Published online Aug 28, 2016. doi: 10.3748/wjg.v22.i32.7301
Peer-review started: March 26, 2016
First decision: April 14, 2016
Revised: May 4, 2016
Accepted: May 23, 2016
Article in press: May 23, 2016
Published online: August 28, 2016
Processing time: 151 Days and 13.3 Hours
Pancreatic duct adenocarcinoma is one of the most fatal malignancies, with R0 resection remaining the most important part of treatment of this malignancy. However, pancreatectomy is believed to be one of the most challenging procedures and R0 resection remains the only chance for patients with pancreatic cancer to have a good prognosis. Some surgeons have tried minimally invasive pancreatic surgery, but the short- and long-term outcomes of pancreatic malignancy remain controversial between open and minimally invasive procedures. We collected comparative data about minimally invasive and open pancreatic surgery. The available evidence suggests that minimally invasive pancreaticoduodenectomy (MIPD) is as safe and feasible as open PD (OPD), and shows some benefit, such as less intraoperative blood loss and shorter postoperative hospital stay. Despite the limited evidence for MIPD in pancreatic cancer, most of the available data show that the short-term oncological adequacy is similar between MIPD and OPD. Some surgical techniques, including superior mesenteric artery-first approach and laparoscopic pancreatoduodenectomy with major vein resection, are believed to improve the rate of R0 resection. Laparoscopic distal pancreatectomy is less technically demanding and is accepted in more pancreatic centers. It is technically safe and feasible and has similar short-term oncological prognosis compared with open distal pancreatectomy.
Core tip: Minimally invasive pancreaticoduodenectomy is as safe and feasible as open pancreaticoduodenectomy (OPD) and shows some superiority. The short-term oncological results are similar between laparoscopic pancreaticoduodenectomy (LPD) and OPD. However, in some experienced hands, better prognosis is detected in the LPD group because the patients can receive adjuvant therapy faster because of the benefits of minimal invasiveness. Minimally invasive distal pancreatectomy is a well-established procedure and widely accepted. It is safe, feasible, and has similar short-term oncological results compared with open distal pancreatectomy.
- Citation: Zhang YH, Zhang CW, Hu ZM, Hong DF. Pancreatic cancer: Open or minimally invasive surgery? World J Gastroenterol 2016; 22(32): 7301-7310
- URL: https://www.wjgnet.com/1007-9327/full/v22/i32/7301.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i32.7301
Pancreatic cancer ranks as the 4th highest cause of cancer-related death in the United States and the 5-year survival is about 6%[1]. Surgical R0 resection is the best chance for a cure and remains the cornerstone of treatment of pancreatic malignancy[2,3]. However, pancreatic surgery is believed to be one of the most challenging procedures because of the high risks of postoperative morbidity and mortality associated with intraoperative bleeding and postoperative complications including pancreatic fistula[2,4,5]. Another key point for surgical treatment of pancreatic malignancy is oncological adequacy. R0 resection is the best chance for patients to have a good prognosis[3,6].
Minimally invasive techniques, including laparoscopic and robotic approaches, have rapidly evolved and include a variety of abdominal surgical procedures[7-10]. They provide the patients with better short-term outcomes, including smaller incisions, shorter hospital stay and less blood loss. Some surgeons in large-volume pancreatic centers have tried minimally invasive pancreatic surgery[11-16]. However, the short- and long-term outcomes of pancreatic malignancy remain controversial, especially for oncological prognosis.
Many pancreatic surgeons doubt the safety and oncological adequacy of minimally invasive pancreatic surgery. Here, we collected and analyzed the published data about minimally invasive pancreatic surgery.
Following the first report of laparoscopic pancreaticoduodenectomy (LPD) in 1994[17], Gagner and Pomp[18] subsequently published a series of 10 patients in 1997. In Gagner’s series, the conversion rate was 40% and the operating time was 8.5 h. Dependent upon these results, the authors concluded that the minimally invasive approach was not advocated because there was no apparent advantage over traditional open approaches. After that, surgeons spent a decade improving their laparoscopic skills, until a large LPD cohort was reported in France in 2005[12] and then in India in 2009[15]. During 1994-2009, several surgeons tried to apply hybrid, laparoscopic-open approaches to avoid the complexity of a purely laparoscopic procedure[13,19]. Although these approaches may overcome some of the limitations, they may reduce the potential benefits of purely laparoscopic approaches, including less pain, improved postoperative recovery and shorter hospital stay. After Palanivelu et al[15] reported 75 cases of LPD in 2009, large cohorts of LPD have been reported in the United States[11,20,21], South Korea[16], China[22], Italy[23] and France[24]. LPD eventually gained momentum, following its 30 years’ development, and it has emerged as a well-established procedure with acceptable morbidity and mortality rates in some specialized high-volume pancreatic centers[12,15,16,20,22,23]. Although LPD has been accepted in many specialized minimally invasive pancreatic centers, the short- and long-term results remain controversial. We collected clinical reports with comparative data between minimally invasive PD (MIPD) and open PD (OPD) (Table 1).
| Ref. | Year | Country | Technique | Cases | Operating time, min | EBL, mL | LHS, D | CD≥III | PF | DGE | Readmission rate | Mortality |
| Sharp et al[21] | 2015 | United States | LPD | 384 | NR | NR | NR | NR | NR | NR | 5.0% | 5.2% (30 D) |
| OPD | 4037 | NR | NR | NR | NR | NR | NR | 9.0% | 3.7% (30 D) | |||
| Song et al[16] | 2015 | SouthKorea | LPPPD | 93 | 482.5 ± 117.6 | 609 ± 375 | 14.3 ± 7.8 | 7.5% | 6 (6.5) | 3.2% | 5 (5.4) | NR |
| OPPPD | 93 | 347.9 ± 87.2 | 570 ± 448 | 19.2 ± 8.8 | 5.4% | 6 (6.5) | 7.5% | 3 (3.2) | NR | |||
| Chen et al[34] | 2015 | China | RPD | 60 | 410 ± 103 | 400 (200-600) | 20 ± 7.4 | 11.7% | 13.3% | 8.3% | NR | 1.7% |
| OPD | 120 | 323 ± 80 | 500 (350-800) | 25 ± 11.2 | 13.3% | 24.9% | 15.0% | NR | 2.5% | |||
| Dokmak et al[24] | 2015 | France | LPD | 46 | 342 (240-540) | 368 (50-1200) | 25 (6-104) | 28.0% | 48.0% | 17.0% | 9.0% | 2.0% |
| OPD | 46 | 264 (120-400) | 293 (50-1200) | 23 (7-115) | 20.0% | 41.0% | 15.0% | 9.0% | 0 | |||
| Baker et al[31] | 2015 | United States | RPD | 22 | 454 (294-529) | 425 (50-2200) | 7 (4-25) | 13.6% | 4.6% | 13.6% | 22.7% | 0 |
| OPD | 49 | 364 (213-948) | 650 (150-6100) | 9 (5-48) | 20.4% | 12.2% | 30.6% | 29.8% | 4.1% | |||
| Tran et al[74] | 2015 | United States | LPD | 681 | NR | NR | 12 (9-20) | NR | NR | NR | NR | 3.8% |
| OPD | 14893 | NR | NR | 11 (8-16) | NR | NR | NR | NR | 5.0% | |||
| Tan et al[75] | 2015 | China | LPD | 30 | 513.17 ± 56.13 | NR | 9.97 ± 3.74 | NR | 10/30 | 2/30 | NR | 0 |
| OPD | 30 | 371.67 ± 85.53 | NR | 11.87 ± 4.72 | NR | 6/30 | 3/30 | NR | 1/30 | |||
| Adam et al[32] | 2015 | United States | MIPD | 983 | NR | NR | NR | NR | NR | NR | 4.8% | NR |
| OPD | 6078 | NR | NR | NR | NR | NR | NR | 3.7% | NR | |||
| Chalikonda et al[33] | 2014 | United States | HPD | 30 | 476.00 | 485 | 9.79 | 30.0% | NR | NR | NR | 4.0% |
| OPD | 30 | 366.48 | 775 | 13.26 | 43.0% | NR | NR | NR | 0 | |||
| Bao et al[76] | 2014 | United States | RPD | 28 | 431 (340-628) | 100 (50-300) | 7.4 (5.5-17.1) | NR | 29.0% | NR | 25.0% | 7.0% (90 D) |
| OPD | 28 | 410 (190-621) | 300 (100-800) | 8.1 (6.5-15.3) | NR | 29.0% | NR | 25.0% | 7.0% (90 D) | |||
| Croome et al[20] | 2014 | United States | LPD | 108 | 379.4 ± 93.5 | 492.4 ± 519.3 | 6 (4-118) | 5.6% (≥ IIIb) | 11% (B/C) | 9% (B/C) | NR | 1.0% (I H) |
| OPD | 214 | 387.6 ± 91.8 | 866.7 ± 733.7 | 9 (5-73) | 13.6% (≥ IIIb) | 12% (B/C) | 18% (B/C) | NR | 2.0% (I H) | |||
| Speicher et al[77] | 2014 | United States | LPD | 25 | 381 (342-465) | 200 (100-425) | 8.5 (7-11.2) | NR | 16% (B/C) | NR | 30.4% | 0 (30 D) |
| HPD | 31 | 442 (386.5-486.5) | 600 (312.5-700) | 12 (8.5-18.5) | NR | 35.5% | NR | 35.5% | 3.2%(30 D) | |||
| OPD | 84 | 425.5 (345.8-478.8) | 425 (300-700) | 10 (8-14) | NR | 22.6% | NR | 39.3% | 1.2% (30 D) | |||
| Asbun et al[11] | 2012 | United States | LPD | 53 | 541 ± 88 | 195 ± 136 | 8.0 ± 3.2 | NR | 16.7% | 11.3% | NR | 5.7% (100 D) |
| OPD | 215 | 401 ± 108 | 1032 ± 1151 | 12.4 ± 8.5 | NR | 17.3% | 15.3% | NR | 8.8% (100 D) | |||
| Lai et al[28] | 2012 | China | RPD | 20 | 491.5 ± 94.0 | 247 (50-889) | 13.7 ± 6.1 | NR | 35.0% | 5% | NR | 0% |
| OPD | 67 | 264.9 ± 63.7 | 774.8 (50-8000) | 25.8 ± 23.1 | NR | 17.9% | 11.9% | NR | 3% |
PD is a complex procedure because of the dissection around important vessels and three complex reconstructions. Moreover, it is a procedure with high morbidity[25]. Although LPD has been accepted in some specialized centers, it is still a challenging operation for most pancreatic surgeons; still, there has been a rapid increase in the number of LPDs performed in different centers. Some large-volume centers have published their comparative studies between LPD and OPD[11,15,16,20,21,24,26-31], demonstrating the safety of LPD; although, long-term oncological benefits of this approach remain debatable.
Croome et al[20] reviewed their data for patients with pancreatic ductal adenocarcinoma (PDAC) undergoing LPD (n = 108) and OPD (n = 214). A significantly reduced blood loss and blood transfusion requirement and a shorter postoperative stay (6 d vs 9 d) were observed in the LPD group compared with the OPD group.
A case match study was performed by Dokmak et al[24] comparing 46 LPD and OPD procedures. Patients were matched for demographic data, associated comorbidity and underlying disease. The results suggested that a high rate of severe morbidity due to severe pancreatic fistula was detected in patients with a high risk of pancreatic fistula. In a subgroup of patients with a low risk of pancreatic fistula, the outcome of the two approaches was similar. The result of this study suggested that, in a subgroup of patients with a high risk of pancreatic fistula, LPD was associated with high morbidity. Thus, it should be considered only in patients with a dilated pancreatic duct and a hard pancreas texture, who are believed to have a low risk of pancreatic fistula.
Adam et al[32] reviewed patients undergoing PD from the National Cancer Database between 2010 and 2011, including 983 MIPDs and 6078 LPDs. Their results suggested that, for patients with PDAC, no difference was detected in number of lymph nodes (LNs) removed, rate of R0 resection, length of hospital stay or readmission. However, the 30-d mortality was lower in the OPD group than in the MIPD group. The authors suggested that the widespread adoption of the technique should be paused. MIPD is a complex procedure that needs comprehensive protocols outlining criteria for implementation.
Asbun and Stauffer[11] presented retrospective clinical data from Mayo Clinic of 215 OPD and 53 LPD patients. They also showed significantly better results in LPD groups, such as less blood loss (P < 0.001) and blood transfusion requirements (P < 0.001), and a shorter postoperative hospital stay (P < 0.001). While a significantly longer operating time was observed in LPD (P < 0.001), the LPD patients had a greater number of LNs removed than the OPD patients (P = 0.007). This series also demonstrated that LPD is safe and feasible and showed some benefits for patients.
Results from another cohort with PDAC in the United States treated with LPD were presented at the Western Surgical Association 122nd Scientific Session[21]. The researchers compared 4037 OPDs with 384 LPDs and showed significant differences favoring LPD for length of hospital stay and unplanned readmission. A lower risk of 30-d mortality was found in high-volume centers and in centers with experience of performing more than 10 LPDs; moreover, the 30-d mortality for LPD was similar to that for OPD. Finally, the researchers demonstrated that there is a learning curve for LPD.
Song et al[16] compared 137 laparoscopic pylorus-preserving PDs (LPPPDs) with 2055 open PPPDs (OPPPDs) in South Korea. They found that operating time was longer for LPPPD than for OPPPD, and the perioperative complications were similar in both groups. Fewer analgesic injections were administrated in the LPPPD group (P < 0.001). The oncological results were similar between the two groups, including number of LNs removed and long-term survival.
In addition to LPD, a few studies have compared robotic PD (RPD) with OPD. Chalikonda et al[33] from the Cleveland Clinic reviewed the results of 30 matched laparoscopic RPD (LRPD) and OPD procedures. LRPD and OPD groups were matched with demographics. A similar estimated blood loss and rate of reoperation were found in the two groups. However, there was a significant increase in operating time and shorter hospital stay for the LRPD group.
We found that most of the clinical studies showed that LPD is as safe and feasible as OPD technically, and has some of the superiority associated with minimally invasive surgery, such as less estimated blood loss and shorter hospital stay. However, some authors have suggested that MIPD should be advocated in a subgroup of patients with lower risk of pancreatic fistula. In our opinion, LPD is as safe as OPD. However, due to the complexity of LPD, it is a technically demanding procedure with a learning curve. In small clinical cohorts of LPD at the beginning of the learning curve, there might be higher morbidity and mortality for LPD than for OPD. The problem now is how to reduce the risks of LPD at the beginning of the learning curve. Apart from technical feasibility, the major arguments against LPD are oncological adequacy, especially for patients with PDAC.
Pancreatic cancer still has a high fatality rate. Radical resection is required for a good prognosis. Many clinical studies have reported LPD; however, most of those studies have included a variety of diseases requiring LPD. To the best of our knowledge, few studies have compared the oncological prognosis of PDAC treated with LPD or OPD (Table 2).
| Ref. | Year | Country | Technique | No. of PDAC cases | Rate of R0 resection | No. of LN | Positive LN | Tumor size, cm |
| Sharp et al[21] | 2015 | United States | LPD | 384 | 80.0% | 18 ± 9.7 | NR | 3.2 ± 1.3 |
| OPD | 4037 | 74.0% | 16 ± 9.6 | NR | 3.3 ± 2.4 | |||
| Song et al[16] | 2015 | South Korea | LPPPD | 11 | 72.7% | 15 ± 10 | 0.8 ± 1.2 | 2.8 ± 0.6 |
| OPPPD | 261 | 81.0% | 16.2 ± 9.6 | 1.5 ± 2.2 | 3.0 ± 1.2 | |||
| Dokmak et al[24] | 2015 | France | LPD | 15 | 60.0% | 20 (8-59) | 4.7 (0-32) | 2.4 (1.5-4) |
| OPD | 14 | 50.0% | 25 (8-47) | 2.2 (0-12) | 2.8 (2.5-4) | |||
| Chen et al[34] | 2015 | China | RPD | 19 | 94.7% | 18.1 ± 6.6 | NR | 3.0 ± 0.9 |
| OPD | 38 | 92.1% | 17.8 ± 7.1 | NR | 3.1 ± 1.0 | |||
| Croome et al[20] | 2014 | United States | LPD | 108 | 77.8% | 21.4 ± 8.1 | 73.1% | 3.3 ± 1.0 |
| OPD | 214 | 76.6% | 20.1 ± 7.5 | 72.0% | 3.3 ± 1.3 |
Song et al[16] compared the oncological results of pancreatic cancer treated with OPPPD (n = 261) and LPPPD (n = 11). TMN stage, R0 resection rate, in-hospital stay and the overall survival were similar between the two groups. In a case-control study from France[24], the results for LPD (n = 15) in patients with PDAC were similar to those with OPD (n = 14) with regard to tumor size, number of LNs harvested and rate of R0 resection. Croome et al[20] reported a large single center study of pancreatic carcinoma treated with LPD. Clinical data of 108 cases of LPD were reviewed retrospectively and compared with 214 cases of OPD performed in the same period at their center. The short-term oncological results, including tumor size, LN positivity, R0 resection and overall survival, were similar between the two groups, and significantly longer progression-free survival was found in the LPD group. The authors thought that this difference might have been because the patients who underwent LPD had the advantage of minimal invasiveness and recovered faster from the operation. This allowed the patients to receive adjuvant therapy in a timely manner and probably led to better prognosis.
A large LPD cohort study[21] from the National Cancer Data Base involved 384 LPDs and 4039 OPDs. The results showed no difference between the LPD and OPD groups with regard to length of stay, margin-positive resection, LN count and readmission rate.
Chen et al[34] compared the oncological results of pancreatic cancer treated with RPD (n = 19) and OPD (n = 38). There was no difference in the R0 resection rate, number of LNs resected, cancer stage, overall survival and disease-free survival between the two groups.
All the results above show that in most of the experienced minimally invasive pancreatic centers, LPD has similar short-term oncological results as OPD. However, Croome et al[20] reported the long-term prognostic benefit in the LPD group because of the advantages of minimal invasiveness. Furthermore, Croome et al[20] presented the largest cohort with pancreatic cancer treated with LPD, thus, we can probably form the hypothesis that, if surgeons acquire enough experience of LPD, LPD can yield the benefits of minimal invasiveness as well as long-term oncological benefit, compared with OPD. To obtain oncological adequacy, some technical tips are suggested for application during the operation.
Superior mesenteric artery-first approach: To improve the long-term prognosis of patients with PDAC, curative (R0) resection is required initially. Many reports have discussed the value of R0 resection in prognosis of PDAC. The consensus among pancreatic surgeons is that positive surgical margins are associated with poor survival[35-38]. The primary site of positive margins is from the right side of the superior mesenteric artery (SMA) (N14) to the right side of the celiac trunk (N9), including the mesopancreas[39]. To improve R0 resection, the SMA-first approach was advocated in OPD. The artery-first approach has been proven as effective in reducing the risk of bleeding and improving the rate of R0 resection in pancreatic cancer.
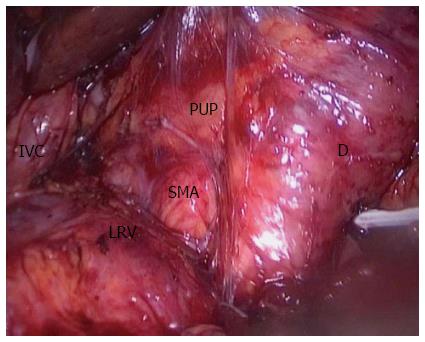
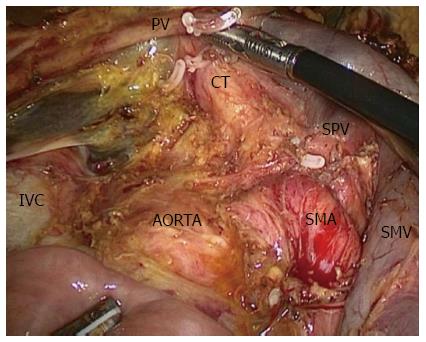
However, few publications have reported the SMA-first approach in LPD. To the best of our knowledge, only two publications have described laparoscopic SMA-first approaches[40,41]. Pittau et al[40] reported the right posterior approach. The authors performed this procedure exactly like the Pessaux procedure in OPD[42], and they dissected the SMA after complete kocherization, including mobilization of the right colon. Cho et al[41] described the left posterior SMA-first approach. They dissected the SMA at the ligament of Treitz without mobilization of the duodenum or right colon[43]. In our center, we perform the right posterior SMA-first approach, as described by Pittau et al[40]. We expose the SMA from the right side after complete kocherization (Figure 1). After exposure of the SMA, it would be easy to decide the resectability of the tumor. Another benefit is that this approach makes resection of the uncinate process from the SMA easier, and warrants complete removal of the neuro laminar tissue at the right side of the SMA up to the celiac axis (Figure 2).
Major vein resection: Involvement of the portal vein in locally advanced tumor is no longer a contradiction for surgical resection of pancreatic malignancy using traditional open procedures. A lot of data from larger pancreatic centers have provided evidence indicating that en bloc resection of tumor with involved vessels is safe and feasible, and can improve the rate of R0 resection[44-51]. Patients who have en bloc resection with the involved vein have similar long-term oncological prognosis compared with patients who do not have vascular involvement[44,45,48-50].
Kendrick et al[52] reported the first example of LPD with vein resection. Later in that same year, Giulianotti et al[53] published data of RPD with major vein resection. Kendrick et al[52] reported 11 patients who underwent laparoscopic pancreatectomy with major venous resection. In their series, one segmental resection and 10 tangential venous resections were described. Giulianotti et al[53] described three cases of robot-assisted distal pancreatectomy (DP) with vascular resection (two cases of celiac truck resection and one of portal vein resection) and two cases of RPD with portal vein resection. These initial results show that, for surgeons with considerable experience of minimally invasive pancreatic surgery, major vein resection during pancreatectomy is a safe and feasible adjunctive procedure. Kendrick et al[52] consequently reported a series of LPD with major vein resection. Thirty-one patients who underwent LPD with vascular resection were compared with 58 patients who underwent OPD with major vessel resection. The LPD group had decreased blood loss and shorter length of hospital stay, but there was no difference between LPD and OPD with regard to severe complications, mortality or overall survival. The authors concluded that LPD with vein resection is safe and feasible, and can achieve similar outcomes compared to patients undergoing OPD with vein resection. Most of the minimally invasive pancreatic centers considered LPD with vein resection a contraindication. However, for further application of LPD in patients with pancreatic malignancy with vein involvement, it is necessary for surgeons to master the minimally invasive technique of vein resection and reconstruction.
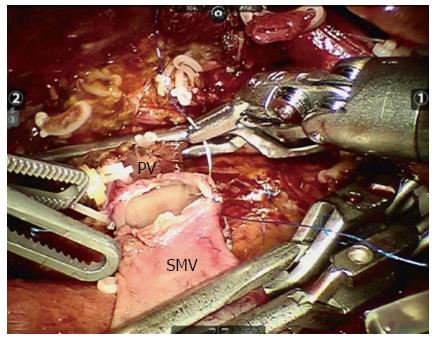
In our center, we have performed five MIPD procedures with major vein resection: four patients underwent LPD with tangential venous resection, and one underwent robotic vein segment resection (Figure 3). In our limited experience of LPD with major vein resection, tangential venous resections can be performed safely laparoscopically. However, for segmentectomy of the major vein, a robotic system is advocated. The first RPD was performed by Giulianotti et al[54] in 2001. RPD has since been proven to be feasible and safe, with the minimally invasive advantages compared with open procedures[30,31,33,34,55,56]. It is believed that the robotic surgical system provides surgeons with enhanced dexterity, superior magnified high-resolution 3D visualization, and greater precision and ergonomic comfort. This approach enables surgeons to control the surgical instruments with accuracy, flexibility and a wide range of motion, which is suggested for procedures that require complicated resection and reconstruction, such as prostatectomy, coronary surgery and PD. In our opinion, the application of robotic systems in PD with major vein resection can improve the quality of vein reconstruction, and we advocate them if possible.
DP is widely accepted as an option for PDAC located in the distal pancreas. However, in past decades, laparoscopic DP (LDP) has been accepted increasingly with evidence of minimally invasive benefits. Compared with LPD, LDP is less technically demanding because there is limited dissection around the vessels and no reconstruction is required[57,58]. So, more surgeons accept LDP than LPD.
A recently published meta-analysis[59-63] indicated that LDP was a safe and feasible option in terms of operating time and postoperative mortality and morbidities, such as postoperative bleeding and pancreatic fistula. Moreover, minimally invasive superiority was found in LDP, including significantly decreased estimated blood loss, time to first oral intake and length of hospital stay[59-63]. These results clearly show that LDP is as safe and feasible as ODP.
Microscopically, R0 resection is the most important part of treatment of resectable pancreatic cancer. Some non-comparative cohorts have shown that R0 resection of pancreatic cancer can be achieved by laparoscopic resection[64,65]. Most of the comparative studies have shown that there is no difference in the rate of R0 resection in the final pathological results between LDP and ODP[58,66,67]. To the best of our knowledge, only DiNorcia et al[68] have reported a decrease in R1 resection in the laparoscopic group; however, their series had mixed pathology, including neuroendocrine tumor and pancreatic adenocarcinoma. Another important short-term oncological marker is LN retrieval. A minimum of 12 LNs is required for resection of pancreatic adenocarcinoma[69,70]. N0 patients with > 12 LNs have better survival than N0 patients with < 12 LNs (P < 0.001)[70]. Most studies have found that the number of LNs harvested in laparoscopic and open procedures is similar[58,66-68,71]. The data here demonstrate that most of the minimally invasive pancreatic surgeons have a consensus that LDP has the same short-term oncological results as ODP.
Only a few studies have described long-term prognosis after LDP, and few comparative data are reported. Mabrut et al[64] reported 16 patients with pancreatic malignancy, 4 of whom had pancreatic adenocarcinoma, and 23% of these patients had recurrence during 15 mo. Fernández-Cruz et al[72] reported 10 cases of laparoscopic radical antegrade modular pancreatosplenectomy (RAMPS), with 3 having died within a year and a median survival period of 14 mo. Rehman et al[67] found a similar 3-year overall survival between 8 LDP and 14 ODP procedures for PDAC. Kooby et al[58] reported similar median survival (16 mo) after LDP and ODP in a matched study. Kim et al[73] reported 11 LDPs with diagnosis of malignancy in their postoperative pathological results, including 5 cases of PDAC; only 1 patient died of cancer during the follow-up period (3-60 mo). The results to date suggest that the long-term prognosis of LDP for adenocarcinoma is similar to that for open procedures. It was also found that there was no difference in short-term oncological markers, including tumor size, radiological stage, margin-negative resection, power of LN retrieval and LN metastasis between the two groups. The authors concluded that LDP is acceptable for patients with pancreatic malignancy. However, further larger studies are required to give solid evidence of the long-term oncological benefit of LDP.
After initial reports of LPD and LDP in the 1990s, laparoscopic pancreatectomy finally became a well-established procedure following 30 years’ development of laparoscopic skills and equipment. The data here suggest that minimally invasive pancreatectomy is safe and feasible and has adequate evidence of good short-term outcome. However, randomized controlled trials and long-term oncological results are still lacking. The long-term oncological results should be further addressed by randomized controlled trials. Another problem now is how to generalize this procedure from experienced hands to other centers.
We wish to thank Tang Wei and Song Peipei from Tokyo University for their kind help and critical review of the manuscript.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kleeff J S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Ma S
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 12991] [Article Influence: 1443.4] [Reference Citation Analysis (2)] |
| 2. | Gao JJ, Song PP, Tamura S, Hasegawa K, Sugawara Y, Kokudo N, Uchida K, Orii R, Qi FH, Dong JH. Standardization of perioperative management on hepato-biliary-pancreatic surgery. Drug Discov Ther. 2012;6:108-111. [PubMed] |
| 3. | Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2078] [Cited by in RCA: 2206] [Article Influence: 147.1] [Reference Citation Analysis (2)] |
| 4. | Shimoda M. Upon completing the 7th Sino-Japanese Symposium on Hepato-Pancreato-Biliary Disease. Biosci Trends. 2008;2:96. [PubMed] |
| 5. | Yamashita S, Sakamoto Y, Kaneko J, Tamura S, Aoki T, Sugawara Y, Hasegawa K, Kokudo N. Resection of the second portion of the duodenum sacrificing the minor papilla but preserving the pancreas for a recurrent duodenal adenocarcinoma: report of a case. Biosci Trends. 2012;6:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Flattet Y, Yamaguchi T, Andrejevic-Blant S, Halkic N. Pancreatic adenocarcinoma: the impact of preneoplastic lesion pattern on survival. Biosci Trends. 2015;9:402-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Hong D, Cheng J, Wang Z, Shen G, Xie Z, Wu W, Zhang Y, Zhang Y, Liu X. Comparison of two laparoscopic splenectomy plus pericardial devascularization techniques for management of portal hypertension and hypersplenism. Surg Endosc. 2015;29:3819-3826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Hong D, Liu Y, Peng S, Sun X, Wang Z, Cheng J, Shen G, Zhang Y, Huang D. Binding pancreaticogastrostomy in laparoscopic central pancreatectomy: a novel technique in laparoscopic pancreatic surgery. Surg Endosc. 2016;30:715-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 9. | Hong DF, Gao M, Bryner U, Cai XJ, Mou YP. Intraoperative endoscopic sphincterotomy for common bile duct stones during laparoscopic cholecystectomy. World J Gastroenterol. 2000;6:448-450. [PubMed] |
| 10. | Hong DF, Xin Y, Chen DW. Comparison of laparoscopic cholecystectomy combined with intraoperative endoscopic sphincterotomy and laparoscopic exploration of the common bile duct for cholecystocholedocholithiasis. Surg Endosc. 2006;20:424-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg. 2012;215:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 12. | Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc. 2006;20:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Kimura Y, Hirata K, Mukaiya M, Mizuguchi T, Koito K, Katsuramaki T. Hand-assisted laparoscopic pylorus-preserving pancreaticoduodenectomy for pancreas head disease. Am J Surg. 2005;189:734-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Lee SH, Kang CM, Hwang HK, Choi SH, Lee WJ, Chi HS. Minimally invasive RAMPS in well-selected left-sided pancreatic cancer within Yonsei criteria: long-term (>median 3 years) oncologic outcomes. Surg Endosc. 2014;28:2848-2855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Palanivelu C, Rajan PS, Rangarajan M, Vaithiswaran V, Senthilnathan P, Parthasarathi R, Praveen Raj P. Evolution in techniques of laparoscopic pancreaticoduodenectomy: a decade long experience from a tertiary center. J Hepatobiliary Pancreat Surg. 2009;16:731-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Song KB, Kim SC, Hwang DW, Lee JH, Lee DJ, Lee JW, Park KM, Lee YJ. Matched Case-Control Analysis Comparing Laparoscopic and Open Pylorus-preserving Pancreaticoduodenectomy in Patients With Periampullary Tumors. Ann Surg. 2015;262:146-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 17. | Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408-410. [PubMed] |
| 18. | Gagner M, Pomp A. Laparoscopic pancreatic resection: Is it worthwhile? J Gastrointest Surg. 1997;1:20-25; discussion 25-26. [PubMed] |
| 19. | Ammori BJ. Laparoscopic hand-assisted pancreaticoduodenectomy: initial UK experience. Surg Endosc. 2004;18:717-718. [PubMed] |
| 20. | Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, Kendrick ML. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg. 2014;260:633-638; discussion 638-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 350] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 21. | Sharpe SM, Talamonti MS, Wang CE, Prinz RA, Roggin KK, Bentrem DJ, Winchester DJ, Marsh RD, Stocker SJ, Baker MS. Early National Experience with Laparoscopic Pancreaticoduodenectomy for Ductal Adenocarcinoma: A Comparison of Laparoscopic Pancreaticoduodenectomy and Open Pancreaticoduodenectomy from the National Cancer Data Base. J Am Coll Surg. 2015;221:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 22. | Wang M, Zhang H, Wu Z, Zhang Z, Peng B. Laparoscopic pancreaticoduodenectomy: single-surgeon experience. Surg Endosc. 2015;29:3783-3794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Corcione F, Pirozzi F, Cuccurullo D, Piccolboni D, Caracino V, Galante F, Cusano D, Sciuto A. Laparoscopic pancreaticoduodenectomy: experience of 22 cases. Surg Endosc. 2013;27:2131-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Dokmak S, Ftériche FS, Aussilhou B, Bensafta Y, Lévy P, Ruszniewski P, Belghiti J, Sauvanet A. Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg. 2015;220:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 25. | Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 896] [Cited by in RCA: 970] [Article Influence: 51.1] [Reference Citation Analysis (34)] |
| 26. | Wei H, Wei B, Zheng Z, Huang Y, Huang J, Fang J. Comparative study of outcomes after laparoscopic versus open pancreaticoduodenectomy. Zhonghua Weichang Waike Zazhi. 2014;17:465-468. [PubMed] |
| 27. | Langan RC, Graham JA, Chin AB, Rubinstein AJ, Oza K, Nusbaum JA, Smirniotopoulos J, Kayser R, Jha R, Haddad N. Laparoscopic-assisted versus open pancreaticoduodenectomy: early favorable physical quality-of-life measures. Surgery. 2014;156:379-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy--a comparative study. Int J Surg. 2012;10:475-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 29. | Hakeem AR, Verbeke CS, Cairns A, Aldouri A, Smith AM, Menon KV. A matched-pair analysis of laparoscopic versus open pancreaticoduodenectomy: oncological outcomes using Leeds Pathology Protocol. Hepatobiliary Pancreat Dis Int. 2014;13:435-441. [PubMed] |
| 30. | Buchs NC, Addeo P, Bianco FM, Ayloo S, Benedetti E, Giulianotti PC. Robotic versus open pancreaticoduodenectomy: a comparative study at a single institution. World J Surg. 2011;35:2739-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 155] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 31. | Baker EH, Ross SW, Seshadri R, Swan RZ, Iannitti DA, Vrochides D, Martinie JB. Robotic pancreaticoduodenectomy: comparison of complications and cost to the open approach. Int J Med Robot. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Adam MA, Choudhury K, Dinan MA, Reed SD, Scheri RP, Blazer DG, Roman SA, Sosa JA. Minimally Invasive Versus Open Pancreaticoduodenectomy for Cancer: Practice Patterns and Short-term Outcomes Among 7061 Patients. Ann Surg. 2015;262:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 199] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 33. | Chalikonda S, Aguilar-Saavedra JR, Walsh RM. Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc. 2012;26:2397-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 34. | Chen S, Chen JZ, Zhan Q, Deng XX, Shen BY, Peng CH, Li HW. Robot-assisted laparoscopic versus open pancreaticoduodenectomy: a prospective, matched, mid-term follow-up study. Surg Endosc. 2015;29:3698-3711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 35. | Konstantinidis IT, Warshaw AL, Allen JN, Blaszkowsky LS, Castillo CF, Deshpande V, Hong TS, Kwak EL, Lauwers GY, Ryan DP. Pancreatic ductal adenocarcinoma: is there a survival difference for R1 resections versus locally advanced unresectable tumors? What is a “true” R0 resection? Ann Surg. 2013;257:731-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 307] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 36. | Neoptolemos JP, Stocken DD, Dunn JA, Almond J, Beger HG, Pederzoli P, Bassi C, Dervenis C, Fernandez-Cruz L, Lacaine F. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg. 2001;234:758-768. [PubMed] |
| 37. | Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, Hodgin MB, Sauter PK, Hruban RH, Riall TS. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199-1210; discussion 1210-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1123] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 38. | Howard TJ, Krug JE, Yu J, Zyromski NJ, Schmidt CM, Jacobson LE, Madura JA, Wiebke EA, Lillemoe KD. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon’s contribution to long-term survival in pancreatic cancer. J Gastrointest Surg. 2006;10:1338-1345; discussion 1345-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 242] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 39. | Gaedcke J, Gunawan B, Grade M, Szöke R, Liersch T, Becker H, Ghadimi BM. The mesopancreas is the primary site for R1 resection in pancreatic head cancer: relevance for clinical trials. Langenbecks Arch Surg. 2010;395:451-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 40. | Pittau G, Sànchez-Cabùs S, Laurenzi A, Gelli M, Cunha AS. Laparoscopic Pancreaticoduodenectomy: Right Posterior Superior Mesenteric Artery “First” Approach. Ann Surg Oncol. 2015;22 Suppl 3:S345-S348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Cho A, Yamamoto H, Kainuma O. Tips of laparoscopic pancreaticoduodenectomy: superior mesenteric artery first approach (with video). J Hepatobiliary Pancreat Sci. 2014;21:E19-E21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | Pessaux P, Varma D, Arnaud JP. Pancreaticoduodenectomy: superior mesenteric artery first approach. J Gastrointest Surg. 2006;10:607-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 43. | Kurosaki I, Minagawa M, Takano K, Takizawa K, Hatakeyama K. Left posterior approach to the superior mesenteric vascular pedicle in pancreaticoduodenectomy for cancer of the pancreatic head. JOP. 2011;12:220-229. [PubMed] |
| 44. | Tseng JF, Raut CP, Lee JE, Pisters PW, Vauthey JN, Abdalla EK, Gomez HF, Sun CC, Crane CH, Wolff RA. Pancreaticoduodenectomy with vascular resection: margin status and survival duration. J Gastrointest Surg. 2004;8:935-49; discussion 949-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 407] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 45. | Takahashi S, Ogata Y, Tsuzuki T. Combined resection of the pancreas and portal vein for pancreatic cancer. Br J Surg. 1994;81:1190-1193. [PubMed] |
| 46. | Shibata C, Kobari M, Tsuchiya T, Arai K, Anzai R, Takahashi M, Uzuki M, Sawai T, Yamazaki T. Pancreatectomy combined with superior mesenteric-portal vein resection for adenocarcinoma in pancreas. World J Surg. 2001;25:1002-1005. [PubMed] |
| 47. | Nakao A, Takeda S, Sakai M, Kaneko T, Inoue S, Sugimoto H, Kanazumi N. Extended radical resection versus standard resection for pancreatic cancer: the rationale for extended radical resection. Pancreas. 2004;28:289-292. [PubMed] |
| 48. | Müller SA, Hartel M, Mehrabi A, Welsch T, Martin DJ, Hinz U, Schmied BM, Büchler MW. Vascular resection in pancreatic cancer surgery: survival determinants. J Gastrointest Surg. 2009;13:784-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 49. | Martin RC, Scoggins CR, Egnatashvili V, Staley CA, McMasters KM, Kooby DA. Arterial and venous resection for pancreatic adenocarcinoma: operative and long-term outcomes. Arch Surg. 2009;144:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 50. | Kaneoka Y, Yamaguchi A, Isogai M. Portal or superior mesenteric vein resection for pancreatic head adenocarcinoma: prognostic value of the length of venous resection. Surgery. 2009;145:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 51. | Harrison LE, Klimstra DS, Brennan MF. Isolated portal vein involvement in pancreatic adenocarcinoma. A contraindication for resection? Ann Surg. 1996;224:342-347; discussion 347-349. [PubMed] |
| 52. | Kendrick ML, Sclabas GM. Major venous resection during total laparoscopic pancreaticoduodenectomy. HPB (Oxford). 2011;13:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 53. | Giulianotti PC, Addeo P, Buchs NC, Ayloo SM, Bianco FM. Robotic extended pancreatectomy with vascular resection for locally advanced pancreatic tumors. Pancreas. 2011;40:1264-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 54. | Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 772] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 55. | Narula VK, Mikami DJ, Melvin WS. Robotic and laparoscopic pancreaticoduodenectomy: a hybrid approach. Pancreas. 2010;39:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Giulianotti PC, Sbrana F, Bianco FM, Elli EF, Shah G, Addeo P, Caravaglios G, Coratti A. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc. 2010;24:1646-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 266] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 57. | Vijan SS, Ahmed KA, Harmsen WS, Que FG, Reid-Lombardo KM, Nagorney DM, Donohue JH, Farnell MB, Kendrick ML. Laparoscopic vs open distal pancreatectomy: a single-institution comparative study. Arch Surg. 2010;145:616-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 58. | Kooby DA, Hawkins WG, Schmidt CM, Weber SM, Bentrem DJ, Gillespie TW, Sellers JB, Merchant NB, Scoggins CR, Martin RC. A multicenter analysis of distal pancreatectomy for adenocarcinoma: is laparoscopic resection appropriate? J Am Coll Surg. 2010;210:779-785, 786-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 239] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 59. | Jin T, Altaf K, Xiong JJ, Huang W, Javed MA, Mai G, Liu XB, Hu WM, Xia Q. A systematic review and meta-analysis of studies comparing laparoscopic and open distal pancreatectomy. HPB (Oxford). 2012;14:711-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 60. | Mehrabi A, Hafezi M, Arvin J, Esmaeilzadeh M, Garoussi C, Emami G, Kössler-Ebs J, Müller-Stich BP, Büchler MW, Hackert T. A systematic review and meta-analysis of laparoscopic versus open distal pancreatectomy for benign and malignant lesions of the pancreas: it’s time to randomize. Surgery. 2015;157:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 61. | Pericleous S, Middleton N, McKay SC, Bowers KA, Hutchins RR. Systematic review and meta-analysis of case-matched studies comparing open and laparoscopic distal pancreatectomy: is it a safe procedure? Pancreas. 2012;41:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 62. | Ricci C, Casadei R, Taffurelli G, Toscano F, Pacilio CA, Bogoni S, D’Ambra M, Pagano N, Di Marco MC, Minni F. Laparoscopic versus open distal pancreatectomy for ductal adenocarcinoma: a systematic review and meta-analysis. J Gastrointest Surg. 2015;19:770-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 63. | Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg. 2012;255:1048-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 388] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 64. | Mabrut JY, Fernandez-Cruz L, Azagra JS, Bassi C, Delvaux G, Weerts J, Fabre JM, Boulez J, Baulieux J, Peix JL, Gigot JF; Hepatobiliary and Pancreatic Section (HBPS) of the Royal Belgian Society of Surgery; Belgian Group for Endoscopic Surgery (BGES); Club Coelio. Laparoscopic pancreatic resection: results of a multicenter European study of 127 patients. Surgery. 2005;137:597-605. [PubMed] |
| 65. | Marangos IP, Buanes T, Røsok BI, Kazaryan AM, Rosseland AR, Grzyb K, Villanger O, Mathisen Ø, Gladhaug IP, Edwin B. Laparoscopic resection of exocrine carcinoma in central and distal pancreas results in a high rate of radical resections and long postoperative survival. Surgery. 2012;151:717-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 66. | Jayaraman S, Gonen M, Brennan MF, D’Angelica MI, DeMatteo RP, Fong Y, Jarnagin WR, Allen PJ. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg. 2010;211:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 67. | Rehman S, John SK, Lochan R, Jaques BC, Manas DM, Charnley RM, French JJ, White SA. Oncological feasibility of laparoscopic distal pancreatectomy for adenocarcinoma: a single-institution comparative study. World J Surg. 2014;38:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 68. | DiNorcia J, Schrope BA, Lee MK, Reavey PL, Rosen SJ, Lee JA, Chabot JA, Allendorf JD. Laparoscopic distal pancreatectomy offers shorter hospital stays with fewer complications. J Gastrointest Surg. 2010;14:1804-1812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 69. | House MG, Gönen M, Jarnagin WR, D’Angelica M, DeMatteo RP, Fong Y, Brennan MF, Allen PJ. Prognostic significance of pathologic nodal status in patients with resected pancreatic cancer. J Gastrointest Surg. 2007;11:1549-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 246] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 70. | Slidell MB, Chang DC, Cameron JL, Wolfgang C, Herman JM, Schulick RD, Choti MA, Pawlik TM. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 71. | Mehta SS, Doumane G, Mura T, Nocca D, Fabre JM. Laparoscopic versus open distal pancreatectomy: a single-institution case-control study. Surg Endosc. 2012;26:402-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 72. | Fernández-Cruz L, Cosa R, Blanco L, Levi S, López-Boado MA, Navarro S. Curative laparoscopic resection for pancreatic neoplasms: a critical analysis from a single institution. J Gastrointest Surg. 2007;11:1607-1621; discussion 1621-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 73. | Kim J, Han HS, Yoon YS, Cho JY, Ahn KS, Kwon Y. Outcomes of the patients who were postoperatively diagnosed as malignancy after laparoscopic distal pancreatectomy. Surg Laparosc Endosc Percutan Tech. 2012;22:467-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Tran TB, Dua MM, Worhunsky DJ, Poultsides GA, Norton JA, Visser BC. The First Decade of Laparoscopic Pancreaticoduodenectomy in the United States: Costs and Outcomes Using the Nationwide Inpatient Sample. Surg Endosc. 2016;30:1778-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 75. | Tan CL, Zhang H, Peng B, Li KZ. Outcome and costs of laparoscopic pancreaticoduodenectomy during the initial learning curve vs laparotomy. World J Gastroenterol. 2015;21:5311-5319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 76. | Bao PQ, Mazirka PO, Watkins KT. Retrospective comparison of robot-assisted minimally invasive versus open pancreaticoduodenectomy for periampullary neoplasms. J Gastrointest Surg. 2014;18:682-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 77. | Speicher PJ, Nussbaum DP, White RR, Zani S, Mosca PJ, Blazer DG, Clary BM, Pappas TN, Tyler DS, Perez A. Defining the learning curve for team-based laparoscopic pancreaticoduodenectomy. Ann Surg Oncol. 2014;21:4014-4019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |