Published online Aug 14, 2016. doi: 10.3748/wjg.v22.i30.6925
Peer-review started: April 13, 2016
First decision: May 12, 2016
Revised: June 7, 2016
Accepted: June 28, 2016
Article in press: June 28, 2016
Published online: August 14, 2016
Processing time: 115 Days and 1.3 Hours
AIM: To evaluate the risk factors for ischemic-type biliary lesion (ITBL) after ABO-incompatible (ABO-I) adult living donor liver transplantation (ALDLT).
METHODS: Among 141 ALDLTs performed in our hospital between 2008 and 2014, 27 (19%) were ABO-I ALDLT and 114 were ABO-identical/compatible ALDLT. In this study, we extensively analyzed the clinico-pathological data of the 27 ABO-I recipients to determine the risk factors for ITBL after ABO-I ALDLT. All ABO-I ALDLT recipients underwent an identical B-cell depletion protocol with preoperative rituximab, plasma exchange (PE), and operative splenectomy. The median follow-up period after transplantation was 26 mo. The clinical outcomes of the 27 ABO-I ALDLT recipients were compared with those of 114 ABO-identical/compatible ALDLT recipients.
RESULTS: ITBL occurred in four recipients (14.8%) between 45 and 112 d after ABO-I ALDLT. The overall survival rates were not different between ABO-I ALDLT and ABO-identical/compatible ALDLT (P = 0.303). Among the ABO-I ALDLT recipients, there was no difference between patients with ITBL and those without ITBL in terms of B-cell and T-cell count, serum isoagglutinin titers, number of PEs, operative time and transfusion, use of graft infusion therapy, or number of remnant B-cell follicles and plasma cells in the spleen. However, the perioperative NK cell counts in the blood of patients with ITBL were significantly higher than those in the patients without ITBL (P < 0.05). Preoperative NK cell count > 150/μL and postoperative NK cell count > 120/μL were associated with greater relative risks (RR) for development of ITBL (RR = 20 and 14.3, respectively, P < 0.05).
CONCLUSION: High NK cell counts in a transplant recipient’s blood are associated with ITBL after ABO-I ALDLT. Further research is needed to elucidate the molecular mechanism of NK cell involvement in the development of ITBL.
Core tip: Despite application of B-cell depletion protocols for ABO-incompatible (ABO-I) adult living donor liver transplantation (ALDLT), ischemic-type biliary lesion (ITBL) remains one of the major complications associated with graft failure. However, no study could yet evaluate the risk factors affecting development of ITBL after ABO-I ALDLT because of limited data availability. We have extensively analyzed clinico-pathological data from ABO-I ALDLT patients in this study to identify the risk factors for ITBL. Our results suggest that NK cells in the recipient’s blood may be associated with development of ITBL after ABO-I ALDLT, which was supported by serological and pathological findings.
- Citation: Bang JB, Kim BW, Kim YB, Wang HJ, Lee HY, Sim J, Kim T, Lee KL, Hu XG, Mao W. Risk factor for ischemic-type biliary lesion after ABO-incompatible living donor liver transplantation. World J Gastroenterol 2016; 22(30): 6925-6935
- URL: https://www.wjgnet.com/1007-9327/full/v22/i30/6925.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i30.6925
ABO-blood type incompatible (ABO-I) adult living donor liver transplantation (ALDLT) has been increasingly used over the last decade because of recent developments in clinical protocols that overcome the ABO-blood barrier between the liver graft and the recipient. However, antibody-mediated rejection (AMR) still remains a major cause of graft failure after ABO-I ALDLT[1,2]. AMR following ABO-I LDLT present with various clinical features, from transient graft dysfunction to ischemic-type biliary lesion (ITBL) or massive hepatic necrosis, according to the severity of microvascular circulatory disturbance in the graft, which results from an inflammatory process triggered by the graft’s endothelial reaction to the recipient’s isoagglutinin[3].
Most ABO-I ALDLT programs nowadays employ a the protocol composed of B-cell depletion using an anti-CD20 antibody (rituximab) and elimination of serum isoagglutinin using plasma exchange (PE)[4]. Adopting the B-cell depletion protocol could effectively prevent hepatic necrosis, the most severe form of AMR[5,6]. However, the incidence of ITBL after ABO-I ALDLT remains high, and is usually followed by graft dysfunction and serious septic complications, such as recurrent cholangitis, liver abscesses, and septicemia. Therefore, the development of ITBL could significantly lower a patient’s quality of life and chance of long-term survival.
To our knowledge, no study has yet evaluated the risk factors affecting development of ITBL after ABO-I ALDLT. In this study, we evaluated the clinico-pathological outcomes of ABO-I ALDLT patients and analyzed the data to determine the risk factors for ITBL.
The clinico-pathological data of liver transplantation patients were prospectively recorded in our institutional database. Between May 2008 and December 2014, a total of 141 adult patients received ALDLT in our institute. Among those 141 patients, 27 recipients (19.1%) underwent ABO-I ALDLT with the B-cell depletion protocol, whereas the remaining 114 recipients received ABO-identical (n = 85) or -compatible ALDLT (n = 29). The B-cell depletion protocol in our institute consisted of preoperative rituximab prophylaxis, PE for elimination of serum isoagglutinin, and operative splenectomy. In this study, we retrospectively analyzed the perioperative data of the 27 cases of ABO-I ALDLT to determine the risk factors for ITBL.
All 27 recipients were admitted to our institute 17 d before the day of scheduled operation (D-17) and receive a single dose of rituximab 170 mg/BSA (m2) on D-14. Preoperative PE was performed according to the serum isoagglutinin titer. The target serum isoagglutinin levels were the same or less than 1:16. PE was performed with 1.0 or 1.5 times the patient’s plasma volume, and the replacement fluid was composed of equal amounts of albumin and fresh frozen plasma (FFP) of blood type AB.
Lymphocyte subsets were routinely measured on the day of a patient’s admission (Lф-1), 4 d after rituximab administration (Lф-2), and 2 wk after ABO-I ALDLT (Lф-3). Immunophenotyping for lymphocyte subsets was performed by 4-color flow cytometry analysis with the tetraCXP system on fresh peripheral blood samples. Lymphocyte gating was performed on the basis of a primary CD45 antigen using the side scatter procedure. Among the CD45+ cells, CD3+ cells were counted as T-cells, CD3+CD4+ as Th-cells, CD3+CD8+ as Tc-cells, CD3-CD19+ as B-cells, CD3-CD56+ as natural killer (NK) cells.
Serum isoagglutinin levels were preoperatively measured on the day of the patient’s admission and after every PE. In the post-transplant period, isoagglutinins were measured daily during the first week, and then twice a week until discharge. The methods used to measure isoagglutinin titer were tube techniques including the immediate spin tube method for IgM and the tube with anti-human globulin method for IgG[7].
The recipient and donor operations were performed as previously described[8]. All 27 ABO-I ALDLTs were performed using right lobe grafts from 27 living donors. All recipients underwent duct-to-duct biliary reconstruction with a 4-french external biliary stent[9]. After biliary reconstruction, splenectomy was performed. The spleen was mobilized and divided with a vessel sealing system and stapling device[10]. Hepatic artery infusion therapy (HAIT) was performed in 6 patients. The HAIT regimen consisted of prostaglandin E1 (0.01 g/kg per minute), methylprednisolone (1 mg/kg per day), and gabexate mesylate (1 mg/kg per day), infused continuously for 21 postoperative days (POD).
Immunosuppression was started with intravenous infusion of methylprednisolone (1 g) during the anhepatic phase of the operation, followed by 200 mg/d given intravenously on POD 1. The dose was gradually tapered to 40 mg/d by POD 7, and then converted to oral prednisolone until it was discontinued after 3 mo. Tacrolimus was started immediately after the operation to reach a target serum trough level of 15 ng/mL until POD 7, then reduced to around 10 ng/mL; that level was maintained until discharge, at which point it was reduced to 5-8 ng/mL. Mycophenolate mofetil (MMF, 500 mg twice a day) was added to a drug combination with steroid and tacrolimus after POD 3. The dose of MMF was adjusted according to the level of tacrolimus in the blood and white blood cell (WBC) count. All recipients received 2 g/kg of intravenous immunoglobulin (IVIg) for the first 3 d after transplantation.
Isoagglutinin titers were measured every other day until POD 14, and twice a week thereafter until discharge. Lymphocyte subset was measured routinely on POD 14 (Lф -3). Post-transplant PE was performed when isoagglutinin titer increased to 1:64 or above or when AMR was diagnosed on a liver biopsy.
Liver function test (LFT) and complete blood cell count were measured daily until the date of discharge from the hospital. Surveillance cultures for bacteria and fungi from blood, urine, stool, sputum, and body fluid drainage were performed weekly until discharge. Also, cytomegalovirus (CMV) infection was assessed weekly with a CMV antigenemia assay. Preemptive anti-CMV therapy was performed using intravenous gancyclovir when the CMV infected WBC count was above 4/200000 WBCs.
Vascular flow in the graft was assessed daily using Doppler ultrasound for the first week and then twice a week until POD 28. Abdominal computed tomogram (CT) images were taken regularly once a week until POD 28. A direct cholangiography was performed routinely on POD 21 via the external biliary stent. A liver biopsy was performed only when the patient had signs of AMR or acute cellular rejection (ACR) and after excluding other causes for abnormal graft dysfunction. All liver biopsy procedures were performed with a trans-hepatic approach under ultrasound guidance.
ITBL was suspected when a recipient showed abnormal LFT with a cholestatic profile and/or dilatation of the intrahepatic bile duct on imaging studies during the post-transplant course. Then, a cholangiogram was taken via the external biliary stent in order to diagnose ITBL. In this study, ITBL after ABO-I ALDLT was defined when dilatation or stricture of the intrahepatic bile ducts with irregularity were observed on a cholangiogram after exclusion of hepatic artery thrombosis by Doppler ultrasound or angiogram.
Management of the ITBL was mainly composed of interventional and supportive treatment. Percutaneous transhepatic biliary drainage (PTBD) was performed to reduce biliary stasis and bile sludge. Supportive treatment consisted of ursodeoxycholic acid and antibiotics.
All liver biopsy specimens were pathologically evaluated for evidence of AMR, ACR, or other findings. Routine staining methods included H&E, Masson’s trichrome, and immunohistochemistry (IHC) with CD56 for NK cells and complement C4d. A pathologic finding of AMR was defined as periportal edema and necrosis or hemorrhage with neutrophil infiltration. Positive staining for C4d in the small vessels or periportal sinusoid supported the finding of AMR[11].
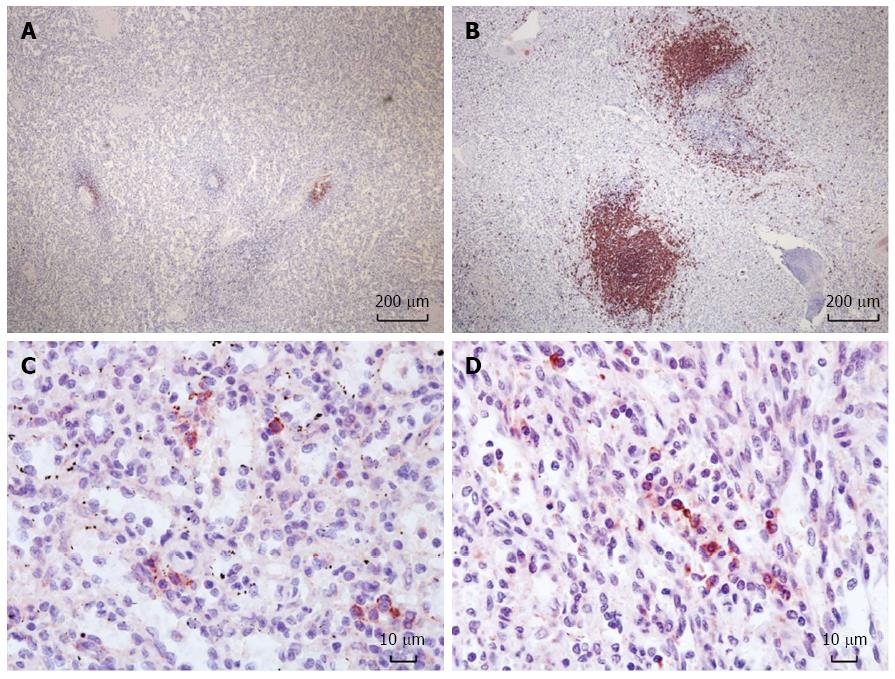
Spleen specimens were routinely stained with H&E and IHC for CD20 for B-cells and CD138 for plasma cell. Each spleen was reviewed by a pathologist, who measured the size of the B-cell follicles and counted the number of plasma cells. B-cell follicles in the spleen were semi-quantitatively scored according to the number and size of CD20+ follicles. Scoring ranged from 0 to 5, with 0 corresponding to an absence of B-cell follicles larger than 10 μm; 1, occasional staining of small follicles less than 100 μm; 2, frequent staining of small follicles less than 100 μm; 3, presence of medium follicles less than 300 μm; 4, presence of medium-to-large follicles less than 500 μm; 5, presence of large follicles more than 500 μm. The number of plasma cells is reported as the sum of CD138+ cells counted in 10 random fields under 400 × magnification using a light microscope. Six spleen specimens for control pathology were obtained from 6 adult recipients who received ABO-compatible liver transplants.
All data were analyzed using SPSS statistical software (Ver 22.0; SPSS Inc., Chicago, IL, United States). Data are expressed as mean ± SD or median values, ranges, and percentages. Univariate analysis was performed by the Mann-Whitney U test or Fisher’s exact test. Survival analysis was performed using the Kaplan-Meier method and survival rates were compared using a log-rank test. Logistic regression analysis was performed to assess the risk factors of ITBL in terms of the odds ratio and 95%CI. P-values of less than 0.05 were considered statistically significant.
The characteristics of all 27 ABO-I ALDLT patients are listed in Table 1. The median age was 50 years old (range, 31-65), and 21 were male (77.8%). The underlying diseases were hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) (n = 17, 62.9%), HBV-related liver cirrhosis (n = 6, 22.2%), alcoholic liver cirrhosis (n = 2, 7.4%), hepatitis C virus-related HCC (n = 1, 3.7%), and Wilson’s disease (n = 1, 3.7%). The median value of the Model for End-Stage Liver Disease score was 11 (6-34).
| Case No. | Age/sex | Etiology | MELD | Donor age/relationship | Blood type (R/D) | Initial B-cell | Initial isoagglutinin titer IgM/IgG | Actual GRWR | Graft ischemic time1 (min) | HAIT |
| 1 | 48/M | HBV + HCC | 10 | 24/Son | B/A | 16% | 1:16/1:2 | 0.86 | 355 | Y |
| 2 | 52/F | HBV | 25 | 24/Daughter | B/AB | 13% | 1:16/1:8 | 0.6 | 210 | Y |
| 3 | 53/M | Alcohol | 33 | 21/Son | O/A | 14% | 1:64/1:256 | 1.14 | 220 | Y |
| 4 | 50/F | HBV + HCC | 13 | 53/Husband | O/A | 21% | 1:256/1:32 | 0.81 | 203 | Y |
| 5 | 53/M | HCV | 13 | 18/Daughter | B/A | 22% | 1:32/1:8 | 0.97 | 130 | Y |
| 6 | 42/M | HBV + HCC | 13 | 39/Wife | B/A | 9% | 1:64/1:256 | 0.67 | 175 | Y |
| 7 | 65/M | HBV + HCC | 10 | 38/Daughter | O/A | 19% | 1:64/1:256 | 1.04 | 128 | N |
| 8 | 51/M | HBV + HCC | 9 | 23/Son | A/AB | 14% | 1:32/1:32 | 1.36 | 85 | N |
| 9 | 43/M | HBV | 34 | 29/Wife | O/B | 18% | 1:32/1:32 | 1.02 | 157 | N |
| 10 | 49/M | HBV + HCC | 7 | 21/Son | A/AB | 21% | 1:32/1:32 | 0.87 | 180 | N |
| 11 | 49/M | HBV + HCC | 13 | 21/Son | O/A | 18% | 1:256/1:1024 | 0.74 | 201 | N |
| 12 | 53/M | Alcohol | 13 | 21/Son | A/B | 14% | 1:64/1:512 | 1.07 | 225 | N |
| 13 | 59/M | HBV + HCC | 12 | 28/Son | O/A | 16% | 1:64/1:512 | 0.93 | 120 | N |
| 14 | 47/M | HBV | 14 | 37/Wife | O/A | 29% | 1:128/1:512 | 1.17 | 167 | N |
| 15 | 31/F | Wilson’s disease | 10 | 29/Sister | O/A | 19% | 1:32/1:32 | 0.95 | 80 | N |
| 16 | 52/M | HBV + HCC | 9 | 25/Daughter | O/B | 9% | 1:128/1:512 | 0.71 | 143 | N |
| 17 | 57/M | HBV + HCC | 9 | 44/Nephew | B/A | 16% | 1:16/1:32 | 0.91 | 136 | N |
| 18 | 48/M | HBV | 17 | 24/Daughter | O/B | 32% | 1:32/1:64 | 0.62 | 180 | N |
| 19 | 50/M | HBV + HCC | 9 | 21/Daughter | B/A | 15% | 1:16/1:16 | 0.64 | 190 | N |
| 20 | 56/M | HBV + HCC | 6 | 22/Son | B/AB | 21% | 1:16/1:16 | 1.14 | 154 | N |
| 21 | 45/F | HBV | 13 | 43/Husband | O/A | 6% | 1:32/1:32 | 1.48 | 100 | N |
| 22 | 49/M | HBV + HCC | 9 | 21/Son | O/A | 11% | 1:64/1:128 | 1.12 | 131 | N |
| 23 | 53/F | HBV + HCC | 11 | 19/Daughter | B/AB | 6% | 1:8/1:2 | 1.21 | 115 | N |
| 24 | 43/F | HBV | 22 | 50/Husband | B/A | 10% | 1:32/1:32 | 1.55 | 155 | N |
| 25 | 47/M | HBV + HCC | 9 | 19/Daughter | O/A | 4% | 1:32/1:1024 | 0.89 | 200 | N |
| 26 | 52/M | HBV + HCC | 10 | 24/Son | A/B | 31% | 1:32/1:32 | 1.01 | 162 | N |
| 27 | 35/M | HBV + HCC | 6 | 35/Wife | O/A | 20% | 1:64/1:256 | 0.85 | 175 | N |
The median age of donors was 24 (18-53) years, and 13 donors were male (48.1%). There were 20 donors who had blood type-A barrier to recipients and 7 with blood type-B barrier. Donors with A or AB blood type were placed in the A1 or A1B subgroup. The mean body mass index of the donors was 22.6 ± 3.1 (17.6-31.5). All donors recovered from right hepatectomy without serious complications and were discharged with normal LFT. The median length of hospital stay after donor hepatectomy was 9 d (7-11).
The mean weight of the 27 grafts was 667.9 ± 113.3 g (460-895), and the graft-to-recipient weight ratio was 0.97 ± 0.25 (0.60-1.55). The degree of macro-vesicular steatosis of the grafts was a median of 0% with a range of 0% to 5%. The cold and warm ischemic time of the grafts was 119.6 ± 51.6 min (25-290) and 46.2 ± 12.1 min (18-65), respectively. The total volume of RBC intraoperatively transfused to the recipients was 2.7 ± 3.7 units of RBC. The recipients’ operation took 658.1 ± 84.5 min (524-850).
The lymphocyte subset counts of the recipients at Lф-1 showed a B-cell count of 149.0 ± 108.7 per μL in blood, which was 16.4% ± 7.1% of total lymphocytes. B-cell depletion was successfully achieved in all recipients at Lф-2 and Lф-3. None of the recipients needed an additional dosage of rituximab before or after the operation.
The median titers of IgM and IgG isoagglutinin of the recipients at the time of admission were 1:32 (1:8-1:256) and 1:32 (1:2-1:1024), respectively. The median number of PEs was 2 (0-15) before the operation. All recipients achieved isoagglutinin titer ≤ 1:16 on the day of transplantation. The median peak titers of isoagglutinin IgM and IgG after transplantation were 1:4 (1:1-1:256) and 1:8 (1:0-1:256), respectively. Six recipients underwent PEs after transplantation.
The mean semi-quantitative score of the B-cell follicles in the spleen of ABO-I ALDLT recipients was 2.8 ± 1.1 (0-4). All control spleens had a score of 5. The scores of B-cell follicles were significantly decreased after B-cell depletion in the recipients compared to the control patients (P = 0.001). The plasma cell count was 76.3 ± 44.6 (10-168) in the ABO-I ALDLT recipients and 72.5 ± 30.3 (20-107) in the control patients (P = 0.846) (Figure 1).
The median follow-up time of the 27 recipients was 26 mo (8-84). The median length of hospital stay after ABO-I ALDLT was 28 d (20-75). Post-transplant laboratory tests showed that the peak levels of serum aspartate transaminase, alanine transaminase, and total bilirubin were 342.5 ± 268.9 IU/L, 342.5 ± 268.9 IU/L, and 4.2 ± 1.6 mg/dL, respectively. Graft function normalized between POD 12 and 21. All recipients showed satisfactory blood flows in the graft’s hepatic vein, portal vein, and hepatic artery without stenosis or obstruction on Doppler ultrasound and CT scan. None of 6 recipients with HAIT experienced infusion catheter-related complications. There were 4 recipients with intra-abdominal hemorrhage that developed between POD 2 - 16. The condition was were managed with angiographic embolization (n = 2) and abdominal exploration (n = 2). Post-transplant transfusion of packed RBC was performed in 17 recipients using 4.1 ± 4.4 units.
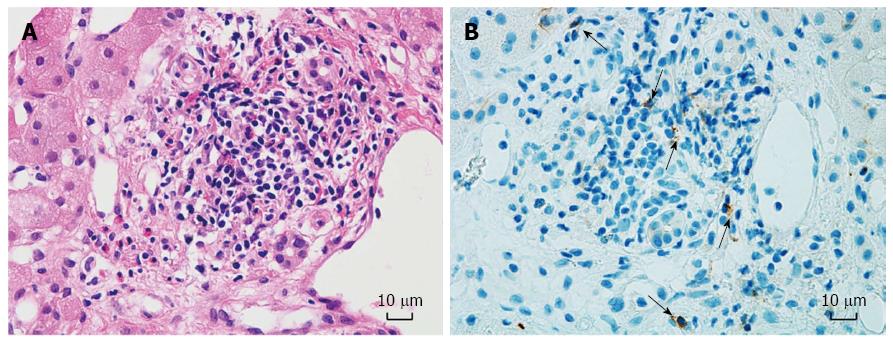
Eleven of the 27 recipients (40.7%) underwent a liver biopsy after ABO-I ALDLT. The total number of biopsy procedures was 13, because 2 patients underwent a second biopsy owing to repetitive graft dysfunction. Among the 13 biopsy results, 1 showed a finding compatible with AMR, 6 showed ACR, and 6 showed non-specific findings. One patient with non-specific inflammatory finding on the liver biopsy showed NK cells embedded in the liver tissue (Figure 2). Six of the 27 recipients (22.2%) experienced CMV infection after ABO-I ALDLT in our series, which was successfully treated with IV or oral gancyclovir. One patient developed MRSA pneumonia and ARDS followed by in-hospital mortality on POD 70. Five recipients passed away during the follow-up period from recurrent HCC (n = 3), post-transplant lymphoproliferative disease (n = 1), and recurrent cholangitis and liver abscess (n = 1). The 1-year, 3-year and 5-year survival rates of the 27 recipients after ABO-I ALDLT were 92.6%, 81.5%, and 73.0%, respectively.
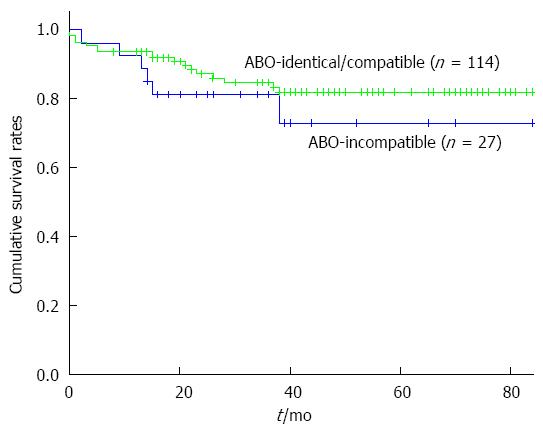
Among the recipients with ABO-identical or compatible ALDLT, 4 recipients (3.5%, 4 of 114) experienced in-hospital mortality after surgery due to bacterial pneumonia sepsis (n = 3), and pulmonary Aspergillosis (n = 1). Thirteen recipients died during the follow-up period after normal discharge due to HCC recurrence (n = 10), chronic alcohol abuse (n =1), and cerebrovascular infarction (n = 1). The 1-year, 3-year and 5-year survival rates of the ABO-identical or compatible ALDLT recipients were 93.9%, 84.8% and 82%, respectively. The survival rates of recipients with ABO-I ALDLT and ABO-identical/compatible ALDLT are compared in Figure 3 (P = 0.303).
No cases of ITBL developed among the 114 recipients with ABO-identical or -compatible ALDLT in this study. However, ITBL developed in 4 recipients (14.8%, 4 of 27) after ABO-I ALDLT during the period between POD 45 and POD 112 (Table 2). ITBL was confirmed by a cholangiogram via the external biliary stent (Figure 4). One of 4 recipients with ITBL had biopsy-proven AMR prior to development of ITBL; however, three had no evidence of AMR on biopsy. All patients with ITBL were treated with radiological intervention and supportive treatment. Among them, one died of recurrent cholangitis, liver abscess, and biliary sepsis 14 mo postoperatively. The remaining 3 patients underwent serial PTBD for more than 1 year. Two patients completed the interventional treatment, one with favorable liver function and the other with abnormal LFT due to diffuse intrahepatic portal vein thrombosis. One patient remains on PTBD treatment and has shown sustained hyperbilirubinemia for 20 mo.
| Case No. | No. of pre-LT PE | Post-LT isoagglutinin peak titer (IgM/IgG) | No. of post-LT PE | Post-LT infection | Post-LT Liver Bx day | Pathology of liver Bx | LOS after LT (d) | ITBL free time (d) | Tx for ITBL | Tx period for ITBL (mo) | Outcome of ITBL tx | Patient's survival | Patient's survival time (mo) |
| 2 | 1 | 1:4/1:8 | 0 | No | 27 | ACR | 35 | 90 | PTBD | 12 | Graft failure | Dead | 14 |
| 12 | 5 | 1:256/1:512 | 4 | No | 22 | NSI | 28 | 45 | PTBD | 5 | Graft dysfunction, PVT | Alive | 33 |
| 14 | 4 | 1:16/1:128 | 4 | No | 27 | AMR | 51 | 60 | PTBD | 11 | Normal graft function | Alive | 31 |
| 19 | 0 | 1:32/1:32 | 0 | No | 25 | NSI | 43 | 112 | PTBD | 20 | Graft dysfunction | Alive | 23 |
One recipient developed anastomosis stricture (AS) after ABO-I ALDLT in this series. The AS occurred on POD 135, and was successfully treated without sequelae by serial dilatation of the stricture site using a percutaneous transhepatic approach.
The clinicopathological characteristics of the 4 patients with ITBL were compared with those of the 23 recipients without ITBL. There was no difference in the perioperative characteristics of donors and recipients between the two groups (Table 3). The lymphocyte subsets analyses are compared between the recipients with and without ITBL in Table 4. The results at Lф-1, Lф-2, and Lф-3 showed no difference in terms of total lymphocyte count, B-cell count, T-cell count, and T4/T8 ratio between the recipients with ITBL and those without ITBL (P > 0.05). However, the NK cell counts were significantly higher in the recipients with ITBL than in those without ITBL at Lф-2 (P = 0.034) and Lф-3 (P = 0.027). ROC curve analysis of the NK cell counts showed optimal cut-off values of 150/μL at Lф-2 and 120/μL at Lф-3 for the development of ITBL. The sensitivity and specificity were 75% and 87% for the NK cell counts at Lф-2 and 75% and 83% for the counts at Lф-3, respectively. The relative risks of ITBL after ABO-I ALDLT according to NK cell count at Lф-2 and Lф-3 are given in Table 5.
| ITBL group (n = 4) | non-ITBL group (n = 23) | P value | |
| Preoperative characteristics | |||
| Recipient age (yr) | 51 (47-53) | 49 (31- 65) | 0.721 |
| Recipient sex (M/F) | 3/1 | 18/5 | 1.000 |
| Donor age (yr) | 22 (21-37) | 24 (18-53) | 0.545 |
| Donor BMI | 21.6 ± 2.5 | 22.8 ± 3.2 | 0.495 |
| Blood type-A barrier | 3 (75%) | 17 (73.9%) | 1.000 |
| MELD | 13 (9-25) | 10 (6-34) | 0.567 |
| CTP score | 7 (5-11) | 5 (5-12) | 0.322 |
| Initial isoagglutinin titer | |||
| IgG (≥ 1:64) | 2 (50%) | 10 (43.5%) | 1.000 |
| IgM (≥ 1:64) | 2 (50%) | 9 (39.1%) | 1.000 |
| No. of PE (preoperative) | 2.5 (0-5) | 2 (0-15) | 0.802 |
| Initial B-cell (%) | 17.7 ± 3.7 | 16.2 ± 1.5 | 0.945 |
| Operative outcomes | |||
| Operation time (min) | 678.3 ± 28.8 | 645.6 ± 90.8 | 0.336 |
| Actual graft weight (g) | 585.5 ± 135.6 | 682.3 ± 105.9 | 0.117 |
| Actual GRWR (%) | 0.87 ± 0.29 | 0.99 ± 0.24 | 0.373 |
| Cold ischemic time (min) | 149.3 ± 28.7 | 114.4 ± 53.3 | 0.219 |
| Warm ischemic time (min) | 48.7 ± 11.8 | 45.8 ± 12.3 | 0.659 |
| Intra-operative transfusion (unit) | |||
| RBC | 1.7 ± 1.5 | 2.9 ± 3.9 | 0.572 |
| FFP | 1.7 ± 2.1 | 2.3 ± 2.7 | 0.678 |
| P-conc. | 2.0 ± 4.0 | 7.1 ± 6.7 | 0.159 |
| HAIT | 1 (25%) | 5 (21.7%) | 1.000 |
| Postoperative outcomes | |||
| Peak level of AST (IU/L) | 330.2 ± 240.7 | 223.5 ± 151.3 | 0.258 |
| Peak level of ALT (IU/L) | 435.0 ± 444.5 | 323.0 ± 230.5 | 0.462 |
| Peak level of total bilirubin (mg/dL) | 5.5 ± 2.2 | 3.9 ± 1.4 | 0.071 |
| Peak isoagglutinin titer | |||
| IgG (≥ 1:64) | 2 (50%) | 4 (17.4%) | 0.204 |
| IgM (≥ 1:64) | 1 (25%) | 3 (13.1%) | 0.279 |
| No. of PE (postoperative) | 2 (0-4) | 0 (0-6) | 0.090 |
| Post-operative transfusion (unit) | |||
| RBC | 2.3 ± 2.6 | 2.8 ± 4.2 | 0.811 |
| FFP | 0.5 ± 1.0 | 4.8 ± 7.5 | 0.264 |
| P-conc. | 9.0 ± 2.0 | 11.5 ± 13.7 | 0.721 |
| Post-LT ICU stay (d) | 4 (1-5) | 4 (3-6) | 0.900 |
| Post-LT hospital stay (d) | 39 (28-51) | 28 (20-75) | 0.277 |
| BPACR | 1 (25%) | 5 (21.7%) | 1.000 |
| BPAMR | 1 (25%) | 0 | 0.148 |
| Complications | |||
| Post-LT hemorrhage | 0 | 4 (17.4%) | 1.000 |
| CMV infection | 0 | 7 (30.4%) | 0.545 |
| Bacterial infection | 0 | 1 (4.3%) | 1.000 |
| B-cell follicle score in spleen | 3.7 ± 0.5 | 2.6 ± 1.1 | 0.067 |
| Plasma cell count in spleen | 73.0 ± 19.0 | 76.8 ± 47.9 | 0.878 |
| ITBL group (n = 4) | Non-ITBL group (n = 23) | P value | |
| At patient's admission (Lф -1) | |||
| WBC counts (/μL) | 3750.0 ± 1658.3 | 4213.0 ± 2015.5 | 0.767 |
| Lymphocyte counts (/μL) | 1075.0 ± 471.7 | 965.2 ± 527.1 | 0.531 |
| B-cell counts (/μL) | 197.5 ± 121.5 | 140.6 ± 106.9 | 0.336 |
| T-cell counts (/μL) | 647.0 ± 300.9 | 675.3 ± 387.6 | 0.818 |
| T4/T8 ratio | 2.5 ± 0.6 | 2.2 ± 1.3 | 0.272 |
| NK cell counts (/μL) | 182.3 ± 98.7 | 100.0 ± 64.5 | 0.069 |
| After the administration of rituximab (Lф -2) | |||
| WBC counts (/μL) | 4500.0 ± 3213.5 | 4234.8 ± 2177.1 | 0.921 |
| Lymphocyte counts (/μL) | 887.5 ± 480.2 | 739.1 ± 428.3 | 0.576 |
| T-cell counts (/μL) | 646.7 ± 500.8 | 574.5 ± 267.9 | 0.974 |
| T4/T8 ratio | 2.1 ± 0.5 | 1.9 ± 0.7 | 0.974 |
| NK cell counts (/μL) | 158.7 ± 71.5 | 77.6 ± 59.2 | 0.034 |
| After the transplantation (Lф -3) | |||
| WBC counts (/μL) | 8800.0 ± 2412.5 | 8500.0 ± 6925.1 | 0.448 |
| Lymphocyte counts (/μL) | 687.5 ± 243.9 | 700.0 ± 342.5 | 0.974 |
| T-cell counts (/μL) | 429.0 ± 213.1 | 575.9 ± 320.4 | 0.448 |
| T4/T8 ratio | 1.3 ± 0.4 | 2.5 ± 2.8 | 0.111 |
| NK cell counts (/μL) | 170.0 ± 98.2 | 76.0 ± 63.7 | 0.027 |
| n | RR | 95%CI | P value | |
| NK cell count at Lф-2 | ||||
| ≥ 150 /μL | 6 | 20 | 1.5-260.8 | 0.022 |
| < 150 /μL | 21 | 1 | ||
| NK cell count at Lф-3 | ||||
| ≥ 120 /μL | 7 | 14.3 | 1.2-174.8 | 0.038 |
| < 120 /μL | 20 | 1 |
Several studies have shown that B-cell depletion using rituximab effectively removes a recipient's circulating B-cells without serious complications, and improves graft's survival rate after ABO-I ALDLT[2,6]. Nevertheless, there are issues concerning timing and dose of rituximab prophylaxis. A previous study reported that early administration of rituximab (more than 7 d before the transplantation) was more effective than late administration in preventing a post-transplant surge of serum isoagglutinin and AMR by depleting B-cells in blood and tissues[12]. The reason for this phenomenon has not been clearly identified, but early prophylaxis with rituximab could provide enough time for disappearance of unstimulated circulating plasma cells with short life expectancies of less than 7 d[13]. The dose of rituximab prophylaxis remains controversial. Previous studies introduced high-dose rituximab (375 mg/m2) to recipients, which is an amount that has been used to treat B-cell lymphoma patients[4,14]. However, recent studies reported that low-dose rituximab prophylaxis can achieve excellent B-cell depletion in the blood and spleen, similar to high-dose rituximab[15,16]. Considering these reports and the results of the present study, our protocol with a single-dose of 170 mg/m2 rituximab prophylaxis 14 d before operation could be acceptable for circulating B-cell depletion.
Splenectomy for the recipients receiving rituximab prophylaxis has also been a controversial issue in cases of ABO-I ALDLT. In this study, we routinely performed splenectomy during ABO-I ALDLT for the following reasons. First, rituximab administration could not eliminate B-cells from the lymphoid tissue completely, and the spleen contains one quarter of peripheral lymphoid tissue[17]. Second, most liver transplant recipients had enlarged spleens secondary to portal hypertension, which may have more lymphoid cells including B-cells and plasma cells than normal spleens. And, last, a safe and bloodless technique for splenectomy can be applied that uses a vessel sealing system and vascular stapler[10]. In this series, no patients experienced serious complications, including infection or vascular thrombosis, related to splenectomy during the follow-up period. The authors of this study suggest that further clinical and immunological investigations are required to determine the effect and feasibility of splenectomy in ABO-I ALDLT.
Local infusion therapy (LIT) using steroids, prostaglandins, and/or protease inhibitors via the hepatic artery and/or portal vein was introduced to prevent features of hepatic disseminated intravascular coagulation after ABO-I liver transplantation[18,19]. Previous studies reported that LIT significantly improved the outcomes after ABO-I ALDLT and AMR. However, the use of LIT in ABO-I ALDLT is controversial because of potential procedure-related complications[2]. Also, LIT is not regarded as an immunological intervention for AMR. Recent studies showed successful ABO-I ALDLTs using systemic infusion of IVIg for modulation of the recipient's immune reaction instead of LIT[4,20]. The present study did not demonstrate significant difference of ITBL incidence between ABO-I ALDLTs with and without HAIT. The use of LIT should be further investigated.
In spite of advancement of B-cell depletion protocols, ITBL remains as one of the major concerns in ABO-I ALDLT. ITBL is characterized by intrahepatic biliary strictures and dilatations in the absence of vascular complications such as hepatic artery thrombosis, portal thrombosis, and chronic ductopenic rejection[21]. It is thought that the mechanism of ITBL development is thrombotic obstruction of the hepatic arteriole by accumulation of isoagglutinin-complement complex on the endothelial cells of an ABO-I liver graft. Previous studies reported that high isoagglutinin titers were closely associated with AMR after ABO-I liver transplantation[2,22]. However, a recent study with a large number of recipients reported that serum isoagglutinin titer was not associated with development of ITBL[23]. Yet, no previous study has determined the significant risk factors for the development of ITBL.
If a graft survives the first 3 wk after ABO-I solid organ transplantation, isoagglutinin titer has no correlation with episodes of AMR. It had been demonstrated repeatedly that a graft may function normally even if both serum isoagglutinin titer and an intact complement system are present in the circulation of the recipient. This condition has been termed accommodation[24]. In ABO-I ALDLT, accommodation indicates avoidance of isoagglutinin-complement reaction in the graft. In this series, 4 of 27 (14.8%) ABO-I ALDLT recipients developed ITBL and were clinically diagnosed on 76 ± 30 d after transplantation (45-112 d). The isoagglutinin titers of the enrolled patients were not associated with development of ITBL. This result may suggest that ITBL can also develop after establishment of accommodation. It was repeatedly reported in previous studies that ITBL occurred later on, after ABO-I ALDLT, and was not associated with isoagglutinin titer[23,25].
There are two likely reasons for the late development of ITBL following ABO-I ALDLT. First, the clinical diagnosis of ITBL could be delayed owing to asymptomatic AMR on the graft’s biliary system. Second, there could be another immunological mechanism for development of ITBL other than AMR via the isoagglutinin-complement pathway. In this study, all recipients had normal biliary trees in the grafts as confirmed by routine cholangiography on POD 21 and were discharged with normal liver function. Therefore, delayed diagnosis of ITBL can be excluded from the possible reason for the late development of ITBL.
In the present study, a high population of NK cells in the blood was found to be the only risk factor for ITBL after ABO-I ALDLT. This may explain the existence of another immunological mechanism, separate from AMR, via the isoagglutinin-complement pathway. Recent studies reported that NK cells have a key role in AMR in allograft transplantation, known as antibody-dependent cellular cytotoxicity (ADCC)[26,27]. ADCC is a complement-independent mechanism of AMR, in which effector cells of the innate immune system, such as NK cells, are activated by binding to the Fc receptor portion of donor specific antibodies[27]. NK cells recognize various NK cell ligands on donor endothelial cells as well as the reaction of FcγRIII (CD16) with donor-specific antibodies (DSA) bound to donor endothelial cell antigens, which brings about direct cytotoxicity and triggers an increase in propagating ADCC. Therefore, authors of this study suggest that ABO-I liver graft recipients with high NK cell counts in the blood have a heightened probability of developing ADCC because ABO-antigens are expressed abundantly on the graft’s endothelial cells with the existence of some degree of serum isoagglutinin, which may behave as a DSA. In addition, some NK cells were detected in a liver biopsy of a recipient with mild graft dysfunction, which was reported as a non-specific finding at the time. However, 23 d after the biopsy, ITBL developed in that recipient. This finding may support our suggestion that ITBL after ABO-I ALDLT may be caused by NK cell-induced ADCC.
One of the limitations of present study was that NK cell activity could not be investigated using molecular analysis of its cytokines in the grafts with ITBL. To clarify the immunologic mechanism of NK cells in the development of ADCC and/or ITBL after ABO-I ALDLT, further molecular investigations are needed. Previous experimental studies showed that NK cell was related to C4d-negative AMR and graft endothelial damage after solid organ transplantation, which could be reduced by anti-NK targeting treatment[28,29]. As, the experimental and clinical evidence is clear that NK cells play an important role in the development of ADCC-induced ITBL after ABO-I ALDLT, anti-NK cell treatment might reduce the incidence of ITBL after ABO-I ALDLT. The other limitation of this study was that it was a retrospective analysis on the relatively small number of patients. However, the appropriate statistical methods were used to assess differences between small data sets.
In conclusion, even though B-cell depletion could prevent severe AMR after ABO-I ALDLT, ITBL remains as a major concern. In our study, high NK cell count in the recipient’s blood may be associated with developing ITBL after ABO-I ALDLT. Further clinical and molecular research is required to elucidate the role and molecular mechanism of NK cells in the development of ITBL after ABO-I ALDLT using the B-cell depletion protocol.
Following the recent advancement in techniques for transplant surgery, the use of ABO-incompatible adult living donor liver transplantation (ABO-I ADLDT) has increased, with improved clinical outcomes. Antibody-mediated rejection (AMR), which is a major cause of the graft’s death after ABO-I ALDLT, seems to be overcome by B-cell depleting protocol using preoperative rituximab prophylaxis and plasma exchange with or without splenectomy. However, ischemic-type biliary lesion (ITBL) remains a problematic issue after ABO-I ALDLT.
There were previously no clearly identified risk factors or immunological mechanisms for the development of ITBL. This study was conducted to determine risk factors for the development of ITBL after ABO-I ALDLT. The authors extensively analyzed the clinico-pathological data from recipients with ABO-I ALDLT, including patient demographics, intraoperative outcomes, postoperative complications, and general serum and blood analysis in the perioperative period. Furthermore, perioperative serum agglutinin titer, blood lymphocyte subset count (B-cell, T-cell, and NK cell count), immunohistochemical staining for B-cells and plasma cells in the spleen and for NK cells in a liver biopsy were specially collected and analyzed for this study.
From our clinico-pathological analyses, the authors found that a perioperative high NK cell level in the blood was associated with the development of ITBL after ABO-I ALDLT. This novel finding could explain the clinical characteristics of ITBL after ABO-I ALDLT by microcirculatory disturbance following NK cell triggering antibody dependent cytotoxicity (ADCC).
The results of this study needs to be expanded through molecular research into NK cell activity in the development of ITBL via further clinical and experimental studies. Anti-NK cell treatment could be used as a preventive measure for the development of ITBL.
ABO-I ALDLT is a method of adult liver transplantation across the blood barrier of ABO-incompatibility using a partial liver graft from a living donor. AMR is a type of graft rejection triggered by an immunological reaction of donor-specific antibodies against graft antigens. ITBL is a radiological diagnosis, characterized by intrahepatic stricture and dilatation of the bile duct on a cholangiogram, in the absence of hepatic artery thrombosis. ADCC is the killing of an antibody coated target cell by a cytotoxic effector cell through a nonphagocytic process.
The authors have presented an interesting study of 27 patients who have undergone ABO incompatible liver transplantation. They present their own immunological protocol and the outcome of the 27 patients concentrating particularly on the incidence of ischaemic biliary injury. These data are very interesting and demonstrate that with careful attention to detail it is possible to achieve a satisfactory outcome in this group of patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bramhall S, Jeng KS, Morioka D S- Editor: Gong ZM L- Editor: A E- Editor: Ma S
| 1. | Demetris AJ, Jaffe R, Tzakis A, Ramsey G, Todo S, Belle S, Esquivel C, Shapiro R, Markus B, Mroczek E. Antibody-mediated rejection of human orthotopic liver allografts. A study of liver transplantation across ABO blood group barriers. Am J Pathol. 1988;132:489-502. [PubMed] |
| 2. | Egawa H, Teramukai S, Haga H, Tanabe M, Fukushima M, Shimazu M. Present status of ABO-incompatible living donor liver transplantation in Japan. Hepatology. 2008;47:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Sanchez-Urdazpal L, Batts KP, Gores GJ, Moore SB, Sterioff S, Wiesner RH, Krom RA. Increased bile duct complications in liver transplantation across the ABO barrier. Ann Surg. 1993;218:152-158. [PubMed] |
| 4. | Ikegami T, Taketomi A, Soejima Y, Yoshizumi T, Uchiyama H, Harada N, Iguchi T, Hashimoto N, Maehara Y. Rituximab, IVIG, and plasma exchange without graft local infusion treatment: a new protocol in ABO incompatible living donor liver transplantation. Transplantation. 2009;88:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 91] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Raut V, Mori A, Kaido T, Ogura Y, Taku I, Nagai K, Sasaki N, Endo K, Hata T, Yagi S. Splenectomy does not offer immunological benefits in ABO-incompatible liver transplantation with a preoperative rituximab. Transplantation. 2012;93:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Tanabe M, Kawachi S, Obara H, Shinoda M, Hibi T, Kitagawa Y, Wakabayashi G, Shimazu M, Kitajima M. Current progress in ABO-incompatible liver transplantation. Eur J Clin Invest. 2010;40:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Walker PS. Identification of antibodies to red cell antigens. Technical manual. Bethesda, Maryland: American Association of Blood Banks 2008; 483-484. |
| 8. | Shin M, Kim SJ. ABO Incompatible Kidney Transplantation-Current Status and Uncertainties. J Transplant. 2011;2011:970421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Kim BW, Bae BK, Lee JM, Won JH, Park YK, Xu WG, Wang HJ, Kim MW. Duct-to-duct biliary reconstructions and complications in 100 living donor liver transplantations. Transplant Proc. 2009;41:1749-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Ikegami T, Toshima T, Takeishi K, Soejima Y, Kawanaka H, Yoshizumi T, Taketomi A, Maehara Y. Bloodless splenectomy during liver transplantation for terminal liver diseases with portal hypertension. J Am Coll Surg. 2009;208:e1-e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Haga H, Egawa H, Shirase T, Miyagawa A, Sakurai T, Minamiguchi S, Yamabe H, Manabe T, Tanaka K. Periportal edema and necrosis as diagnostic histological features of early humoral rejection in ABO-incompatible liver transplantation. Liver Transpl. 2004;10:16-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Egawa H, Ohmori K, Haga H, Tsuji H, Yurugi K, Miyagawa-Hayashino A, Oike F, Fukuda A, Yoshizawa J, Takada Y. B-cell surface marker analysis for improvement of rituximab prophylaxis in ABO-incompatible adult living donor liver transplantation. Liver Transpl. 2007;13:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Browning JL. B cells move to centre stage: novel opportunities for autoimmune disease treatment. Nat Rev Drug Discov. 2006;5:564-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 208] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 14. | Egawa H, Teramukai S, Haga H, Tanabe M, Mori A, Ikegami T, Kawagishi N, Ohdan H, Kasahara M, Umeshita K. Impact of rituximab desensitization on blood-type-incompatible adult living donor liver transplantation: a Japanese multicenter study. Am J Transplant. 2014;14:102-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 141] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 15. | Takagi T, Ishida H, Shirakawa H, Shimizu T, Tanabe K. Evaluation of low-dose rituximab induction therapy in living related kidney transplantation. Transplantation. 2010;89:1466-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Toki D, Ishida H, Horita S, Setoguchi K, Yamaguchi Y, Tanabe K. Impact of low-dose rituximab on splenic B cells in ABO-incompatible renal transplant recipients. Transpl Int. 2009;22:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Llende M, Santiago-Delpín EA, Lavergne J. Immunobiological consequences of splenectomy: a review. J Surg Res. 1986;40:85-94. [PubMed] |
| 18. | Tanabe M, Shimazu M, Wakabayashi G, Hoshino K, Kawachi S, Kadomura T, Seki H, Morikawa Y, Kitajima M. Intraportal infusion therapy as a novel approach to adult ABO-incompatible liver transplantation. Transplantation. 2002;73:1959-1961. [PubMed] |
| 19. | Nakamura Y, Matsuno N, Iwamoto H, Yokoyama T, Kuzuoka K, Kihara Y, Taira S, Sagara T, Jojima Y, Konno O. Successful case of adult ABO-incompatible liver transplantation: beneficial effects of intrahepatic artery infusion therapy: a case report. Transplant Proc. 2004;36:2269-2273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Kim JD, Choi DL, Han YS. Fourteen successful consecutive cases of ABO-incompatible living donor liver transplantation: new simplified intravenous immunoglobulin protocol without local infusion therapy. Transplant Proc. 2014;46:754-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Sanchez-Urdazpal L, Gores GJ, Ward EM, Maus TP, Wahlstrom HE, Moore SB, Wiesner RH, Krom RA. Ischemic-type biliary complications after orthotopic liver transplantation. Hepatology. 1992;16:49-53. [PubMed] |
| 22. | Uchiyama H, Mano Y, Taketomi A, Soejima Y, Yoshizumi T, Ikegami T, Shirabe K, Maehara Y. Kinetics of anti-blood type isoagglutinin titers and B lymphocytes in ABO-incompatible living donor liver transplantation with rituximab and plasma exchange. Transplantation. 2011;92:1134-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, Ha TY, Jung DH, Park GC, Kang SH. Biliary stricture is the only concern in ABO-incompatible adult living donor liver transplantation in the rituximab era. J Hepatol. 2014;61:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Warner PR, Nester TA. ABO-incompatible solid-organ transplantation. Am J Clin Pathol. 2006;125 Suppl:S87-S94. [PubMed] |
| 25. | Nakamura N, Nishida S, Neff GR, Vaidya A, Levi DM, Kato T, Ruiz P, Tzakis AG, Madariaga JR. Intrahepatic biliary strictures without hepatic artery thrombosis after liver transplantation: an analysis of 1,113 liver transplantations at a single center. Transplantation. 2005;79:427-432. [PubMed] |
| 26. | Resch T, Fabritius C, Ebner S, Ritschl P, Kotsch K. The Role of Natural Killer Cells in Humoral Rejection. Transplantation. 2015;99:1335-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Lee CY, Lotfi-Emran S, Erdinc M, Murata K, Velidedeoglu E, Fox-Talbot K, Liu J, Garyu J, Baldwin WM, Wasowska BA. The involvement of FcR mechanisms in antibody-mediated rejection. Transplantation. 2007;84:1324-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Hirohashi T, Chase CM, Della Pelle P, Sebastian D, Alessandrini A, Madsen JC, Russell PS, Colvin RB. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant. 2012;12:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 182] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 29. | Akiyoshi T, Hirohashi T, Alessandrini A, Chase CM, Farkash EA, Neal Smith R, Madsen JC, Russell PS, Colvin RB. Role of complement and NK cells in antibody mediated rejection. Hum Immunol. 2012;73:1226-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |