Published online Aug 21, 2016. doi: 10.3748/wjg.v22.i31.7069
Peer-review started: April 4, 2016
First decision: May 12, 2016
Revised: June 10, 2016
Accepted: June 28, 2016
Article in press: June 28, 2016
Published online: August 21, 2016
Processing time: 132 Days and 21.8 Hours
Obesity and its comorbidities - including diabetes and obstructive sleep apnea - have taken a large and increasing toll on the United States and the rest of the world. The availability of commercial, clinical, and operative therapies for weight management have not been effective at a societal level. Endoscopic bariatric therapy is gaining acceptance as more effective than diet and lifestyle measures, and less invasive than bariatric surgery. Various endoscopic therapies are analogues of the restrictive or bypass components of bariatric surgery, utilizing gastric remodeling or intestinal anastomosis to achieve proven weight loss and metabolic benefits. Others, such as aspiration therapy, employ novel mechanisms of action. Intragastric balloons have recently been approved by the United States Food and Drug Administration, and a number of other technologies have completed large multicenter trials (such as AspireAssist aspiration therapy and Primary Obesity Surgery Endolumenal). Endoscopic sleeve gastroplasty and transoral outlet reduction for endoscopic revision of gastric bypass have proven safe and effective in a number of studies. As devices are approved for use, data will continue to accumulate for safety, effectiveness, and cost effectiveness. Bariatric endoscopists should be prepared to appropriately target and apply various endoscopic bariatric therapies in the context of a comprehensive long-term weight management program.
Core tip: Endoscopic bariatric therapies (EBT) are entering clinical practice. The bariatric endoscopist must be able to provide comprehensive care to patients who are overweight, have obesity, or have weight-related comorbidities. In addition to performing EBT, the endoscopist should be capable of determining appropriateness for EBT, understanding alternatives, ruling out organic causes for weight gain, and recognizing eating disorders. Patients should concurrently be enrolled in a long-term weight management program in order to maintain the benefits of EBT.
- Citation: Kumar N. Weight loss endoscopy: Development, applications, and current status. World J Gastroenterol 2016; 22(31): 7069-7079
- URL: https://www.wjgnet.com/1007-9327/full/v22/i31/7069.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i31.7069
The American Medical Association and other medical societies have stated that obesity is a chronic medical disease[1,2]. The mean body mass index (BMI) in the United States is approximately 28.5 kg/m2 - in the overweight range[3]. Over one-third of Americans are afflicted by obesity - 81 million adults[3]. Far more individuals are overweight. The existence of diet and lifestyle modification programs, medications for weight loss, and bariatric surgery has not reduced the size of the obesity epidemic or the rising burden of weight-related comorbidities such as obstructive sleep apnea and diabetes mellitus. Until recently, patients seeking weight loss therapies have faced a choice between conservative measures (such as diet and lifestyle modification) and bariatric surgery. The former are frequently ineffective over the long term, hampered by complex disadvantageous physiological responses to weight loss[4]. The latter has been relatively underused (47 per 100000 adults in 2012) for reasons including high initial health care utilization, restricted payor coverage in lower BMI ranges, and perceived invasiveness[5].
Endoscopic bariatric therapy (EBT) has the potential to be more effective than conservative measures, and more available and less invasive than bariatric surgery. Several endoscopic therapies for weight loss and control of metabolic comorbidities have been approved by the Food and Drug Administration (FDA), are used outside the United States, are in clinical trials, or are in development (Table 1). Additionally, endoscopic technologies can address weight regain after gastric bypass, averting the need for revisional surgery. This review will focus on endoscopic therapies for weight loss rather than primarily metabolic interventions, although weight loss is closely associated with metabolic benefits. Total weight loss differs from excess weight loss, which is defined as the proportion of excess weight (weight above ideal weight) lost. Primary and revisional endoscopic bariatric therapies, both available and on the horizon, will be discussed herein.
| Device | Procedure | Mechanism | Regulatory status |
| Orbera | Intragastric balloon | Space-occupying device | FDA-approved |
| Integrated dual balloon | Intragastric balloon | Space-occupying device | FDA-approved |
| OverStitch | Endoscopic sleeve gastroplasty | Gastric remodeling | FDA-approved1 |
| Incisionless operating platform | Primary obesity surgery endolumenal | Gastric remodeling | Under FDA review |
| Articulating circular endoscopic stapler | Gastroplasty | Gastric remodeling | In human trials |
| AspireAssist | Aspiration therapy | Aspiration | FDA-approved |
| Self-assembling magnets | Endoscopic enteral anastomosis | Dual-path enteral bypass | In human trials |
| OverStitch | Transoral outlet reduction for revision of gastric bypass | Anastomotic reduction | FDA-approved1 |
| Incisionless operating platform | Revision obesity surgery endolumenal for revision of gastric bypass | Anastomotic and pouch reduction | FDA-approved1 |
The bariatric endoscopist, whether gastroenterologist or bariatric surgeon, must be able to address the comprehensive care of the patient with overweight, obesity, or weight-related comorbidities. To achieve this, the endoscopist can integrate a team of specialists, retained or available by referral, in nutrition and dietetics, behavioral therapy, and exercise therapy[6]. The endoscopist should be able to assess appropriateness for EBT. This entails understanding of alternatives, including diet and lifestyle therapy, medications for weight loss, and bariatric surgeries, and referral for these when appropriate. Organic causes for weight gain should be ruled out or addressed before EBT is undertaken. Patients should be screened for eating disorders, with referral for management prior to EBT. Before undergoing the procedure, patients should enrolled into a long-term weight management program in order to maintain the benefits of the intervention.
Intragastric balloons are space-occupying devices which replace gastric lumenal volume and may distend the stomach, potentially inducing neurohormonal effects and changes in motility[7]. The gastric balloon was first reported in 1982 and approved for use in the United States three years later. The device was subsequently withdrawn due to a high rate of adverse events, but led to the development of several successful intragastric balloons in the following decades.
Application of intragastric balloon therapy should be performed in the context of a comprehensive weight management program that extends beyond balloon explantation. Although the balloon is removed after six months, weight loss should be maintained to the extent possible using the dietary habits, lifestyle changes, and behavioral modification instilled while the balloon is in place. There are several contraindications to intragastric balloon therapy, including prior gastrointestinal surgery, large hiatal hernia, clotting or bleeding disorders, hepatic cirrhosis, and pregnancy.
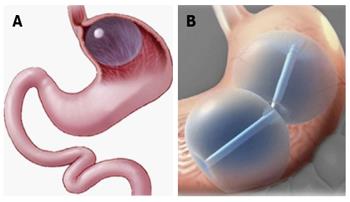
Orbera (Apollo Endosurgery, Austin, TX, United States) is a silicone elastomer balloon designed to be implanted in the stomach, filled with saline, and left in place for 6 mo (Figure 1A)[8]. The device (formerly called BioEnterics Intragastric Balloon) has been used outside the United States in over 200000 patients since the early 1990s, and was approved by the FDA in 2015. The FDA-approved indication is for adults with BMI of 30-40 kg/m2.
During the initial endoscopy, contraindications such as ulcer should be ruled out. To place the balloon, the placement catheter is advanced into the stomach under endoscopic visualization. The balloon is filled with at least 400mL (typically up to 700 mL) of saline. Additionally, injection of 10 mL of methylene blue has been reported, which will change the urine color to green in the event of balloon rupture. A soft food and then liquid diet is required in the days prior to removal, and the patient should be nil per os for at least 12 h prior to removal. Removal is performed using purpose-specific tools that fit through the biopsy channel of the endoscope, including a needle to fully aspirate the saline from the balloon and a grasper to hold the deflated balloon during explantation. The balloon should be removed at 6 mo, and the FDA requires a comprehensive 12-mo weight management program to be employed with Orbera placement.
A systematic review and meta-analysis of Orbera found abdominal pain in 33.7% of patients, nausea in 29%, early removal in 7.5%, balloon migration in 1.4%, and gastrointestinal perforation in 0.1%[9]. The meta-analysis found 25.4% (21.5%-29.4%) excess weight loss and 11.3% (8.2%-14.4%) total weight loss at 12 mo after balloon placement. This safety and efficacy performance met the American Society for Gastrointestinal Endoscopy Preservation and Incorporation of Valuable endoscopic Innovations thresholds for clinical adoption of primary EBT in Class II or III obesity, and for EBT as bridge therapy to reduce the risk of subsequent intervention such as major surgery.
Long-term maintenance of weight loss after Orbera placement has been studied. A study of 500 patients with BMI of 43.7 kg/m2 found that 83% achieved at least 20% excess weight loss at balloon removal[10]. This group had average weight loss of 23.9 ± 9.1 kg and average BMI decrease of 8.3 kg/m2. Five years later, 41% of the group was available for follow-up, and had maintained weight loss of 7.3 ± 5.4 kg and BMI decrease of 2.5 kg/m2.
The effect of Orbera placement on comorbidities has been studied. A multicenter trial of 261 patients with BMI of 27-30 kg/m2 (mean 28.6 ± 0.4 kg/m2) reported excess weight loss of 55.6% at 6 mo and 29.1% at 3 years[11]. The rate of hypertension decreased from 29% to 16%, diabetes from 15 to 10%, and hyperlipidemia from 32 to 21% at 3 years.
The Integrated Dual Balloon System (IDB; Reshape, San Clemente, California) comprises two independent silicone balloons attached to a flexible silicone shaft (Figure 1B). Each balloon is filled with up to 450 mL of saline, or 375 mL in females less than 64 inches in height. A mechanical pump is available to control balloon filling. Removal is accomplished using a purpose-specific catheter. The IDB is FDA-approved for adults with BMI of 30-40 kg/m2 and one or more obesity-related comorbidities. The IDB should be removed at 6 mo, and a 12-mo weight management program is required with device placement.
The prospective, randomized REDUCE trial compared IDB (n = 187) with sham endoscopy (n = 139)[12]. Both groups also underwent a medically-supervised diet and exercise program. The IDB group lost 25.1% of excess weight vs 11.3% in the control group. There was a 9% rate of early removal for nonulcer device intolerance. Although 6% of patients experienced a deflation, there were no balloon migrations due to the presence of two independent balloons. The IDB group experienced a 2.2-inch decrease in waist size at 48 wk. The IDB group experienced significant decreases in blood pressure (-8.3/4.3 mmHg), hemoglobin A1c (-0.2 percentage points), and low-density lipoprotein (-4.1 mg/dL) at 6 mo.
Endoscopic sleeve gastroplasty (ESG) is a restrictive procedure which entails endoscopic suturing for gastric remodeling to reduce lumenal volume. ESG may affect accommodation, motility, or both. The procedure creates a narrow tube, but unlike surgical sleeve gastrectomy, ESG does not entail excision of a portion of the stomach.
ESG was first performed in 2008, using the RESTORe endoscopic suturing device (Davol, Murray Hill, New Jersey)[13]. A version of the device had been previously used to perform an endoscopic version of vertical banded gastroplasty[14]. The RESTORe device was capable of deeper-thickness suturing than its predecessor, and suture reloading without device removal. The trial included 18 patients at two sites. Patients lost 27.7% ± 21.9% of excess weight, or 11.0 ± 10 kg, and an average 12.6 ± 9.5 cm in waist circumference. Change in comorbidites was not assessed. Notably, sutures were partially or completely detached in most patients at endoscopic follow-up.
OverStitch (Apollo Endosurgery, Austin, TX, United States) is a full-thickness endoscopic suturing device capable of rapid deployment of interrupted and running sutures and in vivo suture reloading. OverStitch comprises a needle driver attached to the tip of a double channel endoscope, and an actuating handle attached to the handle of the endoscope. A catheter is passed through one channel of the endoscope to function as a suture anchor, and a tissue helix can be inserted through the other channel to bring tissue into the device for full-thickness tissue acquisition. The device can be used to perform a number of procedures, such as stent fixation and perforation repair[15,16].
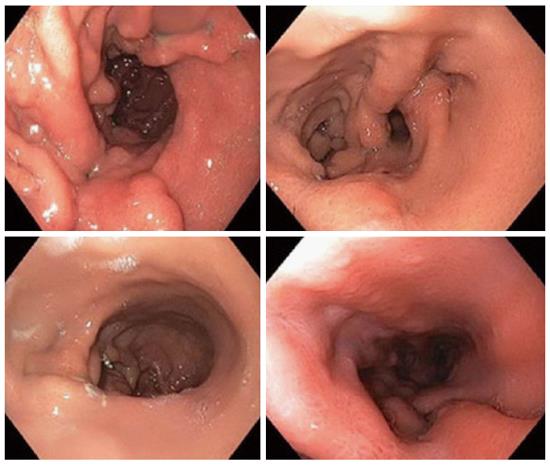
ESG with OverStitch is performed by placing running sutures, with approximately six stitches each, in a triangular fashion from the anterior gastric wall to the greater curvature to the posterior gastric wall. These are placed starting in the antrum and moving proximally to the fundus. The result is the formation of a tubular sleeve from the esophagus to the antrum, along the lesser curvature. The suture lines are then reinforced with interrupted stitches (Figure 2).
The initial cases of ESG with OverStitch were performed in an international procedure development trial in 2012, which studied multiple formulations of the procedure and resulted in the technique described above[17]. This technique has been applied in multiple series, and the Primary Obesity Multicenter Incisionless Suturing Evaluation (PROMISE) trial[18,19].
A series of 10 patients with average BMI of 45.2 kg/m2 reported excess weight loss of 33.0 kg, and 30% excess weight loss, at 6 mo[18]. Postprandial glucose, measured using area under the curve, demonstrated a significant decrease (36%). A prospective study of 25 patients with average BMI of 38.5 ± 4.6 kg/m2 reported total weight loss of 18.7 ± 10.7% and BMI decrease of 7.3 ± 4.2 kg/m2 at 12 mo[19]. The frequency of behavioral and nutritional contacts during the follow-up period was consistent with success. A prospective study of 25 patients with average BMI of 35.5 kg/m2 reported weight loss of 54% ± 40% at 12 mo (n = 10) and 45% ± 41% at 20 mo (n = 8)[20]. Three serious adverse events were reported: perigastric inflammatory serous fluid collection, pulmonary embolism, and pneumoperitoneum with pneumothorax. Four patients underwent detailed analysis of motility and neurohormonal changes. In this group, ESG was found to decrease caloric intake needed to reach maximum satiety by 59%, leading to decrease of meal duration from 35.2 ± 9.9 min to 11.5 ± 2.3 min. Fasting and postprandial ghrelin levels decreased by 29.4% at three-month follow-up. Insulin sensitivity significantly increased at 3 mo. An international multicenter series of 126 patients with BMI of 36.2 kg/m2 reported BMI decrease to 30.9 ± 0.8 kg/m2 at 6 mo and 29.8 ± 1.4 kg/m2 at 1 year[21]. Weight fell from 101.6 ± 2.3 kg initially to 86.9 ± 3.3 kg/m2 at 6 mo, and 81.8 ± 3.8 kg/m2 at 1 year. Further data regarding weight loss and improvement in comorbidities is awaited from ongoing studies.
Primary obesity surgery endolumenal (POSE) is a restrictive gastric procedure. The procedure is performed using the Incisionless Operating Platform (IOP; USGI Medical, San Clemente, CA, United States). The device creates tissue plications by opposing tissue, and then deploying and anchoring full-thickness stitches. The device uses a four-channel platform, with a 4.9 mm visualization endoscope, a rotatable tissue grasper and suture cutter (g-Prox), a tissue helix (g-Lix), and a suture anchor deployment catheter (g-Cath).
To perform the POSE procedure, the IOP is retroflexed and used to create two parallel rows with 4-5 plications each. This reduces the fundic apex to the level of the gastroesophageal junction. After the forward view is restored, a ridge of 3-4 plications is then created at the intersection of the gastric body and gastric antrum, across from the incisura.
A study of 45 patients with average BMI of 36.7 ± 3.8 kg/m2 reported BMI decrease of 5.8 kg/m2 at 6 mo[22]. Excess weight loss was 49.4% ± 21.5% and total weight loss was 15.5% ± 6.1% at 6 mo. Chest pain and low-grade fever were each reported in one case. A study of 147 patients with average BMI of 38.0 ± 4.8 kg/m2 reported total weight loss of 15.1% ± 7.8% and excess weight loss of 44.9% ± 24.4% at one year[23]. Younger patients, and those with higher initial BMI, had more success. No adverse events were reported. The randomized, sham-controlled ESSENTIAL trial has been completed, and data regarding weight loss and resolution of comorbidities are awaited. POSE is under FDA review for approval.
The articulating circular endoscopic (ACE) Stapler (Boston Scientific Corporation, Natick, MA, United States) is a completely rotatable and retroflexable endoscopic stapler. An ultrathin endoscope is used for visualization. Vacuum suction is used to acquire tissue. Full-thickness plications are created by firing 1-cm plastic rings with 8 titanium staples.
A prospective study of gastroplasty with ACE (performed by creating eight fundic and two antral plications) was performed in 17 patients (Figure 3)[24]. Median BMI fell from 40.2 kg/m2 to 34.5 kg/m2. Median excess weight loss was 34.9% (interquartile range 17.8-46.6) at 12 mo. Comorbidities including dyslipidemia, hypertension, diabetes, and obstructive sleep apnea improved. Endoscopy (performed in 11 patients) confirmed 6-9 extant plications in all cases, with durable gastric volume reduction.
AspireAssist (Aspire Bariatrics, King of Prussia, PA, United States) comprises a 30-French percutaneous endoscopic gastrostomy (PEG) tube designed to aspirate gastric contents, a valve port placed at the skin, and a device which connects to the port to flush and then aspirate gastric contents. The device is implanted in a fashion similar to a conventional PEG tube, and the skin port is attached two weeks later. Approximately 20 min after meals, water is infused into the stomach and gastric contents are drained several times. The device was recently approved by the FDA.
Aspiration therapy was studied in a randomized clinical trial of 18 patients (11 AspireAssist, 7 control)[25]. All patients were enrolled in dietary and lifestyle counseling. Of these, 10 AspireAssist (initial BMI 42.0 ± 1.4 kg/m2) and 4 control group patients (initial BMI 39.3 ± 1.1) completed the trial. Total weight loss at 12 mo was 18.6% ± 2.3% with AspireAssist vs 5.9% ± 5.0% in the control group. When given the option, 7/10 remaining AspireAssist patients continued for 12 mo and achieved a total weight loss of 20.1% ± 3.5%. Baseline glucose and lipid values were normal at baseline, and did not show significant changes. No maladaptive eating behaviors, such as increased intake, developed during the study. Complications were primarily related to the PEG tube, including three infections requiring treatment and one persistent fistula after removal which closed without intervention. Abdominal pain at the tube site was successfully addressed by redesigning the device.
A subsequent study of 25 patients with BMI of 39.8 ± 0.9 kg/m2 enrolled patients in a very low calorie diet (VLCD) for 4 wk before implantation of AspireAssist[26]. In per protocol analysis (22 patients), weight loss at 6 mo after aspiration therapy was 16.5 ± 7.8 kg including the VLCD, and 8.0 ± 7.4 kg without VLCD weight loss. Total excess weight loss was 40.8 ± 19.8% at 6 mo, with 14.8% ± 6.3% total weight loss. There was a trend toward improved fasting glucose and hemoglobin A1c, and significant improvement in fasting glucose in patients with type II diabetes mellitus. Three of five patients taking medication for diabetes were able to discontinue it. Early adverse events included post-procedure abdominal pain, intra-abdominal fluid collection, and skin breakdown around the stoma; a later skin infection required treated with antibiotics. Moderate abdominal pain was reported by 52% of patients in the first week, and severe pain by 12%.
The multicenter PATHWAY trial randomized subjects in a 2:1 ratio to AspireAssist with lifestyle counseling, or lifestyle counseling alone[27]. The BMI of the AspireAssist group was 42.0 ± 5.1 kg/m2, and the lifestyle counseling group had BMI of 40.9 ± 3.9 kg/m2. At 52 wk, per-protocol analysis showed 37.2% ± 27.5% excess weight loss in the AspireAssist group vs 13.0% ± 17.6% in the lifestyle counseling group. The most frequently reported adverse events were perioperative abdominal pain and postoperative granulation tissue and peristomal irritation. There was no evidence of increased caloric intake to compensate for aspirated calories, or of new abnormal eating behaviors after AspireAssist placement. Further results regarding change in comorbidities is awaited.
A number of technologies in clinical trials and under development hold the promise of extending EBT beyond gastric interventions. These devices may confer enhanced metabolic benefits, and may be used in combination with gastric or restrictive procedures in the future. Furthermore, the capability to address the small intestine will close the gap between EBT and bariatric surgery.
An endoscopic incisionless anastomosis system (GI Windows, West Bridgewater, Massachusetts) has been developed using self-assembling magnets[28]. Using simultaneous enteroscopy and colonoscopy, two magnets are deployed in the gastrointestinal tract and form octagonal rings. The magnets mate, apply compressive force to the tissue between them, and create a large-bore compression anastomosis over several days (Figure 4). The magnets then disassemble and pass from the gastrointestinal tract. The result is a large-bore anastomosis free of foreign bodies, and a dual-path enteral bypass (with flow of food down both limbs).
Self-assembling magnets for endoscopic anastomosis have been studied in two porcine trials. One trial entailed using the system to create a large jejunocolonic anastomosis in 5 pigs[29]. All anastomoses had formed by day 4, and magnets were expelled by day 12. The anastomoses remained patent and leak-free at 3 mo, with dramatically lower weight than controls. No fibrosis or inflammation was found on histologic examination. A separate study used IAS to create a jejunoileal bypass in 8 pigs[30]. The jejunal magnet was deployed endoscopically, although porcine anatomy required laparoscopic assistance for placement of the ileal magnet. A patent leak-free anastomosis was created by day 10, and necropsy at 90 d revealed lack of significant adhesions. Pressure testing proved the anastomoses to be stronger than native tissue.
The technology is now in human trials. A trial of 10 subjects (6 male) with BMI of 41 kg/m2 reported successful device placement and anastomosis formation in all cases[31]. Transient nausea and diarrhea were reported in most cases. There was no lifestyle counseling or caloric restriction. Mean weight loss at 6 mo was 10.6% total weight loss or 28.3% excess weight loss. Of 4 patients with type II diabetes, hemoglobin A1c declined from 7.8% to 6.0%, and fasting glucose fell to 111 mg/dL (a decline of 66 mg/dL). All diabetic patients were able to discontinue oral diabetic medications within 6 mo.
EndoBarrier (GI Dynamics, Lexington, MA, United States) is a Teflon sleeve 60 cm in length that is anchored by a barbed nickel-titanium ring. The device is anchored in the duodenal bulb, and the sleeve extends into the jejunum. Food travels within the sleeve to the mid-jejunum without making mucosal contact, and pancreaticobiliary secretions travel outside the sleeve without contacting food. Placement is recommended for 12 mo.
EndoBarrier is placed endoscopically with fluoroscopic guidance, under general anesthesia. A guidewire is placed into the duodenum, and the encapsulated device is passed over the guidewire. The sleeve is fully released in the small intestine, and then the anchor is placed in the duodenal bulb just beyond the pylorus. A grasper can be used to collapse the anchor and then remove the device endoscopically (using a foreign body hood).
A systematic review and meta-analysis of EndoBarrier reported 35.3% (24.6%-46.1%) excess weight loss and decrease of hemoglobin A1c by 1.5 percentage points at 12 mo[9]. Adverse events included migration in 4.9%, bleeding in 3.9%, and sleeve obstruction in 3.4%. Enrollment in a US multicenter trial was suspended in 2015 due to the development of hepatic abscess in 4 of 325 patients.
Roux-en-Y gastric bypass (RYGB) is a highly effective bariatric and metabolic surgery, capable of inducing total weight loss of 31.5% at 3 years[32]. Weight-related comorbidities such as diabetes mellitus, obstructive sleep apnea, and hypertension frequently improve or resolve after RYGB. However, some patients do not achieve their weight loss goals, and many regain part of the lost weight[33]. The reasons for weight regain are complex, including physiologic adaptation to weight loss. One addressable anatomic factor is dilation of the gastrojejunal anastomosis, which is linearly correlated with weight regain[34]. Anastomotic dilation may attenuate the restrictive component of RYGB, allowing rapid pouch emptying and transit of intake into the Roux limb. Although surgical revision is possible, it is associated with high adverse event rates, perhaps due to complex anatomy, adhesions, scarring, and older patient age[35-37]. Endoscopic revision of RYGB to restore restriction provides an effective option for patients who have failed dietary and lifestyle modification, without the invasiveness of surgical revision.
Transoral outlet reduction (TORe) is performed by using an endoscopic suturing device to place stitches around the gastrojejunal anastomosis, reducing its aperture. The procedure is performed under general anesthesia with endotracheal intubation. Carbon dioxide should be used for insufflation. A standard upper endoscope is used to examine the esophagus, gastric pouch, gastrojejunal anastomosis, Roux limb, and blind limb. Argon plasma coagulation (end-firing, 30 Watts) is used to ablate the anastomotic margin, approximately 5 mm in thickness on the gastric side. Alternatively, a modified endoscopic submucosal dissection can be performed around the anastomosis to expose muscularis, using submucosal injection followed by incision with a needle knife and insulated tip knife - however, this can be technically challenging due to fibrosis and scarring[38]. Next, an overtube should be inserted. In the most basic iteration of TORe, interrupted stitches are placed at the margins of the anastomosis, crossing its lumen, and tightened to appose the sides of the anastomosis. Gastric pouch volume can be reduced by apposing ridges of tissue.
EndoCinch (Bard Davol, Murray Hill, New Jersey) is a superficial-thickness suturing device which acquires tissue using suction. Endoscopic revision of gastric bypass with EndoCinch was first performed in 2004[39]. RESTORe, a randomized double-blind sham-controlled multicenter trial including 77 patients, established Level 1 evidence for the efficacy of TORe[40]. All patients had anastomotic dilation to > 20 mm. Initial BMI was 47.6 kg/m2. In the TORe group, 89% reached anastomosis aperture < 10 mm. At 6 mo, the TORe group had total weight loss of 3.8%, while the sham group lost 0.3% (P = 0.02). Weight regain was arrested in 96% of the TORe patients during the follow-up period. The TORe group had a significant improvement in systolic and diastolic blood pressure.
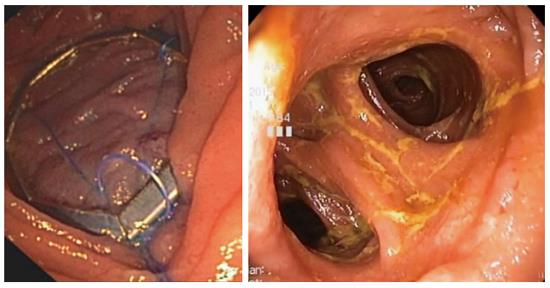
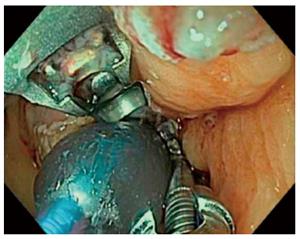
OverStitch (Apollo Endosurgery, Austin, TX, United States) can be used to perform TORe. The device is capable of performing full-thickness endoscopic suturing, and it is also capable of performing a pursestring TORe with a controlled radial expansion balloon (CRE) inserted through the second channel of the endoscope. Full-thickness TORe with OverStitch has proven more effective than TORe performed with EndoCinch in a matched cohort study[41]. The same interrupted TORe technique was performed with each device. There were 59 patients in each group, matched by anastomotic aperture before TORe, BMI, and age. The OverStitch group lost 8.6 ± 2.5 kg at one year, versus 2.9 ± 1.0 kg when EndoCinch was used. Unlike the basic interrupted TORe technique, the pursestring TORe technique enables consistent and precise sizing of the anastomosis, and it reinforces the entire margin of the anastomosis[42]. To perform pursestring TORe, a running stitch is inserted at least one full turn around the anastomosis. Before it is tightened, the CRE balloon is inserted into the anastomosis and inflated to a size of 8-10 mm. The pursestring is then tightened around the CRE balloon and cinched (Figure 5). A series of 25 patients undergoing TORe with OverStitch reported 5.6 ± 6.2 kg or 12.4% excess weight loss at 6 mo, and 7.5 ± 6.4 kg or 17.1% excess weight loss at 1 year[43]. A long-term series of full-thickness TORe including 150 patients who had weight regain despite dietary and lifestyle measures[44]. Weight loss was 10.5 ± 1.2 kg or 24.9 ± 2.6% excess weight loss at 1 year (n = 109), 9.0 ± 1.7 kg or 20.0% ± 6.4% excess weight loss at 2 years (n = 63), and 9.5 ± 2.1 kg or 19.2% ± 4.6% excess weight loss at 3 years (n = 40).The study did not find a benefit to performing gastric pouch volume reduction concurrently with TORe. Further data regarding change in comorbidities is awaited. TORe with OverStitch is an effective and durable procedure to address weight regain after gastric bypass.
Revision obesity surgery endolumenal (ROSE) is performed using the Incisionless Operating Platform (IOP; USGI Medical, San Clemente, CA, United States) described above for POSE. The device has been modified specifically to perform ROSE, with enhanced turning ability and other purpose-specific design changes to ease maneuvering in the small gastric pouch. The procedure reduces gastric pouch volume and anastomotic aperture by placing tissue anchors to create full-thickness tissue plications.
The results of ROSE have been reported in several series. A prospective series of 20 patients (with dilated anastomosis and weight regain) achieved 65% reduction in anastomotic diameter and demonstrated weight loss of 8.8 kg at 3 mo[45]. The device was improved, and a study of 5 patients reported 7.8 kg in weight loss at 3-6 mo[46]. A prospective multicenter series of 116 consecutive patients reported successful procedure completion in 97% of patients, with anastomotic diameter reduction of 50% and pouch length reduction of 44%[47]. Common adverse effects were pharyngitis (41%), nausea and vomiting (12%), and abdominal pain (11%). Additionally, three patients had superficial esophageal injuries, of which one required endoscopic clip placement. At 6 mo, patients reached 18% excess weight loss with loss of 32% of regained weight. A follow-up study was published, reporting weight loss of 5.9 ± 1.1 kg at 1 year, or 14.5% excess weight loss[48]. Endoscopy at 1 year revealed that 92% (of 66 patients available for follow-up) still had anchors in place. Those patients who had initial anastomotic diameter of over 12 mm with reduction to below 10 mm experienced 24% excess weight loss, underscoring the efficacy of outlet reduction. A recent retrospective analysis including 27 patients with initial anastomotic diameter of 21 mm reported long term results: 8% excess weight loss at 1 year (n = 10), -5.8% excess weight loss at 5 years (n = 4), and -4.5% excess weight loss at 6 years (n = 4)[49]. Endoscopy at 1 year found reversion to original anastomotic aperture. Weight regain was attributed to anatomic failure and lack of clinical follow-up. Although data regarding resolution of comorbidities has not been reported, study is ongoing.
Argon plasma coagulation can be used to repeatedly ablate the margin of the gastrojejunal anastomosis, resulting in scarring, decreased anastomotic aperture, and decreased tissue compliance[50]. Further study is ongoing. StomaphyX (EndoGastric Solutions, Redmond, WA, United States) is a full-thickness vacuum-assisted tissue placation platform. The device demonstrated efficacy in some series, but enrollment in a randomized sham-controlled trial was terminated due to failure to achieve the efficacy endpoint[51]. Endoscopic sclerotherapy uses injection of sclerosant (such as 10-25 mL of sodium morrhuate) to reduce anastomotic aperture and compliance; the technique often requires multiple sessions. It demonstrated efficacy in multiple series, but sodium morrhuate is no longer commercially available[52,53].
Obesity is among the most consequential health issues in the United States today, and it is emerging as a global epidemic. The burden of obesity and its comorbidities has not been alleviated by currently available diet and lifestyle modification techniques or bariatric surgery. However, a number of endoscopic therapies have developed a track record of safety and efficacy, and others are in clinical trials. Endoscopic revision of gastric bypass is effective and has become commonplace. Primary endoscopic procedures, including intragastric balloons, gastric remodeling devices, aspiration therapy, and small bowel technologies, take advantage of restrictive and bypass techniques that have proven effective in bariatric surgery. In the context of a multidisciplinary weight management program and long-term clinical follow-up, bariatric endoscopists will have multiple options to deliver safe, noninvasive therapy for obesity.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kuo SM, Lin Y, Panduro A S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Allison DB, Downey M, Atkinson RL, Billington CJ, Bray GA, Eckel RH, Finkelstein EA, Jensen MD, Tremblay A. Obesity as a disease: a white paper on evidence and arguments commissioned by the Council of the Obesity Society. Obesity (Silver Spring). 2008;16:1161-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | AMA Report of the Council on Science and Public Health. Available from: http://www.ama-assn.org/assets/meeting/2013a/a13-addendum-refcomm-d.pdf#page=19. |
| 3. | Ward ZJ, Long MW, Resch SC, Gortmaker SL, Cradock AL, Giles C, Hsiao A, Wang YC. Redrawing the US Obesity Landscape: Bias-Corrected Estimates of State-Specific Adult Obesity Prevalence. PLoS One. 2016;11:e0150735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Ochner CN, Barrios DM, Lee CD, Pi-Sunyer FX. Biological mechanisms that promote weight regain following weight loss in obese humans. Physiol Behav. 2013;120:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 5. | Nguyen NT, Vu S, Kim E, Bodunova N, Phelan MJ. Trends in utilization of bariatric surgery, 2009-2012. Surg Endosc. 2016;30:2723-2727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Sullivan S, Kumar N, Edmundowicz SA, Abu Dayyeh BK, Jonnalagadda SS, Larsen M, Thompson CC. ASGE position statement on endoscopic bariatric therapies in clinical practice. Gastrointest Endosc. 2015;82:767-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Evans JT, DeLegge MH. Intragastric balloon therapy in the management of obesity: why the bad wrap? JPEN J Parenter Enteral Nutr. 2011;35:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Abu Dayyeh BK, Edmundowicz SA, Jonnalagadda S, Kumar N, Larsen M, Sullivan S, Thompson CC, Banerjee S. Endoscopic bariatric therapies. Gastrointest Endosc. 2015;81:1073-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Abu Dayyeh BK, Kumar N, Edmundowicz SA, Jonnalagadda S, Larsen M, Sullivan S, Thompson CC, Banerjee S. ASGE Bariatric Endoscopy Task Force systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting endoscopic bariatric therapies. Gastrointest Endosc. 2015;82:425-438.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 288] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 10. | Kotzampassi K, Grosomanidis V, Papakostas P, Penna S, Eleftheriadis E. 500 intragastric balloons: what happens 5 years thereafter? Obes Surg. 2012;22:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 11. | Genco A, López-Nava G, Wahlen C, Maselli R, Cipriano M, Sanchez MM, Jacobs C, Lorenzo M. Multi-centre European experience with intragastric balloon in overweight populations: 13 years of experience. Obes Surg. 2013;23:515-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Ponce J, Woodman G, Swain J, Wilson E, English W, Ikramuddin S, Bour E, Edmundowicz S, Snyder B, Soto F. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis. 2015;11:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 172] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 13. | Brethauer SA, Chand B, Schauer PR, Thompson CC. Transoral gastric volume reduction as intervention for weight management: 12-month follow-up of TRIM trial. Surg Obes Relat Dis. 2012;8:296-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Fogel R, De Fogel J, Bonilla Y, De La Fuente R. Clinical experience of transoral suturing for an endoluminal vertical gastroplasty: 1-year follow-up in 64 patients. Gastrointest Endosc. 2008;68:51-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Fujii LL, Bonin EA, Baron TH, Gostout CJ, Wong Kee Song LM. Utility of an endoscopic suturing system for prevention of covered luminal stent migration in the upper GI tract. Gastrointest Endosc. 2013;78:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Kumar N, Thompson CC. A novel method for endoscopic perforation management by using abdominal exploration and full-thickness sutured closure. Gastrointest Endosc. 2014;80:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Kumar N, Sahdala HN, Shaikh S, Wilson EB, Manoel GN, Zundel N, Thompson CC. Endoscopic sleeve gastroplasty for primary therapy of obesity: Initial human cases. Gastroenterology. 2014;146:S571-S572. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Abu Dayyeh BK, Acosta A, Camilleri M, Mundi MS, Rajan E, Topazian MD, Gostout CJ. Endoscopic Sleeve Gastroplasty Alters Gastric Physiology and Induces Loss of Body Weight in Obese Individuals. Clin Gastroenterol Hepatol. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 197] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 19. | Sharaiha RZ, Kedia P, Kumta N, DeFilippis EM, Gaidhane M, Shukla A, Aronne LJ, Kahaleh M. Initial experience with endoscopic sleeve gastroplasty: technical success and reproducibility in the bariatric population. Endoscopy. 2015;47:164-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Lopez-Nava G, Galvao M, Bautista-Castaño I, Fernandez-Corbelle JP, Trell M. Endoscopic sleeve gastroplasty with 1-year follow-up: factors predictive of success. Endosc Int Open. 2016;4:E222-E227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 21. | Kumar N, Lopez-Nava G, Sahdala H, GalvaoNeto M, Sharaiha RZ, Wilson EB, Shaikh S, Gomez E, Ryan MB, Zundel N. Endoscopic Sleeve Gastroplasty: Multicenter Weight Loss Results. Gastroenterology. 2015;148:S-179. [RCA] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Espinós JC, Turró R, Mata A, Cruz M, da Costa M, Villa V, Buchwald JN, Turró J. Early experience with the Incisionless Operating Platform™ (IOP) for the treatment of obesity: the Primary Obesity Surgery Endolumenal (POSE) procedure. Obes Surg. 2013;23:1375-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | López-Nava G, Bautista-Castaño I, Jimenez A, de Grado T, Fernandez-Corbelle JP. The Primary Obesity Surgery Endolumenal (POSE) procedure: one-year patient weight loss and safety outcomes. Surg Obes Relat Dis. 2015;11:861-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 101] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Verlaan T, Paulus GF, Mathus-Vliegen EM, Veldhuyzen EA, Conchillo JM, Bouvy ND, Fockens P. Endoscopic gastric volume reduction with a novel articulating plication device is safe and effective in the treatment of obesity (with video). Gastrointest Endosc. 2015;81:312-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Sullivan S, Stein R, Jonnalagadda S, Mullady D, Edmundowicz S. Aspiration therapy leads to weight loss in obese subjects: a pilot study. Gastroenterology. 2013;145:1245-1252.e1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Forssell H, Norén E. A novel endoscopic weight loss therapy using gastric aspiration: results after 6 months. Endoscopy. 2015;47:68-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Thompson CC, Abu Dayyeh BK, Kushner K, Sullivan S, Schorr AB, Amaro A, Apovian CM, Fullum T, Zarrinpar A, Jensen MD. The AspireAssist Is an Effective Tool in the Treatment of Class II and Class III Obesity: Results of a One-Year Clinical Trial. Gastroenterology. 2016;4:S86. [DOI] [Full Text] |
| 28. | Ryou M, Cantillon-Murphy P, Azagury D, Shaikh SN, Ha G, Greenwalt I, Ryan MB, Lang JH, Thompson CC. Smart Self-Assembling MagnetS for ENdoscopy (SAMSEN) for transoral endoscopic creation of immediate gastrojejunostomy (with video). Gastrointest Endosc. 2011;73:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 29. | Ryou M, Agoston AT, Thompson CC. Endoscopic intestinal bypass creation by using self-assembling magnets in a porcine model. Gastrointest Endosc. 2016;83:821-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 30. | Ryou M, Aihara H, Thompson CC. Minimally invasive entero-enteral dual-path bypass using self-assembling magnets. Surg Endosc. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Machytka E, Buzga M, Ryou MK, Lautz DB, Thompson CC. Endoscopic Dual-Path Enteral Anastomosis Using Self-Assembling Magnets: First-in-Human Clinical Feasibility. Gastroenterology. 2016;150: S232. [DOI] [Full Text] |
| 32. | Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, Horlick M, Kalarchian MA, King WC, Mitchell JE. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA. 2013;310:2416-2425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 344] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 33. | Powers PS, Rosemurgy A, Boyd F, Perez A. Outcome of gastric restriction procedures: weight, psychiatric diagnoses, and satisfaction. Obes Surg. 1997;7:471-477. [PubMed] |
| 34. | Abu Dayyeh BK, Lautz DB, Thompson CC. Gastrojejunal stoma diameter predicts weight regain after Roux-en-Y gastric bypass. Clin Gastroenterol Hepatol. 2011;9:228-233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 35. | Gagner M, Gentileschi P, de Csepel J, Kini S, Patterson E, Inabnet WB, Herron D, Pomp A. Laparoscopic reoperative bariatric surgery: experience from 27 consecutive patients. Obes Surg. 2002;12:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 117] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Coakley BA, Deveney CW, Spight DH, Thompson SK, Le D, Jobe BA, Wolfe BM, McConnell DB, O’Rourke RW. Revisional bariatric surgery for failed restrictive procedures. Surg Obes Relat Dis. 2008;4:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Müller MK, Wildi S, Scholz T, Clavien PA, Weber M. Laparoscopic pouch resizing and redo of gastro-jejunal anastomosis for pouch dilatation following gastric bypass. Obes Surg. 2005;15:1089-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Abidi WM, Aihara H, Thompson CC. Modified endoscopic submucosal dissection techniques before endoscopic revision of a gastric bypass. Gastrointest Endosc. 2016;83:1281-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Thompson CC, Carr-Locke DL, Saltzman J. Peroral endoscopic repair of staple-line dehiscence in Roux-en-Y gastric bypass: a less invasive approach. Gastroenterology. 2004;126 (Suppl 2). [DOI] [Full Text] |
| 40. | Thompson CC, Chand B, Chen YK, Demarco DC, Miller L, Schweitzer M, Rothstein RI, Lautz DB, Slattery J, Ryan MB. Endoscopic suturing for transoral outlet reduction increases weight loss after Roux-en-Y gastric bypass surgery. Gastroenterology. 2013;145:129-137.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 41. | Kumar N, Thompson CC. Comparison of a superficial suturing device with a full-thickness suturing device for transoral outlet reduction (with videos). Gastrointest Endosc. 2014;79:984-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Kumar N, Thompson CC. The pursestring technique for endoscopic revision of gastric bypass. Gastrointest Endosc. 2015;82:956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Gitelis M, Ujiki M, Farwell L, Linn J, Wang C, Miller K, Sula C, Carbray J, Haggerty S, Denham W. Six month outcomes in patients experiencing weight gain after gastric bypass who underwent gastrojejunal revision using an endoluminal suturing device. Surg Endosc. 2015;29:2133-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Kumar N, Thompson CC. Transoral outlet reduction for weight regain after gastric bypass: long-term follow-up. Gastrointest Endosc. 2016;83:776-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 45. | Mullady DK, Lautz DB, Thompson CC. Treatment of weight regain after gastric bypass surgery when using a new endoscopic platform: initial experience and early outcomes (with video). Gastrointest Endosc. 2009;70:440-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Ryou M, Mullady DK, Lautz DB, Thompson CC. Pilot study evaluating technical feasibility and early outcomes of second-generation endosurgical platform for treatment of weight regain after gastric bypass surgery. Surg Obes Relat Dis. 2009;5:450-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 47. | Horgan S, Jacobsen G, Weiss GD, Oldham JS, Denk PM, Borao F, Gorcey S, Watkins B, Mobley J, Thompson K. Incisionless revision of post-Roux-en-Y bypass stomal and pouch dilation: multicenter registry results. Surg Obes Relat Dis. 2010;6:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Thompson CC, Jacobsen GR, Schroder GL, Horgan S. Stoma size critical to 12-month outcomes in endoscopic suturing for gastric bypass repair. Surg Obes Relat Dis. 2012;8:282-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Gallo AS, DuCoin CG, Berducci MA, Nino DF, Almadani M, Sandler BJ, Horgan S, Jacobsen GR. Endoscopic revision of gastric bypass: Holy Grail or Epic fail? Surg Endosc. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 50. | Baretta GA, Alhinho HC, Matias JE, Marchesini JB, de Lima JH, Empinotti C, Campos JM. Argon plasma coagulation of gastrojejunal anastomosis for weight regain after gastric bypass. Obes Surg. 2015;25:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 51. | Eid GM, McCloskey CA, Eagleton JK, Lee LB, Courcoulas AP. StomaphyX vs a sham procedure for revisional surgery to reduce regained weight in Roux-en-Y gastric bypass patients: a randomized clinical trial. JAMA Surg. 2014;149:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 52. | Giurgius M, Fearing N, Weir A, Micheas L, Ramaswamy A. Long-term follow-up evaluation of endoscopic sclerotherapy for dilated gastrojejunostomy after gastric bypass. Surg Endosc. 2014;28:1454-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Abu Dayyeh BK, Jirapinyo P, Weitzner Z, Barker C, Flicker MS, Lautz DB, Thompson CC. Endoscopic sclerotherapy for the treatment of weight regain after Roux-en-Y gastric bypass: outcomes, complications, and predictors of response in 575 procedures. Gastrointest Endosc. 2012;76:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |