DISCUSSION
IBD clinical manifestations and treatment strategies
Clinical manifestations of UC include diarrhea, with or without blood, abdominal pain, tenesmus, and fecal urgency while the manifestations of CD are more variable depending on the extent and location of the GI inflammation. CD with predominantly colonic involvement often presents in similar fashion to UC whereas in small bowel CD, diarrhea and rectal bleeding are seen less frequently and symptoms such as fever, fatigue and weight loss are common[8]. Symptoms of IBD often wax and wane, defining periods of “flare” and “remission”, respectively. Extraintestinal manifestations, such as arthritis/arthralgias, skin and ocular involvement and hepatobiliary conditions like primary sclerosing cholangitis, occur in more than 1/3 of IBD patients[9]. Linear growth attenuation and pubertal delay are common among children with IBD. Up to 30% of children with CD have linear growth failure[10] and decreased height velocity often precedes the onset of GI symptoms[11], highlighting the importance of prompt diagnosis and close monitoring of growth in patients with established disease as losses in linear growth are not always recoverable and can result in permanent stunting. Additionally, malabsorption of calories and micronutrients can lead to malnutrition and vitamin and mineral deficiencies, which can exacerbate IBD symptoms.
The diagnosis of IBD is most commonly made on the basis of clinical features mentioned above in combination with findings on endoscopy and histopathology. Laboratory abnormalities such as anemia, hypoalbuminemia and elevation in erythrocyte sedimentation rate and C-reactive protein (CRP) can be supportive but are not specific for IBD and are normal in up to 50% of IBD patients at the time of diagnosis[12,13]. Small bowel imaging modalities like upper GI series with small bowel follow through (UGI-SBFT), computed tomography (CT) and magnetic resonance enterography (MRE), as well as wireless capsule endoscopy are able to assess for active inflammation in areas of the GI tract not reached by traditional upper endoscopy and ileocolonoscopy and can be helpful in distinguishing CD from UC.
There is no medical cure for CD or UC, but medical therapy is effective in many cases, both at reducing symptoms and at reducing intestinal inflammation. The classes of medications most commonly used to treat IBD include corticosteroids, antibiotics, anti-inflammatory agents (5-aminosalicylates), immunomodulators (6-mercaptopurine, azathioprine and methotrexate) and newer biologic therapies. Specific treatment depends on disease location, severity and phenotype with consideration of potential benefits and risks of both specific therapies and of inadequate treatment of active disease which can lead to disease progression and complications. Surgery in IBD is typically reserved for medically refractory cases. In UC, total colectomy with ileal pouch-anal anastomosis is often performed for medically refractory cases or for patients with IBD-associated dysplasia or colorectal cancer but is associated with complications including pouchitis and pouch malfunction/dysfunction. Intestinal resections in CD are also frequently performed in medically refractory cases but disease recurrence is common if not nearly universal.
While treatment goals in practice have traditionally focused on an improvement in clinical symptoms, this improvement alone may not predict underlying disease activity. In CD in particular, the discrepancy between clinical symptoms and active inflammation can potentially lead to unnecessary treatments for patients whose symptoms are not due to active inflammation and to asymptomatic progression of disease and disease-related complications in patients who are without clinical symptoms despite active inflammation. This discordance has led to a search for additional targets that can be used in combination with clinical symptoms to help guide treatment decisions. Candidate targets include CRP, fecal calprotectin, intestinal appearance on cross-sectional imaging modalities like CT enterography (CTE) and MRE, and mucosal healing based on endoscopic findings, which is becoming a preferred objective measure of disease activity as it correlates well with improved clinical outcomes[14]. In both clinical practice and in clinical trials, mucosal healing is now frequently being included and at times required as an efficacy endpoint[15]. More effective and validated non-invasive biomarkers that can accurately predict mucosal healing are needed and may help obviate the need for repeated invasive procedures in the future.
The natural history of IBD is highly variable. The majority of UC patients are able to achieve clinical remission with medications and often receive intermittent corticosteroid treatment for exacerbations of symptoms that occur at varying intervals. A subset of UC patients never enter remission, even with immunosuppressive therapy, and up to 20%-30% will require colectomy for exacerbation of symptoms during their lifetime[16]. Uncontrolled GI inflammation in UC can lead to chronic complications such as stricture, and dysplasia and colorectal cancer, which occur in up to 16.5% of patients who have had UC for 30 years[17]. Fewer patients with CD achieve lasting remission[18] and there is an increased requirement for immunosuppressive agents, which are a mainstay of therapy. Uncontrolled inflammation in CD can lead to the development of fibrostenotic strictures and penetrating disease (enteric fistulae), which often require surgical intervention. A population based cohort of IBD patients in southeastern Norway recently showed that within the first 10 years of diagnosis, 90% of CD patients had a relapse after a period of remission, 53% developed stricturing or penetrating disease and 38% required surgery[19].
Classic treatment paradigms for IBD have previously been based on a step-up approach, where medicines with lower efficacy but fewer perceived side effect profiles were used first, followed by more effective treatment only if needed. Currently, there is debate over the expanding use of a top-down approach, where more effective medications, that also have more potential side effects, are used earlier in an attempt to induce a more sustained response and mucosal healing particularly in patients with risk factors that may predispose them to a more aggressive phenotype[20]. It is not yet entirely clear who would benefit most from this approach or if a top-down strategy significantly changes the natural history and course of the disease for an individual with IBD.
Current role of imaging in IBD evaluation
Historically, IBD evaluation of the bowel has included imaging to assess the portions of the small bowel that are inaccessible to optical endoscopic visualization. This traditionally was performed using barium fluoroscopic techniques; however, cross-sectional imaging techniques (CT and MRI) are being increasingly utilized for IBD evaluation because they can simultaneously assess mural and extramural IBD manifestations. Current roles of imaging in IBD patients include: (1) at the time of initial diagnosis to distinguish UC from CD; (2) to assess and track progression of extraintestinal IBD manifestations; (3) to visualize penetrating complications of disease that extend outside the bowel wall; and (4) to assess disease activity in patients with known IBD during symptomatic recurrence,
Defining extent of disease: Disease extent is important to define at the time of initial diagnosis of IBD in order to classify patients as having UC or CD. Small bowel involvement, most commonly of the terminal ileum, is diagnostic of CD. Imaging plays an important role in defining overall disease extent as patients with distal ileal disease on colonoscopy can have more proximal involvement in areas that are not accessible to endoscopic visualization. CD involvement of the proximal small bowel is important to recognize because it can be associated with symptoms related to malabsorption (vitamin deficiencies, weight loss, steatorrhea) and is associated with increased risk of stricturing behavior and multiple bowel surgeries[21]. In addition, imaging is helpful for determining overall length of bowel involvement in CD, which can impact the decision whether to perform surgical resection of bowel that is refractory to medical therapy and the potential risk of short gut syndrome[22].
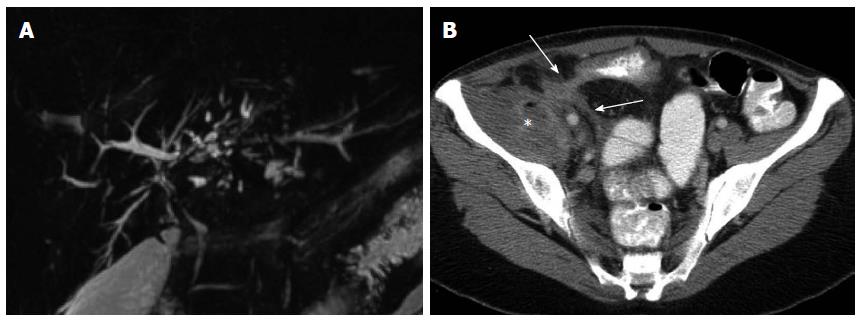
Extraintestinal IBD manifestations: IBD is associated with a wide range of extraintestinal manifestations, a detailed description of which is beyond the scope of this review[23]. Knowledge of some of the more common IBD extraintestinal manifestations is helpful because they can be a source of symptoms and long-term complications. Among the extraintestinal IBD manifestations visible on imaging is primary sclerosis cholangitis (PSC), a progressive inflammatory disease of the biliary tree that affects up to 10% of IBD patients[24]. On CT or MRI, PSC typically appears as multifocal areas of bile duct narrowing and dilation (Figure 1A), with long-term PSC complications including cholangitis, cirrhosis, end-stage liver disease, and an increased risk of cholangioarcinoma. Nephrolithiasis is the most common IBD genitourinary manifestation, occurring in 10%-30% of patients[25]. Nephrolithiasis is common in IBD, particularly in CD with distal ileal involvement or after ileal resection and frequently causes abdominal symptoms, associated with intense flank pain and fever from associated urinary tract infection. Sacroiliitis in IBD patients typically occurs in a bilateral symmetric distribution and can be the source of lower back or pelvic pain[26].
Figure 1 Extraintestinal and extraluminal manifestations of inflammatory bowel disease depicted on imaging.
A: Primary sclerosing cholangitis depicted on magnetic resonance cholangio-pancreatography evidenced as intermittent beading and structuring of the intrahepatic bile ducts; B: Penetrating Crohn’s disease depicted on computed tomography as enhancing fistulous tracks (arrows) of an enterocolonic fistula, with associated abscess formation (asterisk) in the adjacent iliacus musculature.
Extraluminal disease complications: Imaging provides information regarding extraluminal complications of penetrating CD including focal bowel perforation, sinus and/or fistula formation between bowel loops and between bowel and other visceral organs (Figure 1B), and abdominopelvic abscesses[27]. Penetrating complications are important to diagnose because they can be the cause of systemic infection via dissemination of intestinal flora and require urgent intervention. In addition, the presence of penetrating disease can influence the choice of medical therapy[28].
Determination of disease activity: In patients with established IBD, assessment of disease activity is important in order to evaluate adequacy of therapy and potential need for treatment modification. Laboratory and clinical based indices of activity are imperfect due to subjectivity in patient-reported symptoms and serum inflammatory markers that have inadequate sensitivity and specificity[12]. Endoscopy based indices of IBD activity are the current gold standard for assessing efficacy of therapeutics in IBD clinical trials, but are difficult to implement in routine clinical practice because of the invasiveness of serial endoscopic examinations and lack of uniformity in their application[14]. Imaging can provide a noninvasive alternative for assessing IBD activity and response to treatment. The features of active disease observed on different IBD imaging modalities are listed below.
Differentiation from tuberculosis: Tuberculosis is a mimicker of many diseases and thus can prove to be clinically challenging to diagnose and may be confused with many other conditions, including inflammatory bowel disease. Although abdominal tuberculosis is an uncommon form of extrapulmonary tuberculosis, the ileocecal region is the most common area of involvement in the gastrointestinal tract. This is thought to be due to the abundance of lymphoid tissue in this region[29]. The natural course of gastrointestinal tuberculosis may be ulcerative, hypertrophic or ulcerohypertrophic[30,31]. CT may demonstrate circumferential wall thickening of the cecum and terminal ileum associated with adjacent mesenteric lymphadenopathy. Characteristic CT features include asymmetric thickening of the ileocecal valve, exophytic extension engulfing the terminal ileum and massive lymphadenopathy[32-34]. The differential diagnosis for ileocecal tuberculosis includes CD, amebiasis and primary cecal malignancy. When ileocecal wall thickening is observed, mucosal hyperenhancement and associated prominence of the mesenteric vasculature are ancillary imaging features that may be more suggestive of IBD rather than tuberculosis involvement of bowel.
Lymphadenopathy is commonly observed in both IBD and TB[35]. The most common involvement of lymph node chains (mesenteric, celiac, porta hepatis, and peripancreatic lymph nodes) is shared by both diseases and follows the lymphatic drainage of the ileocecal, jejunal, ileal and right colonic regions following active bowel inflammation (IBD) or ingestion of infected material (TB)[36]. Enlarged lymph nodes that demonstrate a necrotic (fluid density and hypoenhancement on either CT or MRI) center would be much more typical for caseating necrosis associated with TB rather than IBD[37]. Abdominal lymphadenopathy secondary to lymphoma, metastasis, pyogenic infection and Whipple’s disease may also demonstrate a similar appearance[29,36].
Involvement of abdominal solid organs in TB occurs in 15%-20% of all patients with abdominal TB[38]. The genitourinary system is the most commonly involved, followed by liver, spleen and pancreas. The mode of spread is via the haematogenous route. Only 15% of patients have concomitant pulmonary tuberculosis[39]. The presence of abdominal visceral organ lesions is much more suggestive of TB than IBD, in which the more common extraintestinal manifestations include primary sclerosing cholangitis, stones in the gallbladder or kidneys, or ankylosing spondylitis. Granulomatous lesions in the abdominal organs would be extremely unlikely in the setting of IBD but may suggest opportunistic infection in an IBD patient being treated with immunosuppressive medical therapy[9]. Peritoneal involvement is another imaging feature much more suggestive of TB than IBD, and may account for 30% of all nonpulmonary tuberculosis in Asia and developing countries[37].
Overview of IBD imaging modalities
Fluoroscopic imaging: Background/technique - Fluoroscopic evaluation of the small bowel with barium has traditionally been the method for assessing IBD involvement in the small bowel. This is particularly important in patients with suspected or newly diagnosed IBD, where small bowel involvement is highly suggestive of CD. In recent years, fluoroscopic small bowel evaluation has largely been replaced by CT and MRI in the established IBD population for evaluation of disease activity. Historically, small bowel enteroclysis was used to evaluate the small bowel, which consists of using a nasojejunal catheter placed under fluoroscopic guidance[40], insufflating the small bowel with barium and air or methylcellulose to create a double contrast, distended view of the small bowel. Double contrast SBE performed using this protocol has high accuracy for detecting mucosal inflammatory changes[41,42]; however, SBE is limited by invasiveness of the nasojejunal catheter often requiring patient sedation. In recent years, SBE has been replaced by barium SBFT, which consists of oral administration of barium over a period of time followed by intermittent spot radiographs tracking passage of contrast through the bowel, with subsequent compression views of the small bowel once contrast has reached the cecum.
Disease features - Fluoroscopic imaging of UC, which predominantly involves the large bowel, has been largely replaced by colonoscopy. Some patients with severe UC may exhibit “backwash” ileitis. In these cases, fluoroscopy can demonstrate a patulous, incompetent ileocecal valve with nodular mucosal pattern of the terminal ileum. This finding is in contrast to CD which is characterized by a stenotic ileocecal valve with luminal narrowing and ulceration of the terminal ileum. The radiological features of CD on fluoroscopy are well described. They include irregular thickening and distortion of the valvulae conniventes, mesenteric and mural thickening causing bowel loop separation and loop adhesions resulting in mass effect[43]. Severe disease produces a “cobblestone” appearance with deep transverse and longitudinal ulcerations bordered by areas of edema creating a checkered mucosal relief pattern. Chronic CD leads to circumferential bowel wall thickening and can progress to fibrotic strictures, in which irreversible deposition of extracellular matrix in the bowel causes impaired peristalsis, fixed luminal narrowing, and bowel obstruction.
Comparison with other modalities
Although conventional enteroclysis offers excellent depiction of small bowel CD due to its uniform small bowel distention, its ability to evaluate disease is limited by intraluminal localization of contrast and patient compliance with nasojejunal intubation. SBFT has similar limitations in evaluation of extraluminal and extraintestinal disease manifestations, but also is limited by the patient’s ability to ingest oral contrast, which can lead to reduced detection of strictures and diseased segments in symptomatic patients. Fluoroscopic small bowel studies in general are also limited by long exam times and poor off-hours availability. These studies have mostly been replaced by cross sectional modalities such as CT and MR, which are available after hours in most emergency rooms and offer mural, extraluminal, and extraintestinal IBD evaluation[44-46].
CT, CT enterography, and CT enteroclysis: Background/technique - CT has become the primary imaging modality for evaluating IBD and its complications in the US for the last 15 years, owing to a combination of rapid scan time, high-resolution evaluation of intestinal and extra-intestinal disease manifestations and 24-h availability in most hospitals[47]. The development of helical multidetector row CT scanners allows high spatial resolution imaging of the entire abdomen and pelvis in just a few seconds, generating isotropic images that can be reconstructed in multiple planes to facilitate visualization of subtle abnormalities. CT scans are typically performed for IBD evaluation following administration of both oral and IV contrast to detect bowel wall abnormalities and abnormal enhancement[48]. Patients are kept NPO for several hours before the study. Conventional CT uses positive enteral contrast agents, usually barium containing solutions, which increase the attenuation of the bowel lumen and conspicuity of bowel wall abnormalities and extraluminal fluid collections. Positive enteric contrast, can, however, obscure IV contrast enhancement of the bowel wall. In addition, positive oral contrast agents opacify but do not always well distend the bowel. IV contrast is administered and images are acquired in the portal venous phase, which is optimized for visceral organ evaluation.
Adequate enteric distension is the primary goal of CT imaging of the small bowel to optimize detection of small bowel disease. CT enterography combines large volume enteral contrast distension of small bowel with intravenous contrast administration. Patients are typically kept NPO for several hours prior to the study. Neutral enteral contrast agents are used which distend but do not opacify the bowel lumen. Using these agents mucosal enhancement patterns are well seen, as are areas of non distensibility such as strictures (Figure 1). In our institution, patients drink 1350 mL of contrast over approximately 45-60 min, with decreased volume required for pediatric patients based on weight. As opposed to conventional CT, CT enterography images are acquired during enteric (45-60 s post-injection) phase which is optimized to evaluate small bowel enhancement.
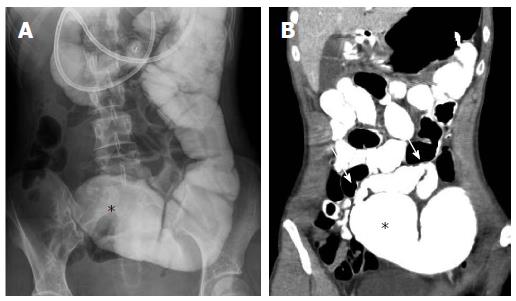
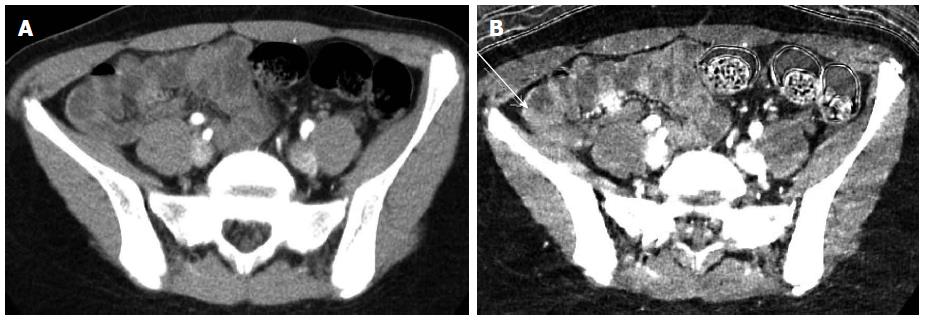
CT enteroclysis is a provocative study similar to conventional enteroclysis to detect small bowel strictures in intermittently symptomatic IBD patients that might be missed on enterography due to limited patient oral contrast intake[49]. A naso-jejunal catheter is usually placed in the fluoroscopy suite, followed by catheter-directed administration of dilute positive enteric contrast under fluoroscopic guidance until either the small bowel is uniformly distended or the patient’s symptoms are reproduced. The patient is then brought to CT for cross-sectional imaging (Figure 2).
Figure 2 Computed tomography enteroclysis depiction of small bowel stricture.
Fluoroscopic (A) and computed tomography (B) images from an enteroclysis demonstrating a 9 cm stricture in the mid small bowel (arrows) with proximal small bowel dilation (asterisks), which was confirmed during subsequent surgical resection.
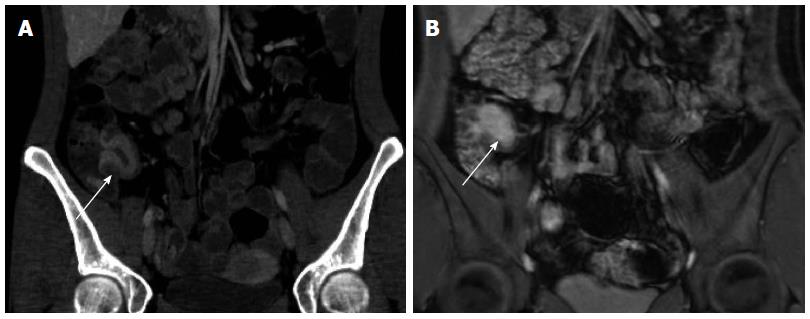
Disease features - As previously discussed, colonoscopy is the primary modality for diagnosing and determining extent of disease in UC. However, complications of UC, for example, toxic megacolon, are an important indication for CT. CT findings include thinning of the colonic wall, luminal distension and pneumatosis. Severe cases can lead to perforation and free air. Common signs of active or inflammatory CD on CTE include bowel wall thickening, increased mural enhancement with hyperenhacing mucosa (Figure 3A), and haziness of the surrounding mesenteric fat. The “comb sign” in CD reflects hypervascularity involving the distal tier of mesenteric arterial arcades and vasa recta of affected segments of small bowel and ascending colon. These changes likely represent increased blood flow and the fibrofatty proliferation in the mesentery and serosa of the affected bowel[50]. Chronic transmural CD, possibly due to chronic inflammatory stimulation, produces a fibrofatty proliferation of the mesenteric fat, also known as the creeping fat sign.
Figure 3 Active Crohn’s terminal ileitis depicted on computed tomography enterography and magnetic resonance enterography in the same patient.
Serial computed tomography enterography (A) and magnetic resonance enterography (B) studies demonstrate marked wall thickening and hyperenhancement (arrows) just proximal to the ileocecal valve consistent with active disease, as confirmed by endoscopy.
Comparison with other modalities
CTE confers several advantages over fluoroscopic small-bowel follow through studies, as outlined above. CTE is highly sensitive and specific for active small intestinal inflammation with better interobserver agreement and more reproducible image quality (improved spatial and contrast resolution) compared with MRE[51,52]. Other advantages over MRE include much more rapid image acquisition (seconds), ability to assess patients with MR-incompatible hardware, and ability to image young patients without the need for sedation. The major drawback of CT is the ionizing radiation exposure to patients, which has received much attention in recent years given that the IBD population (CD in particular) is likely to require multiple imaging studies over the course of their disease[53]. It is important to point out the CT radiation doses continue to decrease due to innovations in CT hardware and image reconstruction technology, and that the potential risk associated with abdominal CT for individual patients is extremely low compared with other activities related to daily living[54].
Assessment of disease activity
A CTE based index of inflammatory severity for CD has been proposed but not widely used, based on the presence of wall thickening, mucosal or mural enhancement, mural stratification, comb sign and involvement of regional nodes[55]. The Lémann score, which uses either CTE or MRE, aims to quantify long term cumulative bowel damage over time. It comprises of extent and severity of bowel damage, including stricturing and penetrating lesions, as well as previous surgery[56].
MRE, MR enteroclysis, and MR fistulography: Background/technique - MRE is a minimally invasive, non-ionizing radiation diagnostic technique with the ability to obtain multiplanar diagnostic information about intra and extra mural involvement of the small bowel in IBD[57]. Because of the need for frequent re-evaluation of disease activity in patients with IBD as well as concern regarding cumulative ionizing radiation of imaging studies, MRE is increasingly utilized over CTE[58], especially in children and young adults. MRE combines large volume oral contrast bowel distention with T2-weighted, balanced steady state free precession, and multiphase T1-weighted fat suppressed contrast-enhanced sequences to optimize detection of bowel wall abnormalities. A multi-channel phased array body coil is used, with imaging field of view extending from the transverse colon superiorly to the bottom of the anal sphincter complex inferiorly. The most commonly used class of MR contrast agents are of the biphasic type, which are hypointense on T1-weighted images and hyperintense on T2-weighted images (e.g., dilute barium with sorbitol, polyethylene glycol, mannitol, water). With these agents one can readily assess the pattern of bowel wall folds on T2 weighted images without losing mucosal enhancement data on T1 weighted images[59]. Nonabsorbable enteric contrast agents are preferred to maintain uniform contrast opacification of small bowel for the duration of the exam (30-45 min). Ingestion of adequate enteric contrast is of the utmost importance for MR enterography. Unlike CT, where underdistended bowel loops can still generally be evaluated, the relative diminished spatial resolution of MRI renders collapsed bowel difficult to assess for disease. Additionally, enteric contrast is needed to displace intraluminal air which can lead to significant susceptibility artifact on gradient echo post-contrast sequences. The total volume of enteric contrast needed to distend the small bowel in adults is similar to CTE and ranges from 1-2 L administered over 45-60 min, with a lower volume given to pediatric patients based on weight. Some institutions administer an anti-peristaltic agent (glucagon or hyoscine butylbromide) to reduce bowel peristalsis and motion artifact, although this can produce nausea in some patients[60,61]. Patient positioning during MRE also varies by institution, with many institutions favoring the supine position for patient comfort and others preferring prone position positioning to compress the bowel and decrease scan times.
Typical MRE pulse sequences include: single shot T2-weighted images and balanced steady state free precession (bSSFP) sequences in the coronal plane to provide motion-free assessment of the bowel wall (Figure 3), mesentery, and extraintestinal regions; cinematic thick slab coronal bSSFP images to evaluate peristalsis and distinguish underdistended from inflamed bowel loops; axial T2-weighted fat suppressed images to assess for bowel well edema and intra-abdominal fluid collections; coronal multiphase 3D T1-weighted fat suppressed images post-contrast to evaluate bowel wall enhancement and mesenteric vascularity; and delayed axial T1-weighted fat suppressed images to evaluate for penetrating disease complications including fistulae and abscesses.
MR enteroclysis is performed in a similar manner to CT enteroclysis with placement of a naso-jejunal catheter and catheter-directed infusion of enteric contrast to facilitate uniform bowel distention. However, with the advent of dynamic thick slab MRI techniques, contrast is now routinely distilled under real-time MR guidance until adequate small bowel distension is achieved[62]. A number of studies have compared MR enterography versus enteroclysis in the detection of Crohn disease[63-67]. In two of these studies[66,67] MR enteroclysis was superior to MR enterography for detecting CD abnormalities, particularly of milder superficial pathology and jejunal disease.
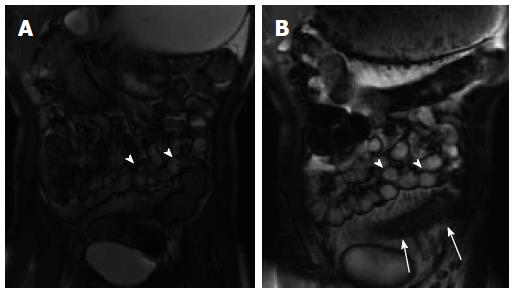
Disease features - The features consistently related to disease activity in inflammatory bowel disease are wall thickness, mural T2 signal intensity and post-contrast enhancement[68,69]. T2 signal intensity of the intestinal wall is directly related to the degree of edema in the submucosal layer as well dilation of submucosal lymphatic vessels, with hyperintensity compared with skeletal muscle considered a sign of active inflammation. MRE, unlike CTE, includes multiphase post-contrast T1-weighted fat suppressed imaging, and the presence of mucosal hyperenhancement on enteric phase images (45-60 s post-contrast injection) followed by progressive transmural enhancement on delayed images is also an MRE sign of active inflammation (Figure 3B). Other MRE signs are similar to CTE and include bowel wall thickening and ulceration, prominent vasa recta (comb sign), and mesenteric fat stranding[63]. Cinematic SSFP images can also be helpful for distinguishing diseased bowel, which exhibits reduced peristalsis, from normal underdistended bowel (Figure 4).
Figure 4 Abnormal bowel peristalsis visualized by cinematic magnetic resonance enterography imaging.
Two static images from a cinematic steady state free precession image series demonstrate multiple normally peristalsing small bowel loops (A, B: arrowheads) as well as a fixed hypoperistaltic loop of inflamed small bowel (B; arrows). This loop also demonstrates wall thickening and mesenteric hypervascularity consistent with active inflammation.
The availability of T2-weighted and multiphase T1-weighted post-contrast images has also led to the use of MRE for distinguishing inflammatory from fibrotic strictures. A common complication of longstanding CD is the development of strictures that produce obstructive symptoms. The distinction between inflammatory and fibrotic strictures is important clinically because of its impact on clinical decision making[70]. Inflammatory strictures are due to active transmural inflammation and typically are treated with anti-inflammatory medications. In contrast, fibrotic strictures are caused by chronic mural deposition of extracellular matrix proteins and are treated mechanically (surgical resection or endoscopic dilation). Both types of strictures appear as focal areas of luminal narrowing with proximal bowel dilation. MRE features associated with intestinal fibrosis include wall thickening, T2 hypointense signal in comparison to skeletal muscle, as well as minimal (no more than mild) mural enhancement on multiphase post-contrast images. MRE has been shown in prospective studies to be superior to CTE for detection of fibrotic bowel strictures in CD[71].
Comparison with other modalities
MRE has gained much more widespread application over MR enteroclysis because of its noninvasiveness and shorter patient time in the scanner. Multiple studies have demonstrated excellent accuracy of MRE compared to CTE and colonoscopy for detection of active disease in IBD patients[51,63,72]. The main advantages of MRE include lack of ionizing radiation, ability to image the bowel repeatedly over time (e.g., cinematic SSFP and multiphase post-contrast imaging) to assess enhancement and function, as well as superior soft tissue contrast for assessing disease activity and penetrating disease complications. The main limitations of MRE are long scan time, which often necessitates sedation for young or neurologically impaired patients, as well as inability to perform in patients with MRI-incompatible devices or metallic foreign bodies.
Assessment of disease activity
The MaRIA (Magnetic Resonance Index of Activity) score was validated for assessing inflammatory activity using MRE in ileocolonic CD based on logistic regression of MRE imaging features of disease[73-77]. Studies have shown good agreement between MaRIA and endoscopic indices of CD activity and treatment response. It is the best validated MRE index of activity; however, the multiparametric nature of the MaRIA scoring system can be labor intensive and, because of this, it currently is not routinely utilized in clinical practice at most institutions.
MR fistulography/MR evaluation of perianal CD
The lifetime risk of perianal fistula formation in CD ranges from 30%-50%[78], with the presence of a fistula leading to significant morbidity due to cutaneous drainage or perianal abscess formation. MRI of the pelvis is the gold standard examination for evaluation of the perianal disease, providing the highest contrast resolution and details of the perianal and sphincter anatomy to help guide management[79,80]. In general, there is no bowel preparation, oral contrast or sedation required; only IV contrast is administered, assuming no contraindications. This study can be performed alone as a pelvic exam or can be combined with an MRE examination. The MRI is performed using a phased array coil with the patient in the supine position. Based on recent pediatric publications and the authors’ experience the following are pulse sequences commonly utilized in a perianal MRI protocol: (1) T2-weighted FSE imaging in the axial and coronal planes to visualize the anal sphincter complex, with the fat in the ischioanal fossae and perirectal space providing intrinsic contrast against the T2-weighted hypointense sphincter complex musculature; (2) T2 -weighted FSE fat suppressed or short tau inversion recovery (STIR) imaging in the axial and coronal planes to visualize fluid-filled fistulous tracks or abscesses; and (3) T1-weighted 2D or 3D gradient echo imaging pre- and post-gadolinium imaging in the axial and coronal planes to visualize perianal inflammatory changes and subtle fistulous tracks that might be missed on fluid-sensitive sequences[80,81]. The T2 fat-suppressed/STIR and T1 fat-suppressed post-contrast images are typically acquired with a smaller field of view (16-22 cm) to increase spatial resolution for identifying fistulous tracks and their relationship to the anal sphincter complex. The coronal plane is especially useful for identifying the anatomic relationship of perianal fistulae with the levator ani complex. Some authors advocate using STIR instead of T2-weighted sequences with frequency-selective fat suppression because of more homogeneous reduction in fat signal intensity in the pelvis[82].
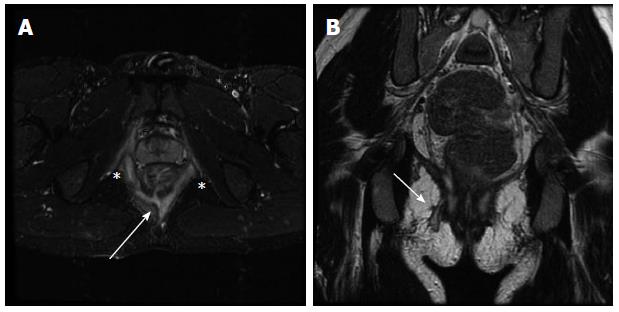
The Parks classification system is commonly used to describe perianal fistulae[83]. This system classifies fistulae as intersphincteric, transsphincteric, suprasphincteric or extrasphincteric based on their anatomical relationship to the anal sphincter complex and levator ani musculature[84]. The St. James classification system is a modified version of the Parks system based on MRI that subclassifies intersphincteric and transsphicteric fistulae based on the presence or absence of associated abscess[85]. Imaging findings range from simple sinus tracts that do not extend to the skin, to simple fistulae without abscess (Figure 5), to complex multiple fistulae communicating with the skin at multiple sites with associated abscess. Perianal fistulae can also communicate with other pelvic organs such as the bladder, vagina, and pelvic small bowel loops. The importance of MR imaging lies in its ability to demonstrate hidden areas of sepsis and secondary extensions, both of which contribute to the high rate of recurrence after surgery[86]. Furthermore, MR imaging can be used to define the anatomic relationships of the fistula to predict the likelihood of postoperative fecal incontinence. MR imaging has been used in monitoring the response of fistulous tracks to medical treatment in patients with Crohn disease, including evaluating the response of perianal Crohn disease to infliximab therapy and determining the extent of obliteration of fistula tracks in patients following treatment[87-90].
Figure 5 Perianal fistulizing Crohn’s disease depicted on magnetic resonance fistulography.
Axial short tau inversion recovery (A) and coronal (B) T2-weighted (B) images demonstrate a complex perianal fistula at the 6 o’clock position (A; arrow) with an intersphincteric component (A; asterisks) as well as a transphincteric track extending to the skin to the right of midline (B; arrow).
A recent publication by Shenoy-Bhangle et al[82] studying MRI predictors of treatment response in pediatric CD patients with perianal disease identified fistula length to be the most significant predictor of response on follow-up pelvic MR. The type of fistula by Parks classification and the presence of an associated abscess both impacted the treatment modality selected but did not significantly affect overall response on MRI.
Ultrasound: Background/technique - The use of ultrasound in the assessment of patients with inflammatory bowel disease is becoming more widespread. Ultrasound is advantageous in that it is low cost, widely available, non-ionizing and allows real time imaging[91]. However, there are several limitations of ultrasound to be considered including the relatively long scan duration that is highly dependent on sonographer/radiologist skill and experience. Ultrasound is also more effective when imaging a patient with known distribution of disease (i.e., with prior MR or CT enterography studies), as it can be difficult to follow the small bowel in its entirety. Patients typically are NPO for several hours prior to the examination and are asked to drink water before the scan to reduce bowel gas that might obscure visualization and to push bowel loops out of the pelvis for easy compression. The current technique employs using anterior and posterior compression with a high resolution (12-18 mHz transducer) probe with graded compression of bowel[48].
Disease features - Normal bowel wall consists of five concentric alternating hyper and hypoechoic layers. Normal bowel wall thickness is 2-5 mm, measured in transverse section from the central hyperechoic line to the outer margin of the edematous wall[92]. Inflammatory bowel disease is manifest on ultrasound as abnormal bowel wall thickening, defined as greater than 3 mm, and loss of definition of the discrete bowel wall layers. Both UC and CD lead to bowel wall thickening. In UC, the sonographic appearance of the deeper bowel wall layers is preserved, unlike in CD, where the sonographic distinction among bowel layers is usually lost. With CD, there may be loss of the bowel wall signature of alternating layers due to fatty infiltration, oedema or fibrosis. The bowel appears diffusely hypoechoic with a central hyperechoic line representing the stenotic lumen. These segments can be angulated, aperistaltic, rigid, incompressible and lack the normal haustrae[93]. Chronic disease can also be identified by sacculations forming on the anti-mesenteric border on US Strictures appear as focal areas of mucosal thickening with proximal dilatation.
Comparison with other modalities
Generally, changes on ultrasound correlate well with endoscopic and histologic changes but have a weak correlation with clinical activity indices or biomarkers[94-97]. The sensitivity and accuracy of ultrasonography in CD depends on disease location, with the highest values found when easily accessed bowel segments (for example the terminal ileum or left colon) are affected by the disease. In contrast, diagnostic accuracy is lowest in the upper small bowel and the rectum[98]. The sensitivity of ultrasonography for diagnosing fistulising lesions ranges from 67% to 87% with a specificity of 90%-100%[99]. The sensitivity of ultrasonography for diagnosing abdominal abscesses is 81%-100% with specificity of 90%-100%. The diagnostic accuracy of ultrasonography for abscesses is lower than for CT or MRI[100] because of false negative cases mainly in the retroperitoneum, or posterior to air-distended bowel loops which limits visualization. Other limitations of US include the ability to evaluate bowel loops deep in the body due to acoustic absorbance in tissues, as well as operator dependence and ability to reproduce images of the entire bowel and compare them with prior studies. In general, US performs best when there is a defined distribution of disease and the patient is thin. CT and MRI are preferred to US at most institutions for evaluating the bowel in larger patients and those in whom the entire bowel needs to be assessed.
Assessment of disease activity
Ultrasound has been used to assess disease activity. Characteristics used for ultrasonography-based indexes include wall thickening, vascularization, loss of bowel wall stratification and reduced peristalsis or compressibility[101]. A sonographic lesion index for CD (SLIC) has been developed for assessment and standardization of disease activity but is not widely utilized[102]. The index takes into account both the extent and severity of small bowel damage, including structuring and penetrating lesions measured by contrast enhanced ultrasonography (CEUS). SLIC has the potential to evaluate the progression of small bowel disease following treatment and over time.
New and emerging directions
Dual energy CT: Dual energy CT (DECT) is an evolving modality with specific capacities beyond single-energy (SE) CT that can translate into improved evaluation in abdominal imaging. The 2 different CT energy spectra are applied to the same tissue, which enables differentiation of tissue composition based on energy-related attenuation characteristics and has the potential to provide better lesion detection, characterization, and monitoring than SECT[103]. The iodine images created represent the amount of iodine present in the tissue and have unique benefit in comparison with single energy conventional acquisition. Iodine images can be a surrogate marker of tissue contrast uptake and may be more sensitive to subtle areas of bowel wall hyperenhancement compared with traditional SECT[104]. Iodine maps acquired from DECT as part of CTE may have a role to play in evaluation of inflammatory lesions in CD (Figure 6). Further research into this is necessary.
Figure 6 Dual energy computed tomography enterography in inflammatory bowel disease.
Standard (A) and Iodine monochromatic (B) images from a dual energy computed tomography enterography demonstrate a polypoid lesion in the distal ileum (arrow) that was only appreciated on the Iodine images and found to represent an inflammatory pseudopolyp at endoscopy.
MR diffusion weighted imaging: Diffusion weighted imaging is an evolving technique in the imaging of IBD. DWI employs the motion of water at a cellular and subcellular level to provide image contrast. The ability of DWI to image microscopic inflammatory changes offers potential for enhancing detection of diseased bowel segments and assessing response to treatment[105,106]. Apparent diffusion coefficient could potentially be used to help assess the degree of inflammation in CD strictures[105]. A prospective study of 96 patients with CD and UC demonstrated DWI hyperintensity correlated well with endoscopic inflammation in CD[107]. The correlation was less than that for UC. This held true for unprepared bowel segments (i.e., no oral or IV preparation) and suggests that there may be a role for DWI in the imaging of patients in whom IV contrast administration is contraindicated.
MR magnetization transfer imaging: Emerging imaging techniques are currently being developed and tested for detecting intestinal fibrosis. Generally, T2 weighted MR imaging reflects the amount of fluid within the pathological wall with better sensitivity and specificity than other imaging modalities, including ultrasonography and CT[108]. T2 hypointense signal of the bowel wall has been shown to correlate with the presence of fibrosis[109], with the amount of collagen and fibroblasts in the submucosal and muscularis propria layers associated with shorter T2 relaxation times exhibited by the bowel wall[108]. However, many fibrotic strictures contain superimposed active inflammation, with the active inflammatory changes masking the fibrosis on imaging[70]. Magnetization transfer imaging may have a role in the imaging of fibrosis in CD. Magnetization transfer imaging reflects the transfer of energy from protons in free water molecules to those associated with large molecules such as collagen, by calculating a ratio of signal intensities based on MR pulse sequences performed with and without an off-resonance saturation pulse. Fibrotic tissues containing collagen demonstrate a high magnetization transfer effect. When applied to rats with peptidoglycan polysaccharide induced fibrosis, the mean magnetization ratio in rats with late phase fibrosis was higher than that of animals with early inflammation and correlated with the amount of tissue fibrosis[110]. In a prospective study of 31 patients, normal bowel wall segments demonstrated an intermediate magnetization transfer ratio of 25.4% ± 3.4%. This ratio was significantly increased in bowel wall segments with fibrotic scarring, while in segments with acute inflammation, the mean magnetization transfer ratio was slightly lower than normal[111]. Further studies will be needed to validate this technique in IBD patients and investigate whether fibrotic strictures can still be imaged when there is superimposed active inflammation.
MR motility imaging: In patients with CD, abnormal bowel segments have decreased motility related to impaired peristalsis from bowel wall thickening, edema, and fibrosis. Motility imaging of the small bowel can be performed with fast cinematic balanced steady state free precession sequences, which allow repeated acquisition of images in a single thick slab over time. These sequences allow qualitative and quantitative assessment of bowel motility[112] One study identified 40 patients and compared the detection of abnormalities with and without cine MRI. Overall, cine MRI depicted 35 more CD related findings than MRI performed with the standard protocol. Quantitative, reproducible methods for assessing bowel peristalsis are currently lacking, and peristalsis on cinematic MRE images at the present time is evaluated on a qualitative basis, with segments of visual evidence of abnormal motility correlated with other MRE findings of inflammation.
MR as a biomarker of therapeutic response: An emerging application of MRI in inflammatory bowel disease is its use as a biomarker of therapeutic response[62]. Recently, treatment of IBD has been revolutionized due to the introduction of biologic therapies. These therapies target molecular pathways thought to contribute to bowel inflammation, such as the proinflammatory cytokine TNF-α, lymphocyte signaling molecules CTLA-4 and CD20, and the α4 integrin adhesion molecule mediating leukocyte migration[113,114]. These agents are more specific for the inflammatory pathways underlying IBD compared with traditional corticosteroids or immunomodulatory agents and are associated with high rates of primary response lasting for a variable duration depending on the patient. The biologic agents currently in clinical practice are either recombinant peptides or chimeric antibodies, which are more expensive to produce compared with traditional compound-based drugs[114]. Therefore, therapy with these agents can be associated with high financial costs to patients and the healthcare system as a whole. A noninvasive biomarker of treatment response/resistance potentially would be of financial benefit to patients undergoing biologic therapy, by ensuring that patients who are refractory to treatment do not remain on medication for longer than necessary. Additionally, such a biomarker would help to minimize toxicities associated with prolonged treatment with biologic agents that include increased risk of serious infections, as well as more rare side effects such as neurologic disorders, CHF, and hematologic malignancies. Finally, the current standard biomarker for treatment response is mucosal healing seen on serial endoscopy[14] and a noninvasive imaging alternative would be preferred by many patients. MRI is particularly well-suited to serve as an imaging biomarker of therapeutic efficacy because of its lack of ionizing radiation, rendering it an ideal modality for repeated assessment before and during treatment. Recent evidence suggests that the MaRIA index of activity correlates well with mucosal healing on endoscopy[115] and future research is aimed at identifying novel MRI biomarkers of microscopic bowel inflammation.
CEUS: CEUS is based on intravenous injection of a microbubble contrast agent that increases echogenic conspicuity of the vascular space, followed by visual assessment with contrast-specific imaging software to evaluate the microcirculation and macrocirculation of the targeted organ[116]. With microbubble contrast agents, it is possible to depict perfusion of the gastrointestinal wall[117]. Using this technology, the percentage of maximal enhancement and the area under the time-intensity curve are substantially reduced in fibrotic ileal loops[118]. Correlation between MRI and CEUS for assessing disease activity is good; however, MRI currently is considered superior to CEUS due to the high number of false-positive results for ultrasonography. High correlation was found between these two techniques when assessing thickness of the small bowel wall, lymph nodes and comb sign; good correlation was depicted in assessing layered wall appearance, disease extension and fibroadipose proliferation[119]. CEUS has similar limitations to standard bowel US, including evaluation of patients with large body habitus and difficulty of following the bowel in its entirety. The emergence of targeted US microbubble contrast agents with increased predilection for neovascularization may help to improve its performance for IBD activity evaluation.