Published online Jan 21, 2016. doi: 10.3748/wjg.v22.i3.961
Peer-review started: August 11, 2015
First decision: September 29, 2015
Revised: September 30, 2015
Accepted: November 19, 2015
Article in press: November 19, 2015
Published online: January 21, 2016
Processing time: 157 Days and 1.9 Hours
Inflammatory bowel disease (IBD) is associated with increased risk of colorectal cancer (CRC). The risk is known to increase with longer duration of the disease, family history of CRC, and history of primary sclerosing cholangitis. The diagnosis of the neoplastic changes associated with IBD is difficult owing to the heterogeneous endoscopic appearance and inter-observer variability of the pathological diagnosis. Screening and surveillance guidelines have been established which aim for early detection of neoplasia. Several surgical options are available for the treatment of IBD-associated neoplasia. Patients’ morbidities, risk factors for CRC, degree and the extent of neoplasia must be considered in choosing the surgical treatment. A multidisciplinary team including the surgeon, gastroenterologist, pathologist, and the patient who has a clear understanding of the nature of their disease is needed to optimize outcomes.
Core tip: This review summarizes the natural history of inflammatory bowel disease associated dysplasia and colorectal cancer. An up to date review of risk factors for inflammatory bowel disease associated colorectal cancer is included. Highlights include surgeon specific factors to aid in joint decision making with the patient regarding further management of their disease. These factors include the management options of continued appropriate endoscopic surveillance and the different disease specific surgical options. Finally, it summarizes the long-term surveillance program and the long-term prognosis following surgery for inflammatory bowel disease associated neoplasia.
- Citation: Althumairi AA, Lazarev MG, Gearhart SL. Inflammatory bowel disease associated neoplasia: A surgeon’s perspective. World J Gastroenterol 2016; 22(3): 961-973
- URL: https://www.wjgnet.com/1007-9327/full/v22/i3/961.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i3.961
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’s disease (CD), is a life-long immune-mediated chronic inflammatory disorder of the gastrointestinal tract. Both types of IBD are characterized by chronic inflammation with episodes of remission and exacerbation[1,2]. Clinical courses can vary from mild to severe and debilitating and often exert a major impact on an individual’s quality of life and consume a large share of health care resources[3]. In recent years, there has been an emphasis on intensive medical therapy and close disease monitoring with the use of imaging and biomarkers to reduce disease activity[4]. The greatest impact has been the development of biological therapy which has proven to have efficacy for both UC and CD with remission rates that approach 40% at one year[5,6]. With the use of newer medical therapies, surgical resection rates have been reported to be as low as 13% at one year for CD and 2% at one year for UC[7].
In 1952, Crohn and Rosenberg reported the association between IBD and colorectal cancer (CRC)[8]. The risk of CRC in patients with IBD is driven by the extent, duration, and severity of the colonic inflammation[9]. Currently, IBD associated CRC (IBD-CRC) accounts for 1%-2% of all cases of CRC, which accounts for 10%-15% of all deaths in IBD patients[10]. Previous studies have suggested an excess risk of CRC at a rate of 1% per year increase beginning after 8 years of disease[11]. However, more recent studies have shown that with better control of intestinal inflammation, this excess risk has declined[12]. In contrast to sporadic CRC, IBD-CRC may arise from dysplasia in flat mucosa which may be difficult to identify during endoscopic examination. Furthermore, since the peak incidence for the diagnosis of IBD is among young people, IBD-CRC frequently develops at a younger age compared to sporadic CRC (40-50 year vs 60 year)[13]. This review will include a discussion on the natural history of IBD-associated neoplasia (dysplasia and cancer), the special risk factors for CRC in IBD patients, screening and prevention methods, management options, long-term surveillance program, and the importance of the patients understanding of the complex nature of their disease.
As defined by the inflammatory bowel disease-dysplasia morphology study group in 1983, IBD-associated dysplasia is an unequivocal neoplastic change of the intestinal mucosa without invasion of the epithelium in the setting of IBD[14]. In contrast to the adenoma to carcinoma sequence seen in sporadic CRC patients, IBD patients develop carcinoma through an inflammation to carcinoma sequence[15,16]. IBD-associated neoplasia develops in areas of active or chronic inflammation[17,18]. Dysplasia may occur in any part of the colon, can be unifocal or multifocal, and cancer is often detected in the same area as the dysplasia[19,20].
IBD patients are at 2 to 5 times higher risk of CRC than the general population in the same age group[21]. Moreover, IBD-CRC has a higher rate of synchronous and metachronous tumors. Ali et al[22] used the national cancer registry in Ireland to compare patients who developed sporadic CRC and IBD-CRC over 11 years. Twenty-two thousand three hundred and thirty-five patients had sporadic CRC and 170 patients had IBD-CRC. Patients in IBD-CRC group were on average 7.7 years younger (P = 0.001), less likely to be smokers (P = 0.002), and less likely to have metastatic disease at the time of diagnosis (12% vs 22%, P < 0.001).
In 1983 Riddell et al[14] established the standard microscopic classification of dysplasia in the setting of IBD and divided them into four categories which are still in use today: negative for dysplasia, indefinite for dysplasia, low grade dysplasia (LGD) and high grade dysplasia (HGD). The definition of dysplasia in IBD has been modified as newer methods of detection have been developed. Historically, dysplasia has been classified into flat or raised based on the macroscopic endoscopic findings. Raised lesions were further classified as Dysplasia Associated Lesion or Mass or Adenoma Like Lesion[23]. However, these terms were often considered confusing. In 2015, an international consensus statement was published by the American Gastrointestinal Association and the American Society for Gastrointestinal Endoscopy which provided current guidelines for the classification of dysplasia in IBD (SCENIC)[24]. Currently, detection of dysplasia in IBD has increased with the use of high definition endoscopes[25]. Therefore, dysplasia should be first characterized as visible or invisible. Invisible dysplasia is only found on random biopsies. Visible dysplasia is then subdivided into polypoid and non-polypoid. Polypoid and non-polypoid lesions are considered endoscopically resectable or not resectable. For a lesion to be considered resectable, it must have the following characteristics: (1) identifiable distinct margins; (2) the lesion appears to be completely removed after resection on visible endoscopic inspection; (3) histological inspection of the lesion is consistent with complete removal; and (4) biopsy specimens taken from the mucosa immediately adjacent to the resection site are free of dysplasia on histological examination[24].
Dysplasia is present in 75%-90% of patients with IBD-CRC[26], however IBD-CRC can develop without prior history of dysplasia[27]. It was historically assumed that dysplasia progressed in a step-wise fashion: no dysplasia, indefinite dysplasia, LGD, HGD, and cancer. However, recent data suggests that sequential progression from inflammation to LGD, HGD, then CRC does not always occur[26,28], and it is also not clear whether dysplasia will progress or regress once developed[2,29]. Furthermore, the absence of dysplasia on follow up surveillance colonoscopy does not negate the risk of IBD-CRC[26].
During pathological evaluation of IBD-associated neoplasia, several problems may be encountered. Sampling error with under sampling of the colonic mucosa during the surveillance colonoscopy has been reported[30]. There is great variability in the interpretation of the grade of dysplasia even among expert gastrointestinal pathologist with particular difficulties in the differentiation between regenerative changes from indefinite or LGD, and between HGD and cancer[14,31,32]. Odze et al[31] assessed the intra-observer variation in diagnosing dysplasia. Thirty-eight UC cases with the diagnosis of dysplasia were evaluated by four expert gastrointestinal pathologists, the overall degree of agreement was fair (k = 0.40) among the four reviewing pathologists. The poorest level of agreement was in the indefinite (k = 0.18) and LGD (k = 0.36) categories, and it was fair to good in HGD (k = 0.54). Due to this great variability, the current recommendation is to confirm the diagnosis of any degree of dysplasia by two expert gastrointestinal pathologists.
Some small studies have shown an increase risk of IBD-CRC in patients diagnosed at a younger age[33,34]. However, a nationwide, long-term survey from Netherland included 251 patients with IBD-CRC, found that the median age at diagnosis of IBD was 40.1 years (IQR = 24-28), and the median age at diagnosis of IBD-CRC was 56.4 years (IQR = 44-65), they found that the mean time from IBD diagnosis to CRC was 12 years, and IBD diagnosis at an older age was a risk factor for the development of CRC earlier (HR for 10 years older 2.25; 95%CI: 1.29-2.63)[35].
The most important risk factor for IBD-CRC is the disease duration (Table 1). Most studies define the disease duration from the endoscopic and pathological diagnosis. However, the consensus on CRC screening and surveillance in inflammatory bowel disease published by the Crohn’s and Colitis Foundation of America Colon Cancer in IBD Study Group defined the duration of disease from the onset of symptoms not from the date of establishing the diagnosis[34]. Historically, the pooled cumulative incidence of IBD-CRC in a meta-analysis by Eaden et al[11] was 2% at 10 years, 8% at 20 years, and 18% after 30 years of disease. Others have suggested that the incidence is lower. Choi et al[36], in a study from St Mark’s Hospital analyzed their results on an endoscopic surveillance program for CRC in patients with long standing IBD. A total of 1375 UC patients were followed up for a median of 11 year per patient, the cumulative incidence of CRC was 0.7% at 10 years, 2.9% at 20 years, 6.7% at 30 years, 10.0% at 40 years, and 13.6% at 50 years of disease. Winther et al[37], in a population based study of 1160 patients with UC in Denmark followed for 19 years. This study demonstrated that the cumulative probability of developing CRC in this cohort was 0.4% at 10 years, 1.1% at 20 years, and 2.1% at 30 years. The authors suggested that this lower rate could be attributed to aggressive surgical intervention and the long-term maintenance use of 5-ASA medications.
| Risk Factors for IBD-CRC |
| Older age of onset of IBD |
| Duration of disease |
| Extent of disease |
| Severity of inflammation |
| Primary Sclerosing cholangitis |
| Family History of CRC |
With greater extent of the disease the risk of developing cancer increases[38]. Patients with pancolitis have increased risk of CRC compared to patients with limited colitis[39,40]. Söderlund et al[41] examined the impact of the extent of the disease on the risk of developing CRC. The relative risk (RR) of CRC was 5.6 for pancolitis, as compared to 1.7 for proctitis only. In a large cohort study from three centers in England and Sweden which included 823 patients, the RR of CRC in patients with pancolitis was 19 fold greater than the general population, as compared to a RR of 3.6 in patients with left-sided colitis[42]. Furthermore, Beaugerie et al[43] prospectively followed 2841 patients characterized with having long-standing extensive colitis (involving > 50% of the colon for > 10 years). In this cohort of patients, the incidence ratio for the development of CRC was 7.0 as compared to 1.1 ratio in patients without long-standing inflammation.
Severity of inflammation identified both on endoscopic evaluation and histologically is associated with increased risk of neoplastic changes. Rutter et al[38] examined the relationship between the endoscopic and histological severity of inflammation and risk of CRC. They found a greater risk of CRC in endoscopic (OR = 2.5, P < 0.001) and histological (OR = 5.1, P < 0.001) active inflammation. The increased risk of CRC associated with histological active inflammation remained significant in a multivariable analysis of other associated risk factors including duration and extent of disease, family history, and a history of primary sclerosing cholangitis (OR = 4.7, P < 0.001)[38].
Both UC and CD patients are at risk of developing primary sclerosing cholangitis (PSC). IBD patients who have PSC are at increased risk of developing neoplasia[44]. All patients presented with PSC who were not known to have IBD need to undergo screening colonoscopy with random biopsies of the mucosa looking for inflammatory changes[34]. Soetikno et al[45] in a meta-analysis that included 11 studies found that patients who have concomitant US and PSC have increased risk of IBD-associated neoplasia (OR = 4.7, 95%CI: 3.58-6.41). Kornfeld et al[46] found that the cumulative risk of CRC was higher in patients with IBD and PSC, 33% at 20 years, and 40% at 30 years.
A positive family history of CRC has been shown to increase the risk of developing IBD-CRC. Askling et al[47] conducted a population-based study of 19876 IBD patients and found that a positive family history of CRC was associated with more than 2-fold risk of CRC (adjusted RR = 2.5, 95%CI: 1.4-4.4). Patients with a first-degree relative diagnosed with CRC before 50 years of age had a higher RR of 9.2, (95%CI: 3.7-23) and the highest absolute risk (29%).
Early detection of neoplastic changes in IBD patients is crucial to decrease the morbidity and mortality associated with the development of CRC. Currently, this is best achieved by screening and surveillance colonoscopy during disease remission[48]. Newer advanced endoscopic techniques such as high-resolution endoscopy and chromoendoscopy have improved neoplasia detection. These newer techniques allow for more targeted biopsies of any abnormal appearing epithelium which is more easily visible with these new techniques.
Screening guidelines have been established by many societies. Recently, an international consensus statement was published to clarify best practices on surveillance and the management of dysplasia in IBD[24]. Historically, most gastroenterologists would take minimum of 32 random biopsies as well as targeted biopsies in areas where abnormal epithelium appeared. According to the SCENIC international consensus statement, high-definition and chromoendoscopy is superior to traditional white light endoscopy. If surveillance is performed with traditional white light endoscopy, chromoendoscopy with targeted biopsies should also be performed[24]. However, there are some concerns regarding the use of chromoendoscopy which could be a potential barrier to the initiation of these guidelines. These concerns include the need for advanced training in this technique and the development of quality metrics to assess performance after training. Furthermore, chromoendoscopy requires additional preparation and procedure time. Finally, there is an increased facility and provider cost with no procedure specific code for reimbursement[24].
With improvements in endoscopic imaging, more dysplasia will be visible. According to the SCENIC guidelines, continued surveillance colonoscopy is recommended rather than colectomy following complete removal of both polypoid and non-polypoid dysplastic lesions. It is expected that the threshold for colectomy will increase from both patients and physicians perspectives. Of note, in a survey to evaluate patient preferences for colectomy or surveillance in the presence of dysplasia, Siegel et al[49] found that 60% of the patients will refuse colectomy if they were told that the CRC risk is 20%. On average, patients would agree on colectomy if their risk of CRC was 73%.
High resolution endoscopy allows for the magnification of the camera lens to increase up to 150 fold compared to conventional endoscopy. Several complementary techniques have been developed along with high-resolution endoscopy which allows the endoscopist to obtain a virtual histology at the time of endoscopy. One of these techniques is confocal Laser Endoscopy (CLE). This technique provides real-time microscopic imaging and histological evaluation of the mucosa[43]. It illuminates tissue with low power laser and it uses either reflected white light or reflected fluorescent light, after injection of 10% fluorescein, to generate magnified images which allows visualization of cell structures at the organelle level[50-52].
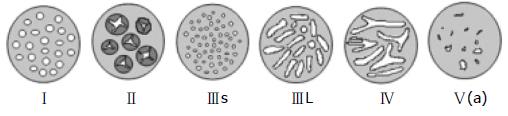
Chromoendoscopy is an enhanced imaging technique to detect mucosal abnormalities by using various dyes that are sprayed on the colonic mucosa by means of a catheter which is passed through the working channel of the endoscope. The frequently used dyes are indigo carmine and methylene blue[53-55]. This technique can be performed using a high resolution magnifying endoscope. The ability to differentiate between neoplastic and non-neoplastic lesions using chromoendoscopy has been well studied using the Kudo criteria (Table 2 and Figure 1)[56]. Use of the Kudo criteria has been shown to assist in the differentiation between neoplastic and non-neoplastic lesions with a 98% sensitivity and a 92% specificity. Subramanian et al[57] performed a meta-analysis of published studies to compare the use of chromoendoscopy against standard white light endoscopy in patients with IBD undergoing surveillance. In this meta-analysis, 6 studies were identified which included a total of 1277 patients. In this group of patients, there was noted to be a 7% difference in the detection of dysplasia that favored chromoendoscopy over standard white light endoscopy. The study concluded that 14 patients were needed to treat in order to identify one patient that would benefit from the use of chromoendoscopy. For this reason, the study recommended that any high risk patient should undergo surveillance with chromoendoscopy.
| Pit type | Description | Pit size (mm) | Associated histological findings |
| I | Round (normal) | 0.07 (0.02) | Non-neoplastic |
| II | Papillary/Stella | 0.09 (0.02) | Hyperplastic |
| IIIs | Round or tubular (small) | 0.03 (0.01) | Neoplastic |
| IIIL | Round or tubular (large) | 0.22 (0.09) | Neoplastic |
| IV | Gyrus-like | 0.93 (0.32) | Neoplastic |
| V(a) | Amorphous structure/irregular arrangement | NA | Invasive cancer |
The management of IBD-associated neoplasia is complex and is often based on the histological degree of dysplasia, whether the dysplasia is visible endoscopically or not, whether it is unifocal or multifocal, and on patient’s factors. The management decision requires a multidisciplinary team approach involving the surgeon, the gastroenterologist, the pathologist, and the patient. Furthermore, the patients’ understanding of the nature of their disease and their involvement in the management decision is crucial.
With the advanced endoscopic techniques, raised or polypoid lesions can be identified and managed endoscopically. If raised lesions are identified during endoscopic surveillance, endoscopic resection is performed with biopsy of the surrounding mucosa. When polyp removal occurs, the area should be tattooed for further reference. In the absence of dysplasia in the mucosa surrounding the polyp or elsewhere in the colon, endoscopic resection of the polyp is adequate. Vieth et al[58] followed 87 patients with UC and a sporadic adenoma for 53 mo following polypectomy. The risk of colitis-associated neoplasia developing in this group was 4.6%. Importantly, all new neoplastic changes developed distant to the original polypectomy. He concluded that the risk of continued neoplasia is low but that close follow up in mandated. A recent systematic review demonstrated that the risk of CRC after resection of a dysplastic polyp in IBD is low (5.3 cases/1000 years). However, patients with a history of a dysplastic polyp have a 10-fold greater risk of developing further dysplasia and should undergo close endoscopic follow up[59]. If a dysplastic polyp has evidence of dysplasia in the surrounding mucosa, is found in an area of active colitis, or is not resectable; colectomy is recommended due to high risk of associated CRC[60-62].
If dysplasia is identified in random biopsies during screening and surveillance endoscopies, the diagnosis must be confirmed by two expert gastrointestinal pathologists. If the presence of dysplasia is confirmed; then the histological degree (LGD or HGD) and whether it is unifocal or multifocal needs to be determined. There are controversies in the management of invisible LGD due to the reported variability in the risk of progression to HGD or cancer (0%-50%)[63,64]. The decision to undergo colectomy or surveillance is challenging and cases should be discussed in a multidisciplinary IBD clinic, and joint decision with the patient after a thorough discussion about the risk and benefits of surgery vs continued surveillance is made. Consideration should be given to the risk of progression to CRC, patient age, presence of other risk factors of CRC, and comorbidities. In the presence of repeat unifocal LGD on follow up endoscopy, or the presence of multifocal LGD, patient should be counseled about the risk of progression to cancer and colectomy should strongly be considered. If endoscopically invisible HGD is detected, the rate of presence of synchronous CRC range from 32% to 42% and therefore colectomy should be performed[63-65].
With the chronic inflammatory process, finding of colonic strictures is not uncommon. In CD, they are usually benign and commonly occur at the terminal ileum, ileocolic valve, and at the ileocolic anastomosis. It is also not uncommon for patients with CD to have chronic anal strictures that also require surveillance. In UC, stricture formation is believed to result from contraction and hypertrophy of the muscularis mucosa[66]. Regardless, if the strictures are symptomatic, in the setting of UC, endoscopy and biopsy should be performed to evaluate for neoplasia[53]. Strictures in the presence of UC are associated with 30-fold risk of CRC and in the presence of CD, a 3-fold increased risk of CRC[67]. To evaluate a stricture, it must be fully traversed by the endoscope and adequately biopsied[68]. If complete evaluation cannot be performed by endoscopy and biopsy, surgery should be strongly recommended[53].
If colon or rectal cancer is found during screening or surveillance colonoscopy, preoperative assessment of patient and staging are performed to decide the best surgical option. This includes a detailed history of symptoms, previous history of IBD-associated neoplasia, comorbidities, and family history of CRC. Physical examination including digital rectal exam to assess the sphincter function and tumor distance from the anal verge in case of rectal cancer. Imaging to assess cancer stage is performed to assess the extent of the tumor, nodal involvement, and presence of distant metastasis. Endorectal ultrasound or magnetic resonance imaging (MRI) are frequently used to assess for local tumor extension and nodal involvement in the case of rectal cancer[69,70]. Computerized tomography (CT) is performed to assess the presence of distant metastasis to decide which treatment plan is best for the patient[71]. Following the complete preoperative assessment and staging, management options, expected outcomes, and prognosis are discussed with the patient.
Surgical management varies by the diagnosis of UC or CD, the location of the neoplasia, and by the patient’s comorbidities (Table 3). All options follow the same oncological principles for removal of the neoplasia and associated lymph nodes. In order to offer the best surgical option, complete assessment of the patient’s medical condition is performed; this includes medical comorbidities, previous surgery, tumor stage, and sphincter function. The goal of surgical intervention in IBD-associated neoplasia is achieving oncological resection with preservation of the quality of life.
| Procedure type | Indications | Relative contraindications |
| Total proctocolectomy with end ileostomy | UC, CD refractory disease or neoplasia | None |
| Restorative proctocolectomy (RP) with Ileoanal pouch | UC | Fecal incontinence |
| Direct sphincter invasion by tumor | ||
| Stage IV CRC | ||
| Subtotal colectomy with ileorectal anastomosis | UC or CD with contraindication for RP | Rectal neoplasia |
| Active rectal inflammation | ||
| Segmental resection | UC or CD with Unifocal dysplasia or cancer and no active disease | Acute or chronic colonic inflammation |
| CRC with comorbidity and age > 65 yr | ||
| Diverting ostomy | UC or CD with Stage IV CRC with obstruction and/or severe comorbidity | None |
Surgical options in UC-associated neoplasia are broadly classified into: (1) total proctocolectomy with end ileostomy; (2) restorative proctocolectomy (RP)or total proctocolectomy with ileal pouch anal anastomosis; (3) subtotal colectomy with ileorectal anastomosis (IRA); (4) segmental resection; and (5) and palliative procedures such as diverting colostomy or ileostomy. Surgical options in CD are dependent upon the location of the cancer. In small bowel adenocarcinoma, radical resection of the diseased segment with adequate lymphadenectomy is recommended. However, in CRC, similarities between UC and CD exist and the utility of more extensive resections is being evaluated.
Historically, total proctocolectomy with mesenteric lymphadenectomy and mesorectal excision with an end ileostomy that removes all at risk colonic mucosa has been the procedure of choice for IBD-associated neoplasia. This procedure is considered the least likely to compromise oncological resection, avoids the issues of chemoradiation and poor pouch function, and is a single stage operation. However, up to 25% of patients may require stoma revision and 50% of patients may develop stoma related complications including leakage and skin irritation[72]. Furthermore, one must also consider the psychological impact of permanent stoma and that most patients would prefer to maintain their intestinal continuity. For these reasons, restorative procedures for patient with colitis-associated neoplasia are the preferred procedure of choice.
As experience has increased and complications decreased with the RP, utilization of this procedure in patients with IBD related dysplasia and CRC has become more accepted. RP preserves the intestinal continuity and this option can be offered to patients with colitis associated neoplasia[73]. Technical recommendations and the justifications regarding the necessity of each technique are outlined below. First, high ligation of the major colonic vessels as well as a total mesorectal excision should be performed for all patients with dysplasia or cancer so that an appropriate lymphadenectomy can be performed[74]. In the treatment of CRC, at least 12 peritumor lymph nodes should be removed at the time of surgery to allow for adequate tumor staging[75]. Second, mucosectomy with removal of all at risk mucosa can be selectively performed based upon oncological principles of at least a 1-2 cm resection margin. Historically, preservation of the 2-3 cm of anal mucosa was considered contraindicated in patients with UC-associated dysplasia or CRC. However, more recently, Al-Sukhni et al[76] demonstrated in a retrospective review of 81 patients that performing a stapled anastomosis did not increase the risk of the development of adenocarcinoma in the retained rectum, in fact, there were 2 patients with adenocarcinoma in the anal area following RP and both of these patients had undergone a mucosectomy. Third, patients who require pelvic radiation or who have stage IV disease may be better served with proctocolectomy and end ileostomy. Snelgrove et al[74] demonstrated 100% pouch failure rates in patients with IBD and rectal cancer who had an RP and underwent postoperative radiation for local control. Furthermore, Wu et al[77] demonstrated an increased risk of long-term pouchitis leading to pouch failure in patients who underwent neoadjuvant radiation prior to RP. The effects of ongoing chemotherapy on pouch function has not been well studied, however, there is a concern for increased bowel frequency. Moreover, inflammation of the retained rectal mucosa (cuffitis) can occur following surgery in about 15% of patients. Only a small number of patients become symptomatic with symptoms of increased bowel movement, abdominal pain, tenesmus, and rectal bleeding[78,79]. In a series of 61 patients who underwent RP for UC, Shen et al[80] found that only 4 (6.5%) patients who developed cuffitis had rectal bleeding. All patients responded to 2-wk medical treatment with topical mesalamine or hydrocortisone without the need for surgical intervention[80]. Patients with cuffitis will have an inflamed rectal cuff seen on endoscopy with ulceration and neutrophil infiltration in biopsy, the pouch mucosa is typically normal with no signs of inflammation[80].
Following these guidelines, patients can expect a similar survival to patients with sporadic non-IBD associated CRC and a similar quality of life to those patients who underwent RP for refractory disease[68].
Included in careful consideration of an RP is a discussion with the patient and the patient’s family regarding the known associated complications with the procedure and their likelihood of occurrence. It is well-known that pelvic sepsis and anastomotic failure have been reported to occur in up to 5%-10% of all patients undergoing an RP and this risk may increase 2 fold in patients who receive radiation[81,82]. Associated with these complications are long-term incontinence and difficulty with evacuation due to anastomotic strictures. There is also a risk of postoperative sexual dysfunction with a reported incidence of 3.4%. Long-term complications include pouchitis which can occur in 19%-26% of patients[81,82] (Table 4).
In patients with unifocal neoplasia, with medical comorbidities, with stage IV CRC, or for patients who find the risks of a RP unacceptable, alternative surgical options may be considered[68,72]. The most common alternative is the total colectomy with ileorectalanastomosis (IRA). The advantage of this procedure is that it is a less extensive procedure which avoids a pelvic dissection and its’ associated risks of neural injury or subsequent sexual and bladder dysfunction. Patients with minimal rectal involvement by their disease and no rectal dysplasia can be considered for IRA. As with the RP, it is important to ascertain anal sphincter function prior to surgery to determine if there would be good continence of stool. However, the IRA does carry a risk of anastomotic leak with sepsis with reported rates up to 9%[72]. da Luz Moreira et al[83] reported long term outcomes on 86 patients who underwent IRA at the Cleveland Clinic. The majority of these patients had a diagnosis of UC and there was a median follow up of 11 years. Rectal dysplasia and cancer developed in 15 (17%) and 7 (8%) of 86 patients, respectively. The cumulative probability of developing dysplasia or cancer in the rectum at 5, 10, 15, and 20 years was 7%, 9%, 20% and 25% for dysplasia, respectively and 0%, 2%, 5%, and 14% for rectal cancer, respectively. There were 22 patients available for follow up telephone surveys that were matched with 66 RP patients. The patients with an IRA reported significant fewer bowel movements per day and less night-time seepage, but often greater urgency. Although, the quality of life was similar among the IRA and RP patients, the IRA patients reported more dietary restrictions and work restrictions.
The use of segmental resection in UC-associated neoplasia has been restricted secondary to the concern for the development of secondary malignancies in other areas of the colon. Choi et al[36] reported that IBD-CRC is accompanied by a synchronous or metachronous CRC or spatially distinct dysplasia in at least 30% of patients. However, we have shown using the Surveillance, Epidemiology, and End Results Medicare database, that patients with IBD-CRC over the age of 65 had similar survival independent of the surgical procedure performed (segmental vs total proctocolectomy)[84]. We concluded that consideration could be given if quality of life mandates it, to a segmental resection for IBD-CRC in patients over the age of 65. Other palliative procedures such as diverting colostomy or ileostomy can be considered in very high surgical risk patients who are not fit to undergo colectomy, or in patients who present with a very advanced stage disease to relive their symptoms.
Intestinal neoplasia in CD is rare. von Roon et al[85] determined the incidence of small bowel adenocarcinoma was 1.55 per 100000 patients and the mean duration of disease before the onset of carcinoma was 9 years. However, the incidence of CRC in CD is higher. Lovasz et al[86] reported the probability of developing CRC in patients with CD to be 5.5% at 5 years and 7.5% at 10 years. Historically, segmental resection is generally indicated in CD patients whether the neoplasia is found in the small or large intestine[68,72]. In the small intestine, neoplasia frequently occurs in the ileocecal area, and it is commonly undiagnosed at the time of surgical exploration[87]. There is more likely to be neoplasia in areas in the colon and small bowel where long-standing stenosis or strictures are found[86]. This includes the perianal area where frequently strictures are found. Unfortunately, since the diagnosis of adenocarcinoma is not known at the time of resection, an appropriate oncological resection is not performed as the surgeon’s goal is to preserve bowel length. If cancer is suspected due to risk factors or preoperative biopsies indicating any neoplasia, performance of an adequate lymphadenectomy with removal of wide margins is necessary. Furthermore, recent retrospective reviews and histological analysis have indicated that neoplasia in Crohn’s colitis behaves similar to UC[88]. This determination is based upon the findings that the incidence of dysplasia distant to the CRC in both UC and CD is similar (39% vs 37.5% retrospectively). Therefore, if the diagnosis of cancer is suspected at the time of surgery, consideration for a more extensive resection should be given.
RP for colitis associated neoplasia, preserves the intestinal continuity and eliminates the diseased colon at risk of neoplasia. However, this procedure does not abolish the risk of IBD-associated neoplasia at the anal transitional zone and pouch body. In a study that used the Dutch Pathology Registry, 1200 patients were identified with IBD and had undergone a RP with a median follow up of 6.5 years. In these 1200 patients, 25 (1.83%) developed pouch neoplasia, with 16 adenocarcinomas. The cumulative incidences at 5, 10, 15, and 20 years for overall pouch dysplasia were 0.3%, 0.5%, 1.6%, and 3.7%, respectively, and for pouch carcinoma were 0.6%, 1.4%, 2.1%, and 3.3%[89].These results are similar to what has been reported from the Cleveland Clinic[90]. In their review of 3202 patients, the cumulative incidences for pouch neoplasia at 5, 10, 15, and 20 years were 0.9%, 1.3%, 1.9%, and 4.2%. In their series, only 11 (0.36%) patients developed adenocarcinoma of the anal canal or pouch. The only independent risk factor for pouch neoplasia was a history of dysplasia or CRC prior to RP.
To date there are no consensus on whether and when to perform surveillance pouchoscopy. Liu et al[91] preformed a review to determine the prevalence of pouch neoplasia, its risk factors, and to establish guidelines for surveillance. Seventy-seven cases of pouch dysplasia were included in this review that have been reported in the literature; 6 with DNA aneuploidy of the pouch, 7 with both DNA aneuploidy and dysplasia, 49 with LGD, 8 with HGD, and 7 with dysplasia of unspecified grade. The pooled prevalence of dysplasia was 1.9% at the anal transitional zone and 4.2% at the pouch body. In the same review, 50 cases of pouch cancer have been reported, of which 42 were adenocarcinoma, 2 lymphoma, 3 squamous cell cancer, and 3 non-specified cancer. In regards to the location of pouch adenocarcinoma, 27 (64%) had cancer of the anal transitional zone, 8 (19%) in the pouch body, 2 (5%) in the anal transitional zone and pouch body, 1 (2%) in the afferent limb and proximal pouch, and an unspecified location in 4 patients (10%). Risk factors for pouch neoplasia included in their review were: history of preoperative or intraoperative diagnosis of UC-associated dysplasia or cancer, the presence of type C mucosa of the pouch, concurrent PSC, a family history of colon cancer, long duration of UC, and history of chronic pouchitis. They concluded that high risk patient for pouch neoplasia should undergo pouchoscopy with biopsy every 1 to 3 years (Table 5).
| History of preoperative or intraoperative diagnosis of UC-associated dysplasia or cancer |
| Presence of type C mucosa of the pouch |
| Concurrent PSC |
| Family history of colon cancer |
| Long duration of UC |
| History of chronic pouchitis |
Similar to the surveillance of colonic dysplasia, there is no consensus on the management of pouch dysplasia. The finding of HGD in the pouch is concerning for the presence of synchronous cancer and is considered an indication for pouch excision[92]. The management of LGD in the pouch is controversial, the current recommendations consist of surgical intervention for multifocal or persistent LGD, and endoscopic surveillance for unifocal LGD[91].
Following subtotal or segmental resection for IBD-neoplasia, there is risk of local and distant recurrence, which is highest during the first 3 to 5 years following surgery[93]. A strict surveillance program should be followed to detect recurrent disease at an early stage in order to provide optimum patient care and improve the oncological outcomes. The interval of follow up surveillance depends on any new neoplastic endoscopic findings. In general, history and physical examination is performed every 3 to 6 mo in the first 3 years following surgery, every 6 mo in the 4th and 5th year, and annually afterward. Serum carcinoembryonic antigen (CEA) is obtained every 3 mo in the first 3 years, then every 6 mo to one year up to the 5th year postoperatively. CT of chest/abdomen/pelvis is performed annually for the first 5 years in patients with high risk for recurrence. Positron emission tomography (PET) and/or MRI may be performed in patients with rising CEA and equivocal CT[93-95].
In both sporadic and IBD associated CRC, the prognosis and survival depend on the pathological stage at diagnosis. Some studies have shown that the 5-year survival rates are similar in sporadic and IBD-CRC, while others have shown that IBD-CRC is associated with lower 5-year survival rate[95-98] (Table 6). A study from Ireland used the National Cancer Registry data from 1994 to 2005 to compare the survival between IBD-CRC and sporadic-CRC, the analysis included 170 patients with IBD-CRC and 22155 patients with sporadic-CRC. They found that patients with IBD-CRC had 3 years longer median survival time (P < 0.001), however, after adjusting for comorbidities and tumor stage, the apparent advantage in survival in patients with IBD-CRC was deemed non-significant (P = 0.097)[22]. Another nationwide study from Denmark that used the Danish Medical Registry data from 1977 to 2009 included 653 patients with UC-CRC, 238 patients with CD-CRC, and 107024 patients with sporadic-CRC. The 5-year adjusted mortality rate ratio was 1.14 (95%CI: 1.03-1.27) for patients with UC-CRC, and 1.26 (95%CI: 1.07-1.49) for patients with CD-CRC compared with patients with sporadic-CRC[97]. In 2006, a study from Mayo Clinic compared the survival between 290 patients with IBD-CRC and 290 patients with sporadic-CRC who were evaluated between 1976 and 1996. The 5-year survival rates were 54% in IBD-CRC and 53% in sporadic-CRC (P = 0.94)[99]. A recent study by Adams et al[98] used Colon Cancer Family Registry data between 1997 and 2009 to compare the survival between IBD-CRC and sporadic-CRC. Patients with IBD-CRC had worse prognosis with adjusted hazard ratio of (aHR = 1.36, 95%CI: 1.05-1.76). With the adherence to the surveillance programs and the use of newer endoscopic methods of screening, IBD-associated neoplasia is most likely to be detected in an earlier stage which may results in better prognosis.
Clearly, the management of IBD-associated neoplasia is complex and requires collaborative effort of surgeons, gastroenterologist, pathologists, and the patients themselves to optimize outcomes. In order for patients to be actively involved in the management of their disease, they need to gain understanding about the nature and the complexity of IBD-associated neoplasia so that appropriate informed decisions can be made regarding treatment plans. One important example how we can improve understanding is to share with patients what the expected final pathological outcome will be once their colon is removed for IBD-associated neoplasia. We have previously shown that the final pathological diagnosis on the specimen of colon that is surgically removed poorly correlates with the preoperative endoscopic pathological diagnosis[100].In this study of 81 patients with preoperative biopsy confirmed IBD-associated neoplasia, overall agreement between preoperative biopsies and final whole-specimen pathology was 41% and no dysplasia was identified in 16 (20%) patients. The poorest agreement was seen among patients with polypoid LGD. It was noted that a repeat endoscopic exam at our institution was associated more commonly with the findings of neoplasia[100]. These diagnostic issues must be effectively explained to the patients by their health care providers, along with addressing other concerns such as social, psychological, and financial concerns.
IBD- associated neoplasia is a very complex disease and is very challenging for both patients and surgeons. Understanding of the nature of the disease and its progression is crucial to provide the optimum oncological care. Adherence with the screening and surveillance programs along with the use of new endoscopic methods will help in the identification of the neoplastic changes in early stages. Surgical options should follow standard oncological principles regardless of the procedure type. Effort in patients’ education about the disease nature and the need of continued surveillance is vital as it will help in engaging them in management decision making.
P- Reviewer: Day AS, Koch TR S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011;4:53-61. [PubMed] |
| 2. | Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 215] [Cited by in RCA: 224] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 3. | Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 1860] [Article Influence: 186.0] [Reference Citation Analysis (1)] |
| 4. | Nielsen OH. New strategies for treatment of inflammatory bowel disease. Front Med (Lausanne). 2014;1:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Rutgeerts P, Van Assche G, Sandborn WJ, Wolf DC, Geboes K, Colombel JF, Reinisch W, Kumar A, Lazar A, Camez A. Adalimumab induces and maintains mucosal healing in patients with Crohn’s disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102-1111.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 451] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 6. | D’haens G, Van Deventer S, Van Hogezand R, Chalmers D, Kothe C, Baert F, Braakman T, Schaible T, Geboes K, Rutgeerts P. Endoscopic and histological healing with infliximab anti-tumor necrosis factor antibodies in Crohn’s disease: A European multicenter trial. Gastroenterology. 1999;116:1029-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 512] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 7. | Niewiadomski O, Studd C, Hair C, Wilson J, Ding NS, Heerasing N, Ting A, McNeill J, Knight R, Santamaria J. Prospective population-based cohort of inflammatory bowel disease in the biologics era: Disease course and predictors of severity. J Gastroenterol Hepatol. 2015;30:1346-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Crohn BB, Rosenberg H. The sigmoidoscopic picture of chronic ulcerative colitis. Am J Med Sci. 1925;170:220-228. |
| 9. | Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372:1441-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 418] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 10. | Vagefi PA, Longo WE. Colorectal cancer in patients with inflammatory bowel disease. Clin Colorectal Cancer. 2005;4:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 2075] [Article Influence: 86.5] [Reference Citation Analysis (1)] |
| 12. | Jess T, Simonsen J, Jørgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375-381.e1; quiz e13-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 384] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 13. | Munkholm P, Loftus EV, Reinacher-Schick A, Kornbluth A, Mittmann U, Esendal B. Prevention of colorectal cancer in inflammatory bowel disease: value of screening and 5-aminosalicylates. Digestion. 2006;73:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, Ahren C, Correa P, Hamilton SR, Morson BC. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983;14:931-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1348] [Cited by in RCA: 1213] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 15. | Sharan R, Schoen RE. Cancer in inflammatory bowel disease. An evidence-based analysis and guide for physicians and patients. Gastroenterol Clin North Am. 2002;31:237-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Ullman TA. Dysplasia and colorectal cancer in Crohn’s disease. J Clin Gastroenterol. 2003;36:S75-S78; discussion S94-S96. [PubMed] |
| 17. | Goldman H. Significance and detection of dysplasia in chronic colitis. Cancer. 1996;78:2261-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Riddell RH. Premalignant and early malignant lesions in the gastrointestinal tract: definitions, terminology, and problems. Am J Gastroenterol. 1996;91:864-872. [PubMed] |
| 19. | Ullman T, Croog V, Harpaz N, Sachar D, Itzkowitz S. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology. 2003;125:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 246] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 20. | Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 311] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 21. | Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 658] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 22. | Ali RA, Dooley C, Comber H, Newell J, Egan LJ. Clinical features, treatment, and survival of patients with colorectal cancer with or without inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:584-9.e1-584-9.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | DeRoche TC, Xiao SY, Liu X. Histological evaluation in ulcerative colitis. Gastroenterol Rep (Oxf). 2014;2:178-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 145] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 24. | Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015;81:489-501.e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 270] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 25. | Iacucci M, Uraoka T, Fort Gasia M, Yahagi N. Novel diagnostic and therapeutic techniques for surveillance of dysplasia in patients with inflammatory bowel disease. Can J Gastroenterol Hepatol. 2014;28:361-370. [PubMed] |
| 26. | Farraye FA, Odze RD, Eaden J, Itzkowitz SH, McCabe RP, Dassopoulos T, Lewis JD, Ullman TA, James T, McLeod R. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 379] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 27. | Woolrich AJ, DaSilva MD, Korelitz BI. Surveillance in the routine management of ulcerative colitis: the predictive value of low-grade dysplasia. Gastroenterology. 1992;103:431-438. [PubMed] |
| 28. | Itzkowitz SH. Molecular biology of dysplasia and cancer in inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:553-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 29. | Harpaz N, Talbot IC. Colorectal cancer in idiopathic inflammatory bowel disease. Semin Diagn Pathol. 1996;13:339-357. [PubMed] |
| 30. | Rubin CE, Haggitt RC, Burmer GC, Brentnall TA, Stevens AC, Levine DS, Dean PJ, Kimmey M, Perera DR, Rabinovitch PS. DNA aneuploidy in colonic biopsies predicts future development of dysplasia in ulcerative colitis. Gastroenterology. 1992;103:1611-1620. [PubMed] |
| 31. | Odze RD, Goldblum J, Noffsinger A, Alsaigh N, Rybicki LA, Fogt F. Interobserver variability in the diagnosis of ulcerative colitis-associated dysplasia by telepathology. Mod Pathol. 2002;15:379-386. [PubMed] |
| 32. | Eaden J, Abrams K, McKay H, Denley H, Mayberry J. Inter-observer variation between general and specialist gastrointestinal pathologists when grading dysplasia in ulcerative colitis. J Pathol. 2001;194:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 158] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Basseri RJ, Basseri B, Ippoliti A, Vasiliauskas EA, Melmed GY, Targan SR, Papadakis KA. The efficacy of colorectal cancer surveillance in Crohn’s colitis. Gastroenterology. 2010;138:S443. [DOI] [Full Text] |
| 34. | Itzkowitz SH, Present DH. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:314-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 395] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 35. | Baars JE, Kuipers EJ, van Haastert M, Nicolaï JJ, Poen AC, van der Woude CJ. Age at diagnosis of inflammatory bowel disease influences early development of colorectal cancer in inflammatory bowel disease patients: a nationwide, long-term survey. J Gastroenterol. 2012;47:1308-1322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 36. | Choi CH, Rutter MD, Askari A, Lee GH, Warusavitarne J, Moorghen M, Thomas-Gibson S, Saunders BP, Graham TA, Hart AL. Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview. Am J Gastroenterol. 2015;110:1022-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 37. | Winther KV, Jess T, Langholz E, Munkholm P, Binder V. Long-term risk of cancer in ulcerative colitis: a population-based cohort study from Copenhagen County. Clin Gastroenterol Hepatol. 2004;2:1088-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 209] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 38. | Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, Williams C, Price A, Talbot I, Forbes A. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 895] [Cited by in RCA: 882] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 39. | Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357-359. [PubMed] |
| 40. | Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, Bodian C, Ullman T. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099-1105; quiz 1340-1341. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 570] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 41. | Söderlund S, Brandt L, Lapidus A, Karlén P, Broström O, Löfberg R, Ekbom A, Askling J. Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology. 2009;136:1561-1567; quiz 1818-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 42. | Gyde SN, Prior P, Allan RN, Stevens A, Jewell DP, Truelove SC, Lofberg R, Brostrom O, Hellers G. Colorectal cancer in ulcerative colitis: a cohort study of primary referrals from three centres. Gut. 1988;29:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 324] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 43. | Beaugerie L, Svrcek M, Seksik P, Bouvier AM, Simon T, Allez M, Brixi H, Gornet JM, Altwegg R, Beau P. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology. 2013;145:166-175.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 44. | Jayaram H, Satsangi J, Chapman RW. Increased colorectal neoplasia in chronic ulcerative colitis complicated by primary sclerosing cholangitis: fact or fiction? Gut. 2001;48:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002;56:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 383] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 46. | Kornfeld D, Ekbom A, Ihre T. Is there an excess risk for colorectal cancer in patients with ulcerative colitis and concomitant primary sclerosing cholangitis? A population based study. Gut. 1997;41:522-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 160] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Askling J, Dickman PW, Karlén P, Broström O, Lapidus A, Löfberg R, Ekbom A. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120:1356-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 283] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 48. | Guagnozzi D, Lucendo AJ. Colorectal cancer surveillance in patients with inflammatory bowel disease: What is new? World J Gastrointest Endosc. 2012;4:108-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Siegel CA, Schwartz LM, Woloshin S, Cole EB, Rubin DT, Vay T, Baars J, Sands BE. When should ulcerative colitis patients undergo colectomy for dysplasia? Mismatch between patient preferences and physician recommendations. Inflamm Bowel Dis. 2010;16:1658-1662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Kantsevoy SV, Adler DG, Conway JD, Diehl DL, Farraye FA, Kaul V, Kethu SR, Kwon RS, Mamula P, Rodriguez SA. Confocal laser endomicroscopy. Gastrointest Endosc. 2009;70:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 51. | Yoshida S, Tanaka S, Hirata M, Mouri R, Kaneko I, Oka S, Yoshihara M, Chayama K. Optical biopsy of GI lesions by reflectance-type laser-scanning confocal microscopy. Gastrointest Endosc. 2007;66:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Kiesslich R, Neurath MF. Endomicroscopy is born--do we still need the pathologist? Gastrointest Endosc. 2007;66:150-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Leighton JA, Shen B, Baron TH, Adler DG, Davila R, Egan JV, Faigel DO, Gan SI, Hirota WK, Lichtenstein D. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc. 2006;63:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 161] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 54. | Ullman TA. Should chromoendoscopy be the standard of care in ulcerative colitis dysplasia surveillance? Gastroenterol Hepatol (N Y). 2010;6:616-620. [PubMed] |
| 55. | Wong Kee Song LM, Adler DG, Chand B, Conway JD, Croffie JM, Disario JA, Mishkin DS, Shah RJ, Somogyi L, Tierney WM. Chromoendoscopy. Gastrointest Endosc. 2007;66:639-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Hurlstone DP, Cross SS, Adam I, Shorthouse AJ, Brown S, Sanders DS, Lobo AJ. Efficacy of high magnification chromoscopic colonoscopy for the diagnosis of neoplasia in flat and depressed lesions of the colorectum: a prospective analysis. Gut. 2004;53:284-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 104] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 57. | Subramanian V, Mannath J, Ragunath K, Hawkey CJ. Meta-analysis: the diagnostic yield of chromoendoscopy for detecting dysplasia in patients with colonic inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:304-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 58. | Vieth M, Behrens H, Stolte M. Sporadic adenoma in ulcerative colitis: endoscopic resection is an adequate treatment. Gut. 2006;55:1151-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Wanders LK, Dekker E, Pullens B, Bassett P, Travis SP, East JE. Cancer risk after resection of polypoid dysplasia in patients with longstanding ulcerative colitis: a meta-analysis. Clin Gastroenterol Hepatol. 2014;12:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 60. | van den Broek FJ, Fockens P, van Eeden S, Stokkers PC, Ponsioen CY, Reitsma JB, Dekker E. Narrow-band imaging versus high-definition endoscopy for the diagnosis of neoplasia in ulcerative colitis. Endoscopy. 2011;43:108-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 61. | Rutter MD, Saunders BP, Wilkinson KH, Kamm MA, Williams CB, Forbes A. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointest Endosc. 2004;60:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 215] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 62. | Rutter MD. Importance of nonpolypoid (flat and depressed) colorectal neoplasms in screening for CRC in patients with IBD. Gastrointest Endosc Clin N Am. 2014;24:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 63. | Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology. 1994;107:934-944. [PubMed] |
| 64. | Jess T, Loftus EV, Velayos FS, Harmsen WS, Zinsmeister AR, Smyrk TC, Tremaine WJ, Melton LJ, Munkholm P, Sandborn WJ. Incidence and prognosis of colorectal dysplasia in inflammatory bowel disease: a population-based study from Olmsted County, Minnesota. Inflamm Bowel Dis. 2006;12:669-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 65. | Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet. 1994;343:71-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 362] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 66. | Goulston SJ, McGovern VJ. The nature of benign strictures in ulcerative colitis. N Engl J Med. 1969;281:290-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 46] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Greenstein AJ. Cancer in inflammatory bowel disease. Mt Sinai J Med. 2000;67:227-240. [PubMed] |
| 68. | Coviello LC, Stein SL. Surgical management of nonpolypoid colorectal lesions and strictures in colonic inflammatory bowel disease. Gastrointest Endosc Clin N Am. 2014;24:447-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 69. | Akbari RP, Wong WD. Endorectal ultrasound and the preoperative staging of rectal cancer. Scand J Surg. 2003;92:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Saunders TH, Mendes Ribeiro HK, Gleeson FV. New techniques for imaging colorectal cancer: the use of MRI, PET and radioimmunoscintigraphy for primary staging and follow-up. Br Med Bull. 2002;64:81-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 71. | Sosna J, Morrin MM, Kruskal JB, Farrell RJ, Nasser I, Raptopoulos V. Colorectal neoplasms: role of intravenous contrast-enhanced CT colonography. Radiology. 2003;228:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 72. | Stucchi AF, Aarons CB, Becker JM. Surgical approaches to cancer in patients who have inflammatory bowel disease. Gastroenterol Clin North Am. 2006;35:641-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Panis Y. Is there a place for ileal pouch-anal anastomosis in patients with Crohn’s colitis? Neth J Med. 1998;53:S47-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 74. | Snelgrove R, Brown CJ, O’Connor BI, Huang H, Victor JC, Gryfe R, MacRae H, Cohen Z, McLeod RS. Proctocolectomy for colorectal cancer--is the ileal pouch anal anastomosis a safe alternative to permanent ileostomy? Int J Colorectal Dis. 2014;29:1485-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 75. | Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, Hammond ME, Henson DE, Hutter RV, Nagle RB. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979-994. [PubMed] |
| 76. | Al-Sukhni W, McLeod RS, MacRae H, O’Connor B, Huang H, Cohen Z. Oncologic outcome in patients with ulcerative colitis associated with dyplasia or cancer who underwent stapled or handsewn ileal pouch-anal anastomosis. Dis Colon Rectum. 2010;53:1495-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 77. | Wu XR, Kiran RP, Remzi FH, Katz S, Mukewar S, Shen B. Preoperative pelvic radiation increases the risk for ileal pouch failure in patients with colitis-associated colorectal cancer. J Crohns Colitis. 2013;7:e419-e426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 78. | Shen B, Lashner BA, Bennett AE, Remzi FH, Brzezinski A, Achkar JP, Bast J, Bambrick ML, Fazio VW. Treatment of rectal cuff inflammation (cuffitis) in patients with ulcerative colitis following restorative proctocolectomy and ileal pouch-anal anastomosis. Am J Gastroenterol. 2004;99:1527-1531. [PubMed] |
| 79. | Thompson-Fawcett MW, Mortensen NJ, Warren BF. “Cuffitis” and inflammatory changes in the columnar cuff, anal transitional zone, and ileal reservoir after stapled pouch-anal anastomosis. Dis Colon Rectum. 1999;42:348-355. [PubMed] |
| 80. | Shen B, Achkar JP, Lashner BA, Ormsby AH, Brzezinski A, Soffer EE, Remzi FH, Bevins CL, Fazio VW. Irritable pouch syndrome: a new category of diagnosis for symptomatic patients with ileal pouch-anal anastomosis. Am J Gastroenterol. 2002;97:972-977. [PubMed] |
| 81. | Hueting WE, Buskens E, van der Tweel I, Gooszen HG, van Laarhoven CJ. Results and complications after ileal pouch anal anastomosis: a meta-analysis of 43 observational studies comprising 9,317 patients. Dig Surg. 2005;22:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 223] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 82. | de Zeeuw S, Ahmed Ali U, Donders RA, Hueting WE, Keus F, van Laarhoven CJ. Update of complications and functional outcome of the ileo-pouch anal anastomosis: overview of evidence and meta-analysis of 96 observational studies. Int J Colorectal Dis. 2012;27:843-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 83. | da Luz Moreira A, Kiran RP, Lavery I. Clinical outcomes of ileorectal anastomosis for ulcerative colitis. Br J Surg. 2010;97:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 84. | Gearhart SL, Nathan H, Pawlik TM, Wick E, Efron J, Shore AD. Outcomes from IBD-associated and non-IBD-associated colorectal cancer: a Surveillance Epidemiology and End Results Medicare study. Dis Colon Rectum. 2012;55:270-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 85. | von Roon AC, Reese G, Teare J, Constantinides V, Darzi AW, Tekkis PP. The risk of cancer in patients with Crohn’s disease. Dis Colon Rectum. 2007;50:839-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 86. | Lovasz BD, Lakatos L, Golovics PA, David G, Pandur T, Erdelyi Z, Balogh M, Szita I, Molnar C, Komaromi E. Risk of colorectal cancer in Crohn’s disease patients with colonic involvement and stenosing disease in a population-based cohort from Hungary. J Gastrointestin Liver Dis. 2013;22:265-268. [PubMed] |
| 87. | Michelassi F, Testa G, Pomidor WJ, Lashner BA, Block GE. Adenocarcinoma complicating Crohn’s disease. Dis Colon Rectum. 1993;36:654-661. [PubMed] |
| 88. | Svrcek M, Cosnes J, Beaugerie L, Parc R, Bennis M, Tiret E, Fléjou JF. Colorectal neoplasia in Crohn’s colitis: a retrospective comparative study with ulcerative colitis. Histopathology. 2007;50:574-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 89. | Derikx LA, Kievit W, Drenth JP, de Jong DJ, Ponsioen CY, Oldenburg B, van der Meulen-de Jong AE, Dijkstra G, Grubben MJ, van Laarhoven CJ. Prior colorectal neoplasia is associated with increased risk of ileoanal pouch neoplasia in patients with inflammatory bowel disease. Gastroenterology. 2014;146:119-128.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 90. | Kariv R, Remzi FH, Lian L, Bennett AE, Kiran RP, Kariv Y, Fazio VW, Lavery IC, Shen B. Preoperative colorectal neoplasia increases risk for pouch neoplasia in patients with restorative proctocolectomy. Gastroenterology. 2010;139:806-812, 812.e1-e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 91. | Liu ZX, Kiran RP, Bennett AE, Ni RZ, Shen B. Diagnosis and management of dysplasia and cancer of the ileal pouch in patients with underlying inflammatory bowel disease. Cancer. 2011;117:3081-3092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Heuschen UA, Heuschen G, Autschbach F, Allemeyer EH, Herfarth C. Adenocarcinoma in the ileal pouch: late risk of cancer after restorative proctocolectomy. Int J Colorectal Dis. 2001;16:126-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Schwartz RW, McKenzie S. Update on postoperative colorectal cancer surveillance. Curr Surg. 2005;62:491-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 94. | Pfister DG, Benson AB, Somerfield MR. Clinical practice. Surveillance strategies after curative treatment of colorectal cancer. N Engl J Med. 2004;350:2375-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 95. | Anthony T. Colorectal cancer follow-up in 2005. Surg Oncol Clin N Am. 2006;15:175-193, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 96. | Solomon MJ, Schnitzler M. Cancer and inflammatory bowel disease: bias, epidemiology, surveillance, and treatment. World J Surg. 1998;22:352-358. [PubMed] |
| 97. | Ording AG, Horváth-Puhó E, Erichsen R, Long MD, Baron JA, Lash TL, Sørensen HT. Five-year mortality in colorectal cancer patients with ulcerative colitis or Crohn’s disease: a nationwide population-based cohort study. Inflamm Bowel Dis. 2013;19:800-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 98. | Adams SV, Ahnen DJ, Baron JA, Campbell PT, Gallinger S, Grady WM, LeMarchand L, Lindor NM, Potter JD, Newcomb PA. Survival after inflammatory bowel disease-associated colorectal cancer in the Colon Cancer Family Registry. World J Gastroenterol. 2013;19:3241-3248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Delaunoit T, Limburg PJ, Goldberg RM, Lymp JF, Loftus EV. Colorectal cancer prognosis among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2006;4:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 100. | Althumairi AA, Monn MF, Derck JE, Wick EC, Efron JE, Lazarev MG, Montgomery EA, Geahart SL. Poor Agreement between Preoperative Biopsies and Pathological Resection Findings in IBD-Associated Dysplasia. Austin J Gastroenterol. 2015;2:1032. |
| 101. | Fazio VW, Ziv Y, Church JM, Oakley JR, Lavery IC, Milsom JW, Schroeder TK. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg. 1995;222:120-127. [PubMed] |
| 102. | Farouk R, Dozois RR, Pemberton JH, Larson D. Incidence and subsequent impact of pelvic abscess after ileal pouch-anal anastomosis for chronic ulcerative colitis. Dis Colon Rectum. 1998;41:1239-1243. [PubMed] |
| 103. | Gecim IE, Wolff BG, Pemberton JH, Devine RM, Dozois RR. Does technique of anastomosis play any role in developing late perianal abscess or fistula? Dis Colon Rectum. 2000;43:1241-1245. [PubMed] |
| 104. | Fazio VW, Kiran RP, Remzi FH, Coffey JC, Heneghan HM, Kirat HT, Manilich E, Shen B, Martin ST. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg. 2013;257:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 526] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 105. | Francone TD, Champagne B. Considerations and complications in patients undergoing ileal pouch anal anastomosis. Surg Clin North Am. 2013;93:107-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 106. | Hrabe JE, Byrn JC, Button AM, Zamba GK, Kapadia MR, Mezhir JJ. A matched case-control study of IBD-associated colorectal cancer: IBD portends worse outcome. J Surg Oncol. 2014;109:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 107. | Renz BW, Thasler WE, Preissler G, Heide T, Khalil PN, Mikhailov M, Jauch KW, Kreis ME, Rentsch M, Kleespies A. Clinical outcome of IBD-associated versus sporadic colorectal cancer: a matched-pair analysis. J Gastrointest Surg. 2013;17:981-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 108. | Kiran RP, Khoury W, Church JM, Lavery IC, Fazio VW, Remzi FH. Colorectal cancer complicating inflammatory bowel disease: similarities and differences between Crohn’s and ulcerative colitis based on three decades of experience. Ann Surg. 2010;252:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |