Published online Aug 14, 2016. doi: 10.3748/wjg.v22.i30.6906
Peer-review started: February 2, 2016
First decision: March 7, 2016
Revised: April 2, 2016
Accepted: June 13, 2016
Article in press: June 13, 2016
Published online: August 14, 2016
Processing time: 184 Days and 22.4 Hours
Locoregional spread of abdominopelvic malignant tumors frequently results in peritoneal carcinomatosis (PC). The prognosis of PC patients treated by conventional systemic chemotherapy is poor, with a median survival of < 6 mo. However, over the past three decades, an integrated treatment strategy of cytoreductive surgery (CRS) + hyperthermic intraperitoneal chemotherapy (HIPEC) has been developed by the pioneering oncologists, with proved efficacy and safety in selected patients. Supported by several lines of clinical evidence from phases I, II and III clinical trials, CRS + HIPEC has been regarded as the standard treatment for selected patients with PC in many established cancer centers worldwide. In China, an expert consensus on CRS + HIPEC has been reached by the leading surgical and medical oncologists, under the framework of the China Anti-Cancer Association. This expert consensus has summarized the progress in PC clinical studies and systematically evaluated the CRS + HIPEC procedures in China as well as across the world, so as to lay the foundation for formulating PC treatment guidelines specific to the national conditions of China.
Core tip: Cytoreductive surgery (CRS) + hyperthermic intraperitoneal chemotherapy (HIPEC) has been considered as the standard treatment for selected patients with peritoneal carcinomatosis (PC) in many established cancer centers worldwide. This Chinese expert consensus summarizes the mechanism of CRS + HIPEC to treat PC and its clinical efficacy in gastric cancer, colorectal cancer, ovarian cancer, pseudomyxoma peritonei, malignant peritoneal mesothelioma, and peritoneal sarcoma. Furthermore, a clinical pathway of CRS + HIPEC to treat PC has also been formulated.
- Citation: Li Y, Zhou YF, Liang H, Wang HQ, Hao JH, Zhu ZG, Wan DS, Qin LX, Cui SZ, Ji JF, Xu HM, Wei SZ, Xu HB, Suo T, Yang SJ, Xie CH, Yang XJ, Yang GL. Chinese expert consensus on cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal malignancies. World J Gastroenterol 2016; 22(30): 6906-6916
- URL: https://www.wjgnet.com/1007-9327/full/v22/i30/6906.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i30.6906
Locoregional spread of abdominopelvic malignancies such as gastric cancer, colorectal cancer, ovarian cancer, pseudomyxoma peritonei (PMP), malignant peritoneal mesothelioma and primary peritoneal carcinoma, frequently results in peritoneal surface malignancies, generally known as peritoneal carcinomatosis (PC). At present, PC is generally regarded as a form of systemic and widespread metastasis and the terminal stage of disease that deserves only palliative care, with a median overall survival (OS) of about 6 mo[1-3].
With research into tumor biological behavior and advances in cancer treatment technology, revolutionary changes have taken place in understanding PC, which is currently regarded as a kind of locoregional cancer progression, but no longer a widespread terminal-stage cancer metastasis. Accordingly, an integrated treatment strategy of cytoreductive surgery (CRS) + hyperthermic intraperitoneal chemotherapy (HIPEC) has been developed by pioneering oncologists, with proved efficacy and safety in selected PC patients with PMP[4,5], gastric cancer[6,7], colorectal cancer[8], and ovarian cancer[9]. Gradually, CRS + HIPEC has been established and promoted in many cancer centers in Europe, America and Asia-Pacific regions[7,10,11].
This CRS + HIPEC treatment combines the advantages of surgical resection, locoregional chemotherapy, hyperthermal therapy and large-volume abdominal perfusion washing, in which CRS removes the peritoneal and abdominopelvic gross tumor, and the synergistic effects of HIPEC eradicate residual tumor nodules, micrometastases and free cancer cells. It is so far the most effective strategy to treat PC[12,13]. This Chinese expert consensus is to summarize the progress in PC clinical studies and systematically evaluate the CRS + HIPEC procedure, so as to lay the foundation for formulating PC treatment guidelines specific to the national conditions of China.
PC is not an uncommon clinical condition. Serosal layer invasion of advanced gastric cancer is prone to forming PC, with 15%-50% of gastric cancer patients developing various degrees of PC at first diagnosis, and 35%-50% of postoperative cancer recurrence mainly in the form of PC[14]. About 10% of colorectal cancer patients develop PC at first treatment, 4%-19% of patients develop PC during follow-up after radical resection, and PC is the only form of recurrence in 25%-35% of the patients[15]. All of the epithelial ovarian cancer beyond FIGO (International Federation of Gynecology and Obstetrics) stage IIB will develop PC as a natural course of disease progression. The clinical pathological process of primary peritoneal carcinoma and peritoneal mesothelioma are typical PC disease courses. PMP is a rare condition, and mostly originates from the appendix; the clinical pathological process is also typical PC[16].
The mechanisms of HIPEC to treat PC cover several aspects. (1) Pharmacokinetic advantages. The peritoneum-plasma barrier prevents the peritoneum from absorbing large-molecular-weight drugs, leading to high concentrations of HIPEC drugs in the abdominal perfusion solution, and relatively lower drug concentrations in peripheral blood. As a result, HIPEC increases the direct cytotoxic effects of drugs on peritoneal surface tumors, and reduces the systemic adverse effects at the same time. The concentration ratio of common chemotherapy drugs in abdominal perfusion solution and peripheral blood is summarized in Table 1[17]; (2) the tolerance of normal tissue and cancer tissue to hyperthermia is different. Hyperthermia has multiple adverse effects on cancer cells. First, hyperthermia causes tumor microvessel embolism at the tissue level, resulting in ischemic necrosis of tumor tissue. Second, hyperthermia disturbs cancer cell homeostasis and energy metabolism, activates the lysosomes, destroys the cytoplasm and nucleus, directly killing cancer cells in S and M phase of the cell cycle. Third, hyperthermia also disrupts cancer cell membrane proteins at the molecular level, and interferes with the synthesis of DNA, RNA and protein; and (3) the synergistic effects of hyperthermia and chemotherapy could be dramatically increased at 42 °C, significantly enhancing the cytotoxic effects of many chemotherapeutic agents such as oxaliplatin, cisplatin and mitomycin C[18-20].
| Drug | Molecular weight | Ascites to plasma concentration ratio |
| Doxorubicin | 579.99 | 230 |
| Melphalan | 305.20 | 93 |
| Mitomycin C | 334.30 | 23.5 |
| Cisplatin | 300.10 | 7.8 |
| Gemcitabine | 299.50 | 500.0 |
| Mitoxantrone | 517.41 | 115-255 |
| Oxaliplatin | 397.30 | 16 |
| Etoposide | 588.58 | 65 |
| Paclitaxel | 853.90 | 1000 |
| Docetaxel | 861.90 | 552 |
| 5-Flurouracil | 130.08 | 250 |
| Floxuridine | 246.20 | 75 |
| Carboplatin | 371.25 | 10 |
The timing of HIPEC treatment is crucial. The effect of postoperative peritoneal chemotherapy is inferior to the intraoperative chemotherapy because of the abdominal adhesions and catheter complications. HIPEC should be performed immediately after the completion of CRS, because at this time there is no peritoneal adhesion, minimal residual tumor burden, and homogeneous distribution of chemotherapy perfusion solutions in the abdominal cavity.
For PC originating from abdominopelvic tumors, such as gastric cancer, colorectal cancer, appendiceal cancer, ovarian cancer, primary peritoneal cancer and peritoneal mesothelioma, if the primary tumor could be radically resected or optimal cytoreduction could be achieved and there is no widespread systemic metastases, HIPEC is recommended as the treatment of choice on the following conditions: (1) age 20-75 years; (2) Karnofsky performance status scale > 70; (3) positive free cancer cells in ascites or abdominal lavage solution; (4) peritoneal metastasis with peritoneal cancer index (PCI) < 20; and (5) patients with high risk of peritoneal dissemination, such as tumor perforation, complete bowl obstruction, or tumor invading the serosa layer or adjacent organs. The contraindications are: (1) age < 20 or > 75 years; (2) any lung, liver, brain or bone metastasis, or prominent retroperitoneal lymph node metastasis during preoperative assessment; (3) moderate-severe contraction of mesentery; and (4) obvious contraindications for routine operation.
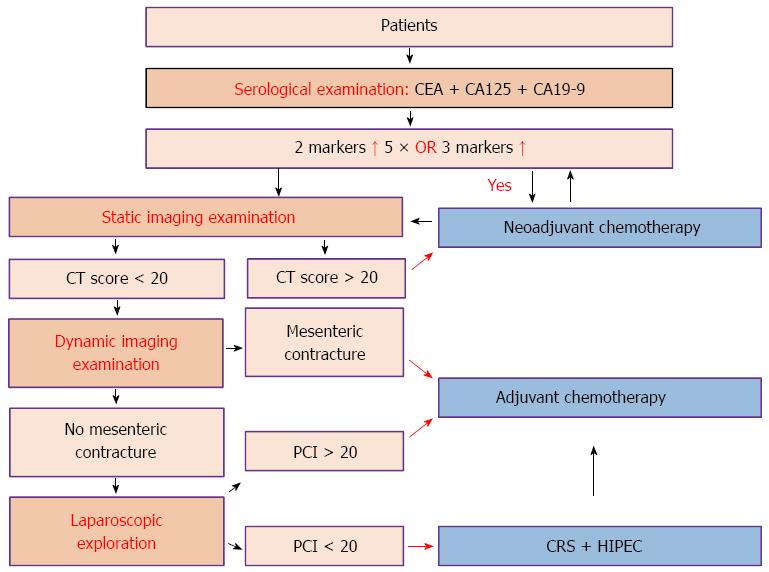
Complete preoperative imaging could help select appropriate patients for CRS + HIPEC treatment and to formulate CRS procedures. Two major forms of preoperative imaging examinations are particularly useful. (1) Static imaging examination: after proper abdominopelvic preparation, the patients undergo abdominopelvic multi-detector row computed tomography (CT) plus multiplanar reconstruction. The overall sensitivity and specificity of such high-resolution three-dimensional CT examination could reach 78.1% and 92.3%, respectively. The detection sensitivity could be 90% for lesions ≥ 0.5 cm in diameter, but reduced to 42.6% for lesions < 0.5 cm in diameter. The degree of fitness between the CT-PCI and the intraoperative PCI is 0.384-0.640[21]. Typical CT signs of PC include peritoneal thickening with contrast enhancement, thickening of the greater omentum dotted with nodules, streaks and cloud-like forms caused by uneven contrast enhancement, uneven distribution of the small intestines with enlarged or narrow lumens, contrast-enhanced nodules on the intestine mesentery showing a pepper-like sign, and ascites. Preoperative CT-PCI score could be estimated according to the typical imaging signs and lesion size, so as to determine the extent of PC. Apart from routine CT examination, positron emission tomography-CT examination is an alternative consideration; and (2) dynamic imaging: oral gastrografin radiography of the whole digestive tract could be used to observe the intestinal peristalsis, distribution status and the duration of the contrast medium to pass through the small intestine, so as to evaluate gastrointestinal motility, intestinal obstruction and mesenteric contracture. The following three imaging characteristics indicate that it is hard to achieve complete cytoreduction: (1) intestinal segmental obstruction; (2) intermingling existence of tumor, small intestine and mesentery; and (3) tumor nodules > 5 cm on the intestinal surface or mesentery. HIPEC should be carefully weighed if the above-mentioned features are obvious. Laparoscopic exploration is a proper choice when it is necessary to score the PCI, estimating whether the CCR0-1[22] resection is achievable before the final determination of whether the CRS + HIPEC procedure should be performed.
Apart from routine hematological examinations, the detection of serum tumor markers is necessary and the combined detection of carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 125 and CA19-9 is the first choice. CEA, CA125 and CA19-9 can also be used, respectively, to judge the extent of tumor invasion, ascites and peritoneal tumor burden, and proliferative activity of tumor cells in ascites or primary tumor[23-31].
In order to determine more accurately cancer stage, better evaluate the abdominal organ involvement, and determine the feasibility of complete cytoreduction, laparoscopic exploration is helpful if diagnosis cannot be established through imaging. Exfoliative cytology examination and pathological biopsy are both also important to establish the disease stage and treatment strategy. According to the examination results, the recommended clinical pathway is shown in Figure 1.
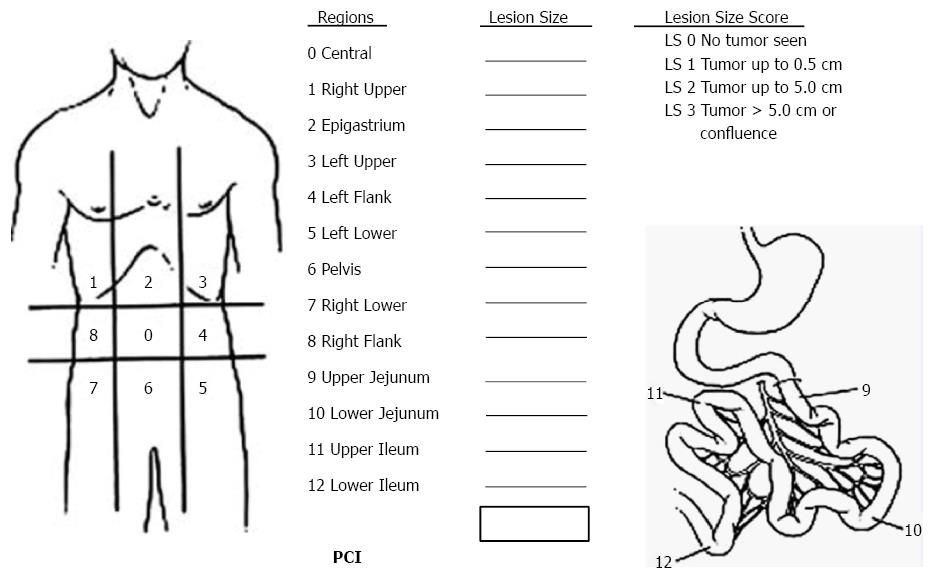
Sugarbaker’s PCI is the standardized intraoperative staging system to determine the PC burden[22]. The abdomen is divided into 13 areas, and the total score of each area is the PCI (Figure 2). PCI score is important to select appropriate patients for CRS + HIPEC.
CRS is performed under general anesthesia. The patient is placed in the lithotomy position, and pressurized inflatable insulating protective bags are wrapped around both lower extremities to prevent the formation of deep vein thrombosis. A preoperative conventional nasogastric tube and urinary catheter are installed.
The long midline xyphoid-pubic incision is made for proper abdominal exposure, so as to evaluate the PCI score thoroughly (Figure 2). Generally, CRS is performed in the following order: round ligament of liver, greater/lesser omentum, right/left upper quadrant, right/left diaphragmatic copula peritoneum, parietal peritoneum, right/left iliac fossa peritoneum, pelvic peritoneum, and small intestine mesentery. The optimal CRS also includes stripping the whole parietal peritoneum, resecting the visceral peritoneum and tumor-involved intestinal tract. Cholecystectomy, splenectomy, rectectomy, hysterectomy and bilateral salpingo-oophorectomy are all necessary if tumor implants are observed in the gallbladder fossa, spleen fossa and Douglas cavity. A ball-tipped electrotome or electric evaporator could be used to carbonize the tumor tissue if the tumor is adhered to important organs and cannot be removed. At the completion of CRS, CC score is evaluated and recorded according to the Sugarbaker’s criteria on the completeness of cytoreduction[22].
After CRS, open or closed HIPEC is performed. The chemotherapy drugs commonly used for HIPEC are cisplatin (20 mg/L), oxaliplatin (25 mg/L), mitomycin C (5 mg/L) and docetaxel (20 mg/L); each dissolved in 3 L warmed nature saline at 43 ± 0.5 °C and then delivered into the abdominal cavity from an automatic hyperthermia chemotherapy perfusion device through the inflow tube placed under the diaphragm at a speed of 400 mL/min. The temperature of the perfusion solution in the peritoneal space is monitored with a thermometer in real time. The total HIPEC time is 60-90 min; after which the perfusion solution is removed through the suction tube, and the abdominal cavity is washed with 2-3 L warm normal saline. Reconstruction of the gastrointestinal system is made before or after HIPEC, and an intestinal stoma is made if necessary. The abdominal wound is closed using a double-layer relaxing suture. After operation, the patient is delivered to the intensive care unit for recovery. When the condition becomes stabilized, usually 24-48 h later, the patient is transferred to the surgical oncology ward and receives early postoperative intraperitoneal chemotherapy.
The extent of CRS determined by Sugarbaker’s criteria on the completeness of cytoreduction (CC)[22] is closely correlated with OS. A score of CC-0 indicates no residual peritoneal disease after CRS; CC-1, < 2.5 mm of residual disease; CC-2, residual tumor between 2.5 mm and 2.5 cm; and CC-3, > 2.5 cm of residual tumor or the presence of a sheet of unresectable tumor nodules.
The incidence of adverse events of CRS + HIPEC is 27%-56%[32], mainly including abdominal abscess, anastomotic leakage, biliary leakage, intestinal leakage, intestinal obstruction, incision dehiscence, pulmonary infection, hematological toxicity, deep vein thrombosis, pleural effusion, congestive heart failure, cerebral infarction, and moderate to severe hypoalbuminemia. These adverse events are correlated with PCI score, operation duration, the number of anastomoses and organ or peritoneum resected[33].
The perioperative mortality of CRS + HIPEC is 0%-11% in the US, with the most common causes being intestinal leakage, bone marrow suppression, respiratory failure, infection by methicillin-resistant Staphylococcus aureus and pulmonary embolism. Poor risk factors include massive malignant ascites, poor performance status, and intestinal obstruction[32]. In a randomized controlled clinical study for gastric carcinoma PC from China[7], nine serious adverse events occurred in 68 cases; four in the CRS group (11.7%) and five in the CRS + HIPEC group (14.7%, P = 0.839), and the median survival was 5.0 and 3.0 mo, respectively. Serious adverse events are independent negative prognostic factors for survival.
There are two interesting phenomena observed after CRS + HIPEC: (1) few patients require a second operation for intra-abdominal adhesion; and (2) Iitra-abdominal adhesion is less than expected for patients who undergo second-look surgery.
Although the incidence of adverse events is high for CRS + HIPEC, the prognosis of these patients would be even worse without this procedure[34].
Data from single center phase II studies of CRS + HIPEC for colorectal cancer PC (Table 2)[35] showed a 3-year survival rate of 21%-40%, which is significantly better than for systemic chemotherapy. A systematic review by Glehen et al[36] on 506 patients with colorectal cancer PC treated by CRS + HIPEC revealed a median overall survival rate of 19.2 mo, and 3- and 5-year survival rates of 39% and 19%, respectively.
| Ref. | Year | Patients | Mean follow-up (mo) | Overall survival in years | ||||
| 1 | 2 | 3 | 4 | 5 | ||||
| Schneebaum et al | 1996 | 15 | 15 | - | - | - | - | - |
| Elias et al | 1997 | 23 | 12 | 88% | 55% | 40% | - | - |
| Fujimura et al | 1999 | 14 | - | 51% | - | 21% | - | - |
| Loggie et al | 2000 | 38 | 27 | 60% | 39% | 24% | - | - |
| Cavaliere et al | 2000 | 14 | 30 | - | 64% | - | - | - |
| Witkamp et al | 2000 | 29 | 38 | 82% | 45% | 23% | - | - |
| Beaujard et al | 2000 | 21 | 12 | 50% | - | - | - | - |
| Piso et al | 2001 | 17 | 39 | - | - | - | 75% | - |
| Elias et al | 2001 | 64 | 36 | 60% | 47% | 36% | 27% | |
| Culliford et al | 2001 | 47 | 17 | - | - | - | - | 28% |
| Zoetmulder et al | 2002 | 35 | - | - | - | - | - | 20% |
| Shen et al | 2003 | 40 | 52 | 60% | - | 24% | - | - |
| Pilati et al | 2003 | 34 | 14 | - | 31% | - | - | - |
| Pestieau et al | 2003 | 99 | - | 100% | - | - | - | 30% |
| Glehen et al | 2004 | 53 | - | 55% | - | - | - | 11% |
| Total | - | 543 | 10-52 | - | > 40% | - | - | 20% |
The Netherlands Cancer Institute conducted the first phase III prospective randomized controlled clinical study, which randomly divided colorectal PC patients into palliative surgery plus systemic chemotherapy (5-fluorouracil/leucovorin) group (n = 51) and CRS + HIPEC + systemic chemotherapy group (n = 54). The median OS was 12.6 and 22.4 mo (P = 0.032) for the two groups, respectively. Although the complete cytoreduction rate for the study group was < 40%, the survival rate was higher than any other treatment strategies so far, and the result is convincing[8]. When the median follow-up time extended to 8 years (72-115 mo), the median OS was still 12.6 mo for the former group, but the latter was 22.2 mo (P = 0.028)[37], proving once again that CRS + HIPEC can prolong the survival time of colorectal carcinoma PC patients.
A series of clinical studies on colorectal cancer PC treatment has been conducted at Zhongnan Hospital of Wuhan University and Hubei Provincial Cancer Clinical Study Center[38]. Retrospective case-control results showed that the median OS of the treatment group was 13.7 mo (95%CI: 5.0-16.5 mo), significantly higher than that of 8.5 mo (95%CI: 4.7-12.4 mo) in the control group (P = 0.02)[39]. A phase II clinical study showed that the 1-, 2-, 3- and 5-year survival rates can reach 70.5%, 34.2%, 22% and 22%, respectively.
At present, CRS + HIPEC have been widely accepted in many European countries and Australia as standard care for selected patients with colorectal PC. The 5-year survival rates for such patients treated by CRS + HIPEC was > 50% in the Netherlands, about 25% in the United Kingdom, 30% in France, 35% in Australia and > 30% in the United States. Therefore, the Peritoneal Surface Oncology Group International (PSOGI) considers it imperative to perform prophylactic HIPEC for patients with colorectal cancer to reduce the risk of peritoneal metastases, so as to evaluate how effective such an approach is in reducing the risk of peritoneal metastases as well as liver metastases. Currently, prospective controlled clinical studies on prophylactic HIPEC for patients with colorectal cancer with a high risk of peritoneal metastases have been carried out by several cancer treatment centers to assess the safety and feasibility of this approach for prevention of peritoneal recurrence of colorectal cancer.
There are several non-randomized clinical studies of CRS + HIPEC for treatment of gastric PC (Table 3)[40-50]. Yonemura et al[50] conducted the largest series of studies, which demonstrated that the 1- and 5-year survival rate for the 83 patients with gastric PC treated with CRS + HIPEC (mitomycin C, etoposide and cisplatin) was 43% and 11%, respectively. The Lyon Research Center reported that 1- and 5-year survival rate was 48% and 16%, respectively, and the median survival was 10.3 mo[35].
| Ref. | Patients | Staging criteria | CCR0 n (%) | HIPEC | Morbidity n (%) | Mortality n (%) | Median follow-up (mo) | Median survival (mo) | Overall survival in years | ||
| 1 | 2 | 5 | |||||||||
| Yonemura et al[42] | 107 | Japanese General Rules for Gastric Cancer Study | 47 (43.9) | Open technique, MMC 30 mg, DDP 300 mg, Etoposide 150 mg, 8 L of normal saline, 42-43 °C, 60 min | 23 (15.9) | 3 (2.8) | 46 | 11.5 | 35.5% | 13.1% | 6.7% |
| CCR 0: 19.2 | |||||||||||
| CCR 1-3: 7.8 | |||||||||||
| Yonemura et al[50] | 83 | Japanese General Rules for Gastric Cancer Study | 28 (33.7) | Open technique, MMC 30 mg, DDP 300 mg, Etoposide 150 mg, 8 L of normal saline, 42-43 °C, 60 min | - | - | 46 | CCR 0: 13.9 | 43.0% | - | 11.0% |
| CCR 1-3: 6.8 | |||||||||||
| Yonemura et al[43] | 48 | Japanese General Rules for Gastric Cancer Study | - | Open technique, MMC 30 mg, DDP 300 mg, 8 L of normal saline, 42-43 °C, 60 min | 9 (19.0) | 2 (4.0) | - | - | - | - | 61.0% |
| Scaringi et al[44] | 26 | Gilly's Classification | 8 (30.8) | Close technique, MMC 120 mg, DDP 200 mg, 6 L of normal saline, 42-43 °C, 90-120 min | 10 (38.5) | 1 (3.8) | - | 6.6 | - | - | - |
| CCR 0: 15 | |||||||||||
| CCR 1-3: 3.9 | |||||||||||
| Fujimoto et al[45] | 15 | - | - | Close technique, MMC 30-50 mg, 44.7-48.7 °C, 120 min | 2 (13.3) | 0 | - | 7.2 ± 4.6 | - | - | - |
| Fujimoto et al[6] | 71 | TNM Classification | 71 (100.0) | Close technique, MMC 10 mg/mL, 44.5-45 °C, 120 min | 2 (2.8) | 0 | 7 | - | 88.0% | 76.0% | 2.0% |
| Hall et al[46] | 34 | - | 7 (21.0) | Close technique, MMC 10 mg/mL, 40 °C, 120 min | 12 (35.0) | 0 | - | 8 | 27.0% | 23.0% | 6.0% |
| Fujimura et al[47] | 31 | Japanese General Rules for Gastric Cancer Study | 2 (16.0) | Open technique, MMC 20 mg/m2, DDP 200 mg/m2, 6 L of normal saline, 42-52 °C, 90-120 min | 6 (19.4) | 0 | - | 9 | 33.3% | 8.3% | 0.0% |
| Hamazoe et al[48] | 42 | - | 40 (95.0) | Close technique, MMC 10 μg/mL, inflow temperature 40-45 °C, outflow temperature 40-42 °C, 60 min | 2 (4.8), | 0 | > 6 | 77 | 90.0% | 80.0% | 64.3% |
| Kim et al[49] | 52 | TNM Classification | - | Close technique, MMC 10 μg/mL, inflow temperature 44 °C, 20 min | 19 (36.5) | 0 | 38 | 36 | - | - | 32.7% |
| Yang et al[7] | 68 | Sugarbaker’s Classification | 20 (58.5) | Open technique, MMC 30 mg, DDP 120 mg, 42 °C, 120 min | 5 (14.7) | 0 | 32 | PCI ≤ 20 13.5 PCI > 20 10.2 | |||
| Chen et al[40] | 500 | - | - | Open technique, Chlorhexidine diacetate hydrate 0.6 g, 4 L of distilled water, 43 °C, 4 min | - | - | - | - | 88.7% | 66.2% | 63.6% |
| Zhu et al[41] | 52 | - | - | Open technique, DDP 50 mg/L, MMC 5 mg/L, 43 °C, 60 min | - | - | 72 | - | 76.9% | 69.2% | 55.2% |
Chen et al[40] at China Medical University conducted clinical studies on intraperitoneal chemotherapy to treat gastric cancer, in which 500 patients with gastric cancer who underwent radical resection were divided into three groups: Group A (n = 198) treated by peritoneal lavage with 4 L of distilled water at 43 °C for 10 min after radical resection; Group B (n = 89) treated by peritoneal lavage with 0.6 g chlorhexidine acetate dissolved in 4 L distilled water at 43 °C for 4 min after radical resection; and Group C (n = 213) treated by peritoneal lavage with 4 L normal saline for 4 min at room temperature after radical resection. The results showed that the effect was the same between Groups A and B, with no significant difference. The 5-year survival rates for the first two lavage groups and control group were 63.8% and 51.2%, respectively.
Zhu et al[41] at Shanghai Ruijin Hospital also studied the clinical efficacy of HIPEC to treat advanced gastric cancer. The 1-, 2- and 4-year survival rates for patients who underwent surgery plus HIPEC were 85.7%, 81.0% and 63.9%, respectively, which was better than that of those who underwent simple surgery (77.3%, 61.0% and 50.8%).
Yang et al[7,14,34] at Zhongnan Hospital of Wuhan University have conducted a series of clinical studies on CRS + HIPEC to treat gastric cancer PC. A phase I clinical study demonstrated the safety of the therapy. In a phase II study, 28 patients who underwent CRS + HIPEC treatment showed a 6-, 12-, 18- and 24-mo survival rate of 75%, 50%, 43% and 43%, respectively. For patients with PCI ≤ 20 vs > 20, the median OS was 27.7 mo (95%CI: 15.2-40.3 mo) vs 6.4 mo (95%CI: 3.8-8.9 mo) (P < 0.001), respectively. For patients with CCR0, CCR1 and CCR2-3, the median OS was 43.4 mo (95%CI: 26.9-59.9 mo), 9.4 mo (95%CI: 7.4-11.4 mo), and 8.3 mo (95%CI: 3.0-13.6 mo) (P = 0.001), respectively. A prospective randomized phase III clinical study showed that the median survival time in the control group (n = 34) and treatment group (n = 34) was 6.5 mo (95%CI: 4.8-8.2 mo) and 11.0 mo (95%CI: 5.0-11.9 mo) (P = 0.046), respectively. The median OS for synchronous gastric cancer PC was 12 mo (95%CI: 8.1-15.9 mo) and there was no significant difference in the rate of serious adverse events between the two groups.
There is no standard treatment for advanced ovarian cancer. The conventional treatment of stage III/IV ovarian cancer is based on optimal CRS followed commonly by intravenous platinum/taxane-based chemotherapy, but the majority of patients will relapse within 5 years. A phase II clinical study conducted by the Italian National Cancer Institute on 27 patients with recurrent ovarian cancer who were treated with HIPEC (cisplatin + mitomycin C) showed a 2-year survival rate of 55%, and a median survival time to tumor local progression time of 21.8 mo[51]. It is worth noting that a phase III clinical study containing 415 patients with advanced ovarian cancer showed that the median survival for intravenous plus intraperitoneal combined chemotherapy group was 65.6 mo, compared with 49.7 mo in the systemic intravenous chemotherapy group[52]. This study was evaluated as showing significant progress in clinical gynecological oncology by American Society of Clinical Oncology, and the United States National Cancer Institute also issued a statement to recommend intravenous and intraperitoneal chemotherapy for these patients. Two case-control studies reported recently the advantage of CRS + HIPEC in ovarian cancer. Cascales-Campos et al[53] reported a case-control study of 87 patients with stage IIIC/IV ovarian cancer. Of the 87 patients, 52 were treated with HIPEC (paclitaxel 60 mg/m2, 60 min, 42 °C) and 35 were in the control group. The result showed that the 1-year disease-free survival was 81.0% vs 66.0% and 3-year disease-free survival was 63.0% vs 18.0% (P < 0.05). Multivariate analysis revealed that HIPEC was an independent prognostic factor for OS. In another case-control study by Safra et al[54] from Israel, patients with recurrent ovarian cancer were included in the proportion of 1:3; 27 patients had recurrent epithelial ovarian cancer treated with CRS + HIPEC and 84 matched control patients only had systemic chemotherapy. The median progression-free survival was 15 mo in the HIPEC group and 6 mo in the systemic chemotherapy group (P < 0.01). The 5-year survival rate was significantly higher in the HIPEC group than in the controls (79% vs 45%, P < 0.05).
More importantly, Spiliotis et al[9] from Greece conducted a double-blind prospective phase III clinical trial on CRS + HIPEC in patients with recurrent ovarian cancer. All 120 patients had stage IIIC/IV ovarian cancer and experienced disease recurrence after initial surgical treatment and first-line systemic chemotherapy. They were randomized into two groups. Group A comprised 60 patients treated with CRS + HIPEC and then systemic chemotherapy. Group B comprised 60 patients treated with CRS only and systemic chemotherapy. The mean survival was 26.7 mo in Group A vs 13.4 mo in Group B (P < 0.01), and the 3-year survival was 75.0 % for Group A vs 18.0% for Group B (P < 0.01). In the HIPEC group, the median survival did not differ between patients with platinum-resistant disease and platinum-sensitive disease (26.6 mo vs 26.8 mo).
PMP is the best indication for HIPEC. Four studies evaluating the efficacy of CRS + HIPEC to treat PMP found that the 5-year survival rates were 66%-97%, the adverse events rates were 27%-44%, and the mortality rates were 2.7%-13%[55-57]. Recently, a French multicenter clinical study including 301 PMP patients treated by CRS + HIPEC revealed that the 5-year survival rate was 73% and the disease-free survival rate was 56%, and CRS + HIPEC has become the standard treatment for PMP[5].
Twenty studies (Table 4) have evaluated the efficacy of CRS + HIPEC to treat malignant peritoneal mesothelioma. The median OS for patients treated with CRS + HIPEC was 29.5-100 mo[58], which was significantly higher than in historical controls (12-17 mo) as previously reported. HIPEC, no deep tissue invasion, age < 60 years and optimal CRS are independent prognostic factors for survival improvement[59]. These data suggest that patients with malignant peritoneal mesothelioma are good candidates for CRS + HIPEC.
| Ref. | Patients | Country | Median follow-up (mo) | Median OS (mo) | Median DFS (mo) | Morbidity | Mortality |
| Baratti et al | 12 | Italy | 27 | - | 24 | - | 0% |
| Baratti et al | 12 | Italy | 64 | - | 11 | 8.3% | 0% |
| Blackham et al | 34 | United States | 72 | 40.8 | 9.1 | - | - |
| Brigand et al | 15 | France | 46.7 | 35.6 | - | - | 0% |
| Chua et al | 20 | Australia | 18.1 | 29.5 | 7.2 | 65.0% | 5% |
| Sebbag et al | 33 | United States | 21.3 | 31 | - | 33.0% | 3% |
| Tudor et al | 20 | Australia | 18 | 30 | 8 | 65.0% | 5% |
| Deraco et al | 61 | Italy | 20 | - | 28 | 23.0% | 0% |
| Deraco et al | 116 | Italy | - | 31.4 | 14.4 | 41.3% | 2.6% |
| Loggie et al | 12 | United States | 45.2 | 34.2 | - | 33.0% | 8% |
| Ma et al | 12 | Turkey | 10 | - | - | 90.0% | 20% |
| Macuks et al | 12 | Turkey | - | - | - | - | - |
| Markman et al | 19 | United States | 25 | 19 | - | - | - |
| Feldman et al | 49 | United States | - | 92 | 17 | 25.0% | - |
| Chua et al | 26 | Australia, Italy, France, United States, United Kingdom, Germany | 54 | - | - | 26.9% | 0% |
| Schaub et al | 104 | United States | 49.4 | 52 | 20.8 | - | - |
| Yan et al | 401 | Australia, Italy, France, United States, United Kingdom, Germany | 33 | 53 | - | 46.0% | 2% |
| Yano et al | 17 | United Kingdom | 13 | - | - | 41.0% | 12% |
| Yonemura et al | 21 | Japan | - | - | - | 46.2% | - |
| Elias et al | 26 | France | 54 | > 100 | 40 | 54.0% | 4% |
Even with radical surgical resection as initial treatment, the recurrence rate of peritoneal sarcoma can reach 58%-85%[60]. Currently, there is no evidence to indicate that adjuvant therapy improves the survival of these patients. A phase I study including 60 patients with peritoneal sarcoma from Italy evaluated the efficacy of HIPEC in peritoneal sarcoma. The results showed the median time to tumor partial progression was 22 mo, and OS was 34 mo[61]. Histopathology stage and optimal CRS are the key factors for survival improvement.
The comprehensive treatment strategy of CRS + HIPEC is an integration of technical advantages of CRS to reduce the tumor burden and HIPEC to eradicate the residual tumor foci, micrometastases and peritoneal free cancer cells, so as to completely eliminate both the primary tumor and metastases[15,62]. Several lines of evidence from well designed clinical studies have indicated that CRS + HIPEC is an effective strategy to treat PC. At the Ninth International Congress on Peritoneal Surface Malignancies held in Amsterdam, the Netherlands, 2014, the PSOGI officially proposed the International Recommendations for Cytoreductive Surgery (CRS) and Hyperthermic Intraperitoneal Chemotherapy (HIPEC). CRS + HIPEC is recommended as the standard treatment for appendiceal mucinous cancer, colorectal PC, and malignant peritoneal mesothelioma, and it is a recommended therapy for ovarian cancer and gastric cancer with PC. The PSOGI also emphasized the necessity to carry out strictly designed prospective multicenter randomized clinical studies to improve the treatment strategy and efficacy, and to promote this comprehensive treatment approach in clinical oncology.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Nowacki M S- Editor: Qi Y L- Editor: Ma JY E- Editor: Wang CH
| 1. | Blair SL, Chu DZ, Schwarz RE. Outcome of palliative operations for malignant bowel obstruction in patients with peritoneal carcinomatosis from nongynecological cancer. Ann Surg Oncol. 2001;8:632-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 649] [Cited by in RCA: 598] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 3. | Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 4. | Ogden GR, Cowpe JG, Chisholm DM, Lane EB. DNA and keratin analysis of oral exfoliative cytology in the detection of oral cancer. Eur J Cancer B Oral Oncol. 1994;30B:405-408. [PubMed] |
| 5. | Elias D, Gilly F, Quenet F, Bereder JM, Sidéris L, Mansvelt B, Lorimier G, Glehen O. Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol. 2010;36:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Fujimoto S, Shrestha RD, Kokubun M, Ohta M, Takahashi M, Kobayashi K, Kiuchi S, Okui K, Miyoshi T, Arimizu N. Intraperitoneal hyperthermic perfusion combined with surgery effective for gastric cancer patients with peritoneal seeding. Ann Surg. 1988;208:36-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 164] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, Zhou YF, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 492] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 8. | Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1511] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 9. | Spiliotis J, Halkia E, Lianos E, Kalantzi N, Grivas A, Efstathiou E, Giassas S. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol. 2015;22:1570-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 271] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 10. | Li Y. My standpoint of perioneal carcinomatosis study. Zhongguo Zhongliu Linchang. 2012;39:1685-1686. [DOI] [Full Text] |
| 11. | Lambert LA. Looking up: Recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J Clin. 2015;65:284-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 12. | Sticca RP. Peritoneal carcinomatosis: a final frontier. Ann Surg Oncol. 2003;10:484-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Pilati P, Mocellin S, Rossi CR, Foletto M, Campana L, Nitti D, Lise M. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy for peritoneal carcinomatosis arising from colon adenocarcinoma. Ann Surg Oncol. 2003;10:508-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Yang XJ, Li Y, al-shammaa Hassan AH, Yang GL, Liu SY, Lu YL, Zhang JW, Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival in selected patients with peritoneal carcinomatosis from abdominal and pelvic malignancies: results of 21 cases. Ann Surg Oncol. 2009;16:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Yang XJ, Li Y, Yang GL. Intraoperative peritoneal hyperthermic chemotherapy for treatment of peritoneal carcinomatosis from colo-reactal cancer. J Int Oncol. 2007;34:384-386. |
| 16. | Moran B, Baratti D, Yan TD, Kusamura S, Deraco M. Consensus statement on the loco-regional treatment of appendiceal mucinous neoplasms with peritoneal dissemination (pseudomyxoma peritonei). J Surg Oncol. 2008;98:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Sugarbaker PH, Mora JT, Carmignani P, Stuart OA, Yoo D. Update on chemotherapeutic agents utilized for perioperative intraperitoneal chemotherapy. Oncologist. 2005;10:112-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Glehen O, Osinsky D, Cotte E, Kwiatkowski F, Freyer G, Isaac S, Trillet-Lenoir V, Sayag-Beaujard AC, François Y, Vignal J. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol. 2003;10:863-869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 274] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 19. | Elias D, Bonnay M, Puizillou JM, Antoun S, Demirdjian S, El OA, Pignon JP, Drouard-Troalen L, Ouellet JF, Ducreux M. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol. 2002;13:267-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | El-Kareh AW, Secomb TW. A theoretical model for intraperitoneal delivery of cisplatin and the effect of hyperthermia on drug penetration distance. Neoplasia. 2004;6:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Mei LJ, Wang LW, Zhou YF, Xie CH, Liu JF, Yang XJ, Liu SP, Li Y. Role of contrast-enhanced multi-detector row computed tomography and multiplanar reconstruction in diagnosing peritoneal carcinomatosis. Zhongguo Zhongliu Linchang. 2012;39:1745-1749. [DOI] [Full Text] |
| 22. | Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 1128] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 23. | Yang XQ, Chen C, Peng CW, Liu SP, Li Y. Carbohydrate antigen 242 highly consists with carbohydrate antigen 19-9 in diagnosis and prognosis of colorectal cancer: study on 185 cases. Med Oncol. 2012;29:1030-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Yang XQ, Li Y, Chen C, Peng CW, Liu SP, Liu Y. Preoperative serum carbohydrate antigen 125 level is an independent negative prognostic marker for overall survival in colorectal cancer. Med Oncol. 2011;28:789-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 25. | Yang XQ, Chen C, Hou JX, Peng CW, Huang CQ, Li Y. Preoperative serum carbohydrate antigen 242 is a useful predictive and prognostic marker in colorectal cancer. Hepatogastroenterology. 2011;58:377-382. [PubMed] |
| 26. | Hou JX, Yang XQ, Chen C, Jiang Q, Yang GL, Li Y. Screening the gastric cancer related tumor markers from multi-tumor markers protein chip with kappa coefficient and cost-effectiveness analysis. Hepatogastroenterology. 2011;58:632-636. [PubMed] |
| 27. | Zhang YH, Li Y, Chen C, Peng CW. Carcinoembryonic antigen level is related to tumor invasion into the serosa of the stomach: study on 166 cases and suggestion for new therapy. Hepatogastroenterology. 2011;56:1750-1754. [PubMed] |
| 28. | Yang XQ, Yan L, Chen C, Hou JX, Li Y. Application of C12 multi-tumor marker protein chip in the diagnosis of gastrointestinal cancer: results of 329 surgical patients and suggestions for improvement. Hepatogastroenterology. 2009;56:1388-1394. [PubMed] |
| 29. | Chen C, Chen LQ, Yang GL, Li Y. The application of C12 biochip in the diagnosis and monitoring of colorectal cancer: systematic evaluation and suggestion for improvement. J Postgrad Med. 2008;54:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Chen C, Chen LQ, Chen LD, Yang GL, Li Y. Evaluation of tumor markers biochip C12 system in the diagnosis of gastric cancer and the strategies for improvement: analysis of 100 cases. Hepatogastroenterology. 2008;55:991-997. [PubMed] |
| 31. | Chen C, Chen LQ, Yang GL, Li Y. Value of tumor markers in diagnosing and monitoring colorectal cancer and strategies for further improvement: analysis of 130 cases. Ai Zheng. 2007;26:1221-1226. [PubMed] |
| 32. | Shen P, Levine EA, Hall J, Case D, Russell G, Fleming R, McQuellon R, Geisinger KR, Loggie BW. Factors predicting survival after intraperitoneal hyperthermic chemotherapy with mitomycin C after cytoreductive surgery for patients with peritoneal carcinomatosis. Arch Surg. 2003;138:26-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Abrignani S, Houghton M, Hsu HH. Perspectives for a vaccine against hepatitis C virus. J Hepatol. 1999;31 Suppl 1:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 34. | Yang XJ, Li Y, Yonemura Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy to treat gastric cancer with ascites and/or peritoneal carcinomatosis: Results from a Chinese center. J Surg Oncol. 2010;101:457-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Gómez Portilla A, Cendoya I, López de Tejada I, Olabarría I, Martínez de Lecea C, Magrach L, Gil A, Echevarría J, Valdovinos M, Larrabide I. Peritoneal carcinomatosis of colorectal origin. Current treatment. Review and update. Rev Esp Enferm Dig. 2005;97:716-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284-3292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 881] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 37. | Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426-2432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 765] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 38. | Huang CQ, Yang XJ, Yu Y, Wu HT, Liu Y, Yonemura Y, Li Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival for patients with peritoneal carcinomatosis from colorectal cancer: a phase II study from a Chinese center. PLoS One. 2014;9:e108509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Huang CQ, Feng JP, Yang XJ, Li Y. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from colorectal cancer: a case-control study from a Chinese center. J Surg Oncol. 2014;109:730-739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Chen J, Wang S, Xu H. [Curative effect of radical gastrectomy combined with peritoneal lavage with thermal hypoosmotic solution in treatment of gastric cancer]. Zhonghua Yixue Zazhi. 2001;81:730-732. [PubMed] |
| 41. | Zhu ZG, Tang R, Yan M, Chen J, Yang QM, Li S, Yao XX, Zhang J, Yin HR, Lin YZ. [Clinical effect of intraoperative peritoneal hyperthermic chemotherapy for advanced gastric cancer]. Zhonghua Weichang Waike Zazhi. 2006;9:26-30. [PubMed] |
| 42. | Yonemura Y, Kawamura T, Bandou E, Takahashi S, Sawa T, Matsuki N. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg. 2005;92:370-375. [PubMed] |
| 43. | Yonemura Y, de Aretxabala X, Fujimura T, Fushida S, Katayama K, Bandou E, Sugiyama K, Kawamura T, Kinoshita K, Endou Y. Intraoperative chemohyperthermic peritoneal perfusion as an adjuvant to gastric cancer: final results of a randomized controlled study. Hepatogastroenterology. 2001;48:1776-1782. [PubMed] |
| 44. | Scaringi S, Kianmanesh R, Sabate JM, Facchiano E, Jouet P, Coffin B, Parmentier G, Hay JM, Flamant Y, Msika S. Advanced gastric cancer with or without peritoneal carcinomatosis treated with hyperthermic intraperitoneal chemotherapy: a single western center experience. Eur J Surg Oncol. 2008;34:1246-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 45. | Fujimoto S, Takahashi M, Mutou T, Kobayashi K, Toyosawa T. Successful intraperitoneal hyperthermic chemoperfusion for the prevention of postoperative peritoneal recurrence in patients with advanced gastric carcinoma. Cancer. 1999;85:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 46. | Hall JJ, Loggie BW, Shen P, Beamer S, Douglas Case L, McQuellon R, Geisinger KR, Levine EA. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for advanced gastric cancer. J Gastrointest Surg. 2004;8:454-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Fujimura T, Yonemura Y, Fushida S, Urade M, Takegawa S, Kamata T, Sugiyama K, Hasegawa H, Katayama K, Miwa K. Continuous hyperthermic peritoneal perfusion for the treatment of peritoneal dissemination in gastric cancers and subsequent second-look operation. Cancer. 1990;65:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 48. | Hamazoe R, Maeta M, Kaibara N. Intraperitoneal thermochemotherapy for prevention of peritoneal recurrence of gastric cancer. Final results of a randomized controlled study. Cancer. 1994;73:2048-2052. [PubMed] |
| 49. | Kim JY, Bae HS. A controlled clinical study of serosa-invasive gastric carcinoma patients who underwent surgery plus intraperitoneal hyperthermo-chemo-perfusion (IHCP). Gastric Cancer. 2001;4:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 50. | Yonemura Y, Fujimura T, Nishimura G, FallaR T, Katayama K, Tsugawa K, Fushida S, Miyazaki I, Tanaka M, Endou Y. Effects of intraoperative chemohyperthermia in patients with gastric cancer with peritoneal dissemination. Surgery. 1996;119:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 51. | Deraco M, Rossi CR, Pennacchioli E, Guadagni S, Somers DC, Santoro N, Raspagliesi F, Kusamura S, Vaglini M. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion in the treatment of recurrent epithelial ovarian cancer: a phase II clinical study. Tumori. 2001;87:120-126. [PubMed] |
| 52. | Armstrong DK, Bundy B, Wenzel L. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2030] [Cited by in RCA: 1949] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 53. | Cascales-Campos PA, Gil J, Gil E, Feliciangeli E, González-Gil A, Parrilla JJ, Parrilla P. Treatment of microscopic disease with hyperthermic intraoperative intraperitoneal chemotherapy after complete cytoreduction improves disease-free survival in patients with stage IIIC/IV ovarian cancer. Ann Surg Oncol. 2014;21:2383-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 54. | Safra T, Grisaru D, Inbar M, Abu-Abeid S, Dayan D, Matceyevsky D, Weizman A, Klausner JM. Cytoreduction surgery with hyperthermic intraperitoneal chemotherapy in recurrent ovarian cancer improves progression-free survival, especially in BRCA-positive patients- a case-control study. J Surg Oncol. 2014;110:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol. 1999;6:727-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 430] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 56. | Witkamp AJ, de Bree E, Kaag MM, van Slooten GW, van Coevorden F, Zoetmulder FA. Extensive surgical cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in patients with pseudomyxoma peritonei. Br J Surg. 2001;88:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 138] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 57. | Deraco M, Baratti D, Inglese MG, Allaria B, Andreola S, Gavazzi C, Kusamura S. Peritonectomy and intraperitoneal hyperthermic perfusion (IPHP): a strategy that has confirmed its efficacy in patients with pseudomyxoma peritonei. Ann Surg Oncol. 2004;11:393-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 58. | Helm JH, Miura JT, Glenn JA, Marcus RK, Larrieux G, Jayakrishnan TT, Donahue AE, Gamblin TC, Turaga KK, Johnston FM. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: a systematic review and meta-analysis. Ann Surg Oncol. 2015;22:1686-1693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 168] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 59. | Feldman AL, Libutti SK, Pingpank JF, Bartlett DL, Beresnev TH, Mavroukakis SM, Steinberg SM, Liewehr DJ, Kleiner DE, Alexander HR. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol. 2003;21:4560-4567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 252] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 60. | Berthet B, Sugarbaker TA, Chang D, Sugarbaker PH. Quantitative methodologies for selection of patients with recurrent abdominopelvic sarcoma for treatment. Eur J Cancer. 1999;35:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 61. | Rossi CR, Deraco M, De Simone M, Mocellin S, Pilati P, Foletto M, Cavaliere F, Kusamura S, Gronchi A, Lise M. Hyperthermic intraperitoneal intraoperative chemotherapy after cytoreductive surgery for the treatment of abdominal sarcomatosis: clinical outcome and prognostic factors in 60 consecutive patients. Cancer. 2004;100:1943-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Li Y, Yang GL, Yang XJ. Cytoreductive surgery with intraoperative peritoneal hyperthermo-chemotherapy for peritoneal carcinomatosis. Zhongguo Zhongliu Linchang. 2007;34:1257-1260. |