-β-muricholic acid (Gly-MCA), improved metabolic parameters, high-fat diet-induced and genetic obesity, insulin resistance and hepatic steatosis in mice[40].
Bile acids and FXR also regulate lipid metabolism. FXR regulates lipogenesis by inhibiting LRH-1 and LXRα[41]. In addition, activation of FXR induces expression of Apolipoprotein C-II and Apolipoprotein A-V (apoA-V) and suppresses expression of Apolipoprotein C-III, which results in an increase in lipoprotein synthesis and a decrease in plasma triglycerides[42]. Peroxisome proliferator-activated receptor α, which is involved in lipid, lipoprotein and fatty acid metabolism, is also regulated by bile acids via FXR[43]. Taken together, these results show that bile acids and FXR regulate lipid metabolism in direct and indirect manners.
Although the regulation of bile acid synthesis and bile acid metabolism is complex, clinical evidence suggests that adjusting the flow rate and composition of bile acids can improve metabolic disorders. Bypass surgery and bile acid sequestrant improve insulin resistance, obesity and hyperlipidemia, although the mechanism of these two treatments is unclear. Bile acid binding resins function by sequestering bile acids, which suppresses absorption and increases excretion of bile acids in the feces. Despite the complexity of the regulation of bile acids and their receptors, bile acid binding resins improve insulin resistance in diet-induced rat models of obesity[44]. In bile acid binding resin-treated groups, plasma glucose levels decreased to baseline values throughout the oral glucose tolerance test, a parameter of insulin resistance, and insulin levels declined to baseline as well. These findings indicated that bile acids modulate glucose metabolism and insulin sensitivity. Another approach for improving metabolic disorder is bariatric surgery, as mentioned above. In contrast to bile acid binding resins, bariatric surgery increases plasma bile acids to improve metabolic parameters, although the underlying mechanism remains to be determined. However, bariatric surgery did not decrease the standardized incidence of obesity-related cancers but increased the incidence of colon cancer with time after the surgery[45,46]. This study included a large sample size cohort, 15095 and 62016 in the surgery and control cohort, respectively, and long-term follow up (up to 30 years). It provided a very convincing result, that bariatric surgery provided a short-term benefit for metabolic disorders but increased colorectal cancers instead over time. It is still unclear why and how bariatric surgery changed the incidence of colorectal cancer. We hypothesize that changing the anatomy of the intestine leads to bile acid flow and composition alteration and results in turnover of the population of gut microbiota. Studies have shown that gastrointestinal bypass surgery may lead to changes in the intestinal and fecal microbiota, resulting in colonic mucosa exposure to increased toxicity of the feces and increased incidence of colon cancer[47].
Takeda G-protein receptor 5 (TGR5) is a membrane receptor of bile acids, and it belongs to the superfamily of G-protein coupled receptors. TGR5 is expressed in the gallbladder, intestine, human spleen and mononuclear and white blood cells, as well as in liver cells, brown adipose tissue, skeletal muscle and the nervous system[48]. Bile acids activate TGR5 with different potency, and LCA > DCA > CDCA > CA[49]. In TGR5-/- mice, the bile acid pool was decreased by increasing fecal bile acid excretion[50]. Because TGR5 is expressed in the gallbladder, it also regulates bile composition by induction of chloride secretion[51]. In addition, TGR5 regulates contraction of smooth muscles of the gallbladder, participating in gallstone disease development[52]. However, the exact mechanism of how TGR5 regulates synthesis and the bile acid pool is still unknown. Similar to FXR, TGR5 also plays a role in glucose metabolism. By binding to TGR5, bile acids induce intestinal glucagon-like peptide-1 (GLP-1) and GLP-2 release, which results in secretion of insulin[53]. One possible mechanism is that GLP stimulates oxidative phosphorylation, resulting in an increase in the ATP/ADP ratio, membrane depolarization and Ca2+ mobilization, leading to insulin secretion from pancreatic β-cells. Hyperinsulinemia is associated with insulin resistance and type 2 diabetes. Interestingly, in female TGR5-/- mice, insulin sensitivity increases but not in male mice[54], indicating that alternative regulatory pathways exist and that TGR5 regulates glucose metabolism and insulin sensitivity.
Insulin regulates the production and activity of insulin-like growth factor 1 (IGF1) by down-regulating insulin-like growth factor-binding protein 1 (IGFBP1) and IGFBP2, which inhibit the activity of IGF1[55]. High plasma concentration of IGF1 and low concentration of IGFBP1 are observed in type 2 diabetes. The main function of IGF1 is to promote cell proliferation and to inhibit cell apoptosis[56]. Both the IGF1 receptor and insulin receptor belong to the family of transmembrane receptor tyrosine kinases. They are structurally and functionally related in cancers[57]. The insulin receptor is highly expressed in insulin-sensitive tissues, such as the liver, skeletal muscle and white adipose tissue, and shows low expression in other tissues, such as the brain, heart, kidney, lung, pancreatic acini, platelets, endothelial cells, monocytes, megakaryocytes and fibroblasts. Insulin does not activate the insulin receptor in these tissues at normal concentrations[58]. Insulin abnormally activates these receptors due to hyperinsulinemia in diabetes. Moreover, in cancer patients, the tumor cells often highly express the insulin receptor, which results in non-metabolic effects. The non-metabolic effects include promotion of cell mitosis, proliferation, and metastasis[59]. The PI3K pathway and MAPK pathway play important roles in pancreatic cancer formation. Appleman et al[60] showed that the insulin receptor and IGF receptor could be activated by their ligands and in turn activated MAPK signaling and PI3K signaling. The insulin receptor and IGF receptor, along with the kras mutation, facilitate pancreatic cancer development[61]. Additionally, another study revealed that there was cross-talk between the insulin receptor and IGF receptor with G-protein coupled receptors, which further activated mTOR signaling and promoted DNA synthesis[62].
Primary bile acids are converted into secondary bile acids by structural modification by the gut microbiota (Figure 2). The gut microbiota has an important impact on the composition of bile acids, and vice versa, as bile acids re-shape the population of bacteria in the intestine. The role of intestinal flora in modulating the host metabolism has received much attention after it was revealed that diabetic patients had changes in intestinal flora, with increases in the Firmicutes to Bacteroidetes ratio. Then, obese patient were also found to have a similar composition shift[63]. Gram-negative bacteria, which belong to Bacteroidetes and Proteobacteria, are enriched in type 2 diabetes[64]. Organic acids decrease luminal pH and damage bacterial cell membranes, which strongly affect the bacterial composition, especially after a high-fat diet[65]. Rats were fed a high-CA diet, which mimicked bile acids induced by a high-fat diet, and it was found that the fecal DCA concentration was much higher, with the CA/DCA ratio reversed, compared to the control diet group[66]. DCA is ten times more toxic to intestinal bacteria than CA[67]. Firmicutes and Bacteroidetes, which are the two major types of intestinal flora, accounted for 54.1% and 30.7%, respectively, in the control group. In contrast, the proportion of Firmicutes increased to 98.6% in the high-CA group[66]. However, the total number of bacteria decreased in the feces, with an increased bile acid concentration, up to 50% that of the control diet group. Taken together, the results showed that a high-fat diet regulates intestinal flora by affecting bile acid composition. Additionally, bile acids change with the gut microbiota composition shift. A recent study found that oral administration of antibiotics led to changes in the gut microbiota and subsequently, changes in bile acids and glucose metabolism via FGF-19 signaling[68]. Vancomycin had the strongest effect on the Firmicutes and Proteobacteria phyla, with Firmicutes decreasing and Proteobacteria increasing. The Firmicutes phylum, which consists of Gram-positive bacteria, plays a crucial role in primary bile acid modification. Researchers have attributed the promotion of insulin sensitivity to the decrease in the Firmicutes phylum and the increased primary bile acids (CA), which are an activator of intestinal FXR. However, to our knowledge, as mentioned above, activation of intestinal FXR may have negative effects on metabolic disorders. Therefore, the mechanism remains to be confirmed.
Increasing gut permeability, which is controlled by microbiota, is associated with many metabolic diseases[69] and chronic low-grade inflammation[70]. All surfaces of the body, including the skin and the intestinal, oral and vaginal mucosa, are covered with microorganisms that maintain human health, rather than cause diseases. These microorganisms interact with the host to maintain the body’s health. However, when there are changes in the density or species composition of these organisms, it may result in disease. The majority of microorganisms exist in the human intestine as an essential part of mucosal immunity. A long-term, high-fat diet affects intestinal flora density through bile acids, resulting in higher mucosal permeability. The integrity of tight junctions in the intestine and trans-epithelial permeability are regulated by the normal intestinal flora, through redistribution of Toll-like receptor 2 protein[71], a toll-like receptor on epithelial cells, and expression of tight-junction proteins in cell to cell contacts[72]. High permeability has two results: bile acid as a metabolism-regulating molecule will enter the blood circulation, and gut microbes and their products will translocate to the bloodstream, leading to chronic local and systemic inflammation[70].
A large number of experiments confirmed that inflammation provides a suitable environment for tumor initiation and progression. Tumor-associated inflammatory cells and tumor stromal cells work together to promote tumor cell metastasis. Chronic inflammation induces bone marrow-derived mesenchymal stem cells to migrate to the tumor site and inhibits tumor suppressor T cells, thereby inhibiting the body’s anti-cancer immunity[73]. Intestinal polyp patients had higher intestinal permeability compared with normal subjects. IL-6, IL-11, IL-17, IL-22, and IL-23 secreted by ectopic bacteria are required for the development of intestinal polyps[70,73,74]. Intestinal flora also affect tumor formation in distant organs by modulating tumor necrosis factor, oxidative stress and DNA damage repair[70]. A more recent study revealed that a long-term, high-fat diet first affected visceral adipose tissue. This effect was caused by damage of the intestinal mucosal barrier function. The local pro-inflammatory response led to the accumulation of fat due to distant and systemic inflammation[75].
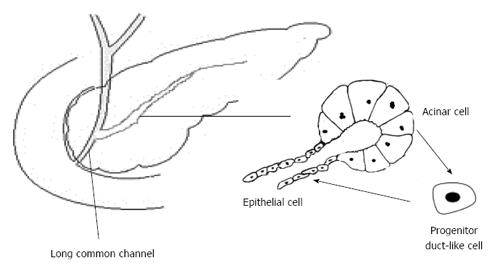
Several risk factors for pancreatic cancer, such as gallstones, pancreaticobiliary maljunction (long common channel) and chronic pancreatitis, share a common pathophysiological feature of bile acid dysmetabolism and bile acid reflux. Consequently, these three are causative factors of pancreatitis. The sphincter of Oddi loses function with a long common channel, resulting in communication of the bile duct and pancreatic duct[76]. The reflux of pancreatic juice into the bile duct leads to a higher incidence of biliary cancer, whereas the reflux of bile juice into the pancreatic duct results in pancreatitis. It is still debatable whether the reflux of pancreatic juice into the bile duct actually occurs. Because pressure in the bile duct is higher than that in the pancreatic duct, and even in the long common channel, there is a greater possibility that pancreatic juice refluxes into the bile duct[77]. However, among the causal factors of acute pancreatitis, pancreatic juice reflux or duct obstruction is the most convincing one. Bile reflux into the pancreatic duct is known to be necessary for the induction of acute pancreatitis[78,79]. Additionally, it has been known for a long time that bile infusion can be used to establish pancreatitis animal models[80]. After a high-fat diet, the secretion pressure of bile may increase to a level high enough to reflux into the pancreatic duct, leading to mild or chronic pancreatitis. Chronic pancreatitis develops from recurrent acute pancreatitis, and it involves pancreatic exocrine and endocrine dysfunction and gradually progresses to malignant tumors and diabetes[81].
Due to different cell sources, pancreatitis and pancreatic cancer were once considered two unrelated disease because pancreatitis predominantly affects pancreatic acinar cells, and pancreatic cancer originates from ductal cells[81]. However, a recent lineage tracing study questioned this hypothesis. Chronic inflammation induces dedifferentiation of acinar cells into progenitor duct-like cells, and the latter could be the source of pancreatic cancer[82]. Whether the bile acids reflux into the pancreatic duct and reach the acinar cells to induce pancreatitis is still controversial. There are two possible ways for bile to reach acinar cells: through bile duct epithelial cells and through cell-cell contacts, with tight junction impairment[83]. Bile acids were originally identified as detergents. Now, they are studied as regulatory molecules. Gpbar1 (the other name of TGR5 mentioned above), a G-protein coupled receptor, is expressed on acinar cells and mediates bile acid-induced pancreatitis. Deletion of this gene reduced hyperamylasemia, edema and inflammation[84]. Acinar cell exposure to bile acids and activation of Gpbar1 cause cell injury mediated by Ca2+ signaling and downstream NF-κB translocation[85]. Ca2+ signaling also mediates intra-acinar cell zymogen activation and in turn damages the acinar cells. In addition to NF-κB signaling, oxidative stress, which is related to bile acid injury[86], is also indispensable in acinar cell necrosis and fibrosis. All these processes produce inflammatory cytokines and chemokines, which activate the immune system. Inflammatory mediators generate secondary oxidative injury and damage cells[81]. Surprisingly, insulin-producing cells develop a malignant phenotype in inflammatory circumstances[87]. Chemokines are inflammatory cues for mesenchymal stem cells from different types of tissues, which can regulate tissue immune response[88]. Mesenchymal stem cells differentiate into fibroblasts or leucocytes infiltrating in the inflammatory lesion. As in the old hypothesis - cancer is just likes a wound that does not heal[89] - duct epithelial cells and acinar cells, as well as stroma and immune cells, function as intrinsic and external factors, respectively, to promote cancer formation (Figure 3).
Removal of cholesterol on the cell membrane can inhibit apoptosis induced by DCA. After staining with filipin, it was found that DCA could cause redistribution of membrane phospholipids. Similarly, DCA could also affect the distribution of plasma membrane caveolin and reduce membrane fluidity. Radiolabeled DCA showed that bile acids are located in the cell membrane microdomains, and different reactions depends on the physical and chemical properties of bile acids. These findings suggest that redistribution of membrane cholesterol is the initial stage of bile acid-induced signaling activation[90]. Additionally, whether the bile acids enter the cell depends on the critical micelle concentration, which is the lowest concentration of surfactant in the solvent molecules to form micelles[91].
Treatment of colonic epithelium with bile acids leads to phospholipid turnover, thereby increasing the release of diacylglycerol, which is a protein kinase C (PKC) activator. Bile acid activation of PKC is mediated by activator protein-1 (AP-1)[92]. PKC activation increased synthesis of DNA and promoted cell proliferation[93]. In addition, ornithine decarboxylase (ODC) activity and DNA synthesis varied with different types of bile acids in an in vitro study. Compared with 12-O-tetradecanoylphorbol-13-acetate (TPA), a tumor-promoting agent, deoxycholic acid (DCA) was a more potent activator of ODC. DCA and TPA both stimulated DNA synthesis within 2 d of treatment, with a peak at 2 h and a decline after 4-12 h. Moreover, the stimulatory activity of bile acids with different structures is different. By analyzing 26 types of bile acid component, bile acids, which are 5β-cholanic acids with two α-hydroxy groups in 3α, 7α, and 12α position and 5β-cholanic acids with a 3 α-hydroxy group, had the strongest activities. Therefore, the composition of bile acids plays an important role in cell proliferation and DNA synthesis in colonic epithelial cells[94].
An in vivo study showed that rats fed a diet containing 2% CA for 18 wk had significantly decreased apoptotic bodies in the normal intestinal epithelium and aberrant crypt foci (ACF) compared to those in the normal diet group (P = 0.0034), and the number of apoptotic bodies in ACF was significantly lower than those in normal intestinal epithelium (P = 0.012). In conclusion, CA simultaneously reduced apoptotic bodies in normal intestine ACF, and ACF are more susceptible to bile acids than normal intestinal mucosa. Bile acids promotes colorectal cancer formation and progression[95], which was consistent with another clinical study that also found the same phenomenon. In patient biopsy specimens, after co-culturing with bile acids, intestinal mucosal cell apoptosis was significantly reduced[96].
DCA and CDCA were found to induce COX-2 expression in the pancreatic cancer cell lines BxPC3 and SU86.86[97] and colon cancer cell lines[98]. Both studies found that bile acids acted in a dose-dependent manner, but the strongest effect was induced by different concentrations (100 μmol/L and 250 μmol/L, respectively) and different reaction times (6-12 h and 24 h, respectively). Glinghammar et al[98] also revealed that bile acids induced COX-2 expression mediated by AP-1, PKC and p38.
The main function of matrix metalloproteinase (MMP) proteins is decomposition of the extracellular matrix proteins, which are involved in cancer metastasis and inflammatory responses. MMP proteins are expressed in a wide range of cancers, including esophageal cancer, stomach cancer, liver cancer, pancreatic cancer, and kidney cancer[99]. Tumors with high expression of MMP7 are more aggressive and have a greater metastatic ability. Apical sodium-dependent bile acid transporter (Asbt)-deficient mice, which show a 10–fold increase in bile acids in the intestinal tract, have 54% more aberrant crypt foci than that in wild-type mice, and the probability of colon cancer development is twice as high as that in the wild-type mice. The study found that increasing the content of bile acids significantly increased MMP7 expression, which is mediated by muscarinic receptors (a G-coupled protein receptor)[100]. This indicates that bile acids also play a role in cancer invasion and migration[101].
Epithelial cells in the gall bladder and bile tract, which directly contact bile acids, highly express erbB2 in their malignant lesions[102,103]. EGFR expression in intrahepatic cholangiocarcinoma, extrahepatic cholangiocarcinoma and gallbladder cancer was 100%, 52.6% and 38.5%, respectively, and HER2 is overexpressed in 10% and 26.3% of gallbladder and extrahepatic cholangiocarcinomas, respectively, suggesting that EGFR and HER2 may contribute to the initiation and development of these cancers[103]. However, whether bile acids directly induce the expression of EGFR and HER2 and activate these receptors was not clear. A recent study confirmed that bile acids induced the expression of EGFR and HER2 directly and activated the EFGR/HER2 pathway and downstream pathways, contributing to cancer formation and progression[104]. The study found that secondary conjugated bile acids, such as taurochenodeoxycholic acid, induced gallbladder cancer cell EGFR/erbB2 expression and activation of EGFR/erbB2 and downstream signaling: bile acid → src → TACE → EGFR/erbB2 → downstream signaling cascade. This induction and activation have also been verified in skin cells. This study was consistent with previous studies showing that in hepatocytes, the bile acids activated the MAPK and PI3K/Akt signaling pathways[105,106], indicating that bile acids have a prolonged effect on the activation of EGFR/erbB2 signaling and finally led to intranuclear effects, rather than acute effects. HER2 is overexpressed in 61.2%-90% of pancreatic cancer patients[107,108], and survival of patients with HER2 overexpression is significantly lower compared with patients with low expression, 14.7 mo and 20.7 mo respectively. In a multivariate analysis, HER2 overexpression is an independent prognostic factor. These findings suggested that a HER2 monoclonal antibody may be beneficial for this subtype of patients[107].
The kras mutation plays an important role in pancreatic cancer initiation. Kras induces endogenous EGFR expression and activation. EGFR inhibitors eliminated kras mediation of pancreatic cancer. In other words, without EGFR activation, kras cannot activate the MEK/ERK pathway and promote tumorigenesis[109]. However, unlike gallbladder and bile tract cancer, whether bile acids induce expression of EGFR and HER2 in pancreatic duct epithelial cells remains unknown. A recent study revealed that there was crosstalk between a bile acid membrane receptor, TGR5, and EGFR signaling. The cell surface protease TACE/ADAM-17, which is required in EGFR activation by its ligands amphiregulin (AREG) and TGF-α, is highly expressed in colorectal cancer and pancreatic cancer. Exposure of colorectal cancer cells and pancreatic cancer cells to bile acids activates EGFR in an AREG-dependent manner. Furthermore, this effect was mediated by a G-protein coupled receptor, TGR5[5,104]. RNA silencing of TGR5 inhibited EGFR, MAPK and STAT3 signaling induced by bile acids.
Although we discussed bile acids as a molecular regulator in metabolic and cancer signaling, we still must note that bile acids are a type of detergent. Exposure of cells or tissues to bile acids at high concentrations (Table 1) primarily causes cell death, whereas activation of signaling pathways is secondary. This may be a reason for the contradictory findings. A study[110] found that DCA and CA increased the proportion of cells in G0 and G1 phase, while GCA and TDCA increased the proportion of cells in S phase. Effective biological effects could not be found, even with different concentrations and different times. After 48 h of treatment, Panc-1 cells showed cell structural damage. Therefore, the researchers concluded that increases in bile acid concentration in the serum might inhibit the progression of pancreatic cancer. The conclusion might be far-fetched. In previous studies (listed in Table 1), the concentration of bile acids and the processing time varied substantially, indicating that bile acid concentration and treatment time are critical factors in research on the biological role of bile acids in cancer. Moreover, even if there is a clear biological effect in an in vitro study, how to simulate the in vivo environment is another issue. In the study of bile acids and pancreatic cancer, determining how bile acids reach the pancreatic duct epithelial cells or acinar cells is a prerequisite for all studies. If the bile acids do not reflux at high concentrations (for example, 500 μmol/L) into the pancreatic parenchyma, how can these studies determine how bile acids affect the development of malignant tumors? Bile acid retrograde infusion into the pancreatic ducts are widely used to induce pancreatitis in vivo[111]. It has been shown that 37 mmol/L of taurocholate acids or 3 mmol/L of tauro-LCS induces maximally severe, acute necrotic pancreatitis but not chronic pancreatitis. For studies on chronic pancreatitis, due to the duration of the study, duct ligation models with bile acid reflux are often used[112]. However, a profile of bile acids is missing in these cases. Therefore, the concentration of bile acids is a crucial factor for both in vivo and in vitro studies. Additionally, the method of contact of bile acids with cells is also important, whether it is by contacting the cell surface (luminal or basal surface) or by disrupting cell-cell connections to enter the pancreatic parenchyma. However, the solution to these questions cannot be determined from in vitro experiments. In vitro studies focus on one type or a few types of cells, and they cannot simulate tissue or organ structures. For example, apical or basolateral membranes of pancreatic ductal epithelium have different cell surface receptors and ion channels[77]. Thus, they have different biological effects caused by contact with bile acids. These effects are different and may even be opposite.
| Ref. | Year | Cell/tissue | Bile acid | Dose | Time | Biological effect |
| Jean-Louis et al[90] | 2006 | HCT 116 | DCA | 500 μmol/L | 5, 15, 30 min | Cholesterol aggregation at membrane |
| 1, 2, 4 h | ||||||
| DCA | 500 μmol/L | 1 h | Internalization of caveolin-1 | |||
| Hirano et al[92] | 1991 | Gastric mucosal primary culture | DCA | 30 min | PKC activation | |
| DeRubertis et al[93] | 1987 | Colonic epithelial cells | DCA | DAG ↑ | ||
| Takano et al[94] | 1984 | Colonic epithelial cells | DCA | 2 d | DNA ↑ | |
| ODC ↑ | ||||||
| Magnuson et al[95] | 1994 | In vivo | CA | 2% in diet | 18 wk | Apoptosis ↓ |
| Garewal et al[96] | 1996 | Biopsies | DCA | 1 mmol/L | 30 °C 3 h | Apoptosis ↓ |
| Tucker et al[97] | 2004 | BxPC3 | CDCA, DCA | 100 μmol/L | 6-12 h | COX-2 ↑ |
| SU86.86 | PGE-2 ↑ | |||||
| Glinghammar et al[98] | 2001 | HCT 116 | Tauro-CDCA | 200-1200 μmol/L | 15 h | AP-1 ↑, COX-2 ↑, PKC(+), P38(+) |
| DCA, CDCA, CA | 250 μmol/L | |||||
| Butyric acid | 0.1-4 mmol/L | |||||
| HT 29 | DCA | 500 μmol/L | 24 h | COX-2 ↑, PCNA ↑ | ||
| Raufman et al[100] | 2015 | In vivo (Asbt-deficient) | Aberrant crypt foci | |||
| Cheng et al[101] | 2007 | H508 | DCT | 50 μmol/L | 24 h | MMP ↑ |
| Kitamura et al[104] | 2015 | Primary culture (BK5 erbB2 mice) | TCDC | 100 μmol/L | 72 h | Cell proliferation ↑, EGFR MAPK Cyclin D1 ↑ |
| Sk-Ch-A-1 | TCDC | 10-200 μmol/L | 72 h | Cell viability ↑ | ||
| CDCA, DCA, TC, TDC | 0.5 mmol/L | 30 min | p-erbB2, p-EGFR, p-MAPK, p-Akt ↑ | |||
| TCDC | 500 μmol/L | 3 h | HB-EGF ↑ | |||
| TCDC | 200 μmol/L | 60 min | TACE activity ↑ | |||
| In vivo (BK5 erbB2 mice) | TCDC | 2.5 mmol/L 200 μL | Twice/wk for 20 wk | Skin tumor ↑ | ||
| Qiao et al[105] | 2001 | hepatocytes | DCA | 50 μmol/L | 5 min | EGFR/Ras/MAPK activation |
| Rao et al[106] | 2002 | Primary rat hepatocytes | TDCA, TCA, DCA | 50 μmol/L | 20 min | p-raf-1↑, MEK ↑, ERK ↑ |
| Nagathihalli et al[5] | 2014 | HCT116, HCA-7, BxPC3, AsPC-1, Capan 2 | DCA | 300 μmol/L | 4 h-6 h | TACE co-localization, TGF-α mRNA ↑ |
After reviewing the role of bile acids in pancreatic cancer formation and progression, more questions are raised. Bile acids enter the bloodstream by enterohepatic circulation. Bile acid receptors, including cell surface receptors and nuclear receptors, are widely distributed in the organs and tissues, including the pancreas. Bile acids regulate endocrine and exocrine functions of the pancreas, and they may be involved in pancreatic cancer formation and progression. We cannot assume that the role of bile acid-induced pancreatic cancer is just due to local effects (reflux); it is more likely to function via systemic effects. Moreover, bile acid receptors in different organs and tissues have different effects (liver FXR and intestine FXR). They simultaneously play a pathogenic role and a protective role, which makes studying these processes very complex.
Bile is composed of a mixture of ingredients, and bile acid is the main component of bile. Bile acid itself has different ingredients, including conjugated bile acids and free bile acids, which have different hydrophilic properties, and their ability to cross the cell membrane is different. Glycine-conjugated bile acids have pKa values of 4.3-5.2, and they constitute greater than 60% of the bile, while taurine-conjugated bile acids have pKa values of 1.8-1.9, accounting for approximately 20% of the bile[113]. Therefore, the ratio of glycine-conjugated bile acids and taurine-conjugated bile acids is approximately 3:1. Taurine-conjugated bile acids are soluble and contact cells with a high frequency. They contribute to the role of bile acids as a carcinogen. Gastrointestinal inflammation and tumorigenic effects caused by different components of bile acids, glycine-conjugated or taurine-conjugated, conjugated or free, are not the same. Non-conjugated bile acids have more significant carcinogenic effects[110]. A novel function of UDP glycosyltransferase 8 (UGT8) has been found; it galactosylates bile acids up to 60-fold more efficiently than its activity towards ceramide[114]. This finding suggested that UGT8 might be involved in modulating bile acid signaling. In contrast, UDCA has anti-neoplastic effects but is also commonly used for clinical treatment of biliary tract disease[90,115]. Therefore, we need to understand the variety in the composition and concentration of bile acids in pancreatic cancer patients to further clarify the role of bile acids in pancreatic cancer.
Bile acids are associated with most risk factors of pancreatic cancer, including alcohol intake, smoking, a high-fat diet, gallstones, a long common channel, and chronic pancreatitis, as well as obesity, diabetes and hypertriglyceridemia. In addition to systemic effects, bile acids have local tissue effects, and they directly activate cancer signaling pathways. Bile acids are likely to be recognized as signaling molecules in pancreatic cancer in the future.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sakai H, Serrano-Luna J S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9957] [Article Influence: 995.7] [Reference Citation Analysis (0)] |
| 2. | Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1346] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 3. | Winter JM, Maitra A, Yeo CJ. Genetics and pathology of pancreatic cancer. HPB (Oxford). 2006;8:324-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589:47-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 458] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 5. | Nagathihalli NS, Beesetty Y, Lee W, Washington MK, Chen X, Lockhart AC, Merchant NB. Novel mechanistic insights into ectodomain shedding of EGFR Ligands Amphiregulin and TGF-α: impact on gastrointestinal cancers driven by secondary bile acids. Cancer Res. 2014;74:2062-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Yee SB, Song YS, Jeong SH, Lee HS, Seo SY, Kim JH, Suh H, Kim ND, Yoo YH. A novel chenodeoxycholic derivative HS-1200 enhances radiation-induced apoptosis in MCF-7 cells. Oncol Rep. 2007;17:919-923. [PubMed] |
| 7. | Kakiyama G, Hylemon PB, Zhou H, Pandak WM, Heuman DM, Kang DJ, Takei H, Nittono H, Ridlon JM, Fuchs M. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2014;306:G929-G937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 154] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 8. | Ackehed G, Hedenborg G, Wisén O, Norman A. Faecal bile acid excretion during detoxification in patients with alcohol abuse. Scand J Gastroenterol. 1996;31:1205-1210. [PubMed] |
| 9. | Axelson M, Mörk B, Sjövall J. Ethanol has an acute effect on bile acid biosynthesis in man. FEBS Lett. 1991;281:155-159. [PubMed] |
| 10. | Chanda D, Kim YH, Li T, Misra J, Kim DK, Kim JR, Kwon J, Jeong WI, Ahn SH, Park TS. Hepatic cannabinoid receptor type 1 mediates alcohol-induced regulation of bile acid enzyme genes expression via CREBH. PLoS One. 2013;8:e68845. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Gut microbiota, cirrhosis, and alcohol regulate bile acid metabolism in the gut. Dig Dis. 2015;33:338-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Suzuki T. Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci. 2013;70:631-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 959] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 13. | Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes. 2013;4:382-387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 271] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 14. | Gnoni A, Licchetta A, Scarpa A, Azzariti A, Brunetti AE, Simone G, Nardulli P, Santini D, Aieta M, Delcuratolo S. Carcinogenesis of pancreatic adenocarcinoma: precursor lesions. Int J Mol Sci. 2013;14:19731-19762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Hermann PC, Sancho P, Cañamero M, Martinelli P, Madriles F, Michl P, Gress T, de Pascual R, Gandia L, Guerra C. Nicotine promotes initiation and progression of KRAS-induced pancreatic cancer via Gata6-dependent dedifferentiation of acinar cells in mice. Gastroenterology. 2014;147:1119-1133.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Müller-Lissner SA. Bile reflux is increased in cigarette smokers. Gastroenterology. 1986;90:1205-1209. [PubMed] |
| 17. | Lindell G, Farnebo LO, Chen D, Nexø E, Rask Madsen J, Bukhave K, Graffner H. Acute effects of smoking during modified sham feeding in duodenal ulcer patients. An analysis of nicotine, acid secretion, gastrin, catecholamines, epidermal growth factor, prostaglandin E2, and bile acids. Scand J Gastroenterol. 1993;28:487-494. [PubMed] |
| 18. | Adachi T, Tajima Y, Kuroki T, Mishima T, Kitasato A, Fukuda K, Tsutsumi R, Kanematsu T. Bile-reflux into the pancreatic ducts is associated with the development of intraductal papillary carcinoma in hamsters. J Surg Res. 2006;136:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Jiao L, Flood A, Subar AF, Hollenbeck AR, Schatzkin A, Stolzenberg-Solomon R. Glycemic index, carbohydrates, glycemic load, and the risk of pancreatic cancer in a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18:1144-1151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Kasbo J, Saleem M, Perwaiz S, Mignault D, Lamireau T, Tuchweber B, Yousef I. Biliary, fecal and plasma deoxycholic acid in rabbit, hamster, guinea pig, and rat: comparative study and implication in colon cancer. Biol Pharm Bull. 2002;25:1381-1384. [PubMed] |
| 21. | Dfanmica H. Obesity and Cancer: Concepts and Challenges. Indian Assoc Surg Oncol. 2015;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Farag YM, Gaballa MR. Diabesity: an overview of a rising epidemic. Nephrol Dial Transplant. 2011;26:28-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 202] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 23. | Hart CL, Hole DJ, Lawlor DA, Davey Smith G. How many cases of Type 2 diabetes mellitus are due to being overweight in middle age? Evidence from the Midspan prospective cohort studies using mention of diabetes mellitus on hospital discharge or death records. Diabet Med. 2007;24:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 24. | Li P, Oh da Y, Bandyopadhyay G, Lagakos WS, Talukdar S, Osborn O, Johnson A, Chung H, Mayoral R, Maris M. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat Med. 2015;21:239-247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 254] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 25. | Pories WJ, Dohm GL. Diabetes: have we got it all wrong? Hyperinsulinism as the culprit: surgery provides the evidence. Diabetes Care. 2012;35:2438-2442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 26. | Haeusler RA, Astiarraga B, Camastra S, Accili D, Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes. 2013;62:4184-4191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 371] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 27. | Li T, Francl JM, Boehme S, Ochoa A, Zhang Y, Klaassen CD, Erickson SK, Chiang JY. Glucose and insulin induction of bile acid synthesis: mechanisms and implication in diabetes and obesity. J Biol Chem. 2012;287:1861-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 28. | Cătoi AF, Pârvu A, Mureşan A, Busetto L. Metabolic Mechanisms in Obesity and Type 2 Diabetes: Insights from Bariatric/Metabolic Surgery. Obes Facts. 2015;8:350-363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Morimoto K, Watanabe M, Houten S, Kaneko-Iwasaki N, Sugizaki T, Horai Y, Mataki C, Arita E, Schoonjans K, Auwerx J. Bile acid binding resins affect diverse molecules to improve metabolic control. Available from: http://www.endocrine-abstracts.org/ea/0029/ea0029p706.htm. |
| 30. | Kohli R, Kirby M, Setchell KD, Jha P, Klustaitis K, Woollett LA, Pfluger PT, Balistreri WF, Tso P, Jandacek RJ. Intestinal adaptation after ileal interposition surgery increases bile acid recycling and protects against obesity-related comorbidities. Am J Physiol Gastrointest Liver Physiol. 2010;299:G652-G660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 120] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365-1368. [PubMed] |
| 32. | Kemper JK. Regulation of FXR transcriptional activity in health and disease: Emerging roles of FXR cofactors and post-translational modifications. Biochim Biophys Acta. 2011;1812:842-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 33. | Penney NC, Kinross J, Newton RC, Purkayastha S. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes (Lond). 2015;39:1565-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 34. | Brendel C, Schoonjans K, Botrugno OA, Treuter E, Auwerx J. The small heterodimer partner interacts with the liver X receptor alpha and represses its transcriptional activity. Mol Endocrinol. 2002;16:2065-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 155] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Zhang M, Chiang JY. Transcriptional regulation of the human sterol 12alpha-hydroxylase gene (CYP8B1): roles of heaptocyte nuclear factor 4alpha in mediating bile acid repression. J Biol Chem. 2001;276:41690-41699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 190] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 36. | Kir S, Zhang Y, Gerard RD, Kliewer SA, Mangelsdorf DJ. Nuclear receptors HNF4α and LRH-1 cooperate in regulating Cyp7a1 in vivo. J Biol Chem. 2012;287:41334-41341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 37. | Fiorucci S, Cipriani S, Baldelli F, Mencarelli A. Bile acid-activated receptors in the treatment of dyslipidemia and related disorders. Prog Lipid Res. 2010;49:171-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Prawitt J, Abdelkarim M, Stroeve JH, Popescu I, Duez H, Velagapudi VR, Dumont J, Bouchaert E, van Dijk TH, Lucas A. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes. 2011;60:1861-1871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 260] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 39. | Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, Qi Y, Fang ZZ, Takahashi S. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 539] [Article Influence: 53.9] [Reference Citation Analysis (0)] |
| 40. | Jiang C, Xie C, Lv Y, Li J, Krausz KW, Shi J, Brocker CN, Desai D, Amin SG, Bisson WH. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun. 2015;6:10166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 435] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 41. | Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819-2830. [PubMed] |
| 42. | Prieur X, Coste H, Rodriguez JC. The human apolipoprotein AV gene is regulated by peroxisome proliferator-activated receptor-alpha and contains a novel farnesoid X-activated receptor response element. J Biol Chem. 2003;278:25468-25480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Pineda Torra I, Claudel T, Duval C, Kosykh V, Fruchart JC, Staels B. Bile acids induce the expression of the human peroxisome proliferator-activated receptor alpha gene via activation of the farnesoid X receptor. Mol Endocrinol. 2003;17:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 361] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 44. | Shang Q, Saumoy M, Holst JJ, Salen G, Xu G. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol. 2010;298:G419-G424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 45. | Ostlund MP, Lu Y, Lagergren J. Risk of obesity-related cancer after obesity surgery in a population-based cohort study. Ann Surg. 2010;252:972-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 46. | Derogar M, Hull MA, Kant P, Östlund M, Lu Y, Lagergren J. Increased risk of colorectal cancer after obesity surgery. Ann Surg. 2013;258:983-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 119] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 47. | Li JV, Reshat R, Wu Q, Ashrafian H, Bueter M, le Roux CW, Darzi A, Athanasiou T, Marchesi JR, Nicholson JK. Experimental bariatric surgery in rats generates a cytotoxic chemical environment in the gut contents. Front Microbiol. 2011;2:183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 48. | Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5: a valuable metabolic target. Dig Dis. 2011;29:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 49. | Sato H, Macchiarulo A, Thomas C, Gioiello A, Une M, Hofmann AF, Saladin R, Schoonjans K, Pellicciari R, Auwerx J. Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies. J Med Chem. 2008;51:1831-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 238] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 50. | Maruyama T, Tanaka K, Suzuki J, Miyoshi H, Harada N, Nakamura T, Miyamoto Y, Kanatani A, Tamai Y. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol. 2006;191:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 223] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 51. | Keitel V, Ullmer C, Häussinger D. The membrane-bound bile acid receptor TGR5 (Gpbar-1) is localized in the primary cilium of cholangiocytes. Biol Chem. 2010;391:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 52. | Lavoie B, Balemba OB, Godfrey C, Watson CA, Vassileva G, Corvera CU, Nelson MT, Mawe GM. Hydrophobic bile salts inhibit gallbladder smooth muscle function via stimulation of GPBAR1 receptors and activation of KATP channels. J Physiol. 2010;588:3295-3305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 53. | Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1223] [Cited by in RCA: 1392] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 54. | Vassileva G, Hu W, Hoos L, Tetzloff G, Yang S, Liu L, Kang L, Davis HR, Hedrick JA, Lan H. Gender-dependent effect of Gpbar1 genetic deletion on the metabolic profiles of diet-induced obese mice. J Endocrinol. 2010;205:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 55. | Frystyk J. Free insulin-like growth factors -- measurements and relationships to growth hormone secretion and glucose homeostasis. Growth Horm IGF Res. 2004;14:337-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 56. | Pollak M. Insulin, insulin-like growth factors and neoplasia. Best Pract Res Clin Endocrinol Metab. 2008;22:625-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 57. | Trajkovic-Arsic M, Kalideris E, Siveke JT. The role of insulin and IGF system in pancreatic cancer. J Mol Endocrinol. 2013;50:R67-R74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 59. | Belfiore A, Malaguarnera R. Insulin receptor and cancer. Endocr Relat Cancer. 2011;18:R125-R147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 60. | Appleman VA, Ahronian LG, Cai J, Klimstra DS, Lewis BC. KRAS(G12D)- and BRAF(V600E)-induced transformation of murine pancreatic epithelial cells requires MEK/ERK-stimulated IGF1R signaling. Mol Cancer Res. 2012;10:1228-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 61. | Tanno S, Tanno S, Mitsuuchi Y, Altomare DA, Xiao GH, Testa JR. AKT activation up-regulates insulin-like growth factor I receptor expression and promotes invasiveness of human pancreatic cancer cells. Cancer Res. 2001;61:589-593. [PubMed] |
| 62. | Rozengurt E, Sinnett-Smith J, Kisfalvi K. Crosstalk between insulin/insulin-like growth factor-1 receptors and G protein-coupled receptor signaling systems: a novel target for the antidiabetic drug metformin in pancreatic cancer. Clin Cancer Res. 2010;16:2505-2511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 63. | Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7222] [Cited by in RCA: 6407] [Article Influence: 337.2] [Reference Citation Analysis (0)] |
| 64. | Suez J, Korem T, Zeevi D, Zilberman-Schapira G, Thaiss CA, Maza O, Israeli D, Zmora N, Gilad S, Weinberger A. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature. 2014;514:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 1360] [Article Influence: 123.6] [Reference Citation Analysis (0)] |
| 65. | Yokota A, Fukiya S, Islam KB, Ooka T, Ogura Y, Hayashi T, Hagio M, Ishizuka S. Is bile acid a determinant of the gut microbiota on a high-fat diet? Gut Microbes. 2012;3:455-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 66. | Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, Ogura Y, Hayashi T, Yokota A. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 596] [Cited by in RCA: 702] [Article Influence: 50.1] [Reference Citation Analysis (0)] |
| 67. | Kurdi P, Kawanishi K, Mizutani K, Yokota A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol. 2006;188:1979-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 257] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 68. | Vrieze A, Out C, Fuentes S, Jonker L, Reuling I, Kootte RS, van Nood E, Holleman F, Knaapen M, Romijn JA. Impact of oral vancomycin on gut microbiota, bile acid metabolism, and insulin sensitivity. J Hepatol. 2014;60:824-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 427] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 69. | Suzuki T, Hara H. Dietary fat and bile juice, but not obesity, are responsible for the increase in small intestinal permeability induced through the suppression of tight junction protein expression in LETO and OLETF rats. Nutr Metab (Lond). 2010;7:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 70. | Dzutsev A, Goldszmid RS, Viaud S, Zitvogel L, Trinchieri G. The role of the microbiota in inflammation, carcinogenesis, and cancer therapy. Eur J Immunol. 2015;45:17-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 203] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 71. | Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1311] [Cited by in RCA: 1210] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 72. | Alaish SM, Smith AD, Timmons J, Greenspon J, Eyvazzadeh D, Murphy E, Shea-Donahue T, Cirimotich S, Mongodin E, Zhao A. Gut microbiota, tight junction protein expression, intestinal resistance, bacterial translocation and mortality following cholestasis depend on the genetic background of the host. Gut Microbes. 2013;4:292-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 73. | Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 960] [Cited by in RCA: 1031] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 74. | Bongers G, Pacer ME, Geraldino TH, Chen L, He Z, Hashimoto D, Furtado GC, Ochando J, Kelley KA, Clemente JC. Interplay of host microbiota, genetic perturbations, and inflammation promotes local development of intestinal neoplasms in mice. J Exp Med. 2014;211:457-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 75. | Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 516] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 76. | Kamisawa T, Ando H, Hamada Y, Fujii H, Koshinaga T, Urushihara N, Itoi T, Shimada H. Diagnostic criteria for pancreaticobiliary maljunction 2013. J Hepatobiliary Pancreat Sci. 2014;21:159-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 77. | Lerch MM, Aghdassi AA. The role of bile acids in gallstone-induced pancreatitis. Gastroenterology. 2010;138:429-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Lerch MM, Saluja AK, Rünzi M, Dawra R, Saluja M, Steer ML. Pancreatic duct obstruction triggers acute necrotizing pancreatitis in the opossum. Gastroenterology. 1993;104:853-861. [PubMed] |
| 79. | Hegyi P, Rakonczay Z. The role of pancreatic ducts in the pathogenesis of acute pancreatitis. Pancreatology. 2015;15:S13-S17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 80. | Lerch MM, Gorelick FS. Models of acute and chronic pancreatitis. Gastroenterology. 2013;144:1180-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 318] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 81. | Pinho AV, Chantrill L, Rooman I. Chronic pancreatitis: a path to pancreatic cancer. Cancer Lett. 2014;345:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 82. | Pinho AV, Rooman I, Reichert M, De Medts N, Bouwens L, Rustgi AK, Real FX. Adult pancreatic acinar cells dedifferentiate to an embryonic progenitor phenotype with concomitant activation of a senescence programme that is present in chronic pancreatitis. Gut. 2011;60:958-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 83. | Mayerle J, Schnekenburger J, Krüger B, Kellermann J, Ruthenbürger M, Weiss FU, Nalli A, Domschke W, Lerch MM. Extracellular cleavage of E-cadherin by leukocyte elastase during acute experimental pancreatitis in rats. Gastroenterology. 2005;129:1251-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 84. | Perides G, Laukkarinen JM, Vassileva G, Steer ML. Biliary acute pancreatitis in mice is mediated by the G-protein-coupled cell surface bile acid receptor Gpbar1. Gastroenterology. 2010;138:715-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 85. | Muili KA, Jin S, Orabi AI, Eisses JF, Javed TA, Le T, Bottino R, Jayaraman T, Husain SZ. Pancreatic acinar cell nuclear factor κB activation because of bile acid exposure is dependent on calcineurin. J Biol Chem. 2013;288:21065-21073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Booth DM, Murphy JA, Mukherjee R, Awais M, Neoptolemos JP, Gerasimenko OV, Tepikin AV, Petersen OH, Sutton R, Criddle DN. Reactive oxygen species induced by bile acid induce apoptosis and protect against necrosis in pancreatic acinar cells. Gastroenterology. 2011;140:2116-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 87. | Gidekel Friedlander SY, Chu GC, Snyder EL, Girnius N, Dibelius G, Crowley D, Vasile E, DePinho RA, Jacks T. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell. 2009;16:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 270] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 88. | Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 577] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 89. | Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3033] [Cited by in RCA: 3098] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 90. | Jean-Louis S, Akare S, Ali MA, Mash EA, Meuillet E, Martinez JD. Deoxycholic acid induces intracellular signaling through membrane perturbations. J Biol Chem. 2006;281:14948-14960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 91. | Batzri S, Harmon JW, Schweitzer EJ, Toles R. Bile acid accumulation in gastric mucosal cells. Proc Soc Exp Biol Med. 1991;197:393-399. [PubMed] |
| 92. | Hirano F, Tanada H, Makino Y, Okamoto K, Hiramoto M, Handa H, Makino I. Induction of the transcription factor AP-1 in cultured human colon adenocarcinoma cells following exposure to bile acids. Carcinogenesis. 1996;17:427-433. [PubMed] |
| 93. | DeRubertis FR, Craven PA. Relationship of bile salt stimulation of colonic epithelial phospholipid turnover and proliferative activity: role of activation of protein kinase C1. Prev Med. 1987;16:572-579. [PubMed] |
| 94. | Takano S, Akagi M, Bryan GT. Stimulation of ornithine decarboxylase activity and DNA synthesis by phorbol esters or bile acids in rat colon. Gan. 1984;75:29-35. [PubMed] |
| 95. | Magnuson BA, Shirtliff N, Bird RP. Resistance of aberrant crypt foci to apoptosis induced by azoxymethane in rats chronically fed cholic acid. Carcinogenesis. 1994;15:1459-1462. [PubMed] |
| 96. | Garewal H, Bernstein H, Bernstein C, Sampliner R, Payne C. Reduced bile acid-induced apoptosis in “normal” colorectal mucosa: a potential biological marker for cancer risk. Cancer Res. 1996;56:1480-1483. [PubMed] |
| 97. | Tucker ON, Dannenberg AJ, Yang EK, Fahey TJ. Bile acids induce cyclooxygenase-2 expression in human pancreatic cancer cell lines. Carcinogenesis. 2004;25:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 98. | Glinghammar B, Rafter J. Colonic luminal contents induce cyclooxygenase 2 transcription in human colon carcinoma cells. Gastroenterology. 2001;120:401-410. [PubMed] |
| 99. | Yokoyama Y, Grünebach F, Schmidt SM, Heine A, Häntschel M, Stevanovic S, Rammensee HG, Brossart P. Matrilysin (MMP-7) is a novel broadly expressed tumor antigen recognized by antigen-specific T cells. Clin Cancer Res. 2008;14:5503-5511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 100. | Raufman JP, Dawson PA, Rao A, Drachenberg CB, Heath J, Shang AC, Hu S, Zhan M, Polli JE, Cheng K. Slc10a2-null mice uncover colon cancer-promoting actions of endogenous fecal bile acids. Carcinogenesis. 2015;36:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 101. | Cheng K, Xie G, Raufman JP. Matrix metalloproteinase-7-catalyzed release of HB-EGF mediates deoxycholyltaurine-induced proliferation of a human colon cancer cell line. Biochem Pharmacol. 2007;73:1001-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 102. | Harder J, Waiz O, Otto F, Geissler M, Olschewski M, Weinhold B, Blum HE, Schmitt-Graeff A, Opitz OG. EGFR and HER2 expression in advanced biliary tract cancer. World J Gastroenterol. 2009;15:4511-4517. [PubMed] |
| 103. | Pignochino Y, Sarotto I, Peraldo-Neia C, Penachioni JY, Cavalloni G, Migliardi G, Casorzo L, Chiorino G, Risio M, Bardelli A. Targeting EGFR/HER2 pathways enhances the antiproliferative effect of gemcitabine in biliary tract and gallbladder carcinomas. BMC Cancer. 2010;10:631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 139] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 104. | Kitamura T, Srivastava J, DiGiovanni J, Kiguchi K. Bile acid accelerates erbB2-induced pro-tumorigenic activities in biliary tract cancer. Mol Carcinog. 2015;54:459-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 105. | Qiao L, Studer E, Leach K, McKinstry R, Gupta S, Decker R, Kukreja R, Valerie K, Nagarkatti P, El Deiry W. Deoxycholic acid (DCA) causes ligand-independent activation of epidermal growth factor receptor (EGFR) and FAS receptor in primary hepatocytes: inhibition of EGFR/mitogen-activated protein kinase-signaling module enhances DCA-induced apoptosis. Mol Biol Cell. 2001;12:2629-2645. [PubMed] |
| 106. | Rao YP, Studer EJ, Stravitz RT, Gupta S, Qiao L, Dent P, Hylemon PB. Activation of the Raf-1/MEK/ERK cascade by bile acids occurs via the epidermal growth factor receptor in primary rat hepatocytes. Hepatology. 2002;35:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 107. | Komoto M, Nakata B, Amano R, Yamada N, Yashiro M, Ohira M, Wakasa K, Hirakawa K. HER2 overexpression correlates with survival after curative resection of pancreatic cancer. Cancer Sci. 2009;100:1243-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 108. | Troiani T, Martinelli E, Capasso A, Morgillo F, Orditura M, De Vita F, Ciardiello F. Targeting EGFR in pancreatic cancer treatment. Curr Drug Targets. 2012;13:802-810. [PubMed] |
| 109. | Ardito CM, Grüner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, Delgiorno KE, Carpenter ES, Halbrook CJ, Hall JC. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22:304-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 430] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 110. | Wu Z, Lü Y, Wang B, Liu C, Wang ZR. Effects of bile acids on proliferation and ultrastructural alteration of pancreatic cancer cell lines. World J Gastroenterol. 2003;9:2759-2763. [PubMed] |
| 111. | Perides G, van Acker GJ, Laukkarinen JM, Steer ML. Experimental acute biliary pancreatitis induced by retrograde infusion of bile acids into the mouse pancreatic duct. Nat Protoc. 2010;5:335-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 112. | Aghdassi AA, Mayerle J, Christochowitz S, Weiss FU, Sendler M, Lerch MM. Animal models for investigating chronic pancreatitis. Fibrogenesis Tissue Repair. 2011;4:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 113. | Setchell KDR, Kritchevsky D, Nair PP. The Bile Acids: Chemistry, Physiology, and Metabolism. Volume 4: Methods and Applications. Springer US: Methods and Applications 2012; . |
| 114. | Meech R, Mubarokah N, Shivasami A, Rogers A, Nair PC, Hu DG, McKinnon RA, Mackenzie PI. A novel function for UDP glycosyltransferase 8: galactosidation of bile acids. Mol Pharmacol. 2015;87:442-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 115. | Pardi DS, Loftus EV, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 355] [Article Influence: 16.1] [Reference Citation Analysis (0)] |