Published online Aug 14, 2016. doi: 10.3748/wjg.v22.i30.6817
Peer-review started: March 16, 2016
First decision: March 31, 2016
Revised: June 16, 2016
Accepted: July 6, 2016
Article in press: July 6, 2016
Published online: August 14, 2016
Processing time: 142 Days and 18.7 Hours
Gastrointestinal neuroendocrine tumors (GI-NETs) are rare neoplasms, like all NETs. However, the incidence of GI-NETS has been increasing in recent years. Gastric NETs (G-NETs) and duodenal NETs (D-NETs) are the common types of upper GI-NETs based on tumor location. G-NETs are classified into three distinct subgroups: type I, II, and III. Type I G-NETs, which are the most common subtype (70%-80% of all G-NETs), are associated with chronic atrophic gastritis, including autoimmune gastritis and Helicobacter pylori associated atrophic gastritis. Type II G-NETs (5%-6%) are associated with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome (MEN1-ZES). Both type I and II G-NETs are related to hypergastrinemia, are small in size, occur in multiple numbers, and are generally benign. In contrast, type III G-NETs (10%-15%) are not associated with hypergastrinemia, are large-sized single tumors, and are usually malignant. Therefore, surgical resection and chemotherapy are generally necessary for type III G-NETs, while endoscopic resection and follow-up, which are acceptable for the treatment of most type I and II G-NETs, are only acceptable for small and well differentiated type III G-NETs. D-NETs include gastrinomas (50%-60%), somatostatin-producing tumors (15%), nonfunctional serotonin-containing tumors (20%), poorly differentiated neuroendocrine carcinomas (< 3%), and gangliocytic paragangliomas (< 2%). Most D-NETs are located in the first or second part of the duodenum, with 20% occurring in the periampullary region. Therapy for D-NETs is based on tumor size, location, histological grade, stage, and tumor type. While endoscopic resection may be considered for small nonfunctional D-NETs (G1) located in the higher papilla region, surgical resection is necessary for most other D-NETs. However, there is no consensus regarding the ideal treatment of D-NETs.
Core tip: There is a significant increase in the incidence of gastric and duodenal neuroendocrine tumors (G-NETs and D-NETs). G-NETs are classified into three distinct subtypes based on tumor etiology. There are important differences between each type in terms of clinical presentation, prognosis, and management strategies. D-NETs include five histological types. The majority of D-NETs are located in the first or second part of the duodenum. The choice of treatment for D-NETs is based on tumor size, location, histological grade, stage, and tumor type.
- Citation: Sato Y, Hashimoto S, Mizuno KI, Takeuchi M, Terai S. Management of gastric and duodenal neuroendocrine tumors. World J Gastroenterol 2016; 22(30): 6817-6828
- URL: https://www.wjgnet.com/1007-9327/full/v22/i30/6817.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i30.6817
Neuroendocrine tumors (NETs), which were first labeled as carcinoid tumors by Oberndorfer in 1907, are rare neoplasms that arise from the peripheral neuroendocrine system dispersed in various organs[1]. Gastrointestinal neuroendocrine tumors (GI-NETs), like all NETs, are being increasingly reported in recent times[1,2]. This increase in the incidence of GI-NETs reflects the widespread use of endoscopy, and an increased awareness of GI-NETs among clinicians and pathologists.
The 2010 WHO classification[3] endorsed the European Neuroendocrine Tumor Society (ENETS) grading system, which is based on the mitotic count and Ki-67 index of NETs[4]. As a result, NETs are classified as NET G1, NET G2, neuroendocrine carcinoma (NEC), mixed adeno-NEC, and hyperplastic and pre-neoplastic lesions, based on their histological proliferation and differentiation (Table 1). Tumors classified as G3 by the ENETS criteria correspond to NEC in the 2010 WHO classification of NETs. These classification and staging systems are useful in predicting prognosis as well as in therapeutic decision-making.
| ENETS grading | Mitotic index (× 10 HPF) | Ki-67 proliferation index | WHO classification 2010 |
| G1 | < 2 | ≤ 2% | NET G1 (carcinoid) |
| G2 | 2-20 | 3%-20% | NET G2 |
| G3 | > 20 | > 20% | NEC G3; large-cell or small-cell type |
This review focuses on the pathogenesis, endoscopic diagnosis and management of the NETs of the stomach [gastric NETs (G-NETs)] and duodenum [duodenal NETs (D-NETs)].
There appears to be a recent increase in the proportion of G-NETs reported among all GI-NETs[5-10]. The incidence of G-NETs as recorded in the period from 2000 to 2006 in England was 0.16 and 0.15 per 100000, in men and women, respectively. This represents a 23-fold increase in men and a 47-fold increase in women as compared to data recorded in 1995[5]. There is a similar increase in the incidence of G-NETs in the United States, from 0.03 (1973-1977) to 0.33 (2003-2007) per 100000[2]. Japanese data also demonstrates an increase in the incidence of foregut (stomach and duodenum) NETs from 1.05 (2005) to 1.67 (2010) per 100000[7,11]. In the latest data review, the prevalence was 3.4 and 1.7 per 100000 people in 10 European countries and the United States, respectively[12].
G-NETs are classified into three distinct subgroups, type I, II, and III[13]. Table 2 summarizes the clinical characteristics of the three types of G-NETs[14-25].
| Characteristic | Type I G-NETs | Type II G-NETs | Type III G-NETs |
| Proportion of all G-NETs | 70%-80% | 5%-10% | 10%-15% |
| Associated disease | Chronic atrophic gastritis | MEN type 1/ZES | None |
| Gender | Women > men | Women = men | Women < men |
| Tumor number | multiple | multiple | single |
| Tumor size | < 10 mm | < 10 mm | > 10 mm |
| Tumor location | fundus or corpus | fundus or corpus | Any region |
| Histology | Usually NET G1 | NET G1/G2 | Usually NEC G3 |
| Invasion depth | Mucosa or submucosa | Mucosa or submucosa | Any depth |
| Serum gastrin | High | High | Normal |
| Gastric pH | High | Low | Normal |
| Risk of Metastasis | 2%-5% | 10%-20% | > 50% |
| Prognosis | Excellent | Good | Poor |
Type I G-NETs comprise 70%-80% of all G-NETs, and occur more frequently in females. Histologically, type I G-NETs are composed of enterochromaffin-like (ECL) cells and are commonly detected in patients with chronic atrophic gastritis (CAG), including autoimmune gastritis and Helicobacter pylori associated atrophic gastritis[24]. It is known that the achlorhydria caused by the loss of fundic glands in CAG results in hypergastrinemia, and hyperplasia of ECL cells. In immunohistochemical staining, NETs cells are positive for chromogranin-A (CgA), synaptophysin, vesicular monoamine transporter 2, and somatostatin receptor 2A[26]. In particular, CgA staining is useful for observing hyperplastic and dysplastic ECL cell changes. ECL cell hyperplasia is characterized by more than six chains of linear hyperplasia per mm, and ECL cell dysplasia, occurring mainly in microinfiltrative lesions, is associated with increased risks of G-NETs[27].
The majority of type I G-NETs present as small, multiple tumors, located in the gastric body or fundus, and limited to the mucosal or submucosal layers of the stomach wall. Since most type I G-NETs are G1 tumors, the metastatic risk is very low, and the prognosis is excellent. Patients with type I G-NETs associated with autoimmune gastritis may present with other autoimmune diseases (type I diabetes mellitus, autoimmune thyroiditis, and primary biliary cirrhosis) or pernicious anemia[23]. Type I G-NETs are generally asymptomatic, and are usually incidentally detected at screening upper gastrointestinal endoscopy.
Type II G-NETs account for 5%-6% of all G-NETs. These tumors are associated with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome (MEN1-ZES). Type II G-NETs are detected in 13%-37% of all patients with MEN1-ZES, and in only 0%-2% of patients with sporadic ZES (without MEN1). Like type I G-NETs, type II G-NETs are associated with hypergastrinemia and originate from ECL cells. They too are small, multiple, and relatively benign tumors. However, unlike type I G-NETs, type II G-NETs occur in males and females with equal proportions. Approximately 30% of type II G-NETs are metastatic at presentation[17], and the survival rates of patients with type II G-NETs are lower than those of patients with type I G-NETs[28]. The increased gastric acid secretion observed in patients with type II G-NETs may result in peptic ulcers. Therefore, though most type II G-NETs by themselves are asymptomatic, patients may present with symptoms of peptic ulcers[29].
Type III G-NETs, representing approximately 10%-15% of all G-NETs, are sporadic tumors that develop independent of gastrin secretion, and are not associated with ECL hyperplasia. The tumors are usually single, and greater than 10 mm in size[19]. Type III G-NETs are more commonly G3 tumors, with a tendency to infiltrate the muscularis propria. The tumors also demonstrate a higher rate of angioinvasive transformation. Type III tumors are usually metastatic at presentation, with spread to regional lymph nodes or liver. These tumors often present with symptoms of anemia, loss of appetite, dyspepsia, gastrointestinal bleeding, and weight loss.
The typical presentation of “carcinoid syndrome” including flushing, tachycardia, and diarrhea, occurs rarely in patients with gastric NETs (< 1%) and is almost exclusively associated with type III tumors, especially those presenting with liver metastasis[30]. It is not commonly associated with any other types of G-NET.
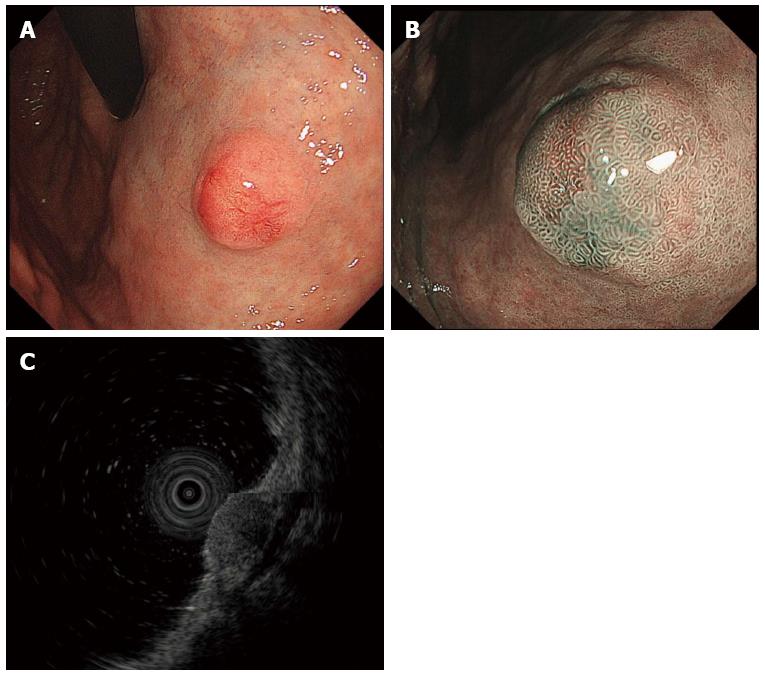
It is important to recognize the endoscopic features of G-NETs, since they are often incidentally detected on screening upper gastrointestinal endoscopy. Type I and II G-NETs appear either as small (< 10 mm) polypoid lesions, or more frequently, as smooth hemispherical submucosal lesions, that may appear yellow or red in color[23]. Each tumor appears as a hemispherical reddish polyp with or without a central depression on endoscopy with white light (Figure 1A). The presence of the central depression indicates subepithelial tumor growth. High resolution magnifying endoscopy (ME) with narrow band imaging may demonstrate gastric pit-like structures present on the surface of the tumor (Figure 1B). Since G-NETs are usually covered by normal mucosa, these details may not be obvious on conventional endoscopy. It has been reported that the pit-like structures are not present in the region of the central depression, and instead, dilated blackish-brown subepithelial vessels with cork-screw capillaries are visible[23,31]. Type III G-NETs are usually single lesions, large in size and with features of ulceration indicating deeper invasion.
Endoscopic ultrasonography (EUS) is useful for assessing the depth of the tumor and its location within the layers of the gastric wall[32]. G-NETs are commonly present in the second (deeper mucosal) or third (submucosal) echo layers, and are identified as hypoechoic intramural structures (Figure 1C).
Computed tomography (CT) or magnetic resonance imaging (MRI) provide additional information regarding local spread and distant metastasis, and aid in tumor staging. The role of fluorodeoxyglucose positron emission tomography (PET) in the assessment of G-NETs is unclear[33].
Octreoscan (somatostatin receptor scintigraphy), is recommended for the detection of liver, bone and lymph node metastasis[34]. However, recent studies concerning PET in NETs using 68Ga-labeled PET tracers (68Ga-DOTATOC, -DOTANOC, and -DOTATATE) have shown promising results, with a higher rate of lesion identification and lower costs than usually achieved with octreoscan[35,36]. However, few studies have demonstrated the utility of 68Ga-labeled PET in patients with G-NETs[35,36], and further studies would be therefore required.
The management of G-NETs is based on the type of tumor, size, and the presence of risk factors such as muscular wall infiltration, increased tumor proliferation and metastasis.
Type I G-NETs: The National Comprehensive Cancer Network (NCCN) guidelines[37] recommend simple surveillance or endoscopic resection (ER) for tumors that are smaller than 20 mm in size, and without features of invasion of muscularis propria or metastasis, regardless of the tumor number. Surgical resection, or ER is recommended for tumors that are greater than 20 mm in size, whether single or multiple. On the other hands, the ENETS guidelines recommend ER for the treatment of type I G-NETs, with surgical resection to be considered only if invasion extends beyond the submucosa, or if lymph nodal or distant metastasis is present. Surgical resection is also recommended for poorly differentiated lesions[34]. Previously, endoscopic mucosal resection (EMR) was recommended for well localized, type I G-NETs. However, since type I G-NETs frequently invade the submucosa, their complete removal by snare polypectomy or conventional EMR is difficult. Endoscopic submucosal dissection (ESD) is useful for the removal of submucosal tumors including type I G-NETs. Studies have demonstrated superior complete resection rates of gastric neuroendocrine tumors by using ESD compared to those achieved with EMR[38,39]. Several recent reports have revealed that no tumor-related deaths were observed in patients with type I G-NETs who were assessed by endoscopic surveillance but not treated[25,40,41]. Therefore, endoscopic surveillance seems to be a reasonable approach in selected patients with type I G-NETs, such as small tumors in elderly patients or those with co-morbidities. However, we[25] have reported four patients with small TIGC (< 1 cm) that were complicated with capillary invasion (lymphatic invasion in two, venous invasion in two), so endoscopic follow-up without treatment must be selected after careful consideration.
Among the surgical options, antrectomy is an option for recurrent type I G-NETs. Antrectomy alleviates G-cell-mediated hypergastrinemia resulting in ECL cell hypertrophy. However, it may not be effective in preventing recurrence or metastasis[42]. Moreover, surgical therapy is more invasive and associated with higher risks of complications[42]. However, patients treated with antrectomy have a lower risk of recurrence and need fewer follow-up EGDs than patients who receive endoscopic resection or EGD surveillance alone[43]. Laparoscopic antrectomy may provide a minimally invasive alternative surgical treatment for type I G-NETs[43,44]. Therefore, in cases of recurrence or persistent G-NETs after ER or local resection, antrectomy or partial/total gastrectomy, along with lymph node dissection, is needed.
Somatostatin analogues (SSAs), which inhibit G cell-mediated gastric secretion and reduce ECL cell hyperplasia, are effective in reducing the number and size of type I and II G-NETS[45-48]. However, the routine use of SSA is not recommended due to their short-term effects, recurrence following cessation of therapy[49], and high treatment costs. Therefore, SSAs therapy should be limited to patients with recurrent or multifocal type I G-NETs[50]. Recent report have revealed that intermittent treatment with SSAs would be safe and effective method of treating recurrent type I G-NETs in patients who do not undergo ER[51].
Netazepide (YF476), a potent gastrin/CCK-B receptor antagonist, has been reported to suppress gastric acid output and reduce serum CgA levels, in addition to the size and number of type I G-NET[52,53]. Therefore, netazepide is a potentially useful drug for the treatment for type I G-NET. However, the levels of CgA mRNAs recovered to pre-treatment levels after stopping treatment[53], so the long-term administration of natazepide needs to be assessed in the context of type I G-NETs.
Type II G-NETs: According to the NCCN guidelines, the management of type II and type I G-NETs are almost similar[37]. However, the ENETS guidelines clearly recommend surgical resection for type II G-NETs, owing to their higher rate of metastasis compared to type I tumors[34]. Moreover, although the prevalence of the different histological classification (diffuse, linear, micronodular) of ECL cell hyperplasia in peritumoral gastric mucosa were approximately equal between patients with MEN-1 associated gastrinoma and sporadic gastrinoma, ECL cell dysplasia and ECL cell NET only were detected in patients with MEN-1[54]. In addition, the resection of the co-existing gastrinoma should be attempted to the extent possible. SSA treatment is often adequate for patients with symptomatic type II G-NET[37].
Type III G-NETs: The ENETS guidelines recommend that type-III G-NETs be managed in the same manner as gastric adenocarcinomas. Therefore, these guidelines recommend surgical resection (partial or total gastrectomy with lymph node dissection) and chemotherapy[34]. Similarly, the NCCN guidelines recommend radical gastric resection with perigastric lymph node dissection for localized type III G-NETs[37]. However, it has been documented that endoscopic or wedge resection can be considered for type III G-NETs < 2 cm in the NCCN guideline. Scherübl et al[19] proposed that small, well differentiated (G1) type III G-NETs be treated conservatively by ER, and Kwon et al[55] also reported that if the tumor is < 2 cm and confined to the submucosal layer with no lymphovascular invasion, then endoscopic treatment should be considered as the initial choice.
According to the NCCN guidelines[37], patients with small (< 20 mm) type I and II G-NETs should be followed up every 6-12 mo after treatment for the first 3 years, and each visit should include patient history, physical examination and esophagogastroduodenoscopy (EGD). Beyond three years, EGD may be performed annually. Patients with type III G-NETs or large (> 20 mm) type I and II G-NETs should be followed-up every 3 to 12 mo following resection, and every 6 to 12 mo thereafter. Imaging studies such as CT or MRI may be considered depending on clinical indications. The ENETS guidelines[34] recommend endoscopic follow-up every 12 mo for patients with recurrent type I G-NETs, and every 24 mo for patients without recurrence. Annual EGD is recommended for patients with type II G-NETs. The follow-up of patients with type III G-NETs following gastrectomy is similar to that for gastric adenocarcinoma.
D-NETs are rare tumors with an overall incidence of 0.19/100000 in the United States[2]. Recent studies suggest an increase in the incidence of D-NETs[56]. These tumors comprise 1%-3% of primary duodenal tumors, 11% of small intestinal NETs, and 5%-8% of all GI-NETs[2,56]. Their overall prevalence is reportedly lower in England (0.04/100000)[5] and higher in Japan (0.17/100000)[7]. D-NETs are slightly more common in males than in females[57-59].
Most D-NETs are located in the first or second part of the duodenum[57,58], with 20% of them occurring in the periampullary region[60,61]. Despite the detection of various gastrointestinal hormones in D-NETs, 90% of the tumors are non-functional[57]. The majority of D-NETs are, therefore, incidentally detected on EGD. Symptoms related to ZES are present in 10% of all patients with D-NETs, while carcinoid syndrome is reported in 3%[34]. D-NETs are generally solitary and small, with 75% of tumors smaller than 2 cm in size[58,61]. Since multiple gastrinomas are found in 60%-95% of patients with MEN1-ZES, the presence of multiple D-NETs, seen in 9%-13% of patients, should raise suspicion of MEN1-ZES[57,59,62,63]. While the majority of D-NETs are limited to the mucosa or the submucosa[64], metastases to the lymph nodes (40%-60%) and liver (less than 10%) are known to occur[57,65-67]. The 5-year survival rate in patients with well-differentiated D-NETs is 80%-85%. However, this rate is only 72% in those with well-differentiated NEC[58,68].
Based on the WHO classification, D-NETs are classified as NET G1 (50%-75%), NET G2 (25%-50%), and NEC (≤ 3%)[9]. D-NETs are also divided into 5 subtypes including duodenal gastrinomas (50%-60% of all D-NETs), somatostatin-producing tumors (15%), nonfunctional serotonin-containing tumors (19%-27%), poorly differentiated neuroendocrine carcinomas (< 3%), and gangliocytic paragangliomas (< 2%)[34,69].
Duodenal gastrinomas are either sporadic or associated with MEN1-ZES[17]. Recent reports have suggested that gastrinomas may be etiologically related to H. pylori associated atrophic gastritis or the use of proton pump inhibitors[70,71]. These tumors are almost always small in size, and are located predominantly in the first or second part of the duodenum. The “gastrinoma triangle” located in the right upper quadrant (duodenal and pancreatic head area) is the location of 70%-85% of these tumors[72]. Sporadic duodenal gastrinoma are solitary, while familial tumors occurring in association with MEN1 are usually multiple and may be easily missed[69]. Despite their small size, lymph node metastases are often observed, and 5%-10% of patients present with liver metastasis. The 10-year survival rate of patients with duodenal gastrinoma is about 60%-80%, which is better than for patients with pancreatic gastrinomas. Fast growing and metastasizing duodenal gastrinoma are rare[64,73].
Somatostatin-producing tumors (somatostatinomas) are exclusively located in the ampullary or periampullary region, and 20%-30% of them are associated with neurofibromatosis-1[74]. These tumors result in obstruction of the duodenal ampulla and present with jaundice or pancreatitis. Functional somatostatinoma syndromes occur very rarely with these tumors[74].
Duodenal gangliocytic paragangliomas also show a preference for the periampullary region. They are often large (> 2 cm) and invade the muscularis propria. However, in contrast to gastrinomas or somatostatinomas, these tumors follow a benign course[69,74].
Poorly differentiated NEC occur primarily in the ampulla of Vater or the periampullary region. They are hormonally inactive, and usually present with lymph node metastasis or liver metastasis at diagnosis[74].
D-NETs at the ampulla of Vater or the periampullary area differ from D-NETs at other locations[57,59,74]. Ampullary D-NETs present at a more advanced stage of the disease and have poorer overall survival than D-NETs located elsewhere in the duodenum[61]. In patients with D-NETs, larger size of the primary tumor, invasion into the muscularis mucosa or beyond, increased mitotic activity, and poor differentiation correlates with the presence of distant metastases or aggressive growth[34]. However, the size of ampullary D-NETs is not correlated with the presence of liver metastases[34].
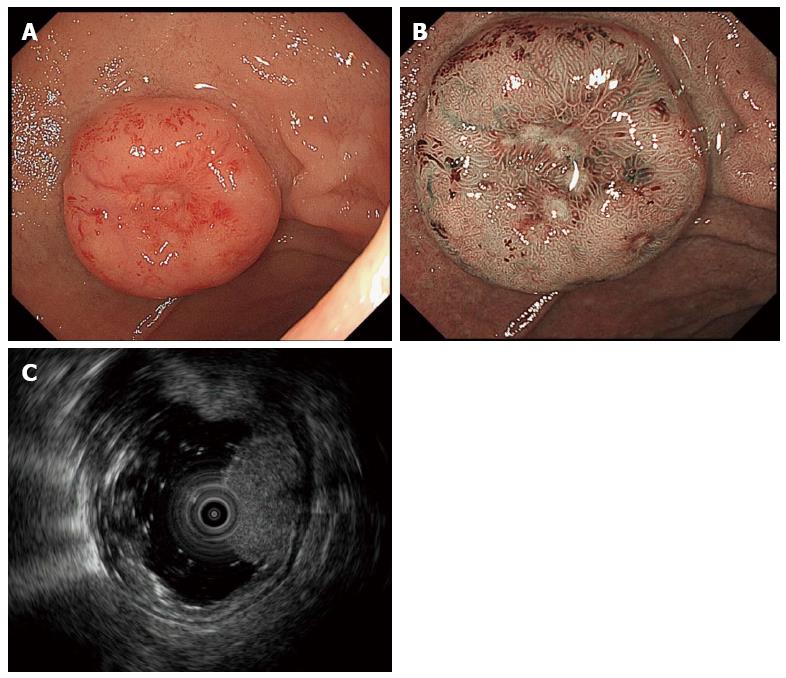
As with G-NETs, D-NETs arise in the deep mucosa and invade the submucosa. Thus, D-NETs have the appearance of submucosal tumors that are either “hemispherical” or “flatly elevated”. However, the protuberances of D-NETs are slightly steeper than those of other submucosal tumors. The surface coloring of the tumors may be identical to the surrounding tissue or appear yellowish. In addition, as the tumor enlarges a depression begins to form in the center, which is eventually replaced by an ulcer crater with tumor progression (Figure 2).
The differential diagnosis of D-NETs includes Brunner’s gland hyperplasia, heterotopic gastric mucosa, adenomas, adenocarcinomas, gastrointestinal stromal tumors, lymphoid hyperplasia, metastatic tumors, neurofibromas and schwannomas[75].
EGD and direct tissue biopsy is the most common method of diagnosing D-NETs. However, it is possible that endoscopic biopsy may not always include tumor tissue due to the location of the tumor within the deep mucosal or the submucosal layer. EUS is essential to confirm tumor size and depth of tumor invasion. Additionally, CT and/or MRI are indicated for patients with suspected or proven advanced disease.
The management of D-NETs is based on tumor size, location, histological grade, stage, and tumor type. However, there is no consensus on the definitive treatment of D-NETs.
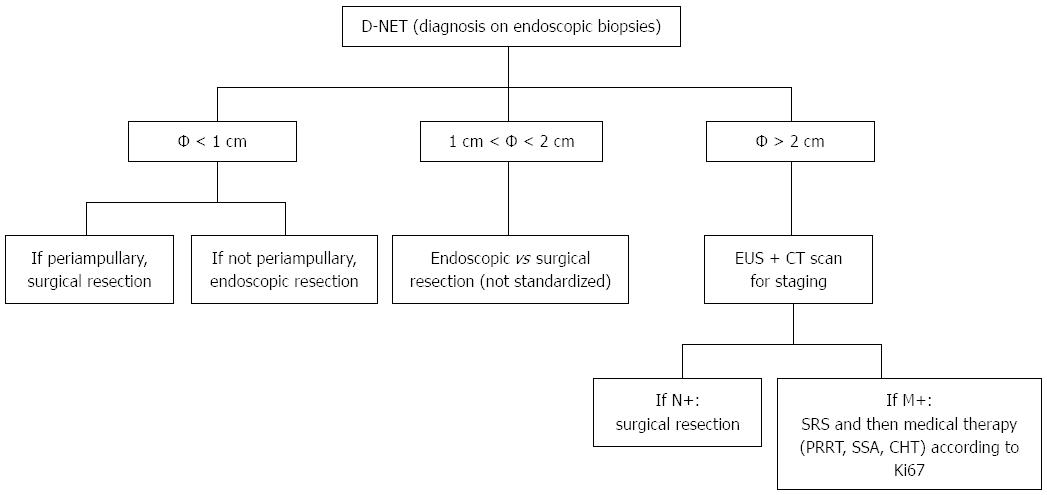
The management protocol of D-NETs based on the ENETS guidelines[34] is shown in Figure 3. Small (≤ 1 cm) G1 D-NETs in nonampullary locations and without metastases or functional hormonal syndromes can be resected by endoscopic techniques. However, if the D-NETs are present in the ampullary region, local surgical resection with lymphadenectomy or picking is recommended. The management of intermediate-sized (1-2 cm) D-NETs is controversial. Large (> 2 cm) D-NETs or D-NETs of any size with lymph node involvement, should be treated by limited surgical resection. According to the NCCN guidelines[37], endoscopic resection is recommended for well-localized D-NETs, whenever possible. Transduodenal local excision with or without lymph node sampling, and pancreatoduodenectomy are other options for primary treatment of non-metastatic D-NETs. To date, endoscopic surveillance for D-NETs, even in cases of small G1 tumors, is not generally recommended because lymph node metastasis and microvascular invasion have been observed in such tumors[76-78].
ER is selected as a safe and minimally invasive method of treatment of D-NETs. Currently, various methods of ER, such as cap technique, EMR, EMR with ligation device (EMR-L), or ESD, are described for superficial duodenal tumors including D-NETs[79-83]. However, the histological complete resection rate of EMR or EMR-L is low[80]. ESD can achieve en bloc resection of tumors larger than 1 cm in diameter, but is associated with a higher rate of complications of bleeding and perforation[81-83]. These complications may be prevented by performing endoscopic closure of mucosal defects following duodenal ESD, by using techniques such as “endoloop with the endoclip”, “over-the-scope clip”, or overlay with polyglycolic acid sheets[82,84,85]. Endoscopic full-thickness resection with laparoscopic assistance is another option for the management of D-NET[86].
Hoteya et al[82] reported that the bleeding rates following ESD for duodenal tumors located in the lower papilla were higher than those for tumors located in the higher papilla. This difference could be attributed to the direct exposure of the lower papilla region to digestive enzymes and bile juice compared to the higher papilla region. In addition, D-NETs in the lower papilla region are derived from the embryological midgut. Midgut (jejunum and ileum) NETs, in the advanced stage of the disease, are often complicated by carcinoid syndrome[74,87,88]. Therefore, extreme caution is essential in the treatment of D-NETs of the lower papilla region.
Surgical resection is recommended for patients with sporadic (non-MEN1) duodenal gastrinomas[89], since lymph node metastases are present in at least half of such cases[57,90,91]. In addition, surgical resection is known to decrease the rate of liver metastasis[66,92] in these cases. The role of curative surgery in patients with MEN1-ZES is controversial. Aggressive surgical resection in this group of patients may not result in increasing disease-free survival rates as most patients are found to have lymph node metastasis at the time of surgery[93,94]. However, several reports have documented that aggressive surgery increases survival[95] or prevents the development of liver metastasis[96]. Imamura et al[97,98] also reported that surgical curative resection, especially pancreas preserving total duodenectomy based on accurate localization using selective arterial secretagogue infection test, is useful for curing MEN1-ZES related duodenal gastrinomas.
As previously mentioned, D-NETs in the region of the ampulla of Vater or the periampullary area are more advanced (invasion of muscularis propria and lymph node metastasis) at presentation and have poorer overall survival than D-NETs at other locations[10,99]. In these cases, surgical resection is preferred to endoscopic papillectomy or resection.
According to the ENETS guidelines[34], when non-functional D-NETs are completely removed by using endoscopic techniques, follow up visits, with EGD, abdominal ultrasound (US) or CT and plasma CgA levels, should be performed at 6, 24, and 36 mo following such treatment. Surgical resection of D-NETs should be followed up by multislice CT, somatostatin receptor scintigraphy (SRS) and CgA levels performed at 6 and 12 mo after surgery, and then annually for a minimum of 3 years. Re-evaluation of untreated patients with metastatic/inoperable D-NETs should be performed at 3- to 6-mo intervals by CgA, CT scan, and/or US and SRS. The NCCN guidelines recommend re-evaluation of patients at 3 to 12 mo following resection, and then, every 6 to 12 mo for a duration of 10 years. Baseline imaging recommendations for patients suspected to have distant metastatic disease include multi-phase CT or MRI. Baseline levels of CgA may also be measured to enable monitoring of patients, and to assess disease progression.
There is a significant increase in the reported incidence of G-NETs and D-NETs. G-NETs are classified into three distinct subtypes (Type I, II, III), and their management is based on the tumor subtype and histological features, the extent of loco-regional spread, and the presence of metastasis. D-NETs include five different types of NETs based on histology. The treatment of D-NETs is based on tumor size, location, histological grade, stage and tumor type. Further studies are required to establish the optimal method of management for G-NETs and D-NETs.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B,B
Grade C (Good): C,C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Boyce M, Imamura M, Khuroo MS, Pritchard DM S- Editor: Gong ZM L- Editor: A E- Editor: Ma S
| 1. | Lawrence B, Gustafsson BI, Chan A, Svejda B, Kidd M, Modlin IM. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 631] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 2. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3247] [Article Influence: 191.0] [Reference Citation Analysis (0)] |
| 3. | Rindi G, Arnold R, Bosman FT, Capella C, Klimstra DS, Klöppel G, Komminoth P, Solcia E. Nomenctlature and classification of neuroendocrine neoplasms of the digestive system. WHO classification of Tumours of the digestive system. Lyon: IARC 2010; 13-14. |
| 4. | Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M, Komminoth P. TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch. 2006;449:395-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1196] [Cited by in RCA: 1086] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 5. | Ellis L, Shale MJ, Coleman MP. Carcinoid tumors of the gastrointestinal tract: trends in incidence in England since 1971. Am J Gastroenterol. 2010;105:2563-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Modlin IM, Lye KD, Kidd M. A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem? Am J Gastroenterol. 2004;99:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Ito T, Sasano H, Tanaka M, Osamura RY, Sasaki I, Kimura W, Takano K, Obara T, Ishibashi M, Nakao K. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol. 2010;45:234-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 261] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 8. | Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European Neuroendocrine Tumour Society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010;17:909-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 323] [Article Influence: 21.5] [Reference Citation Analysis (1)] |
| 9. | Cho MY, Kim JM, Sohn JH, Kim MJ, Kim KM, Kim WH, Kim H, Kook MC, Park do Y, Lee JH. Current Trends of the Incidence and Pathological Diagnosis of Gastroenteropancreatic Neuroendocrine Tumors (GEP-NETs) in Korea 2000-2009: Multicenter Study. Cancer Res Treat. 2012;44:157-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Caldarella A, Crocetti E, Paci E. Distribution, incidence, and prognosis in neuroendocrine tumors: a population based study from a cancer registry. Pathol Oncol Res. 2011;17:759-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Ito T, Igarashi H, Nakamura K, Sasano H, Okusaka T, Takano K, Komoto I, Tanaka M, Imamura M, Jensen RT. Epidemiological trends of pancreatic and gastrointestinal neuroendocrine tumors in Japan: a nationwide survey analysis. J Gastroenterol. 2015;50:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 265] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 12. | Boyce M, Thomsen L. Gastric neuroendocrine tumors: prevalence in Europe, USA, and Japan, and rationale for treatment with a gastrin/CCK2 receptor antagonist. Scand J Gastroenterol. 2015;50:550-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993;104:994-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 370] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Nikou GC, Angelopoulos TP. Current concepts on gastric carcinoid tumors. Gastroenterol Res Pract. 2012;2012:287825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Basuroy R, Srirajaskanthan R, Prachalias A, Quaglia A, Ramage JK. Review article: the investigation and management of gastric neuroendocrine tumours. Aliment Pharmacol Ther. 2014;39:1071-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Li TT, Qiu F, Qian ZR, Wan J, Qi XK, Wu BY. Classification, clinicopathologic features and treatment of gastric neuroendocrine tumors. World J Gastroenterol. 2014;20:118-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Massironi S, Sciola V, Spampatti MP, Peracchi M, Conte D. Gastric carcinoids: between underestimation and overtreatment. World J Gastroenterol. 2009;15:2177-2183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Zhang L, Ozao J, Warner R, Divino C. Review of the pathogenesis, diagnosis, and management of type I gastric carcinoid tumor. World J Surg. 2011;35:1879-1886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Scherübl H, Cadiot G, Jensen RT, Rösch T, Stölzel U, Klöppel G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: small tumors, small problems? Endoscopy. 2010;42:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 20. | Kaltsas G, Grozinsky-Glasberg S, Alexandraki KI, Thomas D, Tsolakis AV, Gross D, Grossman AB. Current concepts in the diagnosis and management of type 1 gastric neuroendocrine neoplasms. Clin Endocrinol (Oxf). 2014;81:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | O’Toole D, Delle Fave G, Jensen RT. Gastric and duodenal neuroendocrine tumours. Best Pract Res Clin Gastroenterol. 2012;26:719-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Dakin GF, Warner RR, Pomp A, Salky B, Inabnet WB. Presentation, treatment, and outcome of type 1 gastric carcinoid tumors. J Surg Oncol. 2006;93:368-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 23. | Sato Y. Endoscopic diagnosis and management of type I neuroendocrine tumors. World J Gastrointest Endosc. 2015;7:346-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 24. | Sato Y, Iwafuchi M, Ueki J, Yoshimura A, Mochizuki T, Motoyama H, Sugimura K, Honma T, Narisawa R, Ichida T. Gastric carcinoid tumors without autoimmune gastritis in Japan: a relationship with Helicobacter pylori infection. Dig Dis Sci. 2002;47:579-585. [PubMed] |
| 25. | Sato Y, Imamura H, Kaizaki Y, Koizumi W, Ishido K, Kurahara K, Suzuki H, Fujisaki J, Hirakawa K, Hosokawa O. Management and clinical outcomes of type I gastric carcinoid patients: retrospective, multicenter study in Japan. Dig Endosc. 2014;26:377-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | La Rosa S, Inzani F, Vanoli A, Klersy C, Dainese L, Rindi G, Capella C, Bordi C, Solcia E. Histologic characterization and improved prognostic evaluation of 209 gastric neuroendocrine neoplasms. Hum Pathol. 2011;42:1373-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 27. | Vanoli A, La Rosa S, Luinetti O, Klersy C, Manca R, Alvisi C, Rossi S, Trespi E, Zangrandi A, Sessa F. Histologic changes in type A chronic atrophic gastritis indicating increased risk of neuroendocrine tumor development: the predictive role of dysplastic and severely hyperplastic enterochromaffin-like cell lesions. Hum Pathol. 2013;44:1827-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Meko JB, Norton JA. Management of patients with Zollinger-Ellison syndrome. Annu Rev Med. 1995;46:395-411. [PubMed] |
| 29. | Nikou GC, Toubanakis C, Nikolaou P, Giannatou E, Marinou K, Safioleas M, Karamanolis D. Gastrinomas associated with MEN-1 syndrome: new insights for the diagnosis and management in a series of 11 patients. Hepatogastroenterology. 2005;52:1668-1676. [PubMed] |
| 30. | Crosby DA, Donohoe CL, Fitzgerald L, Muldoon C, Hayes B, O’Toole D, Reynolds JV. Gastric neuroendocrine tumours. Dig Surg. 2012;29:331-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Singh R, Yao K, Anagnostopoulos G, Kaye P, Ragunath K. Microcarcinoid tumor diagnosed with high-resolution magnification endoscopy and narrow band imaging. Endoscopy. 2008;40 Suppl 2:E12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Karaca C, Turner BG, Cizginer S, Forcione D, Brugge W. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 33. | Bushnell DL, Baum RP. Standard imaging techniques for neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:153-162, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Delle Fave G, Kwekkeboom DJ, Van Cutsem E, Rindi G, Kos-Kudla B, Knigge U, Sasano H, Tomassetti P, Salazar R, Ruszniewski P. ENETS Consensus Guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology. 2012;95:74-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 224] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 35. | Kjaer A, Knigge U. Use of radioactive substances in diagnosis and treatment of neuroendocrine tumors. Scand J Gastroenterol. 2015;50:740-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Johnbeck CB, Knigge U, Kjær A. PET tracers for somatostatin receptor imaging of neuroendocrine tumors: current status and review of the literature. Future Oncol. 2014;10:2259-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 37. | Kulke MH, Shah MH, Benson AB, Bergsland E, Berlin JD, Blaszkowsky LS, Emerson L, Engstrom PF, Fanta P, Giordano T. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw. 2015;13:78-108. [PubMed] |
| 38. | Sato Y, Takeuchi M, Hashimoto S, Mizuno K, Kobayashi M, Iwafuchi M, Narisawa R, Aoyagi Y. Usefulness of endoscopic submucosal dissection for type I gastric carcinoid tumors compared with endoscopic mucosal resection. Hepatogastroenterology. 2013;60:1524-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (1)] |
| 39. | Kim HH, Kim GH, Kim JH, Choi MG, Song GA, Kim SE. The efficacy of endoscopic submucosal dissection of type I gastric carcinoid tumors compared with conventional endoscopic mucosal resection. Gastroenterol Res Pract. 2014;2014:253860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 40. | Ravizza D, Fiori G, Trovato C, Fazio N, Bonomo G, Luca F, Bodei L, Pelosi G, Tamayo D, Crosta C. Long-term endoscopic and clinical follow-up of untreated type 1 gastric neuroendocrine tumours. Dig Liver Dis. 2007;39:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Campana D, Ravizza D, Ferolla P, Faggiano A, Grimaldi F, Albertelli M, Berretti D, Castellani D, Cacciari G, Fazio N. Clinical management of patients with gastric neuroendocrine neoplasms associated with chronic atrophic gastritis: a retrospective, multicentre study. Endocrine. 2016;51:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 42. | Gladdy RA, Strong VE, Coit D, Allen PJ, Gerdes H, Shia J, Klimstra DS, Brennan MF, Tang LH. Defining surgical indications for type I gastric carcinoid tumor. Ann Surg Oncol. 2009;16:3154-3160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Jenny HE, Ogando PA, Fujitani K, Warner RR, Divino CM. Laparoscopic antrectomy: a safe and definitive treatment in managing type 1 gastric carcinoids. Am J Surg. 2016;211:778-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Ozao-Choy J, Buch K, Strauchen JA, Warner RR, Divino CM. Laparoscopic antrectomy for the treatment of type I gastric carcinoid tumors. J Surg Res. 2010;162:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Thomas D, Tsolakis AV, Grozinsky-Glasberg S, Fraenkel M, Alexandraki K, Sougioultzis S, Gross DJ, Kaltsas G. Long-term follow-up of a large series of patients with type 1 gastric carcinoid tumors: data from a multicenter study. Eur J Endocrinol. 2013;168:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Grozinsky-Glasberg S, Kaltsas G, Gur C, Gal E, Thomas D, Fichman S, Alexandraki K, Barak D, Glaser B, Shimon I. Long-acting somatostatin analogues are an effective treatment for type 1 gastric carcinoid tumours. Eur J Endocrinol. 2008;159:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 47. | Campana D, Nori F, Pezzilli R, Piscitelli L, Santini D, Brocchi E, Corinaldesi R, Tomassetti P. Gastric endocrine tumors type I: treatment with long-acting somatostatin analogs. Endocr Relat Cancer. 2008;15:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Manfredi S, Pagenault M, de Lajarte-Thirouard AS, Bretagne JF. Type 1 and 2 gastric carcinoid tumors: long-term follow-up of the efficacy of treatment with a slow-release somatostatin analogue. Eur J Gastroenterol Hepatol. 2007;19:1021-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Jianu CS, Fossmark R, Syversen U, Hauso Ø, Fykse V, Waldum HL. Five-year follow-up of patients treated for 1 year with octreotide long-acting release for enterochromaffin-like cell carcinoids. Scand J Gastroenterol. 2011;46:456-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 50. | Massironi S, Zilli A, Conte D. Somatostatin analogs for gastric carcinoids: For many, but not all. World J Gastroenterol. 2015;21:6785-6793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 51. | Massironi S, Zilli A, Fanetti I, Ciafardini C, Conte D, Peracchi M. Intermittent treatment of recurrent type-1 gastric carcinoids with somatostatin analogues in patients with chronic autoimmune atrophic gastritis. Dig Liver Dis. 2015;47:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 52. | Fossmark R, Sørdal Ø, Jianu CS, Qvigstad G, Nordrum IS, Boyce M, Waldum HL. Treatment of gastric carcinoids type 1 with the gastrin receptor antagonist netazepide (YF476) results in regression of tumours and normalisation of serum chromogranin A. Aliment Pharmacol Ther. 2012;36:1067-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 53. | Moore AR, Boyce M, Steele IA, Campbell F, Varro A, Pritchard DM. Netazepide, a gastrin receptor antagonist, normalises tumour biomarkers and causes regression of type 1 gastric neuroendocrine tumours in a nonrandomised trial of patients with chronic atrophic gastritis. PLoS One. 2013;8:e76462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 54. | La Rosa S, Vanoli A. Gastric neuroendocrine neoplasms and related precursor lesions. J Clin Pathol. 2014;67:938-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 55. | Kwon YH, Jeon SW, Kim GH, Kim JI, Chung IK, Jee SR, Kim HU, Seo GS, Baik GH, Choi KD. Long-term follow up of endoscopic resection for type 3 gastric NET. World J Gastroenterol. 2013;19:8703-8708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 56. | Modlin IM, Champaneria MC, Chan AK, Kidd M. A three-decade analysis of 3,911 small intestinal neuroendocrine tumors: the rapid pace of no progress. Am J Gastroenterol. 2007;102:1464-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Hoffmann KM, Furukawa M, Jensen RT. Duodenal neuroendocrine tumors: Classification, functional syndromes, diagnosis and medical treatment. Best Pract Res Clin Gastroenterol. 2005;19:675-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 58. | Soga J. Endocrinocarcinomas (carcinoids and their variants) of the duodenum. An evaluation of 927 cases. J Exp Clin Cancer Res. 2003;22:349-363. [PubMed] |
| 59. | Burke AP, Sobin LH, Federspiel BH, Shekitka KM, Helwig EB. Carcinoid tumors of the duodenum. A clinicopathologic study of 99 cases. Arch Pathol Lab Med. 1990;114:700-704. [PubMed] |
| 60. | Klöppel G, Arnold R, Capella C, Klimstra DS, Albores-Saavedra J, Solcia E. Rindi G, Komminoth P. Neuroendocrine neoplasms of the ampullary resion. WHO classification of Tumours of the digestive system. Lyon: IARC 2010; 13-14. |
| 61. | Randle RW, Ahmed S, Newman NA, Clark CJ. Clinical outcomes for neuroendocrine tumors of the duodenum and ampulla of Vater: a population-based study. J Gastrointest Surg. 2014;18:354-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 62. | Jensen RT, Berna MJ, Bingham DB, Norton JA. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer. 2008;113:1807-1843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 309] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 63. | Gibril F, Schumann M, Pace A, Jensen RT. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore). 2004;83:43-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 64. | Levy AD, Taylor LD, Abbott RM, Sobin LH. Duodenal carcinoids: imaging features with clinical-pathologic comparison. Radiology. 2005;237:967-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 65. | Weber HC, Venzon DJ, Lin JT, Fishbein VA, Orbuch M, Strader DB, Gibril F, Metz DC, Fraker DL, Norton JA. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995;108:1637-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 302] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 66. | Yu F, Venzon DJ, Serrano J, Goebel SU, Doppman JL, Gibril F, Jensen RT. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J Clin Oncol. 1999;17:615-630. [PubMed] |
| 67. | Chang S, Choi D, Lee SJ, Lee WJ, Park MH, Kim SW, Lee DK, Jang KT. Neuroendocrine neoplasms of the gastrointestinal tract: classification, pathologic basis, and imaging features. Radiographics. 2007;27:1667-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Kirshbom PM, Kherani AR, Onaitis MW, Hata A, Kehoe TE, Feldman C, Feldman JM, Tyler DS. Foregut carcinoids: a clinical and biochemical analysis. Surgery. 1999;126:1105-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Klöppel G. Tumour biology and histopathology of neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007;21:15-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 70. | Grin A, Kim YI, Mustard R, Streutker CJ, Riddell RH. Duodenal gastrinoma with multiple gastric neuroendocrine tumors secondary to chronic Helicobacter pylori gastritis. Am J Surg Pathol. 2012;36:935-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Merchant SH, VanderJagt T, Lathrop S, Amin MB. Sporadic duodenal bulb gastrin-cell tumors: association with Helicobacter pylori gastritis and long-term use of proton pump inhibitors. Am J Surg Pathol. 2006;30:1581-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Levy AD, Sobin LH. From the archives of the AFIP: Gastrointestinal carcinoids: imaging features with clinicopathologic comparison. Radiographics. 2007;27:237-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 73. | Thompson JC, Lewis BG, Wiener I, Townsend CM. The role of surgery in the Zollinger-Ellison syndrome. Ann Surg. 1983;197:594-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 114] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 74. | Scherübl H, Jensen RT, Cadiot G, Stölzel U, Klöppel G. Neuroendocrine tumors of the small bowels are on the rise: Early aspects and management. World J Gastrointest Endosc. 2010;2:325-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 75. | Waisberg J, Joppert-Netto G, Vasconcellos C, Sartini GH, Miranda LS, Franco MI. Carcinoid tumor of the duodenum: a rare tumor at an unusual site. Case series from a single institution. Arq Gastroenterol. 2013;50:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Soga J. Early-stage carcinoids of the gastrointestinal tract: an analysis of 1914 reported cases. Cancer. 2005;103:1587-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 220] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 77. | Mullen JT, Wang H, Yao JC, Lee JH, Perrier ND, Pisters PW, Lee JE, Evans DB. Carcinoid tumors of the duodenum. Surgery. 2005;138:971-977; discussion 977-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 78. | Margonis GA, Samaha M, Kim Y, Postlewait LM, Kunz P, Maithel S, Tran T, Berger N, Gamblin TC, Mullen MG. A Multi-institutional Analysis of Duodenal Neuroendocrine Tumors: Tumor Biology Rather than Extent of Resection Dictates Prognosis. J Gastrointest Surg. 2016;20:1098-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | Karagiannis S, Eshagzaiy K, Duecker C, Feyerabend B, Mozdzanowski E, Faiss S. Endoscopic resection with the cap technique of a carcinoid tumor in the duodenal bulb. Endoscopy. 2009;41 Suppl 2:E288-E289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Kim GH, Kim JI, Jeon SW, Moon JS, Chung IK, Jee SR, Kim HU, Seo GS, Baik GH, Lee YC. Endoscopic resection for duodenal carcinoid tumors: a multicenter, retrospective study. J Gastroenterol Hepatol. 2014;29:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 81. | Matsumoto S, Miyatani H, Yoshida Y. Future directions of duodenal endoscopic submucosal dissection. World J Gastrointest Endosc. 2015;7:389-395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 82. | Hoteya S, Kaise M, Iizuka T, Ogawa O, Mitani T, Matsui A, Kikuchi D, Furuhata T, Yamashita S, Yamada A. Delayed bleeding after endoscopic submucosal dissection for non-ampullary superficial duodenal neoplasias might be prevented by prophylactic endoscopic closure: analysis of risk factors. Dig Endosc. 2015;27:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 83. | Matsumoto S, Miyatani H, Yoshida Y, Nokubi M. Duodenal carcinoid tumors: 5 cases treated by endoscopic submucosal dissection. Gastrointest Endosc. 2011;74:1152-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 84. | Mori H, Shintaro F, Kobara H, Nishiyama N, Rafiq K, Kobayashi M, Nakatsu T, Miichi N, Suzuki Y, Masaki T. Successful closing of duodenal ulcer after endoscopic submucosal dissection with over-the-scope clip to prevent delayed perforation. Dig Endosc. 2013;25:459-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 85. | Takimoto K, Imai Y, Matsuyama K. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to prevent delayed perforation after duodenal endoscopic submucosal dissection. Dig Endosc. 2014;26 Suppl 2:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 86. | Tsujimoto H, Ichikura T, Nagao S, Sato T, Ono S, Aiko S, Hiraki S, Yaguchi Y, Sakamoto N, Tanimizu T. Minimally invasive surgery for resection of duodenal carcinoid tumors: endoscopic full-thickness resection under laparoscopic observation. Surg Endosc. 2010;24:471-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 87. | Hari DM, Goff SL, Reich HJ, Leung AM, Sim MS, Lee JH, Wolin E, Amersi F. Small bowel carcinoid: Location isn’t everything! World J Gastrointest Surg. 2013;5:239-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 88. | Jann H, Roll S, Couvelard A, Hentic O, Pavel M, Müller-Nordhorn J, Koch M, Röcken C, Rindi G, Ruszniewski P. Neuroendocrine tumors of midgut and hindgut origin: tumor-node-metastasis classification determines clinical outcome. Cancer. 2011;117:3332-3341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 195] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 89. | Jensen RT, Niederle B, Mitry E, Ramage JK, Steinmuller T, Lewington V, Scarpa A, Sundin A, Perren A, Gross D. Gastrinoma (duodenal and pancreatic). Neuroendocrinology. 2006;84:173-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 90. | Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger-Ellison syndrome. Ann Surg. 2004;240:757-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 91. | Norton JA, Alexander HR, Fraker DL, Venzon DJ, Gibril F, Jensen RT. Does the use of routine duodenotomy (DUODX) affect rate of cure, development of liver metastases, or survival in patients with Zollinger-Ellison syndrome? Ann Surg. 2004;239:617-625; discussion 626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 118] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 92. | Fraker DL, Norton JA, Alexander HR, Venzon DJ, Jensen RT. Surgery in Zollinger-Ellison syndrome alters the natural history of gastrinoma. Ann Surg. 1994;220:320-328; discussion 328-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 133] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 93. | MacFarlane MP, Fraker DL, Alexander HR, Norton JA, Lubensky I, Jensen RT. Prospective study of surgical resection of duodenal and pancreatic gastrinomas in multiple endocrine neoplasia type 1. Surgery. 1995;118:973-979; discussion 979-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 105] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 94. | Jensen RT, Cadiot G, Brandi ML, de Herder WW, Kaltsas G, Komminoth P, Scoazec JY, Salazar R, Sauvanet A, Kianmanesh R. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95:98-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 374] [Article Influence: 28.8] [Reference Citation Analysis (1)] |
| 95. | Norton JA, Fraker DL, Alexander HR, Gibril F, Liewehr DJ, Venzon DJ, Jensen RT. Surgery increases survival in patients with gastrinoma. Ann Surg. 2006;244:410-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 148] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 96. | Bartsch DK, Fendrich V, Langer P, Celik I, Kann PH, Rothmund M. Outcome of duodenopancreatic resections in patients with multiple endocrine neoplasia type 1. Ann Surg. 2005;242:757-764, discussion 764-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 97. | Imamura M. Recent standardization of treatment strategy for pancreatic neuroendocrine tumors. World J Gastroenterol. 2010;16:4519-4525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Imamura M, Komoto I, Ota S, Hiratsuka T, Kosugi S, Doi R, Awane M, Inoue N. Biochemically curative surgery for gastrinoma in multiple endocrine neoplasia type 1 patients. World J Gastroenterol. 2011;17:1343-1353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 76] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 99. | Makhlouf HR, Burke AP, Sobin LH. Carcinoid tumors of the ampulla of Vater: a comparison with duodenal carcinoid tumors. Cancer. 1999;85:1241-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |