Published online Jan 21, 2016. doi: 10.3748/wjg.v22.i3.974
Peer-review started: May 29, 2015
First decision: September 9, 2015
Revised: October 2, 2015
Accepted: November 19, 2015
Article in press: November 19, 2015
Published online: January 21, 2016
Processing time: 233 Days and 16.2 Hours
In the gut, where billions of non-self-antigens from the food and the microbiota are present, the immune response must be tightly regulated to ensure both host protection against pathogenic microorganisms and the absence of immune-related pathologies. It has been well documented that regulatory T cells (Tregs) play a pivotal role in this context. Indeed, Tregs are able to prevent excessive inflammation, which can lead to the rupture of intestinal homeostasis observed in inflammatory bowel diseases (IBDs). Both the worldwide incidence and prevalence of such diseases have increased throughout the latter part of the 20th century. Therefore, it is crucial to understand how Tregs suppress the colitogenic immune cells to establish new treatments for patients suffering from IBDs. In this review, we will first summarize the results obtained in animal model studies that highlight the importance of Tregs in maintaining intestinal homeostasis and describe the specific suppressive mechanisms involved. Next, our current knowledge about Tregs contribution to human IBDs will be reviewed, as well as the current therapeutic perspective on using Tregs for clinical IBD treatment and the challenges that remain to be resolved to ensure both the safety and effectiveness of these therapies in targeting this critical immune-regulatory cell population.
Core tip: The mucosal immune system must be very well-controlled to avoid immune responses against both microbial and innocuous food antigens. Regulatory T cells (Tregs) constitute a key mechanism to ensure gut homeostasis. In this review, both Treg biology and the suppressive mechanisms that have been identified using animal models of intestinal inflammation are first summarized. Then, we discuss the current knowledge concerning their contribution to human inflammatory bowel diseases (IBDs). Interestingly, these relatively recent advances have led to new therapeutic perspectives for IBD treatment by targeting this potent immunosuppressive cell population.
- Citation: Pedros C, Duguet F, Saoudi A, Chabod M. Disrupted regulatory T cell homeostasis in inflammatory bowel diseases. World J Gastroenterol 2016; 22(3): 974-995
- URL: https://www.wjgnet.com/1007-9327/full/v22/i3/974.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i3.974
Immune responses in the digestive tract are tightly regulated to ensure host protective immunity and prevent the development of immune-mediated pathology. The host immune system must efficiently respond to invading pathogenic microorganisms, while simultaneously and specifically blocking these mechanisms in response to antigens derived from the abundant commensal gut flora and the alimentation. The integrity of the intestinal mucosa is crucial and acts as a physical barrier, but additional tolerance mechanisms are required to prevent the excessive inflammation that can lead to the rupture of intestinal homeostasis observed in inflammatory bowel diseases (IBDs). IBDs are a group of chronic intestinal inflammatory disorders that include Crohn’s disease (CD) and ulcerative colitis (UC). CD generally affects the entire gastrointestinal tract, whereas UC mainly affects the colon and the rectum. IBDs are complex diseases whose aetiology is currently incompletely understood. The current and most accepted hypothesis is that IBDs occur as a result of the complex interactions between genetic and environmental factors, leading to a breakdown in intestinal homeostasis and the development of aberrant inflammatory responses to the intestinal flora. Mouse models of IBDs that mimic features of the human pathology have been useful to better understand and dissect the immuno-pathophysiology of IBDs. They indicate that chronic inflammation may be the result of excessive inflammatory responses or deficiencies in key negative regulatory pathways. In particular, regulatory T cells (Tregs) have been pinpointed as a key immunosuppressive population that is critically involved in the maintenance of the intestinal homeostasis. Here, we will first review the key features of Tregs before exploring their contribution to mouse models of IBDs. Then, we will focus on the implications of Tregs in human IBDs and discuss the current therapeutic perspectives based on the use of this immunosuppressive cell population.
The idea that a specific T cell population with regulatory capacities was able to control the immune system homeostasis and ensure tolerance emerged in the early seventies from experiments showing that neonatal thymectomy leads to the development of autoimmune manifestations[1] that can be prevented by adoptive transfer of T cells[2,3]. Since then, many studies have been performed to precisely define specific phenotypic markers of these suppressor CD4 T cells to analyze their key role in immune tolerance. The differential expression of CD5[3], CD45RB[4,5] and CD25[6] has been successively used to define this potent regulatory population. It is now well-documented that several Treg subsets regulate the immune system, but the best-characterized Treg population is the CD4+ CD25+ phenotype. This subset expresses the transcription factor Foxp3 and plays a central role in preventing pathological immune responses, including autoimmunity, intestinal inflammation and allergy. Thus, Tregs ensure dominant tolerance to self and innocuous environmental antigens. The first and most convincing observation that Tregs play a major role in immune homeostasis was the dramatic autoimmune phenotype observed in the Scurfy mice that bear a mutation in the Foxp3 gene[7,8] and the analogous fatal immune dysregulation, polyendocrinopathy, enteropathy and X-linked inheritance (IPEX) seen in humans with mutations in the Foxp3 gene[9]. The mutations in Foxp3 in Scurfy mice and IPEX patients result in the specific absence of functional CD4+ CD25+ Tregs. Following these seminal observations, the use of genetically modified mice that allow to visualize or ablate Tregs in vivo have rejuvenated the field of T cell-mediated suppression and formally demonstrated that Foxp3 acts in Treg lineage specification[10].
Functional studies require the isolation of a pure Treg population. Tregs are currently defined by the constitutive expression of CD25, but this molecule is also up-regulated by activated effector T cells (Teff). Additionally, although Foxp3 remains the best Treg marker in mice, its intracellular location precludes the use of this marker for the isolation of live human cells. Furthermore, Foxp3 can be expressed by activated human Teff[11,12]. Tregs also constitutively express CTLA-4[13,14] and GITR[15], but those markers are also impacted by T cell activation and do not provide more specificity than CD25. The lack of Treg-specific surface markers can be overcome by the use of Foxp3-reporter mice, but the identification of highly specific markers to distinguish Tregs from activated Teff remains a critical hurdle to studies in humans. The CD127 and CD27 markers have been proposed to increase the specificity of Treg identification. The level of CD127 expression is lower in CD25+ Foxp3+ Tregs than in Teffs[16]. However, CD127 expression is also downregulated following Teff activation[17] and, therefore, is only useful to identify Tregs in non-inflammatory conditions. However, most of the current studies rely on Treg identification through the CD25+ CD127low phenotype. The CD27 expression level in Tregs is higher than that in Teffs and identifies human Tregs under certain inflammatory conditions[18,19].
Foxp3+ Tregs can be divided into two main subsets: thymus-derived Tregs (tTregs), which are generated in the thymus, and peripherally-induced Tregs (pTregs), which can be induced from naive CD4 T cells in the periphery. We will briefly review the similarities and differences between these populations and discuss the relative contribution of tTregs and pTregs to intestinal homeostasis maintenance.
Tregs are generated in the thymus and represent less than 5% of the CD4+ T cell population. Interestingly, tTregs develop from precursors expressing TCRs with high affinity for self-antigens. As a consequence, the TCR affinity of tTregs for self-antigens is higher than that of Teffs. Thus, although a partial overlap exists, the Treg and Teff TCR repertoires are distinct[20]. The actual model of tTreg differentiation consists of 2 steps[21,22]. A strong TCR signal associated with the engagement of costimulatory molecules leads to the upregulation of CD25 at the CD4 single positive stage. Then, signals through CD25, also known as the IL-2 receptor, lead to the expression of Foxp3. Indeed, the transcription factor STAT-5, which is activated downstream of the IL-2 receptor, binds a regulatory sequence in the Foxp3 gene and thus promotes its expression. Several mouse models of IL-2 deficiency demonstrate that IL-2 is a key cytokine for the development and the peripheral maintenance of tTregs[23-26]. Interestingly, the lack of IL-2 in mice promotes colitis[27].
It is assumed that most of the Foxp3+ Tregs recirculating in the lymphoid organs of healthy mice originate from the thymus. However, a large proportion of pTregs derived from conventional T cells (Tconv) are present in the gut (particularly in the lamina propria and the gut-associated lymphoid tissues), where tolerogenic conditions are combined. Indeed, in addition to the relatively high concentration of active TGF-β1, the continuous presence of antigens derived from the commensal microbiota and aliments, together with the presence of tolerogenic CD103+ dendritic cells (DCs), is a favorable environment for pTreg differentiation[28,29]. This conversion of Tconv to Tregs is also mediated in part by retinoic acid (RA)[29,30]. Finally, DCs are able to generate the active form of vitamin D, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), a potent immunomodulatory molecule. Indeed, stimulation of Tconv with both 1,25(OH)2D3 and IL-2 induces both CTLA-4 and Foxp3 expression, conferring immunoregulatory functions to these cells[31]. In addition, the literature describes the importance of the PD-1/PD-L1 signaling pathway for the generation, maintenance and plasticity of Foxp3+ pTreg[32-34]. It has been suggested that the exhausted colitogenic Teffs cells can lose their effector functions and acquire regulatory properties. These suppressor cells are characterized by the expression of PD-1, low expression of CD25[35,36] and constitutive expression of Foxp3 and CTLA-4. This subpopulation of pTregs is able to suppress the proliferation of CD4+ CD25- PD-1- Tconv in vitro and inhibits the development of intestinal inflammation in the transfer model of colitis. These data suggest that the PD-1 and PD-L1 pathway could be one way to generate pTregs cells during IBDs. The butyrate produced by the commensal microorganisms during starch fermentation has been shown to induce pTreg differentiation in the intestinal mucosa and to reduce the disease severity in the T cell transfer model of colitis[37]. In agreement with these data, another study revealed that, in addition to butyrate, propionate, another metabolite generated by the microbiota, exhibits the same function[38]. The composition of the microbiota is crucial for Treg accumulation[39]. Indeed, oral administration of a mix of 17 different gut bacteria strains is sufficient to promote Treg accumulation in the colon of germ-free (GF) mice. Further studies are required to investigate whether these Tregs derive from pre-existing Tregs that have migrated and/or proliferated or from the peripheral differentiation of naive Tconv. Strikingly, this treatment with the bacteria mixture protects the GF mice from developing disease in the T cell transfer model of colitis and in an allergic diarrhea model. In addition, modification of the gut microbiota by oral administration of intestinal Clostridium bacteria strains improves Treg function, indicating that the microbial environment in the colonic mucosa influences Treg function[39,40]. The proposed mechanism is that DCs, and likely intestinal epithelial cells, would lead to the accumulation of IL-10+ CTLA4high pTregs through their production of TGF-β and other Treg-inducing factors. Moreover, the oral administration of Clostridium early in life results in resistance to colitis and systemic immunoglobulin E responses in adult mice. In addition, IBD patients exhibit some shifts in the composition of their intestinal microbiota[41,42]. This observation suggests that the equilibrium of the gut flora is important to prevent IBD development and opens new therapeutic windows. Together, these data strongly imply that the intestinal bacteria promote tolerance by producing molecules that improve Treg-mediated immunosuppression, thus allowing symbiosis between the intestinal flora and the host.
In addition to these two Treg subsets, a third subset of Tregs, referred to as type 1 regulatory T (Tr1) cells, expresses only low and transient levels of Foxp3 and produces high levels of IL-10[43-45]. Tr1 cells have been described as particularly important for maintaining intestinal tolerance. Indeed, the transfer of Tr1 cells can prevent IBDs induced by pathogenic CD4+ CD45RBhigh T cells[43]. The coexpression of CD49b and LAG-3 enables the isolation of a highly suppressive Tr1 population[46]. However, the biology of this Treg population is still poorly understood.
The TCR repertoires of tTregs and pTregs are different. Indeed, tTregs are enriched in autospecific cells compared to the pTregs that are generated from naive T cells that have not been negatively selected in the thymus[20,47]. Therefore, pTregs are thought to be mainly responsible for tolerance to non-self-antigens, such as allergens, diet and fetal antigens[48], whereas tTregs would be preferentially involved in the control of autospecific responses[49].
Because both pTregs and tTregs express CD25 and the key Treg lineage transcription factor Foxp3, the distinction between these 2 populations in the peripheral organs remains challenging. Currently, Nrp-1 and Helios are two candidates for this discrimination. However, these markers do not permit a strict distinction between Teffs, pTregs and tTregs. Higher expression of the transcription factor Helios on tTregs has been proposed to distinguish them from pTregs[50]; however, recent studies have revealed that Helios could also be expressed by pTregs in vivo[51]. Furthermore, a fraction of human tTregs does not express Helios[52]. Nrp-1 expression by tTregs and its absence in pTregs distinguishes these 2 populations under non-inflammatory conditions[53,54]. However, in humans, Nrp-1 expression can be induced in activated Teffs[55]. Thus, the lack of clear markers limits our understanding of the relative contribution of tTregs and pTregs to the disease pathophysiology, particularly in humans.
In the context of IBDs, the relative contribution of tTregs and pTregs is difficult to assess for the reasons described above. The inhibition of pTreg differentiation (by preventing TGF-β-dependent induction of Foxp3) demonstrated that pTregs are necessary to prevent Th2 type pathologies at mucosal sites[56]. In addition, the lack of pTregs modified the composition of the intestinal microbiota. Thus, this study suggested that tTregs are primarily responsible for the control of autoimmune responses, whereas the main role of pTregs would be to prevent immune responses against commensal and dietary antigens. This idea is further supported by another study analyzing the TCR repertoire of colonic Tregs[57]. The comparison of the TCR repertoire of Tregs from this site with other locations revealed that gut antigens shape the Treg TCR repertoire in the intestine. Indeed, a large proportion of colonic Tregs are specific for bacterial antigens, suggesting that pTregs compose a major proportion of the gut Tregs. However, another recent study revealed that the TCR repertoires of intestinal and thymic Tregs are highly similar, suggesting that tTregs also contribute significantly to the intestinal Treg pool[58]. Therefore, both pTregs and tTregs are likely to contribute to the maintenance of intestinal homeostasis, and it remains to be elucidated which population plays a major role.
Effector CD4+ T helper (Th) cells are heterogeneous and differentiate into at least 4 subsets (Th1, Th2, Th17 and Tfh) that differ in their cytokine production and functions. For instance, Th1 cells are defined as producing IFN-γ and expressing the transcription factor T-bet. Th1 cells can enhance the killing potential of macrophages and CD8 T cells. Th2 cells produce IL-4, IL-5 and IL-13, express the transcription factor GATA3 and contribute to eosinophilic inflammation and allergic reactions. Th17 cells release IL-17A, IL-21 and IL-22, express the transcription-factor RORγt and are involved in the activation and recruitment of neutrophils. Follicular helper T cells (Tfh) are characterized by the expression of CXCR5 and the transcription factor Bcl-6. This subpopulation plays a key role in humoral responses by promoting germinal center reactions and providing critical signals that shape the B cell responses. There is now a large body of evidence indicating that Foxp3+ Tregs are also heterogeneous and could be further subdivided into highly specialized subpopulations with different functions. The expression of distinct effector molecules, chemokine receptors and transcription factors varies within Foxp3+ Tregs isolated from distinct anatomical sites[59]. This differential expression of specific molecules endows Tregs with the ability to specifically control the various types of immune responses in different anatomical locations. The mechanisms involved in Treg specialization to control the Th1/Th2/Th17/Tfh responses have been recently elucidated. Expression of the Th1 transcription factor T-bet by a subtype of Tregs controls the expression of CXCR3 and allows Tregs to accumulate at Th1 inflammation sites[60]. The acquisition of T-bet expression by Tregs depends on STAT1 activation. Both IFN-γ and IL-27 are involved in this differentiation by acting in different anatomical sites, and these 2 cytokines also induce distinct gene expression profiles[60-62]. Treg expression of miR-146a, which is involved in the control of STAT1 expression, is also critical for their ability to control the Th1 responses[63]. Treg expression of the Th2 transcription factor IRF4 allows Tregs, in cooperation with Foxp3, to specifically control the Th2 responses. Indeed, specific deletion of IRF4 in Foxp3+ T cells leads to the spontaneous development of a deregulated Th2 response, lymphoproliferation associated with a selective increase in IL-4/IL-5-producing CD4+ T cells, the aberrant production of IgE and tissue lesions[64]. IRF4 physically interacts with Foxp3. Moreover, expression of the protein kinase CK2 is also involved in the Treg-mediated control of the Th2 responses. A Treg-specific CK2 deficiency induces the expression of the ILT3 receptor by a discrete population of Foxp3+ Tregs. These ILT3+ Tregs retain the ability to control the Th1 responses but favor the emergence of Th2-promoting PD-L2+ IRF4+ DCs, leading to a Th2 lymphoproliferative disease[65]. Tregs ability to suppress Th17 responses relies on their expression of STAT3, a key Th17 differentiation factor. Treg-specific ablation of STAT3 induces specific Th17 activation and an intestinal pathology[66]. STAT3 expression by Tregs is involved in the expression of the chemokine receptor CCR6, thus impacting their intestinal location. STAT3 also controls IL-1R and IL-6R expression. Expression of these cytokine receptors is likely to impact Teff polarization by depriving them of the cytokines important for Th17 differentiation. However, this phenomenon has only be demonstrated in vitro. Finally, the expression of the Tfh differentiation factor BCL6 by Tregs induces CXCR5 expression, promoting Treg migration into the germinal center (GC), where they become T follicular regulatory T cells (Tfr). The absence of BCL6 or CXCR5 expression by Tregs leads to increased GC activity[67]. Finally, Tregs can also be separated into subsets of naive and antigen-primed/memory Tregs. These populations are defined by the expression of CD45RA and CD45RO, respectively, and are both suppressive but functionally distinct[68-72]. Naive Tregs have the ability to recirculate between lymphoid tissues and are believed to function at the priming site, where their activation endows them with the ability to migrate into inflamed sites.
The fine-tuning of gene expression is critical for Treg specialization and optimal control of various immune responses, and it has been suggested that Tregs can lose Foxp3 expression and their suppressive function to be reprogrammed into a phenotype resembling effector cells, particularly under inflammatory conditions where these ex-Tregs could contribute to disease development[73-77]. Other studies revealed that these “ex-Tregs”, which are initially found at a high frequency, even in homeostatic conditions[73], are mainly generated in the thymus where transient Foxp3 expression can occur without generating tTregs[78] or by transient expression of Foxp3 in non-Treg cells[79]. Thus, these results support the view that the differentiated Tregs are ultimately a plastic, but stable lineage. Foxp3 controls the activation or repression of genes and plays a key role in the development and suppressive function of Tregs[80]. However, the expression of Foxp3 alone is not sufficient to establish a complete and stable regulatory T cell phenotype. Indeed, ectopic expression of Foxp3 in Teffs induced only one-third of the Treg genetic signature[80-82]. Retroviral transduction of Foxp3 does not induce the activation of the endogenous Foxp3 locus, indicating that Foxp3 cannot activate its own transcription[81,83]. Furthermore, an analysis of the genetic signature of Tregs in Foxp3-GFP-KO mice, where the Foxp3 gene is deleted and replaced by GFP under control of the same promotor, shows that the GFP+ Foxp3- cells express some genes of the Treg signature[83], revealing that if Foxp3 is essential for Treg development, it is not the only factor involved in the acquisition of a complete regulatory T cell phenotype.
The key role of epigenetic modifications in the regulation of cellular differentiation processes and lineage stabilization has been recently revealed in many cell types[84-86]. These epigenetic modifications regulate transcriptional activity and are transmitted during cell division[86]. A specific methylation profile of Tregs has been established. Compared to Teffs, Tregs exhibit hypomethylation of genes coding for key proteins involved in their functions, such as Foxp3, CTLA-4, and GITR[87-89]. In parallel, the Teff-specific genes that are repressed in Tregs are hypermethylated. These epigenetic modifications are initiated in Tregs during tTreg development in the thymus. A partial demethylation profile is indeed found in thymic Foxp3+ cells[87,88]. The establishment of the hypomethylation profile is independent of Foxp3 expression[88,90]. Epigenetic modifications affect the DNA structure and increase the accessibility to Foxp3 binding sites in specific gene promotors involved in Treg functions, thus modifying the transcriptional activity of these genes in a Foxp3-dependent manner[90]. The epigenetic modifications differ between the thymus-derived tTregs and the induced Tregs (iTregs) in vitro[87,88]. The incomplete methylation profile of iTregs compared to tTregs explains the reduced stability of this population, which loses its Foxp3 expression and suppressive functions when restimulated in the absence of TGF-β[87]. Furthermore, iTregs do not express all of the genes of the Treg-specific signature and show different capacities to control the effector responses in vivo[81]. Therefore, the Treg instability and loss of Foxp3 expression observed in inflammatory settings is thought to occur through pTreg conversion rather than through the tTreg population.
In addition to the epigenetic modifications that control Treg stability, numerous other mechanisms are involved, such as the repression of SATB1 transcription factor expression by Foxp3[91], which is essential for maintaining Treg function and preventing the expression of effector cytokines, or the expression of the transcription factors BACH2 and Eos, which ensure the repression of the effector program[92,93]. The miRNAs expressed by Tregs also play a key role in preserving the stability of the Treg program[94,95]. Likewise, the expression of the phosphatase PTEN is critical for maintaining Treg stability, given that PTEN-deficient Tregs can lose Foxp3 expression and up-regulate molecules of the Th1 and Tfh lineages[96,97].
In the context of IBDs, the inflammatory environment might influence Treg stability. Indeed, in mouse models of autoimmunity, Treg functions may be directly influenced by multiple pro-inflammatory cytokines, such as TNF-α, IL-6 and IL-21[98-101]. Of these cytokines, TNF-α is the best example because it has a negative effect on Treg functions in the context of rheumatoid arthritis[102]. It is worth noting that many researchers believe that anti-TNF antibody treatment in patients suffering from autoimmune diseases is so successful because it promotes the recovery of Treg suppressive functions. By contrast, the IL-33 cytokine has been described as positively modulating Treg biology during gut inflammation[103]. Indeed, IL-33 signaling can both potentiate TGF-β1 signaling to favor pTreg differentiation and improve Treg functions in vivo during intestinal inflammation. In the T cell transfer model of colitis, signals through the receptors for the C3a and C5a complement molecules also decrease Treg suppressive functions[104]. The specific suppressive functions of Tregs that are impaired have not been studied, although decreased expression of Foxp3 and CTLA-4 is documented.
TCR signals are absolutely required for thymic development and the suppressive function of Tregs[105-107]. In mice, Ly-6C expression is inversely correlated with the strength of the TCR signals received and divides the Tregs into two subpopulations[108]. Ly-6C- Tregs exhibit better survival and suppressive functions in vitro and in the T cell transfer model of colitis in vivo. These data highlight the requirement for a minimal TCR signal for Treg survival and their suppressive functions, which is consistent with the fact that they recognize self-ligands during their recirculation in the secondary lymphoid organs.
T cell-specific defects in genes of the proximal TCR signaling cascade (such as PLC-γ1 and LAT) lead to a partial block in thymic T cell development[109,110]. As a consequence, the number of both peripheral Tconv and Tregs is reduced and is associated with the development of autoimmune manifestations, particularly those affecting the gut. The deletion of LAT in T cells reduces the expression of Foxp3, CTLA-4, and CD25 in Tregs and impairs their function[110]. This data has been further confirmed in a study that restricted this defect in TCR signaling to the Treg population[111]. Similarly, the LAT-PLC-γ1 interaction in Tregs is necessary for their suppressive functions in vivo, as disruption of this interaction leads to the development of a lymphoproliferative syndrome. Specific mutations resulting in an impaired scaffolding function of ZAP-70 also result in a lymphopenia, a loss of the Treg suppressive functions and the development of rheumatoid factor autoantibodies, but it is not known whether those mice develop intestinal lesions[112]. Interestingly, mutations affecting the catalytic domain of ZAP-70 have no impact on Treg functions[113]. Lck inactivation in Tregs also compromised their homeostatic proliferation in vivo and their regulatory functions in vitro, but it remains to be determined whether this defect in TCR signaling impairs the Treg functions in a colitis model[114]. Moreover, rats deficient in Themis1, a molecule involved in the TCR proximal signaling hub, exhibit CD4 T cell lymphopenia, which affects both the Tconv and Treg cell numbers. This phenotype has been associated with a defect in Treg functions and the spontaneous development of intestinal inflammation[115]. Furthermore, we reported that an epistatic interaction between Themis1 and Vav1 controls regulatory T cell function and IBD development in Themis1-deficient BN rats by modulating TCR signaling strength[116]. It would be interesting to analyze the consequences of a conditional deletion of Themis1 in Treg cells to investigate its role in Treg biology and disease development independently of the defect in thymic T cell development and subsequent lymphopenia.
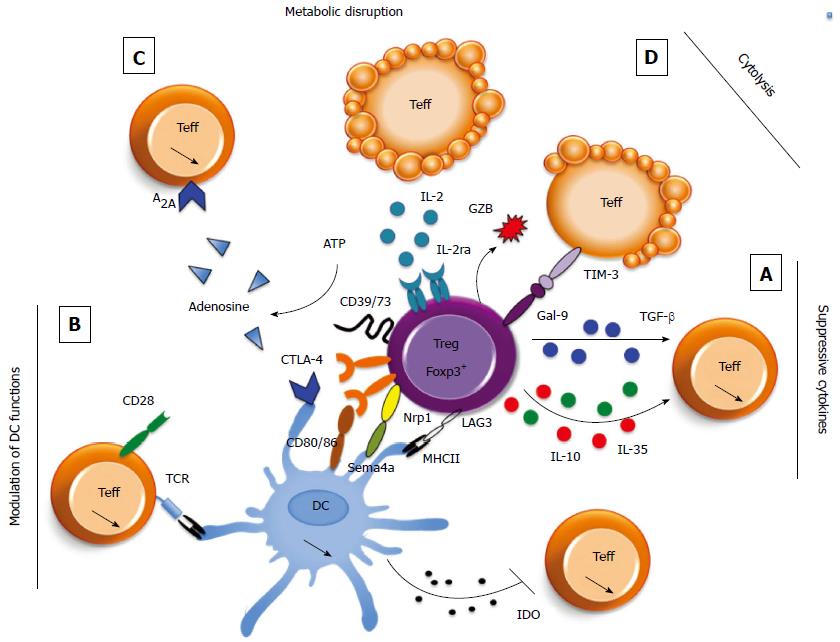
Tregs are equipped with several different suppressor mechanisms that allow them to control both the innate and adaptive immune cells in various pathophysiological contexts. Tregs can modulate the activation of diverse cellular populations, including CD4+ and CD8+ T cells[117], NK cells[118], NK-T cells[119], B cells[120] and macrophages[121,122]. Tregs can exert their functions by directly acting on effector populations or through indirect mechanisms that modulate the antigen presenting cell (APC) phenotype[123]. Tregs suppressive activities can be divided into two main categories, contact-dependent or contact-independent mechanisms, through the secretion of soluble mediators or metabolic disruption (Figure 1). Briefly, Tregs can modulate APC functions by controlling their expression of major histocompatibility complex (MHC) and costimulatory molecules, thereby limiting the APC-Teff contacts and inducing the production of the immunosuppressive molecule IDO. The expression of CTLA-4, Nrp-1 and LAG-3 in Tregs has been shown to mediate this contact-dependent APC modulation. Tregs are also able to produce immunosuppressive molecules, such as IL-10, IL-35 and TGF-β, that can act directly on Teffs to inhibit their activation or via APCs. The expression of the CD39/CD73 ectoenzymes by Tregs can mediate the conversion of extracellular ATP to adenosine, a metabolite that modulates the functions of Teffs and innate immune cells. Tregs are even able to directly kill their target cells. There is some redundancy among those mechanisms because a deficiency in only one does not recapitulate the dramatic phenotype of the Foxp3-deficient mice. The mechanisms that are employed in specific situations and pathologies constitute an active area of research.
Murine models of IBDs are useful tools for the study of the pathogenesis and regulation of intestinal inflammation. They can be divided into 4 relatively distinct categories: chemically induced barrier disruption models, spontaneous models (due to genetic mutations), intestinal pathogen-induced models and the model of naive T cell transfer in immune-deficient mice, which is the most widely used experimental model of IBD. In the last, colitis is induced by an injection of naive (CD4+ CD45RBhigh) T cells into syngeneic immune-deficient (e.g., SCID or RAG-deficient) mice[5]. As a consequence, these animals develop Th1/Th17-mediated colitis that shares some features with human IBDs[124-127]. This intestinal pathology is characterized by severe mononuclear cell infiltration into the colon, which is associated with epithelial hyperplasia and goblet cell depletion[5,128]. In this model, gut inflammation would be driven by the resident commensal bacteria, given that the immune-deficient recipients that have been transferred with naive T cells but raised under a germ-free environment or flora-restricted conditions are partially protected from colitis development. This experiment suggests that T cells specific for enteric bacteria are present in the inoculated CD45RBhigh CD4+ T cells and are most likely responsible for the pathogenesis of the disease[129]. Interestingly, the transfer of Tregs can prevent disease development[130-132], providing a model to discover the factors that control the accumulation and functions of colitogenic T cells as well as Tregs in vivo. Importantly, from a clinical perspective, Tregs can also cure established colitis[4,133]. Thus, in contrast to the in vitro reductionist Treg/Teff coculture model, this mouse model of intestinal inflammation is a potent tool to dissect the Treg immunosuppressive mechanisms in vivo.
TGF-β: The TGF-β family consists of three members, TGF-β1, TGF-β2, and TGF-β3, which all have similar roles in the regulation of immune cells. TGF-β1 is a pleiotropic cytokine that has been described for its broad immunoregulatory effects. This cytokine influences the biology of many different immune cells[134,135]. TGF-β1 can be secreted or expressed at the cell surface by different non-immune and immune cells, including Tregs. Its bioavailability is tightly regulated; to be functional, TGF-β1, which is secreted as an inactive precursor molecule [combined with latency-associated peptide (LAP)], needs to be cleaved by enzymes (such as furin) or modified by integrins (such as αvβ8 and αvβ6)[136,137]. DCs, which express αvβ8 integrins, and Tregs, which express furin, are able to perform this post-translational modification of TGF-β1, again highlighting their potent immunosuppressive function.
TGF-β1 is present in large quantities in the intestine. TGF-β1 is critical for the maintenance of gut homeostasis, because TGF-β1-deficient mice develop lethal multiorgan lymphoproliferative disease, which particularly affects the gut. Of note, this pathology is similar to the one observed in the Foxp3-deficient mice[138,139]. CD4+ T cells are responsible for the phenotype of the TGF-β1-deficient mice, because their depletion improves the animal survival[140,141]. The administration of TGF-β1 blocking antibodies is sufficient to inhibit the Treg control of colitogenic Teffs in the T cell transfer model of colitis[13]. Activated TGF-β1 acts on Teffs rather than on Tregs because the expression of a dominant negative TGF-β-receptor (TGF-βR) type II or overexpression of an endogenous inhibitor of TGF-β1, Smad7, in Teffs impairs Treg-mediated suppression[142,143]. Surprisingly, TGF-β1-deficient Tregs are still able to prevent colitis, unless TGF-β blocking antibodies are administered along with the colitogenic CD45RBhigh CD4+ T lymphocytes[142]. This data highlights the relevance of TGF-β production by cells other than Tregs and even raises the question of the relevance of TGF-β secretion by Tregs. TGF-β1 signaling seems to be important for Treg survival[144], for extra-thymic induction in pTregs[145] and for the inhibition of Th17 cell differentiation[146]. Therefore, these data suggest that TGF-β1 would inhibit Teff functions in the context of IBD development, but would also drive the conversion of Teffs into pTregs. Therefore, further investigation is required to determine whether either of these effects is a dominant immunoregulatory mechanism. In addition, the ability of Tregs to activate TGF-β is crucial in the regulation of TGF-β1 production, particularly in the gut. Indeed, furin-deficient Tregs exhibit impaired regulatory function in the T cell transfer model of colitis[137]. Likewise, myeloid cells (particularly DCs) participate in intestinal homeostasis by promoting pTreg differentiation, in part, by activating TGF-β1 in the intestinal mucosa through the expression of the αv integrin[147,148].
IL-10: IL-10 is a potent anti-inflammatory cytokine that is produced by both immune and non-immune cells. This cytokine exhibits a broad range of target cell types. IL-10 signaling inhibits T cell proliferation and cytokine secretion[149-152]. A Treg-specific IL-10-deficient mouse demonstrated the key role of this cytokine in mucosal homeostasis[153], and its neutralization abolished the Tregs impact on feto-maternal tolerance[154]. The first evidence that IL-10 is a major immunosuppressive cytokine in the gut comes from a study using IL-10-deficient mice[155]. These mice spontaneously develop chronic enterocolitis if they are not housed in a specific pathogen-free environment. This data highlights the importance of IL-10 in maintaining the symbiosis between the gut microbiota and the organism. Additional studies confirmed the critical role of IL-10 in intestinal homeostasis by showing that the administration of recombinant IL-10 or intestinal bacteria able to produce IL-10 can dampen an established intestinal inflammation in mice[124,156]. IL-10 secretion is one of the key Treg suppressive mechanisms responsible for immunological tolerance to self and environmental antigens at environmental interfaces, particularly in the colon, lung and skin[153]. Indeed, Foxp3+ Treg-specific IL-10-deficient mice spontaneously exhibit signs of inflammation in the colon, lung and skin. In the colon, most of the IL-10-producing Tregs express Foxp3[157]. According to these data, in this model, Foxp3+ Tregs, rather than Tr1 cells, would constitute the main IL-10 source, preventing colitis. Moreover, the secretion of IL-10 by Tregs and other cells also controls the activation of proinflammatory macrophages in an IBD mouse model[121,122]. IL-10 can directly suppress the Th1 and Th1+Th17 colitogenic T cells[158], and, interestingly, IL-10 signaling in Tregs is necessary for colitogenic Th17 Teff control[159]. Thus, IL-10 secretion by Tregs might amplify the negative regulatory mechanisms, because IL-10 secretion by Tregs directly inhibits colitogenic cell activation, and IL-10 signaling in Tregs is required for Treg suppressive functions. Taken together, these data demonstrate a crucial role for IL-10 in Treg activation and suppressive functions.
IL-35: IL-35, a member of the heterodimeric IL-12 cytokine family, has been described as a suppressive cytokine produced by Tregs. In T cells, IL-35 signals through the homodimers of the IL-12Rβ2 or gp130 molecules or through IL-12Rβ2-gp130 heterodimers, leading to STAT1 and STAT4 phosphorylation[160,161]. IL-35 is critical for Treg-mediated control of the inflammatory responses in the gut. Indeed, in the T cell transfer model of colitis, mice receiving Tregs from animals deficient in one subunit of the IL-35 receptor were less protected than mice transferred with wild-type (WT) Tregs[160]. It has been proposed that IL-35 exerts its regulatory effects by inducing the conversion of Tconv to pTregs. Indeed, CD4+ T cell activation, which is induced by both IL-35 and TCR signals, promotes the generation of a stable pTreg population[162]. Interestingly, these Foxp3- pTregs are very efficient in controlling inflammation, including colitis, in vivo in an IL-10- and TGF-β1-independent manner.
Tregs highly express the ectoenzymes CD39 and CD73. These two molecules can metabolize extracellular ATP to ADP, 5’-AMP, and eventually adenosine[163,164]. Thus, Tregs are able to generate adenosine, which is an immunomodulatory molecule. Adenosine nucleosides signal through four different receptor subtypes (A2A), which are expressed by diverse cells, particularly immune cells (APC, Teff and Tregs)[165,166]. Thus, Tregs can also moderate inflammatory responses by consuming ATP, a well-described inflammatory molecule, or by generating adenosine, which positively impacts Treg activation but also decreases Teff functions[167-169]. Adenosine can not only inhibit the in vivo development of both Th1 and Th17 CD4+ T responses but may also act on DCs, neutrophils and macrophages[170]. In addition, adenosine signaling can induce the generation of LAG-3+ pTregs[171], allowing Tregs to inhibit DC maturation after interactions with their MHC class II molecules[172]. Because adenosine is widely expressed by diverse cell types, the specific role of the Treg-generated adenosine remains to be determined. In the context of IBDs, the administration of the highly selective A2A adenosine receptor agonist ATL-146e decreases the disease severity in both acute and chronic colitis models[173]. Moreover, the expression of A2A adenosine receptor by colitogenic Teffs is crucial because A2A adenosine receptor-deficient Teffs are resistant to Treg-mediated suppression[174]. Interestingly, it has been shown that CD39 deficiency exacerbates murine intestinal inflammation[175], but to date, it has not been directly shown that CD39 expression by Tregs is absolutely required for them to exert their suppressive functions in this inflammatory context. In contrast, CD73 expression by Tregs plays a nonredundant role in their regulatory effects because CD73-deficient Tregs are unable to control WT colitogenic CD4+ CD45RBhigh Teffs in the T cell transfer model of colitis[176].
In addition to immunoregulatory cytokine production, Tregs may suppress immune responses by killing effector T cells. Tregs are able to induce Teff apoptosis using the granzyme machinery. Indeed, granzyme B is expressed by activated Tregs and promotes immunosuppression by directly killing effector cells or APCs[177,178]. This suppressive mechanism might be implicated in transplantation[179] and tumor[180] models in vivo, but its role in IBDs is still unknown. The constitutive expression of CD25 by Tregs not only promotes their peripheral maintenance but can also permit them to preferentially bind IL-2 and deprive the effector cells of this critical cytokine for T cell proliferation, inducing apoptosis[181]. This mechanism of Teff control has been demonstrated in vitro, but the direct proof that Tregs increase Teff apoptosis though cytokine deprivation in the T cell transfer model of colitis in vivo is lacking and difficult to obtain. Finally, Gal-9 might also be involved in this mechanism. Indeed, Gal-9 is highly expressed in pTregs but not tTregs[182]. Gal-9 deficiency impairs pTreg regulatory functions both in vitro and in vivo; this deficiency also impairs pTreg stability, a feature associated with decreased Foxp3 expression and Smad3 activation[182]. Interestingly, Gal-9-deficient pTregs are unable to control intestinal inflammation in the T cell transfer model of colitis. Gal-9 may function in vivo by regulating Teff apoptosis or exhaustion[183,184].
The maturation of APCs, particularly DCs, is necessary to fully activate Teffs in both physiological and pathological conditions. A key mechanism used by Tregs to suppress immune responses is interference with the DC maturation process. Indeed, in vivo two-photon real-time experiments nicely showed that Teffs and Tregs directly interact with DCs in different contexts[185-189]. Tregs are able to modulate DC maturation and promote the immunosuppressive function of APCs through diverse mechanisms.
The CTLA-4 and CD28 glycoproteins share the CD80 and CD86 ligands expressed by activated APCs (such as DCs and B cells). The CD80 and CD86 molecules are recognized by CTLA-4 with a higher affinity than CD28, allowing CTLA-4 to limit CD28-dependent costimulation and consequently limit T cell activation[190]. Therefore, CTLA-4 is a potent negative regulator of T cell activation. Indeed, the disruption of the CTLA-4 gene in mice results in fatal multiorgan inflammation very early in life, which particularly affects the gut[191,192]. The constitutive expression of CTLA-4 by Tregs plays a key role in their immunosuppressive function. Treg-specific CTLA-4-deficient mice develop a fatal multiorgan pathology characterized by an exacerbated Th1/Th2 response and autoantibody production[193]. CTLA4 expression by Tregs confers at least two main functions to Tregs: the modulation of APC maturation and the induction of indoleamine 2,3-dioxygenase (IDO) in APCs. The engagement of CTLA-4 has been shown to deplete CD80 and CD86 expression on DCs by trans-endocytosis[194]. Given that CTLA-4 and CD28 share the costimulatory CD80 and CD86 ligands expressed by APCs, CTLA-4 engagement reduces the activation potential of DCs by inhibiting costimulation of Teffs through CD28 engagement[193,194]. In addition, CTLA-4 engagement with its ligands expressed on DCs also represents a major regulatory mechanism through the induction of IDO production[195]. The IDO produced by DCs inhibits Teff proliferation by depriving them of IDO-catabolized tryptophan. Furthermore, the metabolites generated during tryptophan catabolism can also induce T cell apoptosis[195]. In the T cell transfer model of colitis, the administration of CTLA-4 blocking antibodies is sufficient to inhibit the control of colitogenic Teffs by Tregs[13]. Interestingly, CTLA-4-deficient Tregs exhibit an impaired ability to inhibit both Teff proliferation and activation in vitro and in vivo in models of gastrointestinal inflammation[193,196]. Moreover, Treg-specific CTLA-4-deficient mice develop systemic lymphoproliferation and fatal T cell-mediated autoimmune disease, pathologies similar to those developed by CTLA-4-deficient mice that also affect the intestine. Together, these data identify the major role of CTLA-4 in Treg function and, more importantly, in the maintenance of immune homeostasis. Of note, a recent study showed that CTLA-4 expression promotes pTreg production and Treg accumulation in the intestine[197].
Neuropilin 1 (Nrp-1) is involved in the physical interaction between Tregs and DCs. Nrp-1 inhibition decreases the interaction time between Tregs and immature DCs, whereas Nrp-1 overexpression in Teffs increases their interactions with APCs[198]. Therefore, Treg-specific Nrp-1 expression enhances their capacities to respond to the low antigen quantities presented in the absence of inflammatory signals and potentially modulates their functions. Nrp-1 expression by Tregs promotes their inhibition of anti-tumor responses[199,200]. Likewise, the interaction between neuropilin expressed by Tregs with semaphorin-4a expressed by the DCs allows the Tregs to modulate DC activation[200]. This mechanism is particularly important for the Treg suppressive functions in the T cell transfer model of colitis because neuropilin-deficient Tregs are unable to cure an established colitis. Tregs can also exert their suppressive function by controlling DC homeostasis, a model of particular interest in the context of IBDs[201].
It seems evident that highly coordinated Treg trafficking is crucial for Tregs to be in the right place at the right time, but several studies also argue that this well-regulated Treg trafficking is necessary for the generation of a tailored phenotype to efficiently address the situation. This trafficking has been extensively studied in the intestine. Both β7 integrin and CCR9 expression play a role in T cell trafficking in the gut[202-204]. The expression of these molecules can be induced by RA secretion by intestinal DCs and non-hematopoietic stromal cells[205-207]. In the intestinal context of oral tolerance, RA is also involved in the conversion of Tconv cells to pTreg cells[208]. Ex vivo pretreatment of Tregs with RA enhances Treg suppressive functions in a model of acute but not chronic colitis[209]. Thus, RA has critical roles in regulating T cell migration towards intestinal inflammatory sites and in promoting pTreg production in the gut. Interestingly, β7 integrin-deficient Tregs retain their ability to suppress colitis, even if they may not populate the intestine, suggesting that, in this model, migration to the mesenteric draining lymph nodes is sufficient to control intestinal inflammation[210]. In agreement with these data, in the T cell transfer model of colitis, the transferred CCR4- or CCR7-deficient Tregs are unable to reach the mesenteric lymph nodes, leading to impaired control of colitogenic Teffs and the development of colitis[211,212]. Taken together, these data indicate that Tregs must enter the mesenteric lymph nodes to exert their suppressive function and prevent IBD development but do not necessarily need to migrate into the intestinal mucosa. However, one study contradicts this hypothesis. Indeed, in the T cell transfer model of colitis, the transferred CCR6-deficient Tregs are less efficient in their migration to the colonic mucosa but not to the mesenteric lymph nodes, and they are also unable to control colitis development compared to WT Tregs[213]. CCR6 is the receptor for the chemokine CCL20. Because CCL20 is highly expressed in the inflamed colon of humans and mice suffering from IBDs, CCR6 expression by Tregs may be mandatory for their migration into the inflamed gut. Thus, it is still an open question whether Tregs need to be present at the inflammatory sites in the context of IBDs.
Experimental models have clearly shown that Tregs can not only prevent the development of intestinal inflammation[5], but they can also control an established IBD[133]. In humans, Tregs also play a key role in controlling intestinal homeostasis and therefore represent an attractive therapeutic target for clinical treatments. The most striking evidence of Treg involvement in the control of intestinal homeostasis is that intestinal inflammation is the most prominent feature of human immune dysregulation, polyendocrinopathy, enteropathy, and X-linked (IPEX) syndrome, which is caused by mutations of the Foxp3 gene[214].
Numerous studies investigating Tregs in human IBDs show that patients exhibit decreased Treg numbers in the blood and increased Treg numbers in the inflamed intestinal mucosa compared to the uninflamed tissues[215-218]. The disease activity has a differential impact on the circulating and mucosal Treg numbers[215,218]. The circulating Treg percentages inversely correlate with disease activity[215,219], and a concomitant increase in the mucosal Treg numbers is associated with a reduction of the circulating Treg population in active disease[220]. The increased Treg localization in the inflamed mucosa could be due to an active recruitment and expansion of this population in inflamed areas in an attempt to regulate the inflammation. Mucosal Treg recruitment could be responsible for the reduction of circulating Tregs. However, Treg accumulation in the inflamed intestines is also observed in other inflammatory conditions, such as diverticulitis or CMV-induced colitis[215,221], where it does not correlate with a reduction in the number of circulatory Tregs. The Treg increase in the inflamed bowel mucosa is not as strong in IBD patients as in other intestinal inflammatory diseases[215]. This suggests that although they are recruited to the inflamed areas, Tregs may still be present in insufficient numbers in IBD lesions to efficiently control the inflammation, or that they exert their immunoregulatory functions in a different location, such as the mesenteric lymph nodes. This question remains unresolved due to obvious limitations in studying the cells from patient and healthy control mesenteric lymph nodes. Increased caspase activation and PARP cleavage is observed in blood Tregs as well as mucosal Tregs in UC and CD patients[222], suggesting that their anti-inflammatory effect might be limited due to increased apoptosis. A more recent study in patients with Crohn’s disease corroborated these findings and also revealed that the apoptosis of circulating Tregs was higher in female than in male patients[223]. Although no causal link can be established from the current knowledge, this increased Treg apoptosis is correlated with the increased prevalence and severity of the disease in females. Retrospective studies examining the evolution of the circulating Treg counts before disease onset and during the disease would be of great interest to determine whether a decreased Treg count precedes the disease occurrence or is secondary to the appearance of symptoms.
In many studies, the Tregs from IBD patients were functional in in vitro suppression assays. This has been shown for both the peripheral blood[215] and mucosal Tregs[217,218,220,224] of CD and UC patients. A potential reason for the development of a deleterious immune response, despite the presence of functional Tregs in the inflamed mucosa, could be the resistance of Teffs to the immunosuppressive effects of TGF-β. The upregulation of SMAD7 in the intestinal mucosa of IBD patients renders the cells resistant to Treg inhibition[225]. However, other studies have reported different results, showing that IBDs can be associated with functional defects of Tregs[226,227]. It is impossible to determine whether these defects are initially responsible for the disease development in those patients or is secondary to the excessive inflammation triggered by other mechanisms. A whole exome sequencing study in a genetic familial case of IBDs identified a mutation in the Foxp3 gene linked to impaired Treg suppressive activity in vitro[227], suggesting that a Treg defect can be a key cofactor for disease development. In addition, human genetic studies have pinpointed that mutations in the IL-10R genes that abrogate IL-10 signaling can lead to inflammatory disease development[228], demonstrating the relevance of this key immunosuppressive cytokine to human pathology. Because IL-10 production by Tregs is critical in mouse colitis models, IL-10 production in human Tregs may also represent a central mechanism for intestinal homeostasis maintenance. Interestingly, disruption of the Themis1 gene in rats leads to a functional Treg defect that is associated with spontaneous IBD development[115], and a Themis1 polymorphism has been linked to Crohn’s disease in humans[229]. It is not known whether any qualitative or quantitative Treg defects caused by Themis1 polymorphisms contribute to disease development in those patients. IBDs are complex, multifactorial diseases that depend on an intricate interplay between genetic and environmental factors. Although genetic Treg defects may not occur in the majority of cases as the primary cause of IBD, the association of Treg defects with other genetic or environmental variables is likely to contribute to disease development and/or severity.
An increase in the number of circulating T cells co-expressing the Treg transcription factor Foxp3 and the Th17 cytokine IL-17 is observed in CD and UC patients[226]. These Foxp3+ IL-17+ T cells also express the Th17 transcription factor RORγt, revealing an intermediate or transitional phenotype between the Treg and Th17 subsets. Foxp3+ IL-17+ T cells can be found in the mucosa of IBD patients. One study identified Foxp3+ IL-17+ double positive T cells in the intestinal mucosa of CD but not UC patients[230], whereas another study reported their presence in UC patients[231]. The Foxp3+ IL-17+ T cells are likely to represent a transitional stage between Tregs and Th17 cells. They would be induced by the inflammatory microenvironment or would come from incompletely differentiated Tregs. Whether these cells retain their suppressive activity remains controversial. A significant decrease in the Tregs ability to suppress autologous T-cell proliferation in vitro was associated with an increased proportion of IL-17+ T cells among the Foxp3+ Tregs[226], while another study concluded that the mucosal Foxp3+ IL-17+ T cells from UC patients showed suppressor capacities in vitro[231]. Interestingly, additional investigations of the mucosal Foxp3+ IL-17+ T cells functional capacities in the latter study revealed that they increased IL-1 and IL-6 inflammatory cytokine production by colonic tissue cultures in an IL-17-dependent manner[231]. Thus, the acquisition of pro-inflammatory capacities by Tregs, concomitant with the loss of suppressive activity is likely to contribute to the uncontrolled inflammation in vivo.
One major caveat for understanding Treg function in IBD pathogenesis is the lack of reliable markers for identifying Tregs. The classical CD25 and Foxp3 markers, which are used in many studies, can also be expressed by activated Teffs[232]. Therefore, drawing firm conclusions will require revisiting those studies using a more refined phenotype for Treg identification. Currently, the identification of human Tregs under non-inflammatory conditions is based on their high CD25 expression and low CD127 expression. However, in rheumatoid arthritis patients, CD127 is also downregulated on activated effector T cells under inflammatory conditions[17].
Some treatments that were not designed to specifically target Tregs exert beneficial effects on the disease and a concomitant impact on Tregs. Lymphocyto-plasmapheresis and Granulocyte/monocyte apheresis (GMA), therapies that deplete the circulating immune cells in patients with active UC so they cannot reach the target organ and promote inflammation, have showed positive clinical results. The clear clinical efficacy of GMA in UC patients with severe disease who are refractory to conventional treatments is associated with a significant increase in the circulating Treg numbers[233]. Furthermore, this increase was specifically observed in responder patients[234,235]. The numbers of mucosal Tregs were concomitantly decreased in the responders, likely because of the reduced inflammation. Together with depletion of the pathogenic populations, this increased circulating Treg ratio is likely to exert a beneficial impact. An anti-TNF treatment associated with clinical benefits also increases the circulating Treg numbers and decreases the mucosal Treg infiltrates of IBD patients, particularly the responders[219,236,237]. The anti-TNF treatment can reverse the increased Treg apoptosis observed in UC patients[222], increase their suppressive functions in vitro[237] and increase the seric levels of TGF-β and IL-10 in responder CD patients[238]. Combined with the neutralization of this important proinflammatory cytokine, the impact of the anti-TNF treatment on Tregs is highly likely to also contribute to its clinical benefits. A low dose IL-2 treatment that selectively targets Treg expansion, likely because of their high expression of IL2Rα (CD25)[239], has shown promising results in patients with type-1 diabetes[240]. A clinical trial investigating the impact of low dose IL-2 treatment in several autoimmune and inflammatory diseases including UC and CD is currently ongoing (TRANSREG, NCT01988506).
Treg transfer can cure intestinal pathology in mice[133]. This prompted the scientific community to use Tregs as a therapeutic modality for IBD treatment. The generation of new protocols allowing the large-scale expansion of Tregs from a small initial population has made possible the use of autologous Treg therapy to treat the disease in humans. This strategy has already been successfully applied to graft-versus-host disease[241] and type-1 diabetes[242] and has shown promising clinical efficiency.
In a first escalating dose phase I and IIa clinical trial, autologous ovalbumin-specific Tregs (OVA-Tregs) were expanded in vitro and injected back into 20 patients with refractory CD[243]. Although ovalbumin is not implicated in intestinal inflammation in animal models or in patients with CD, this common diet antigen was chosen to ensure OVA-Treg antigen-specific activation in the gut. A reduction of CD disease activity was observed in 40% of the patients. This promising study has led to the development of a larger, ongoing, placebo-controlled clinical trial to evaluate the impact of autologous Treg cell therapy in patients with active Crohn’s disease who are refractory to conventional treatments (NCT02327221 Ovasafe).
Treg-based therapies present the risk of adverse effects, such as increased sensibility to infections or cancer development caused by immunosuppression. These off-target effects are likely to be reduced by controlling the antigen specificity of the Tregs. Additionally, the phenotype of the starting population and the culture conditions are critical to obtain the maximal purity of the therapeutic Tregs and ensure phenotypic stability. In this regard, a recent study suggested that CD4+ CD25+ CD127low CD45RA+ Tregs might be the most appropriate population from which to expand Tregs for autologous Treg therapy for CD to reduce the potential deleterious effects of Th17 conversion[244].
There is little doubt that Tregs represent one of the major research areas in immunology. Their critical role in intestinal homeostasis maintenance is now well established because defects affecting Treg development or suppressive functions have clearly been associated with IBD development, and Tregs can be used to cure an established IBD. This has led to an increasing interest in the possibility of using Tregs as targets for therapy to preserve intestinal integrity. However, although substantial progress in understanding Treg biology has been achieved in the past 10 years, currently, no phenotypic marker can be used to isolate pure Tregs for cellular therapy. The identification of such markers, together with developing a better understanding of the extrinsic factors that impact Treg stability and function, represents a great challenge. The recent discovery of the critical role of epigenetic modifications that affect Treg stability constitute an important advance in our understanding of Treg lineage specification. The identification of factors that control these modifications and their maintenance in peripheral Tregs will hopefully pave the way for the design of new strategies aimed at improving Treg stability and functions in the context of Treg transfer therapies.
In addition, when developing new strategies to improve Treg function, we also need to consider the fact that excessive Treg activity could lead to adverse effects by coincidently impairing protective immunity towards pathogens and tumors. Therefore, investigations of how Tregs function, how they are activated/inactivated, and the effector mechanisms that are required for the control of various immune responses are critical to specifically target some disease-specific mechanisms and limit the potential negative side effects.
P- Reviewer: Soares RLS S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Nishizuka Y, Sakakura T. Thymus and reproduction: sex-linked dysgenesia of the gonad after neonatal thymectomy in mice. Science. 1969;166:753-755. [PubMed] |
| 2. | Penhale WJ, Farmer A, McKenna RP, Irvine WJ. Spontaneous thyroiditis in thymectomized and irradiated Wistar rats. Clin Exp Immunol. 1973;15:225-236. [PubMed] |
| 3. | Sakaguchi S, Takahashi T, Nishizuka Y. Study on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement of Lyt-1 cells in normal female mice for the prevention of oophoritis. J Exp Med. 1982;156:1577-1586. [PubMed] |
| 4. | Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J Exp Med. 1993;178:237-244. [PubMed] |
| 5. | Powrie F, Leach MW, Mauze S, Caddle LB, Coffman RL. Phenotypically distinct subsets of CD4+ T cells induce or protect from chronic intestinal inflammation in C. B-17 scid mice. Int Immunol. 1993;5:1461-1471. [PubMed] |
| 6. | Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151-1164. [PubMed] |
| 7. | Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1827] [Cited by in RCA: 1926] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 8. | Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1371] [Cited by in RCA: 1380] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 9. | Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2432] [Cited by in RCA: 2489] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 10. | Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5576] [Cited by in RCA: 5863] [Article Influence: 266.5] [Reference Citation Analysis (0)] |
| 11. | Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, Elferink BG, van der Zanden L, de Vries RR, Huizinga TW. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 314] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 12. | Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983-2990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 628] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 13. | Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295-302. [PubMed] |
| 14. | Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, Sakaguchi S. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303-310. [PubMed] |
| 15. | McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, Byrne MC. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311-323. [PubMed] |
| 16. | Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693-1700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1131] [Cited by in RCA: 1217] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 17. | Aerts NE, Dombrecht EJ, Ebo DG, Bridts CH, Stevens WJ, De Clerck LS. Activated T cells complicate the identification of regulatory T cells in rheumatoid arthritis. Cell Immunol. 2008;251:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Ruprecht CR, Gattorno M, Ferlito F, Gregorio A, Martini A, Lanzavecchia A, Sallusto F. Coexpression of CD25 and CD27 identifies FoxP3+ regulatory T cells in inflamed synovia. J Exp Med. 2005;201:1793-1803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 273] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 19. | Mack DG, Lanham AM, Palmer BE, Maier LA, Fontenot AP. CD27 expression on CD4+ T cells differentiates effector from regulatory T cell subsets in the lung. J Immunol. 2009;182:7317-7324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 556] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 21. | Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 334] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 22. | Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 528] [Cited by in RCA: 517] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 23. | Papiernik M, de Moraes ML, Pontoux C, Vasseur F, Pénit C. Regulatory CD4 T cells: expression of IL-2R alpha chain, resistance to clonal deletion and IL-2 dependency. Int Immunol. 1998;10:371-378. [PubMed] |
| 24. | Almeida AR, Legrand N, Papiernik M, Freitas AA. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850-4860. [PubMed] |
| 25. | Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167-178. [PubMed] |
| 26. | Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 900] [Cited by in RCA: 971] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 27. | Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253-261. [PubMed] |
| 28. | Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775-1785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1408] [Cited by in RCA: 1500] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 29. | Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757-1764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2056] [Cited by in RCA: 2203] [Article Influence: 122.4] [Reference Citation Analysis (0)] |
| 30. | Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765-1774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 625] [Cited by in RCA: 681] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 31. | Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458-5467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 575] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 32. | Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1835] [Cited by in RCA: 1776] [Article Influence: 118.4] [Reference Citation Analysis (0)] |
| 33. | Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3:111ra120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 358] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 34. | Yogev N, Frommer F, Lukas D, Kautz-Neu K, Karram K, Ielo D, von Stebut E, Probst HC, van den Broek M, Riethmacher D. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor(+) regulatory T cells. Immunity. 2012;37:264-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 35. | Totsuka T, Kanai T, Makita S, Fujii R, Nemoto Y, Oshima S, Okamoto R, Koyanagi A, Akiba H, Okumura K. Regulation of murine chronic colitis by CD4+CD25- programmed death-1+ T cells. Eur J Immunol. 2005;35:1773-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Totsuka T, Kanai T, Nemoto Y, Tomita T, Tsuchiya K, Sakamoto N, Okamoto R, Watanabe M. Immunosenescent colitogenic CD4(+) T cells convert to regulatory cells and suppress colitis. Eur J Immunol. 2008;38:1275-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2951] [Cited by in RCA: 3792] [Article Influence: 316.0] [Reference Citation Analysis (0)] |
| 38. | Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2516] [Cited by in RCA: 3435] [Article Influence: 286.3] [Reference Citation Analysis (0)] |
| 39. | Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1875] [Cited by in RCA: 2142] [Article Influence: 178.5] [Reference Citation Analysis (2)] |
| 40. | Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2568] [Cited by in RCA: 2881] [Article Influence: 192.1] [Reference Citation Analysis (0)] |
| 41. | Sokol H, Seksik P, Rigottier-Gois L, Lay C, Lepage P, Podglajen I, Marteau P, Doré J. Specificities of the fecal microbiota in inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 323] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 42. | Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA. 2007;104:13780-13785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3731] [Cited by in RCA: 3432] [Article Influence: 190.7] [Reference Citation Analysis (1)] |
| 43. | Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2752] [Cited by in RCA: 2710] [Article Influence: 96.8] [Reference Citation Analysis (2)] |
| 44. | Bacchetta R, Sartirana C, Levings MK, Bordignon C, Narula S, Roncarolo MG. Growth and expansion of human T regulatory type 1 cells are independent from TCR activation but require exogenous cytokines. Eur J Immunol. 2002;32:2237-2245. [PubMed] |
| 45. | Magnani CF, Alberigo G, Bacchetta R, Serafini G, Andreani M, Roncarolo MG, Gregori S. Killing of myeloid APCs via HLA class I, CD2 and CD226 defines a novel mechanism of suppression by human Tr1 cells. Eur J Immunol. 2011;41:1652-1662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 46. | Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat Med. 2013;19:739-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 634] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 47. | Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1256] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 48. | Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell. 2012;150:29-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 499] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 49. | Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 800] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 50. | Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433-3441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1089] [Cited by in RCA: 1081] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 51. | Gottschalk RA, Corse E, Allison JP. Expression of Helios in peripherally induced Foxp3+ regulatory T cells. J Immunol. 2012;188:976-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 237] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 52. | Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK. Helios+ and Helios- cells coexist within the natural FOXP3+ T regulatory cell subset in humans. J Immunol. 2013;190:2001-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 53. | Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713-1722, S1-S19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 452] [Cited by in RCA: 524] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 54. | Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, Xiong H, Dolpady J, Frey AB, Ruocco MG. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209:1723-1742, S1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 493] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 55. | Milpied P, Renand A, Bruneau J, Mendes-da-Cruz DA, Jacquelin S, Asnafi V, Rubio MT, MacIntyre E, Lepelletier Y, Hermine O. Neuropilin-1 is not a marker of human Foxp3+ Treg. Eur J Immunol. 2009;39:1466-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 56. | Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, Rudensky AY. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature. 2012;482:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 669] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 57. | Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh CS. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 858] [Cited by in RCA: 822] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 58. | Cebula A, Seweryn M, Rempala GA, Pabla SS, McIndoe RA, Denning TL, Bry L, Kraj P, Kisielow P, Ignatowicz L. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497:258-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 306] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 59. | Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci USA. 2010;107:5919-5924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 60. | Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1050] [Cited by in RCA: 1010] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 61. | Hall AO, Beiting DP, Tato C, John B, Oldenhove G, Lombana CG, Pritchard GH, Silver JS, Bouladoux N, Stumhofer JS. The cytokines interleukin 27 and interferon-γ promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 62. | Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor β2. Immunity. 2012;37:501-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 208] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 63. | Lu LF, Boldin MP, Chaudhry A, Lin LL, Taganov KD, Hanada T, Yoshimura A, Baltimore D, Rudensky AY. Function of miR-146a in controlling Treg cell-mediated regulation of Th1 responses. Cell. 2010;142:914-929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 789] [Cited by in RCA: 784] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 64. | Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 788] [Cited by in RCA: 770] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 65. | Ulges A, Klein M, Reuter S, Gerlitzki B, Hoffmann M, Grebe N, Staudt V, Stergiou N, Bohn T, Brühl TJ. Protein kinase CK2 enables regulatory T cells to suppress excessive TH2 responses in vivo. Nat Immunol. 2015;16:267-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 66. | Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 813] [Cited by in RCA: 828] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 67. | Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang YH, Lim H, Reynolds JM, Zhou XH. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983-988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 882] [Cited by in RCA: 914] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 68. | Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, Debes GF, Lauber J, Frey O, Przybylski GK. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199:303-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 498] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 69. | Kleinewietfeld M, Puentes F, Borsellino G, Battistini L, Rötzschke O, Falk K. CCR6 expression defines regulatory effector/memory-like cells within the CD25(+)CD4+ T-cell subset. Blood. 2005;105:2877-2886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 238] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 70. | Fritzsching B, Oberle N, Pauly E, Geffers R, Buer J, Poschl J, Krammer P, Linderkamp O, Suri-Payer E. Naive regulatory T cells: a novel subpopulation defined by resistance toward CD95L-mediated cell death. Blood. 2006;108:3371-3378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 71. | Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622-4631. [PubMed] |
| 72. | Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1588] [Cited by in RCA: 1812] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 73. | Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1083] [Cited by in RCA: 1054] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 74. | Dominguez-Villar M, Baecher-Allan CM, Hafler DA. Identification of T helper type 1-like, Foxp3+ regulatory T cells in human autoimmune disease. Nat Med. 2011;17:673-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 402] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 75. | McClymont SA, Putnam AL, Lee MR, Esensten JH, Liu W, Hulme MA, Hoffmüller U, Baron U, Olek S, Bluestone JA. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2011;186:3918-3926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 348] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 76. | Laurence A, Amarnath S, Mariotti J, Kim YC, Foley J, Eckhaus M, O’Shea JJ, Fowler DH. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity. 2012;37:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 77. | Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H. Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med. 2014;20:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 883] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 78. | Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J, Mathis D, Benoist C, Rudensky AY. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 580] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 79. | Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, Huehn J, Hori S. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 483] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 80. | Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 673] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 81. | Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 507] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 82. | Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T, Sakaguchi S. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18:1197-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 282] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 83. | Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 853] [Cited by in RCA: 916] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 84. | Heintzman ND, Hon GC, Hawkins RD, Kheradpour P, Stark A, Harp LF, Ye Z, Lee LK, Stuart RK, Ching CW. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459:108-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2178] [Cited by in RCA: 1958] [Article Influence: 122.4] [Reference Citation Analysis (0)] |
| 85. | Kim JK, Samaranayake M, Pradhan S. Epigenetic mechanisms in mammals. Cell Mol Life Sci. 2009;66:596-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 317] [Cited by in RCA: 327] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 86. | Gibney ER, Nolan CM. Epigenetics and gene expression. Heredity (Edinb). 2010;105:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 666] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 87. | Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5:e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 922] [Cited by in RCA: 996] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 88. | Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N. T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity. 2012;37:785-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 497] [Cited by in RCA: 583] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 89. | Schmidl C, Klug M, Boeld TJ, Andreesen R, Hoffmann P, Edinger M, Rehli M. Lineage-specific DNA methylation in T cells correlates with histone methylation and enhancer activity. Genome Res. 2009;19:1165-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 90. | Samstein RM, Arvey A, Josefowicz SZ, Peng X, Reynolds A, Sandstrom R, Neph S, Sabo P, Kim JM, Liao W. Foxp3 exploits a pre-existent enhancer landscape for regulatory T cell lineage specification. Cell. 2012;151:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 387] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 91. | Beyer M, Thabet Y, Müller RU, Sadlon T, Classen S, Lahl K, Basu S, Zhou X, Bailey-Bucktrout SL, Krebs W. Repression of the genome organizer SATB1 in regulatory T cells is required for suppressive function and inhibition of effector differentiation. Nat Immunol. 2011;12:898-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 92. | Roychoudhuri R, Hirahara K, Mousavi K, Clever D, Klebanoff CA, Bonelli M, Sciumè G, Zare H, Vahedi G, Dema B. BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis. Nature. 2013;498:506-510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 330] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 93. | Pan F, Yu H, Dang EV, Barbi J, Pan X, Grosso JF, Jinasena D, Sharma SM, McCadden EM, Getnet D. Eos mediates Foxp3-dependent gene silencing in CD4+ regulatory T cells. Science. 2009;325:1142-1146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 255] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 94. | Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983-1991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 439] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 95. | Liston A, Lu LF, O’Carroll D, Tarakhovsky A, Rudensky AY. Dicer-dependent microRNA pathway safeguards regulatory T cell function. J Exp Med. 2008;205:1993-2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 96. | Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat Immunol. 2015;16:178-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 303] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 97. | Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol. 2015;16:188-196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 337] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 98. | Valencia X, Stephens G, Goldbach-Mansky R, Wilson M, Shevach EM, Lipsky PE. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 631] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 99. | Wan S, Xia C, Morel L. IL-6 produced by dendritic cells from lupus-prone mice inhibits CD4+CD25+ T cell regulatory functions. J Immunol. 2007;178:271-279. [PubMed] |
| 100. | Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Bäckström BT, Sobel RA, Wucherpfennig KW, Strom TB. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 625] [Cited by in RCA: 674] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 101. | Clough LE, Wang CJ, Schmidt EM, Booth G, Hou TZ, Ryan GA, Walker LS. Release from regulatory T cell-mediated suppression during the onset of tissue-specific autoimmunity is associated with elevated IL-21. J Immunol. 2008;180:5393-5401. [PubMed] |
| 102. | Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, Mauri C. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 894] [Cited by in RCA: 965] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 103. | Schiering C, Krausgruber T, Chomka A, Fröhlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564-568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 804] [Cited by in RCA: 811] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 104. | Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210:257-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 105. | Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nat Rev Immunol. 2012;12:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 357] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 106. | Levine AG, Arvey A, Jin W, Rudensky AY. Continuous requirement for the TCR in regulatory T cell function. Nat Immunol. 2014;15:1070-1078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 435] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 107. | Vahl JC, Drees C, Heger K, Heink S, Fischer JC, Nedjic J, Ohkura N, Morikawa H, Poeck H, Schallenberg S. Continuous T cell receptor signals maintain a functional regulatory T cell pool. Immunity. 2014;41:722-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 253] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 108. | Delpoux A, Yakonowsky P, Durand A, Charvet C, Valente M, Pommier A, Bonilla N, Martin B, Auffray C, Lucas B. TCR signaling events are required for maintaining CD4 regulatory T cell numbers and suppressive capacities in the periphery. J Immunol. 2014;193:5914-5923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 109. | Fu G, Chen Y, Yu M, Podd A, Schuman J, He Y, Di L, Yassai M, Haribhai D, North PE. Phospholipase C{gamma}1 is essential for T cell development, activation, and tolerance. J Exp Med. 2010;207:309-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 110. | Shen S, Chuck MI, Zhu M, Fuller DM, Yang CW, Zhang W. The importance of LAT in the activation, homeostasis, and regulatory function of T cells. J Biol Chem. 2010;285:35393-35405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 111. | Chuck MI, Zhu M, Shen S, Zhang W. The role of the LAT-PLC-gamma1 interaction in T regulatory cell function. J Immunol. 2010;184:2476-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 112. | Hsu LY, Tan YX, Xiao Z, Malissen M, Weiss A. A hypomorphic allele of ZAP-70 reveals a distinct thymic threshold for autoimmune disease versus autoimmune reactivity. J Exp Med. 2009;206:2527-2541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 113. | Au-Yeung BB, Levin SE, Zhang C, Hsu LY, Cheng DA, Killeen N, Shokat KM, Weiss A. A genetically selective inhibitor demonstrates a function for the kinase Zap70 in regulatory T cells independent of its catalytic activity. Nat Immunol. 2010;11:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 114. | Kim JK, Klinger M, Benjamin J, Xiao Y, Erle DJ, Littman DR, Killeen N. Impact of the TCR signal on regulatory T cell homeostasis, function, and trafficking. PLoS One. 2009;4:e6580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 115. | Chabod M, Pedros C, Lamouroux L, Colacios C, Bernard I, Lagrange D, Balz-Hara D, Mosnier JF, Laboisse C, Vergnolle N. A spontaneous mutation of the rat Themis gene leads to impaired function of regulatory T cells linked to inflammatory bowel disease. PLoS Genet. 2012;8:e1002461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 116. | Pedros C, Gaud G, Bernard I, Kassem S, Chabod M, Lagrange D, Andréoletti O, Dejean AS, Lesourne R, Fournié GJ. An Epistatic Interaction between Themis1 and Vav1 Modulates Regulatory T Cell Function and Inflammatory Bowel Disease Development. J Immunol. 2015;195:1608-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 117. | Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137-1140. [PubMed] |
| 118. | Ghiringhelli F, Ménard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202:1075-1085. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 644] [Cited by in RCA: 714] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 119. | Azuma T, Takahashi T, Kunisato A, Kitamura T, Hirai H. Human CD4+ CD25+ regulatory T cells suppress NKT cell functions. Cancer Res. 2003;63:4516-4520. [PubMed] |
| 120. | Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: direct suppression of B cells by CD4+ CD25+ regulatory T cells. J Immunol. 2005;175:4180-4183. [PubMed] |
| 121. | Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, Mascanfroni ID, Al Adham Z, Lavoie S, Ibourk M. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 450] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 122. | Zigmond E, Bernshtein B, Friedlander G, Walker CR, Yona S, Kim KW, Brenner O, Krauthgamer R, Varol C, Müller W. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40:720-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 451] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 123. | Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2593] [Cited by in RCA: 2471] [Article Influence: 145.4] [Reference Citation Analysis (0)] |
| 124. | Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553-562. [PubMed] |
| 125. | Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1235] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 126. | Izcue A, Hue S, Buonocore S, Arancibia-Cárcamo CV, Ahern PP, Iwakura Y, Maloy KJ, Powrie F. Interleukin-23 restrains regulatory T cell activity to drive T cell-dependent colitis. Immunity. 2008;28:559-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 314] [Cited by in RCA: 324] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 127. | Kobayashi T, Okamoto S, Hisamatsu T, Kamada N, Chinen H, Saito R, Kitazume MT, Nakazawa A, Sugita A, Koganei K. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut. 2008;57:1682-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 441] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 128. | Leach MW, Bean AG, Mauze S, Coffman RL, Powrie F. Inflammatory bowel disease in C.B-17 scid mice reconstituted with the CD45RBhigh subset of CD4+ T cells. Am J Pathol. 1996;148:1503-1515. [PubMed] |
| 129. | Powrie F, Mauze S, Coffman RL. CD4+ T-cells in the regulation of inflammatory responses in the intestine. Res Immunol. 1997;148:576-581. [PubMed] |
| 130. | Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001;2:816-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 841] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 131. | Powrie F, Carlino J, Leach MW, Mauze S, Coffman RL. A critical role for transforming growth factor-beta but not interleukin 4 in the suppression of T helper type 1-mediated colitis by CD45RB(low) CD4+ T cells. J Exp Med. 1996;183:2669-2674. [PubMed] |
| 132. | Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 388] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 133. | Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939-3943. [PubMed] |
| 134. | Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, Sporn MB, Fauci AS. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163:1037-1050. [PubMed] |
| 135. | Gorelik L, Flavell RA. Transforming growth factor-beta in T-cell biology. Nat Rev Immunol. 2002;2:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 618] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 136. | Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 424] [Cited by in RCA: 419] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 137. | Pesu M, Watford WT, Wei L, Xu L, Fuss I, Strober W, Andersson J, Shevach EM, Quezado M, Bouladoux N. T-cell-expressed proprotein convertase furin is essential for maintenance of peripheral immune tolerance. Nature. 2008;455:246-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 138. | Kulkarni AB, Huh CG, Becker D, Geiser A, Lyght M, Flanders KC, Roberts AB, Sporn MB, Ward JM, Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770-774. [PubMed] |
| 139. | Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2256] [Cited by in RCA: 2323] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 140. | Diebold RJ, Eis MJ, Yin M, Ormsby I, Boivin GP, Darrow BJ, Saffitz JE, Doetschman T. Early-onset multifocal inflammation in the transforming growth factor beta 1-null mouse is lymphocyte mediated. Proc Natl Acad Sci USA. 1995;92:12215-12219. [PubMed] |
| 141. | Letterio JJ, Geiser AG, Kulkarni AB, Dang H, Kong L, Nakabayashi T, Mackall CL, Gress RE, Roberts AB. Autoimmunity associated with TGF-beta1-deficiency in mice is dependent on MHC class II antigen expression. J Clin Invest. 1996;98:2109-2119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 168] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 142. | Fahlén L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA, Powrie F. T cells that cannot respond to TGF-beta escape control by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2005;201:737-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 387] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 143. | Fantini MC, Rizzo A, Fina D, Caruso R, Sarra M, Stolfi C, Becker C, Macdonald TT, Pallone F, Neurath MF. Smad7 controls resistance of colitogenic T cells to regulatory T cell-mediated suppression. Gastroenterology. 2009;136:1308-1316, e1-e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 144. | Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 640] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 145. | Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875-1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3466] [Cited by in RCA: 3767] [Article Influence: 179.4] [Reference Citation Analysis (0)] |
| 146. | Gutcher I, Donkor MK, Ma Q, Rudensky AY, Flavell RA, Li MO. Autocrine transforming growth factor-β1 promotes in vivo Th17 cell differentiation. Immunity. 2011;34:396-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 147. | Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT, Roes JT, Savill JS, Hynes RO. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci USA. 2007;104:15823-15828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 148. | Yang Z, Mu Z, Dabovic B, Jurukovski V, Yu D, Sung J, Xiong X, Munger JS. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176:787-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 149. | Taga K, Mostowski H, Tosato G. Human interleukin-10 can directly inhibit T-cell growth. Blood. 1993;81:2964-2971. [PubMed] |
| 150. | de Waal Malefyt R, Yssel H, de Vries JE. Direct effects of IL-10 on subsets of human CD4+ T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754-4765. [PubMed] |
| 151. | Schandené L, Alonso-Vega C, Willems F, Gérard C, Delvaux A, Velu T, Devos R, de Boer M, Goldman M. B7/CD28-dependent IL-5 production by human resting T cells is inhibited by IL-10. J Immunol. 1994;152:4368-4374. [PubMed] |
| 152. | Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4725] [Cited by in RCA: 4965] [Article Influence: 206.9] [Reference Citation Analysis (0)] |
| 153. | Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1254] [Article Influence: 73.8] [Reference Citation Analysis (0)] |
| 154. | Schumacher A, Wafula PO, Bertoja AZ, Sollwedel A, Thuere C, Wollenberg I, Yagita H, Volk HD, Zenclussen AC. Mechanisms of action of regulatory T cells specific for paternal antigens during pregnancy. Obstet Gynecol. 2007;110:1137-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 155. | Kühn R, Löhler J, Rennick D, Rajewsky K, Müller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263-274. [PubMed] |
| 156. | Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, Fiers W, Remaut E. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352-1355. [PubMed] |
| 157. | Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 489] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 158. | Huber S, Gagliani N, Esplugues E, O’Connor W, Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY. Th17 cells express interleukin-10 receptor and are controlled by Foxp3⁻ and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 501] [Cited by in RCA: 482] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 159. | Chaudhry A, Samstein RM, Treuting P, Liang Y, Pils MC, Heinrich JM, Jack RS, Wunderlich FT, Brüning JC, Müller W. Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity. 2011;34:566-578. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 763] [Cited by in RCA: 736] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 160. | Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1324] [Cited by in RCA: 1516] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 161. | Collison LW, Delgoffe GM, Guy CS, Vignali KM, Chaturvedi V, Fairweather D, Satoskar AR, Garcia KC, Hunter CA, Drake CG. The composition and signaling of the IL-35 receptor are unconventional. Nat Immunol. 2012;13:290-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 350] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 162. | Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093-1101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 664] [Cited by in RCA: 657] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 163. | Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D, Bernardi G, Dell’Acqua ML. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 958] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 164. | Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257-1265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1917] [Cited by in RCA: 1844] [Article Influence: 102.4] [Reference Citation Analysis (0)] |
| 165. | Linden J. Regulation of leukocyte function by adenosine receptors. Adv Pharmacol. 2011;61:95-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 166. | Alam MS, Kurtz CC, Wilson JM, Burnette BR, Wiznerowicz EB, Ross WG, Rieger JM, Figler RA, Linden J, Crowe SE. A2A adenosine receptor (AR) activation inhibits pro-inflammatory cytokine production by human CD4+ helper T cells and regulates Helicobacter-induced gastritis and bacterial persistence. Mucosal Immunol. 2009;2:232-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 167. | Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600-1610. [PubMed] |
| 168. | Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073-1080. [PubMed] |
| 169. | Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ, Mosmann TR. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5’-adenosine monophosphate to adenosine. J Immunol. 2006;177:6780-6786. [PubMed] |
| 170. | Kumar V, Sharma A. Adenosine: an endogenous modulator of innate immune system with therapeutic potential. Eur J Pharmacol. 2009;616:7-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 171. | Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 392] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 172. | Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, Flores M, Li N, Schweighoffer E, Greenberg S. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916-5926. [PubMed] |
| 173. | Odashima M, Bamias G, Rivera-Nieves J, Linden J, Nast CC, Moskaluk CA, Marini M, Sugawara K, Kozaiwa K, Otaka M. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology. 2005;129:26-33. [PubMed] |
| 174. | Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765-2769. [PubMed] |
| 175. | Friedman DJ, Künzli BM, A-Rahim YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci USA. 2009;106:16788-16793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 176. | Alam MS, Kurtz CC, Rowlett RM, Reuter BK, Wiznerowicz E, Das S, Linden J, Crowe SE, Ernst PB. CD73 is expressed by human regulatory T helper cells and suppresses proinflammatory cytokine production and Helicobacter felis-induced gastritis in mice. J Infect Dis. 2009;199:494-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 177. | Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174:1783-1786. [PubMed] |
| 178. | Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925-3932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 363] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 179. | Gondek DC, Devries V, Nowak EC, Lu LF, Bennett KA, Scott ZA, Noelle RJ. Transplantation survival is maintained by granzyme B+ regulatory cells and adaptive regulatory T cells. J Immunol. 2008;181:4752-4760. [PubMed] |
| 180. | Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 706] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 181. | Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 891] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 182. | Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C, Zhu C, Hirashima M, Anderson AC, Kuchroo VK. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity. 2014;41:270-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 262] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 183. | Chou FC, Shieh SJ, Sytwu HK. Attenuation of Th1 response through galectin-9 and T-cell Ig mucin 3 interaction inhibits autoimmune diabetes in NOD mice. Eur J Immunol. 2009;39:2403-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 184. | Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1334] [Cited by in RCA: 1590] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 185. | Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, Dustin ML. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 271] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 186. | Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 618] [Cited by in RCA: 626] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 187. | Tadokoro CE, Shakhar G, Shen S, Ding Y, Lino AC, Maraver A, Lafaille JJ, Dustin ML. Regulatory T cells inhibit stable contacts between CD4+ T cells and dendritic cells in vivo. J Exp Med. 2006;203:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 398] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 188. | Lin KL, Fulton LM, Berginski M, West ML, Taylor NA, Moran TP, Coghill JM, Blazar BR, Bear JE, Serody JS. Intravital imaging of donor allogeneic effector and regulatory T cells with host dendritic cells during GVHD. Blood. 2014;123:1604-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 189. | Matheu MP, Othy S, Greenberg ML, Dong TX, Schuijs M, Deswarte K, Hammad H, Lambrecht BN, Parker I, Cahalan MD. Imaging regulatory T cell dynamics and CTLA4-mediated suppression of T cell priming. Nat Commun. 2015;6:6219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 190. | Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 573] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 191. | Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985-988. [PubMed] |
| 192. | Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541-547. [PubMed] |
| 193. | Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T, Miyara M, Fehervari Z, Nomura T, Sakaguchi S. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2013] [Cited by in RCA: 2254] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 194. | Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, Baker J, Jeffery LE, Kaur S, Briggs Z. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1406] [Cited by in RCA: 1315] [Article Influence: 93.9] [Reference Citation Analysis (0)] |
| 195. | Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, Belladonna ML, Fioretti MC, Alegre ML, Puccetti P. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 973] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 196. | Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, Sharpe AH, Powrie F. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376-4383. [PubMed] |
| 197. | Barnes MJ, Griseri T, Johnson AM, Young W, Powrie F, Izcue A. CTLA-4 promotes Foxp3 induction and regulatory T cell accumulation in the intestinal lamina propria. Mucosal Immunol. 2013;6:324-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 198. | Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 275] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 199. | Hansen W, Hutzler M, Abel S, Alter C, Stockmann C, Kliche S, Albert J, Sparwasser T, Sakaguchi S, Westendorf AM. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med. 2012;209:2001-2016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 200] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 200. | Delgoffe GM, Woo SR, Turnis ME, Gravano DM, Guy C, Overacre AE, Bettini ML, Vogel P, Finkelstein D, Bonnevier J. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 458] [Cited by in RCA: 478] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 201. | Darrasse-Jèze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao KH, Masilamani RF, Dustin ML, Rudensky A, Liu K. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med. 2009;206:1853-1862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 324] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 202. | Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282-3293. [PubMed] |
| 203. | Pabst O, Förster R, Lipp M, Engel H, Arnold HH. NKX2.3 is required for MAdCAM-1 expression and homing of lymphocytes in spleen and mucosa-associated lymphoid tissue. EMBO J. 2000;19:2015-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 204. | Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. J Exp Med. 2000;192:761-768. [PubMed] |
| 205. | Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1148] [Cited by in RCA: 1220] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 206. | Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Förster R, Pabst O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483-2490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 207. | Hammerschmidt SI, Friedrichsen M, Boelter J, Lyszkiewicz M, Kremmer E, Pabst O, Förster R. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J Clin Invest. 2011;121:3051-3061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 208. | Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Semin Immunol. 2009;21:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 209. | Menning A, Loddenkemper C, Westendorf AM, Szilagyi B, Buer J, Siewert C, Hamann A, Huehn J. Retinoic acid-induced gut tropism improves the protective capacity of Treg in acute but not in chronic gut inflammation. Eur J Immunol. 2010;40:2539-2548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 210. | Denning TL, Kim G, Kronenberg M. Cutting edge: CD4+CD25+ regulatory T cells impaired for intestinal homing can prevent colitis. J Immunol. 2005;174:7487-7491. [PubMed] |
| 211. | Yuan Q, Bromley SK, Means TK, Jones KJ, Hayashi F, Bhan AK, Luster AD. CCR4-dependent regulatory T cell function in inflammatory bowel disease. J Exp Med. 2007;204:1327-1334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 212. | Schneider MA, Meingassner JG, Lipp M, Moore HD, Rot A. CCR7 is required for the in vivo function of CD4+ CD25+ regulatory T cells. J Exp Med. 2007;204:735-745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 213. | Kitamura K, Farber JM, Kelsall BL. CCR6 marks regulatory T cells as a colon-tropic, IL-10-producing phenotype. J Immunol. 2010;185:3295-3304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 214. | Gambineri E, Perroni L, Passerini L, Bianchi L, Doglioni C, Meschi F, Bonfanti R, Sznajer Y, Tommasini A, Lawitschka A. Clinical and molecular profile of a new series of patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: inconsistent correlation between forkhead box protein 3 expression and disease severity. J Allergy Clin Immunol. 2008;122:1105-1112.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 167] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 215. | Maul J, Loddenkemper C, Mundt P, Berg E, Giese T, Stallmach A, Zeitz M, Duchmann R. Peripheral and intestinal regulatory CD4+ CD25(high) T cells in inflammatory bowel disease. Gastroenterology. 2005;128:1868-1878. [PubMed] |
| 216. | Wang Y, Liu XP, Zhao ZB, Chen JH, Yu CG. Expression of CD4+ forkhead box P3 (FOXP3)+ regulatory T cells in inflammatory bowel disease. J Dig Dis. 2011;12:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 217. | Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T, Nakamura T, Koganei K, Fukushima T, Watanabe M. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173:3119-3130. [PubMed] |
| 218. | Holmén N, Lundgren A, Lundin S, Bergin AM, Rudin A, Sjövall H, Ohman L. Functional CD4+CD25high regulatory T cells are enriched in the colonic mucosa of patients with active ulcerative colitis and increase with disease activity. Inflamm Bowel Dis. 2006;12:447-456. [PubMed] |
| 219. | Dige A, Hvas CL, Deleuran B, Kelsen J, Bendix-Struve M, Dahlerup JF, Agnholt J. Adalimumab treatment in Crohn’s disease does not induce early changes in regulatory T cells. Scand J Gastroenterol. 2011;46:1206-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 220. | Saruta M, Yu QT, Fleshner PR, Mantel PY, Schmidt-Weber CB, Banham AH, Papadakis KA. Characterization of FOXP3+CD4+ regulatory T cells in Crohn’s disease. Clin Immunol. 2007;125:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 221. | Uhlig HH, Coombes J, Mottet C, Izcue A, Thompson C, Fanger A, Tannapfel A, Fontenot JD, Ramsdell F, Powrie F. Characterization of Foxp3+CD4+CD25+ and IL-10-secreting CD4+CD25+ T cells during cure of colitis. J Immunol. 2006;177:5852-5860. [PubMed] |
| 222. | Veltkamp C, Anstaett M, Wahl K, Möller S, Gangl S, Bachmann O, Hardtke-Wolenski M, Länger F, Stremmel W, Manns MP. Apoptosis of regulatory T lymphocytes is increased in chronic inflammatory bowel disease and reversed by anti-TNFα treatment. Gut. 2011;60:1345-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 223. | Poniedziałek B, Rzymski P, Karczewski J. Increased apoptosis of regulatory T cells in Crohn’s disease. Hepatogastroenterology. 2014;61:382-384. [PubMed] |
| 224. | Kelsen J, Agnholt J, Hoffmann HJ, Rømer JL, Hvas CL, Dahlerup JF. FoxP3(+)CD4(+)CD25(+) T cells with regulatory properties can be cultured from colonic mucosa of patients with Crohn’s disease. Clin Exp Immunol. 2005;141:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 225. | Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 458] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 226. | Ueno A, Jijon H, Chan R, Ford K, Hirota C, Kaplan GG, Beck PL, Iacucci M, Fort Gasia M, Barkema HW. Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:2522-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 227. | Okou DT, Mondal K, Faubion WA, Kobrynski LJ, Denson LA, Mulle JG, Ramachandran D, Xiong Y, Svingen P, Patel V. Exome sequencing identifies a novel FOXP3 mutation in a 2-generation family with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014;58:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 228. | Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schäffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1149] [Cited by in RCA: 1092] [Article Influence: 68.3] [Reference Citation Analysis (0)] |
| 229. | Jostins L, Ripke S, Weersma RK, Duerr RH, McGovern DP, Hui KY, Lee JC, Schumm LP, Sharma Y, Anderson CA, Essers J, Mitrovic M, Ning K, Cleynen I, Theatre E, Spain SL, Raychaudhuri S, Goyette P, Wei Z, Abraham C, Achkar JP, Ahmad T, Amininejad L, Ananthakrishnan AN, Andersen V, Andrews JM, Baidoo L, Balschun T, Bampton PA, Bitton A, Boucher G, Brand S, Buning C, Cohain A, Cichon S, D’Amato M, De Jong D, Devaney KL, Dubinsky M, Edwards C, Ellinghaus D, Ferguson LR, Franchimont D, Fransen K, Gearry R, Georges M, Gieger C, Glas J, Haritunians T, Hart A, Hawkey C, Hedl M, Hu X, Karlsen TH, Kupcinskas L, Kugathasan S, Latiano A, Laukens D, Lawrance IC, Lees CW, Louis E, Mahy G, Mansfield J, Morgan AR, Mowat C, Newman W, Palmieri O, Ponsioen CY, Potocnik U, Prescott NJ, Regueiro M, Rotter JI, Russell RK, Sanderson JD, Sans M, Satsangi J, Schreiber S, Simms LA, Sventoraityte J, Targan SR, Taylor KD, Tremelling M, Verspaget HW, De Vos M, Wijmenga C, Wilson DC, Winkelmann J, Xavier RJ, Zeissig S, Zhang B, Zhang CK, Zhao H, International IBDGC, Silverberg MS, Annese V, Hakonarson H, Brant SR, Radford-Smith G, Mathew CG, Rioux JD, Schadt EE, Daly MJ, Franke A, Parkes M, Vermeire S, Barrett JC, Cho JH. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119-124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3979] [Cited by in RCA: 3603] [Article Influence: 277.2] [Reference Citation Analysis (0)] |
| 230. | Hovhannisyan Z, Treatman J, Littman DR, Mayer L. Characterization of interleukin-17-producing regulatory T cells in inflamed intestinal mucosa from patients with inflammatory bowel diseases. Gastroenterology. 2011;140:957-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 285] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 231. | Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Roliński J. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186:4388-4395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 232. | Kmieciak M, Gowda M, Graham L, Godder K, Bear HD, Marincola FM, Manjili MH. Human T cells express CD25 and Foxp3 upon activation and exhibit effector/memory phenotypes without any regulatory/suppressor function. J Transl Med. 2009;7:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 135] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 233. | Yokoyama Y, Fukunaga K, Fukuda Y, Tozawa K, Kamikozuru K, Ohnishi K, Kusaka T, Kosaka T, Hida N, Ohda Y. Demonstration of low-regulatory CD25High+CD4+ and high-pro-inflammatory CD28-CD4+ T-Cell subsets in patients with ulcerative colitis: modified by selective granulocyte and monocyte adsorption apheresis. Dig Dis Sci. 2007;52:2725-2731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 234. | Kamikozuru K, Fukunaga K, Hirota S, Hida N, Ohda Y, Yoshida K, Yokoyama Y, Tozawa K, Kawa K, Iimuro M. The expression profile of functional regulatory T cells, CD4+CD25high+/forkhead box protein P3+, in patients with ulcerative colitis during active and quiescent disease. Clin Exp Immunol. 2009;156:320-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 235. | Hanai H, Iida T, Ikeya K, Abe J, Maruyama Y, Shimura T, Sugimoto K, Watanabe F. A new paradigm in ulcerative colitis: regulatory T cells are key factor which induces/exacerbates UC through an immune imbalance. Mol Immunol. 2013;54:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 236. | Li Z, Arijs I, De Hertogh G, Vermeire S, Noman M, Bullens D, Coorevits L, Sagaert X, Schuit F, Rutgeerts P. Reciprocal changes of Foxp3 expression in blood and intestinal mucosa in IBD patients responding to infliximab. Inflamm Bowel Dis. 2010;16:1299-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 237. | Boschetti G, Nancey S, Sardi F, Roblin X, Flourié B, Kaiserlian D. Therapy with anti-TNFα antibody enhances number and function of Foxp3(+) regulatory T cells in inflammatory bowel diseases. Inflamm Bowel Dis. 2011;17:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 238. | Di Sabatino A, Biancheri P, Piconese S, Rosado MM, Ardizzone S, Rovedatti L, Ubezio C, Massari A, Sampietro GM, Foschi D. Peripheral regulatory T cells and serum transforming growth factor-β: relationship with clinical response to infliximab in Crohn’s disease. Inflamm Bowel Dis. 2010;16:1891-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 239. | Yu A, Snowhite I, Vendrame F, Rosenzwajg M, Klatzmann D, Pugliese A, Malek TR. Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in type 1 diabetes. Diabetes. 2015;64:2172-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 158] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 240. | Rosenzwajg M, Churlaud G, Mallone R, Six A, Dérian N, Chaara W, Lorenzon R, Long SA, Buckner JH, Afonso G. Low-dose interleukin-2 fosters a dose-dependent regulatory T cell tuned milieu in T1D patients. J Autoimmun. 2015;58:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 202] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 241. | Trzonkowski P, Bieniaszewska M, Juścińska J, Dobyszuk A, Krzystyniak A, Marek N, Myśliwska J, Hellmann A. First-in-man clinical results of the treatment of patients with graft versus host disease with human ex vivo expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol. 2009;133:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 536] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 242. | Marek-Trzonkowska N, Mysliwiec M, Dobyszuk A, Grabowska M, Techmanska I, Juscinska J, Wujtewicz MA, Witkowski P, Mlynarski W, Balcerska A. Administration of CD4+CD25highCD127- regulatory T cells preserves β-cell function in type 1 diabetes in children. Diabetes Care. 2012;35:1817-1820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 352] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 243. | Desreumaux P, Foussat A, Allez M, Beaugerie L, Hébuterne X, Bouhnik Y, Nachury M, Brun V, Bastian H, Belmonte N. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology. 2012;143:1207-1217.e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 296] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 244. | Canavan JB, Scottà C, Vossenkämper A, Goldberg R, Elder MJ, Shoval I, Marks E, Stolarczyk E, Lo JW, Powell N. Developing in vitro expanded CD45RA+ regulatory T cells as an adoptive cell therapy for Crohn’s disease. Gut. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |