Published online Jan 21, 2016. doi: 10.3748/wjg.v22.i3.1279
Peer-review started: May 5, 2015
First decision: June 23, 2015
Revised: July 15, 2015
Accepted: November 13, 2015
Article in press: November 13, 2015
Published online: January 21, 2016
Processing time: 261 Days and 22.6 Hours
More evidence has underscored the importance of Hippo signaling pathway in gastrointestinal tissue homeostasis, whereas its deregulation induces tumorigenesis. Yes-associated protein 1 (YAP1) and its close paralog TAZ, transcriptional co-activator with a PDZ-binding motif, function as key effectors negatively controlled by the Hippo pathway. YAP1/TAZ exerts oncogenic activities by transcriptional regulation via physical interaction with TEAD transcription factors. In various cancers, Hippo pathway cross-talks with pro- or anti-tumorigenic pathways such as GPCR, Wnt/β-catenin, Notch and TGF-β signaling and is deregulated by multiple factors including cell density/junction and microRNAs. As YAP1 expression is significantly associated with poor prognosis of gastric and other gastrointestinal cancers, detailed delineation of Hippo regulation in tumorigenesis provides novel insight for therapeutic intervention. In current review, we summarized the recent research progresses on the deregulation of Hippo pathway in the gastrointestinal tract including stomach and discuss the molecular consequences leading to tumorigenesis.
Core tip: Hippo signaling pathway is a gradually emerging pathway that plays a necessary role in homeostasis of gastrointestinal tissues, whereas its deregulation frequently induces the occurrence of cancers. In gastric and other gastrointestinal cancers, the upstream components of Hippo pathway often show decreased expression and their downregulation loses the inhibitory effect on yes-associated protein 1 (YAP1)/TAZ. Thus YAP1/TAZ is translocated into the nucleus and exerts oncogenic function by direct binding with TEAD transcription factors to activate the downstream targets transcriptionally. In this brief review, we summarize the deregulation of Hippo pathway in gastric and other gastrointestinal cancers.
- Citation: Kang W, Cheng AS, Yu J, To KF. Emerging role of Hippo pathway in gastric and other gastrointestinal cancers. World J Gastroenterol 2016; 22(3): 1279-1288
- URL: https://www.wjgnet.com/1007-9327/full/v22/i3/1279.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i3.1279
Gastric cancer (GC)[1] is still one of the most common malignancies worldwide. According to the World Health Organization statistics in 2012, GC counts for the 4th in men and 6th in women for the incidence and mortality. The incidence is exceptionally higher in eastern Asia countries and regions including China, South Korea and Japan than other countries. The potential risk factors of GC include Helicobacter pylori (H. pylori) infection, Epstein-Barr virus (EBV) infection, high-salty pickled food diet, low-vegetable diet, smoking, chronic gastritis with glandularatrophy, intestinal metaplasia, and the most important factor is genetic alterations[2]. The chronic H. pylori infection inducing GC appears to relate to the VacA virulence factor and Th17/Treg mechanisms[3]. Gastric adenocarcinoma is histologically classified as intestinal type and diffuse type. And it is also grouped by molecular classification that defines four major genomic subtypes of GC including EBV-infected tumors, microsatellite instability tumors, genomically stable tumors and chromosomally unstable tumors[4] in The Cancer Genome Atlas project. GC is a multistep carcinogenesis process with genetic and epigenetic alterations. The oncogenes, tumour suppressor genes, mismatch repair genes, cell adhesion molecules and cell cycle regulators showed altered from the DNA, RNA and protein level[5]. Multiple well-established oncogenic signaling pathways such as Wnt/β-catenin, nuclear factor-κB, Sonic Hedgehog (Shh), Notch and epidermal growth factor receptor (EGFR) signaling are involved in gastric carcinogenesis. Better revealing the biological significance of these signaling pathways will provide fundamental knowledge for drug or small molecule screening[6]. Emerging evidence has underscore the Hippo signaling pathway in the developmental process and tumors[7].
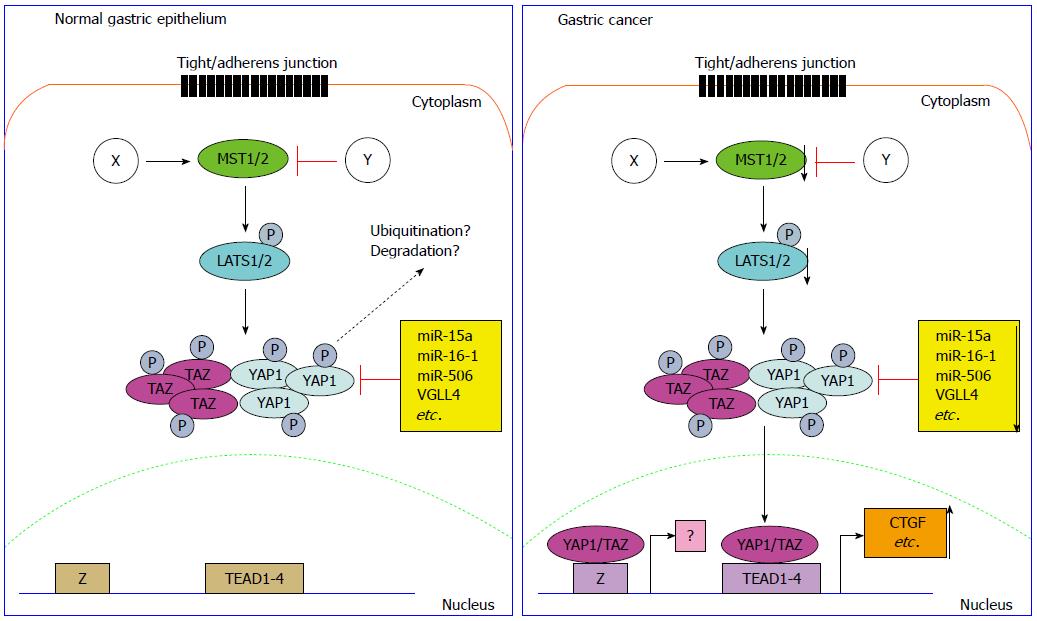
The mammalian Hippo signaling pathway, short for MST1/2-WW45-LATS1/2 signaling, is a critical pathway that determines cell growth rate and organ size[8-9]. MST1/2 (short for mammalian Ste20-like kinase 1 and 2) phosphorylates LATS1/2 (large tumor suppressor 1 and 2) and Mob1 (Mobkl1a/b), leading to their activation[10,11]. LATS1/2 phosphorylates YAP1 (Yes-associated protein 1)[9] and TAZ (WW domain-containing transcription regulator 1)[12] and promotes 14-3-3 binding to phosphorylated YAP1/TAZ, causing YAP1/TAZ cytoplasmic accumulation and sequestering its oncogenic function. The unphosphorylated YAP1 and TAZ are translocated to the nucleus and bind with TEAD1-4 (TEA domain DNA-binding transcription factors 1-4), inducing transcriptional activity for cell proliferation and differentiation[13-15].
As the most important negative regulator of YAP1/TAZ, much studies have provided new findings into the Hippo signaling pathway[9], elucidating novel phosphorylation-dependent[16] and independent mechanisms of YAP1/TAZ inhibition by the Hippo pathway. The key components of Hippo pathways form a phosphokinase cascade and play a crucial role to inhibit the downstream effectors, YAP1 and TAZ. Meanwhile, the Hippo pathway is the same important for homeostasis control and dysregulation of Hippo pathway contributes to carcinogenesis[17].
The tumor suppressor function of Hippo pathway is enhanced by E-cadherin/catenin complex[18,19], AMOT family proteins[20] and LKB1-MARK signaling[21], but is negatively regulated by GPCR (G-protein-coupled receptor) signaling[22]. The protease activated receptors[23] inhibits the LATS1/2 kinase activity via Rho GTPase and G12/13-coupled receptors[24].
The tight junction (TJ)[25] and adherens junction (AJ) components contact with YAP1 and quench its oncogenic function. α-catenin, a component of AJ, binds with 14-3-3 to form complex and phosphorylates YAP1, promoting YAP1 inhibition[19]. AMOT proteins restrict YAP1 activity in a LAST1/2 dependent and LATS1/2 independent manners[20,26]. As YAP1/TAZ is also a mechanotransduction sensor[27], the cell spreading, tension, attachment and detachment modulate YAP1/TAZ activity which is associated with Rho GTPase activity and reorganization of actin cytoskeleton[28]. YAP1/TAZ is also positively or negatively regulated by GPCR signaling pathway through RhoGTPases/actin/LATS1/2 cascade, which is determined by different GPCR ligands. Gα12/13-, Gαq/11-, and Gαi/o-coupled ligands activate YAP1/TAZ but Gαs-coupled ligands suppress YAP1/TAZ activity[22,29].
The deregulated Hippo pathway shares crosstalks with Wnt/β-catenin[30], Notch[31] and TGF-β (transforming growth factor beta)[32] signaling pathways to promote tumorigenesis coordinately. Many studies have highlighted the regulation of Hippo pathway on Wnt/β-catenin pathway. The cytoplasmic accumulation of YAP1/TAZ directly interacts with β-catenin and inhibits β-catenin nuclear translocation[33], whereas in the nuclei YAP1 binds with β-catenin to enhance the tumorigenicity[34]. The subcellular localization and phosphorylation status of YAP1/TAZ also determine the effect of Hippo signaling on TGF-β signaling. In the cytoplasm, YAP1/TAZ binds with Smad protein to suppress the TGF-β induced transcription, but in the nuclei, YAP1/TAZ retains Smad protein and enhances transcription under the TGF-β stimulation[35,36]. The interplay of Hippo pathway and Notch pathway was also demonstrated in mouse intestine. YAP1 induces the expression Notch ligand Jagged-1 and activates Notch signaling[37]. Meanwhile in MST1/2-deficient intestinal epithelium, β-catenin and Notch signaling are strongly activated[38]. In addition, the EGFR ligand amphiregulin (AREG) is another transcriptional target of YAP1, which functions as a contributor for YAP1-mediated proliferation and invasion[39], suggesting that the Hippo pathway also regulates the growth factor receptor tyrosine kinase signaling.
Some other studies focus on findings of new Hippo pathway components and crosstalks, novel YAP1/TAZ target genes and the three-dimensional structure of the YAP1-TEAD complex[40,41]. This provides further evidence and insight for the involvement of the deregulated Hippo pathway and YAP1/TAZ activation in tumorigenesis[42].
YAP1 is located at Chromosome 11q22, a recurrent amplicon region in esophageal squamous cell carcinoma and liver cancer[43,44]. The amplification within this region is also identified in a subset of cervical and lung cancers[45,46]. The modular structure of YAP1 contains a WW domain, TEAD-binding domain, SH3-binding motif and PDZ-binding motif[47]. YAP1 is a modular adaptor protein with multiple protein interaction domains and it was first identified by its affinity to bind the SH3 domain of nonreceptor tyrosine kinase c-Yes, a Src protein kinase[48]. The majority of the interactions are mediated by the WW domain of YAP1. Through WW domain, YAP1, PPxY motif-containing LATS1 kinase, and AMOTL1 protein form functional complex[49,50]. YAP1 binds to the ERBB4 and functions as a transcription co-activator for the cytoplasmic fragment of ERBB4 which is translocated to the nucleus[51]. TEAD family is one of the most important binding partners of YAP1[40] through TEAD-binding domain which is responsible for promoting oncogenic transformation[52] and metastasis[53]. PDZ-binding motif of YAP1 is a critical region for its cytoplasmic-nuclear translocation[54] and accumulation thus to exert its oncogenic function in tumorigenesis.
YAP1 has been concordantly identified as a candidate oncogene that promotes tumorigenesis in many different types of solid tumor[55-59]. In non-transformed mammary epithelial cells, the upregulation and activation of YAP1 promotes epithelial-mesenchymal transition (EMT) and induces growth factor independent growth and anchorage independent proliferation in soft agar[60]. In non-small cell lung cancer, YAP1 binds with Oct4 through its WW domain and transcriptionally induces Sox2 activation thus to endow the stem-like properties[61]. In medulloblastoma, Shh signaling induces YAP1 expression and promotes YAP1 nuclear localization to promote tumorigenesis[62]. In uveal melanoma, Gαq promotes the YAP-dependent growth via a Trio-Rho/Rac signaling circuitry which promotes actin re-polymerization. This process is independent of phospholipase Cβ and the canonical Hippo pathway[63]. In ovarian cancer cell lines, YAP1 can enhance the transformed phenotype and confers drug resistance to chemotherapeutic agents, which are commonly used in clinical ovarian cancer[64].
On the contrary, other studies identify that YAP1 stabilizes p73 and prevents its ubiquitination[65] thus selectively activating the transcription of proapoptotic genes as a consequence of damage to the DNA[66]. Therefore the function of YAP1 in favoring tumor suppression is thought to through apoptosis induction. Promyelocytic leukemia, a tumor suppressor gene and direct transcriptional target of p73/YAP1, directly binds with YAP1 physically via the PVPVY domain and WW domain thus to stabilize YAP1 from degradation[67]. The binding affinity of protein-protein interaction domains is thought to be critical in directing the biological function of YAP1[68]. O’Neill and his colleagues pointed out the molecular background such as loss of RASFF1A expression switches YAP1 from a tumor suppressor to an oncogene[69].
The deregulated Hippo signaling pathway is strongly associated with initiation, development and distant metastasis of human GC[70] (Figure 1). The most upstream of Hippo pathway, MST1/2 and LATS1, is frequently showing downregulation in GC compared with its expression in normal gastric epithelium or adenoma[71,72]. The tumor suppressor RUNX3 is an evolutionarily conserved component of the Hippo pathway. RUNX3 is capable to induce cell apoptosis through cooperation with MST2 and SAV1. It is a very complex circuit. SAV1/WW45 facilitates the tight association of MST2 and RUNX3. In turn, MST2 stimulates the interaction of SAV1 and RUNX3[73]. 3,3-diindolylmethane[74] stimulates RASSF1 binding to the MST1/2-LATS1-Mob1 complex, which will further promote the activation of Hippo signaling pathway and inactivate cell proliferation[75].
As the core downstream effector of Hippo pathway, the expression of YAP1 both in the cytoplasm and nucleus was first described to be dramatically upregulated in high-grade dysplasia, gastric adenocarcinoma, and metastatic gastric disease[76]. The YAP1 nuclear accumulation was correlated with shorter disease specific survival. In early stage GC, the result is more stringent, suggesting the activation of YAP1 is correlated with poor outcome. As YAP1 is up-regulated in GC, siRNA-mediated YAP1 knockdown exhibited suppressive phonotype, such as decreased cell proliferation, inhibited anchorage-dependent monolayer colony formation, reduced cell invasion and migration. In MKN45 cells which show negative YAP1 expression due to the homozygous deletion, ectopic expression of YAP1 promoted anchorage-dependent or -independent colony formation. The upregulation of YAP1 in MKN45 cells also induced more invasive phenotype change and promoted cell proliferation both in vitro and in vivo. The signaling pathway analysis revealed that RAF/MEK/ERK pathway is constitutively activated in cells stably expressing YAP1. YAP1 was further demonstrated to enhance the serum/EGF-inducing c-Fos expression in GC cells[72]. The upregulation of YAP1 in GC exhibits positive expression correlation with survivin[77]. The interaction of YAP1 and RUNX2, a Runt box domain DNA-binding transcription factor, increases oncogenic transformation by repression of p21 protein expression[78]. In gastric and lung adenocarcinoma, receptor tyrosine kinase AXL is the direct functional target of YAP1[79]. The similar oncogenic role of YAP1 in gastric tumorigenesis and metastasis was also comprehensively illustrated by several research groups[80-84].
YAP1 is reported to be negatively regulated by tumor suppressor microRNAs (miRNAs) including miR-15a, miR-16-1[85] and miR-506[86] in GC. VGLL4 is a family member of the Vestigial-like proteins which have been reported to function as tumor suppressors. By competing with YAP1 for the TEADs binding, VGLL4 directly interacts with TEAD transcription factors in GC and inhibits EMT through Wnt/β-catenin signaling pathway[87]. A peptide mimicking this function of VGLL4 potently suppressed tumor proliferation in vitro and in vivo[88], which sheds light on the therapeutic potential of this small molecule.
Several YAP1 related genes exhibit mutation or epigenic modification in gastric carcinogenesis. RhoA, an upstream and activator of YAP1, is found to have recurrent mutations in diffuse type GC[89,90]. TEAD4 transcription factor, the binding partner of YAP1 and a linker between Hippo pathway upstream components and the downstream targets, is significantly hypomethylated in the CpG site cg21637033 and over-expressed in GC tissues compared with the adjacent normal gastric epithelium[91]. TAZ, another key effector of Hippo pathway and transcriptional coactivator with PDZ binding motif, is associated with abnormal overexpression of β-catenin, which correlates with poor prognosis of patients with adenocarcinoma of the esophagogastric junction[92].
The Hippo signaling pathway also plays a important role in other gastrointestinal cancers which was recently comprehensively summarized in a review by Yu et al[93].
In hepatic oval cells, the mammalian Hippo pathway controls the proliferation rate and thereby restricts liver size and prevents the initiation of hepatocellular carcinoma (HCC)[94]. Overexpression of MST1/2 inhibits cell proliferation[95], promotes YAP1 phosphorylation[96] and downregulates the mRNA expression of CTGF, AREG and Survivin[97]. YAP1 plays oncogenic even cancer-driven role in HCC development[98,99]. YAP1 knockdown by siRNA-lipid nanoparticles (siRNA-LNPs) dramatically restores hepatocyte differentiation in advanced HCC and leads to tumor regression[100]. The activation of YAP1 is an early event[101] in HCC and serves as a an independent prognostic factor[102,103]. The PDZ binding motif in YAP1 is crucial for its activation of CTGF, a cell proliferation gene and TEAD-dependent transcription target[104]. CREB (cyclic adenosine monophosphate response element-binding protein) promotes YAP1 transcriptional output through binding to -608/-439, a novel region from the YAP promoter[105,106].
Several binding partners were identified to enhance the oncogenic property exerted by YAP1. YAP1 and PI3K exerts oncogenic cooperation in HCC[107]. The interaction of MEK1-YAP1 is critical for cell proliferation and transformed phenotype maintenance of HCC cells[108]. Amot-p130 was associated with the YAP1-TEAD transcriptional complex and contributed to the regulation of a bunch of YAP1 targeting genes. These targeted genes are associated with liver tumorigenesis[109]. SIRT1 was reported to deacetylate YAP1 protein in HCC cells and SIRT1-mediated deacetylation strengthened the YAP1-TEAD4 interaction, leading to the activation of YAP1/TEAD4 transcriptional ability and stimulating tumor growth in HCC cells[110]. YAP1 up-regulates Jag-1 to activate Notch signaling in HCC cells[37] and it also activates PI3K-mTOR signaling pathway[111], indicating the crosstalks of Hippo pathway and oncogenic pathways. As the same with GC, AXL is a target for YAP1-dependent oncogenic transcription in HCC and has been reported to be a potential therapeutic target[112].
In colorectal cancer (CRC), decreased expression of LATS1 in CRC was associated with promoter hypermethylation and such reduced expression promotes progression of CRC[113]. The Hippo pathway is suppressed by a critical regulator named integrin-linked kinase (ILK) via phosphorylation of MYPT1-PP1, leading to the Merlin inactivation[114]. MST1 and MST2 significantly repress the abundance and activation of YAP1 in normal intestinal epithelium. As tumor suppressors, MST1 and MST2 have an anti-proliferative function, however this function is often overcome by the over-abundance of YAP1 in CRC[38]. YAP1 overexpression in CRC cells is partly due the activation of β-catenin pathway. The β-catenin/TCF4 complexes bind to a DNA enhancer element within the first intron of the YAP1 gene to promote the transcription of YAP1[115]. The interplay between overexpressed YAP1 and β-catenin drives proliferation of colon cancer cells[116]. In KRAS-dependent cells when KRAS was suppressed, overexpression of YAP1 could rescue cell viability, suggesting that YAP1 was required for KRAS-induced cell transformation like EMT in CRC[117]. YAP1 transcription levels positively correlates with 5-fluorouracil resistance[118], relapse and shorter patient survival[119]. TAZ, the paralog of YAP1, regulates AXL in CRC and also plays an critical role in clonogenicity and non-adherent growth[120]. However, YAP1 is negatively regulated by E2A, which encodes two bHLH (basic helix-loop-helix) transcription factors E12 and E47[121].
In pancreatic ductal adenocarcinoma (PDAC), YAP1 was identified as a critical and functional downstream which plays a role in the oncogenic switch between KRAS pathway and MAPK (mitogen-activated protein kinase) pathway. YAP1 promotes the expression of genes which encode secretory factors. These factors accumulatively sustained the neoplastic proliferation, a stromal tumorigenic response from the tumor microenvironment[122]. The recent findings reveal that YAP1 and TEAD2, a TEAD transcriptional factor, interact and function cooperatively with E2F transcription factors to drive KRAS(G12D)-independent tumor initiation. In PDAC, this complex activates cell cycle progression and DNA synthesis program and finally escapes from the oncogenic KRAS addiction[123]. AGR2 up-regulates the expression of AREG, a EGFR ligand with growth-promoting potential, which is mediated by activation of YAP1 in PDAC[124]. YAP1 is negatively regulated by miR-141[125] and miR-375[126], which serves an independent prognostic factor for PDAC patients and exerts tumor suppressor functions.
In conclusion, the activation of Hippo signaling pathway is necessary for the homeostasis maintenance of gastric and other gastrointestinal organs. However, its deregulation causes tumorigenesis in digestive system through loss of control on YAP1/TAZ. The YAP1/TAZ hyperactivation in gastrointestinal cancers seems to drive tumorigenesis and take over carcinogenesis in a RAS-independent manner. Thus it provides a therapeutic target for clinical intervention.
However several issues should be addressed in the following study on Hippo pathway in GC as indicated in Figure 1. First, the detailed regulation mechanism on Hippo pathway in GC is not understood, thus the cell tight/adherens junction, cytoskeleton changes and other factors linked with Hippo pathway need to be addressed. Second, the left regulatory mechanisms on YAP1/TAZ should be addressed including miRNA/lncRNA regulation to facilitate our understanding on the regulation of YAP1/TAZ in tumorigenesis. Third but not the last, exploring novel transcription factors which are required for YAP1/TAZ to exert its function will be helpful to fully understand the oncogenic role of YAP1/TAZ in GC.
P- Reviewer: Loforese G S- Editor: Kong JX L- Editor: A E- Editor: Liu XM
| 1. | Yap P, Pantangco E, Yap A, Yap R. Surgical management of gastric carcinoma. Follow-up results in 465 consecutive cases. Am J Surg. 1982;143:284-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3179] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 3. | Sepulveda AR. Helicobacter, Inflammation, and Gastric Cancer. Curr Pathobiol Rep. 2013;1:9-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4829] [Article Influence: 439.0] [Reference Citation Analysis (2)] |
| 5. | Yasui W, Oue N, Aung PP, Matsumura S, Shutoh M, Nakayama H. Molecular-pathological prognostic factors of gastric cancer: a review. Gastric Cancer. 2005;8:86-94. [PubMed] |
| 6. | Wu WK, Cho CH, Lee CW, Fan D, Wu K, Yu J, Sung JJ. Dysregulation of cellular signaling in gastric cancer. Cancer Lett. 2010;295:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1948] [Cited by in RCA: 1880] [Article Influence: 125.3] [Reference Citation Analysis (0)] |
| 8. | Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 377] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 9. | Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747-2761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2041] [Cited by in RCA: 2456] [Article Influence: 144.5] [Reference Citation Analysis (0)] |
| 10. | Chan EH, Nousiainen M, Chalamalasetty RB, Schäfer A, Nigg EA, Silljé HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 478] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 11. | Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18:311-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 346] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 12. | Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28:2426-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 788] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 13. | Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 553] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 14. | Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962-1971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2068] [Cited by in RCA: 1980] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 15. | Vassilev A, Kaneko KJ, Shu H, Zhao Y, DePamphilis ML. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 2001;15:1229-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 585] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 16. | Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010;24:72-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 1139] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 17. | Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 953] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 18. | Kim NG, Koh E, Chen X, Gumbiner BM. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci USA. 2011;108:11930-11935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 555] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 19. | Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell. 2011;144:782-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 919] [Cited by in RCA: 863] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 20. | Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, Guan KL. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25:51-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 553] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 21. | Mohseni M, Sun J, Lau A, Curtis S, Goldsmith J, Fox VL, Wei C, Frazier M, Samson O, Wong KK. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nat Cell Biol. 2014;16:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 22. | Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1225] [Cited by in RCA: 1305] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 23. | Portela M, Parsons LM, Grzeschik NA, Richardson HE. Regulation of Notch signaling and endocytosis by the Lgl neoplastic tumor suppressor. Cell Cycle. 2015;14:1496-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs). Genes Dev. 2012;26:2138-2143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 237] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 25. | Tielbeek AV, Rosenbusch G, Muytjens HL, Yap SH, Strijk SP, Boetes C. Roentgenologic changes of the colon in Campylobacter infection. Gastrointest Radiol. 1985;10:358-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Wang W, Huang J, Chen J. Angiomotin-like proteins associate with and negatively regulate YAP1. J Biol Chem. 2011;286:4364-4370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 227] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 27. | Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4707] [Cited by in RCA: 4154] [Article Influence: 296.7] [Reference Citation Analysis (0)] |
| 28. | Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 632] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 29. | Kim M, Kim M, Lee S, Kuninaka S, Saya H, Lee H, Lee S, Lim DS. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 2013;32:1543-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 30. | Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill H. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell. 2010;18:579-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 461] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 31. | Chen HJ, Wang CM, Wang TW, Liaw GJ, Hsu TH, Lin TH, Yu JY. The Hippo pathway controls polar cell fate through Notch signaling during Drosophila oogenesis. Dev Biol. 2011;357:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | Fujii M, Toyoda T, Nakanishi H, Yatabe Y, Sato A, Matsudaira Y, Ito H, Murakami H, Kondo Y, Kondo E. TGF-β synergizes with defects in the Hippo pathway to stimulate human malignant mesothelioma growth. J Exp Med. 2012;209:479-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 33. | Imajo M, Miyatake K, Iimura A, Miyamoto A, Nishida E. A molecular mechanism that links Hippo signalling to the inhibition of Wnt/β-catenin signalling. EMBO J. 2012;31:1109-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 321] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 34. | Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, Zack TI, Wang X, Tsherniak A, Schinzel AC. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 644] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 35. | Ferrigno O, Lallemand F, Verrecchia F, L’Hoste S, Camonis J, Atfi A, Mauviel A. Yes-associated protein (YAP65) interacts with Smad7 and potentiates its inhibitory activity against TGF-beta/Smad signaling. Oncogene. 2002;21:4879-4884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 166] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 36. | Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, Rao BM, Dembowy J, Yaffe MB, Zandstra PW, Wrana JL. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10:837-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 546] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 37. | Tschaharganeh DF, Chen X, Latzko P, Malz M, Gaida MM, Felix K, Ladu S, Singer S, Pinna F, Gretz N. Yes-associated protein up-regulates Jagged-1 and activates the Notch pathway in human hepatocellular carcinoma. Gastroenterology. 2013;144:1530-1542.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 269] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 38. | Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci USA. 2011;108:E1312-E1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 390] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 39. | Zhang J, Ji JY, Yu M, Overholtzer M, Smolen GA, Wang R, Brugge JS, Dyson NJ, Haber DA. YAP-dependent induction of amphiregulin identifies a non-cell-autonomous component of the Hippo pathway. Nat Cell Biol. 2009;11:1444-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 40. | Chen L, Chan SW, Zhang X, Walsh M, Lim CJ, Hong W, Song H. Structural basis of YAP recognition by TEAD4 in the hippo pathway. Genes Dev. 2010;24:290-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 41. | Tian W, Yu J, Tomchick DR, Pan D, Luo X. Structural and functional analysis of the YAP-binding domain of human TEAD2. Proc Natl Acad Sci USA. 2010;107:7293-7298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 42. | Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 2010;24:862-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 797] [Cited by in RCA: 931] [Article Influence: 62.1] [Reference Citation Analysis (0)] |
| 43. | Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 904] [Cited by in RCA: 906] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 44. | Imoto I, Yang ZQ, Pimkhaokham A, Tsuda H, Shimada Y, Imamura M, Ohki M, Inazawa J. Identification of cIAP1 as a candidate target gene within an amplicon at 11q22 in esophageal squamous cell carcinomas. Cancer Res. 2001;61:6629-6634. [PubMed] |
| 45. | Imoto I, Tsuda H, Hirasawa A, Miura M, Sakamoto M, Hirohashi S, Inazawa J. Expression of cIAP1, a target for 11q22 amplification, correlates with resistance of cervical cancers to radiotherapy. Cancer Res. 2002;62:4860-4866. [PubMed] |
| 46. | Dai Z, Zhu WG, Morrison CD, Brena RM, Smiraglia DJ, Raval A, Wu YZ, Rush LJ, Ross P, Molina JR. A comprehensive search for DNA amplification in lung cancer identifies inhibitors of apoptosis cIAP1 and cIAP2 as candidate oncogenes. Hum Mol Genet. 2003;12:791-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 126] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Sudol M, Shields DC, Farooq A. Structures of YAP protein domains reveal promising targets for development of new cancer drugs. Semin Cell Dev Biol. 2012;23:827-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 48. | Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9:2145-2152. [PubMed] |
| 49. | Oka T, Mazack V, Sudol M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP). J Biol Chem. 2008;283:27534-27546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 295] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 50. | Paramasivam M, Sarkeshik A, Yates JR, Fernandes MJ, McCollum D. Angiomotin family proteins are novel activators of the LATS2 kinase tumor suppressor. Mol Biol Cell. 2011;22:3725-3733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 51. | Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem. 2003;278:33334-33341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 371] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 52. | Chan SW, Lim CJ, Loo LS, Chong YF, Huang C, Hong W. TEADs mediate nuclear retention of TAZ to promote oncogenic transformation. J Biol Chem. 2009;284:14347-14358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 53. | Lamar JM, Stern P, Liu H, Schindler JW, Jiang ZG, Hynes RO. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proc Natl Acad Sci USA. 2012;109:E2441-E2450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 483] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 54. | Oka T, Remue E, Meerschaert K, Vanloo B, Boucherie C, Gfeller D, Bader GD, Sidhu SS, Vandekerckhove J, Gettemans J. Functional complexes between YAP2 and ZO-2 are PDZ domain-dependent, and regulate YAP2 nuclear localization and signalling. Biochem J. 2010;432:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 55. | Muramatsu T, Imoto I, Matsui T, Kozaki K, Haruki S, Sudol M, Shimada Y, Tsuda H, Kawano T, Inazawa J. YAP is a candidate oncogene for esophageal squamous cell carcinoma. Carcinogenesis. 2011;32:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 56. | Chen D, Sun Y, Wei Y, Zhang P, Rezaeian AH, Teruya-Feldstein J, Gupta S, Liang H, Lin HK, Hung MC. LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med. 2012;18:1511-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 357] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 57. | Baia GS, Caballero OL, Orr BA, Lal A, Ho JS, Cowdrey C, Tihan T, Mawrin C, Riggins GJ. Yes-associated protein 1 is activated and functions as an oncogene in meningiomas. Mol Cancer Res. 2012;10:904-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 58. | Orr BA, Bai H, Odia Y, Jain D, Anders RA, Eberhart CG. Yes-associated protein 1 is widely expressed in human brain tumors and promotes glioblastoma growth. J Neuropathol Exp Neurol. 2011;70:568-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 59. | Lau AN, Curtis SJ, Fillmore CM, Rowbotham SP, Mohseni M, Wagner DE, Beede AM, Montoro DT, Sinkevicius KW, Walton ZE. Tumor-propagating cells and Yap/Taz activity contribute to lung tumor progression and metastasis. EMBO J. 2014;33:468-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 60. | Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103:12405-12410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 673] [Cited by in RCA: 760] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 61. | Bora-Singhal N, Nguyen J, Schaal C, Perumal D, Singh S, Coppola D, Chellappan S. YAP1 Regulates OCT4 Activity and SOX2 Expression to Facilitate Self-Renewal and Vascular Mimicry of Stem-Like Cells. Stem Cells. 2015;33:1705-1718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 62. | Fernandez-L A, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, Taylor MD, Kenney AM. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729-2741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 319] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 63. | Feng X, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A. Hippo-independent activation of YAP by the GNAQ uveal melanoma oncogene through a trio-regulated rho GTPase signaling circuitry. Cancer Cell. 2014;25:831-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 443] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 64. | Zhang X, George J, Deb S, Degoutin JL, Takano EA, Fox SB, Bowtell DD, Harvey KF. The Hippo pathway transcriptional co-activator, YAP, is an ovarian cancer oncogene. Oncogene. 2011;30:2810-2822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 239] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 65. | Levy D, Adamovich Y, Reuven N, Shaul Y. The Yes-associated protein 1 stabilizes p73 by preventing Itch-mediated ubiquitination of p73. Cell Death Differ. 2007;14:743-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 66. | Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, Mantovani F, Damalas A, Citro G, Sacchi A. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol Cell. 2005;18:447-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 306] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 67. | Lapi E, Di Agostino S, Donzelli S, Gal H, Domany E, Rechavi G, Pandolfi PP, Givol D, Strano S, Lu X. PML, YAP, and p73 are components of a proapoptotic autoregulatory feedback loop. Mol Cell. 2008;32:803-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 68. | Strano S, Blandino G. YAP1 meets tumor suppression. Mol Cell. 2007;27:863-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | van der Weyden L, Papaspyropoulos A, Poulogiannis G, Rust AG, Rashid M, Adams DJ, Arends MJ, O’Neill E. Loss of RASSF1A synergizes with deregulated RUNX2 signaling in tumorigenesis. Cancer Res. 2012;72:3817-3827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 70. | Zhou GX, Li XY, Zhang Q, Zhao K, Zhang CP, Xue CH, Yang K, Tian ZB. Effects of the hippo signaling pathway in human gastric cancer. Asian Pac J Cancer Prev. 2013;14:5199-5205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 71. | Xu ZP, Zhu JS, Zhang Q, Wang XY. A breakdown of the Hippo pathway in gastric cancer. Hepatogastroenterology. 2011;58:1611-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Kang W, Tong JH, Chan AW, Lee TL, Lung RW, Leung PP, So KK, Wu K, Fan D, Yu J. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin Cancer Res. 2011;17:2130-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 209] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 73. | Min B, Kim MK, Zhang JW, Kim J, Chung KC, Oh BC, Stein GS, Lee YH, van Wijnen AJ, Bae SC. Identification of RUNX3 as a component of the MST/Hpo signaling pathway. J Cell Physiol. 2012;227:839-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Lay V, Yap J, Sonderegger S, Dimitriadis E. Interleukin 11 regulates endometrial cancer cell adhesion and migration via STAT3. Int J Oncol. 2012;41:759-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Li XJ, Park ES, Park MH, Kim SM. 3,3’-Diindolylmethane suppresses the growth of gastric cancer cells via activation of the Hippo signaling pathway. Oncol Rep. 2013;30:2419-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Lam-Himlin DM, Daniels JA, Gayyed MF, Dong J, Maitra A, Pan D, Montgomery EA, Anders RA. The hippo pathway in human upper gastrointestinal dysplasia and carcinoma: a novel oncogenic pathway. Int J Gastrointest Cancer. 2006;37:103-109. [PubMed] |
| 77. | Da CL, Xin Y, Zhao J, Luo XD. Significance and relationship between Yes-associated protein and survivin expression in gastric carcinoma and precancerous lesions. World J Gastroenterol. 2009;15:4055-4061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 78. | Vitolo MI, Anglin IE, Mahoney WM, Renoud KJ, Gartenhaus RB, Bachman KE, Passaniti A. The RUNX2 transcription factor cooperates with the YES-associated protein, YAP65, to promote cell transformation. Cancer Biol Ther. 2007;6:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 79. | Cui ZL, Han FF, Peng XH, Chen X, Luan CY, Han RC, Xu WG, Guo XJ. YES-associated protein 1 promotes adenocarcinoma growth and metastasis through activation of the receptor tyrosine kinase Axl. Int J Immunopathol Pharmacol. 2012;25:989-1001. [PubMed] |
| 80. | Hu X, Xin Y, Xiao Y, Zhao J. Overexpression of YAP1 is correlated with progression, metastasis and poor prognosis in patients with gastric carcinoma. Pathol Oncol Res. 2014;20:805-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 81. | Zhang J, Yang YC, Zhu JS, Zhou Z, Chen WX. Clinicopathologic characteristics of YES-associated protein 1 overexpression and its relationship to tumor biomarkers in gastric cancer. Int J Immunopathol Pharmacol. 2012;25:977-987. [PubMed] |
| 82. | Zhang J, Xu ZP, Yang YC, Zhu JS, Zhou Z, Chen WX. Expression of Yes-associated protein in gastric adenocarcinoma and inhibitory effects of its knockdown on gastric cancer cell proliferation and metastasis. Int J Immunopathol Pharmacol. 2012;25:583-590. [PubMed] |
| 83. | Song M, Cheong JH, Kim H, Noh SH, Kim H. Nuclear expression of Yes-associated protein 1 correlates with poor prognosis in intestinal type gastric cancer. Anticancer Res. 2012;32:3827-3834. [PubMed] |
| 84. | Zhou Z, Zhu JS, Xu ZP, Zhang Q. Lentiviral vector-mediated siRNA knockdown of the YAP gene inhibits growth and induces apoptosis in the SGC7901 gastric cancer cell line. Mol Med Rep. 2011;4:1075-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 85. | Kang W, Tong JH, Lung RW, Dong Y, Zhao J, Liang Q, Zhang L, Pan Y, Yang W, Pang JC. Targeting of YAP1 by microRNA-15a and microRNA-16-1 exerts tumor suppressor function in gastric adenocarcinoma. Mol Cancer. 2015;14:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 86. | Deng J, Lei W, Xiang X, Zhang L, Yu F, Chen J, Feng M, Xiong J. MicroRNA-506 inhibits gastric cancer proliferation and invasion by directly targeting Yap1. Tumour Biol. 2015;36:6823-6831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 87. | Li H, Wang Z, Zhang W, Qian K, Liao G, Xu W, Zhang S. VGLL4 inhibits EMT in part through suppressing Wnt/β-catenin signaling pathway in gastric cancer. Med Oncol. 2015;32:83. [PubMed] |
| 88. | Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X, He F, Wang Y, Zhang Z, Wang W. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell. 2014;25:166-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 485] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 89. | Kakiuchi M, Nishizawa T, Ueda H, Gotoh K, Tanaka A, Hayashi A, Yamamoto S, Tatsuno K, Katoh H, Watanabe Y. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet. 2014;46:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 419] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 90. | Wang K, Yuen ST, Xu J, Lee SP, Yan HH, Shi ST, Siu HC, Deng S, Chu KM, Law S. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat Genet. 2014;46:573-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 830] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 91. | Lim B, Park JL, Kim HJ, Park YK, Kim JH, Sohn HA, Noh SM, Song KS, Kim WH, Kim YS. Integrative genomics analysis reveals the multilevel dysregulation and oncogenic characteristics of TEAD4 in gastric cancer. Carcinogenesis. 2014;35:1020-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 92. | Sun L, Chen F, Shi W, Qi L, Zhao Z, Zhang J. Prognostic impact of TAZ and β-catenin expression in adenocarcinoma of the esophagogastric junction. Diagn Pathol. 2014;9:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 93. | Yu FX, Meng Z, Plouffe SW, Guan KL. Hippo pathway regulation of gastrointestinal tissues. Annu Rev Physiol. 2015;77:201-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 94. | Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA. 2010;107:8248-8253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 398] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 95. | Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, Chen Y, Park O, Chang J, Simpson RM. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci USA. 2010;107:1431-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 471] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 96. | Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 756] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 97. | Xu C, Liu C, Huang W, Tu S, Wan F. Effect of Mst1 overexpression on the growth of human hepatocellular carcinoma HepG2 cells and the sensitivity to cisplatin in vitro. Acta Biochim Biophys Sin (Shanghai). 2013;45:268-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 98. | Wang C, Zhu ZM, Liu CL, He XJ, Zhang HY, Dong JH. Knockdown of yes-associated protein inhibits proliferation and downregulates large tumor suppressor 1 expression in MHCC97H human hepatocellular carcinoma cells. Mol Med Rep. 2015;11:4101-4108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 99. | Piccolo S, Cordenonsi M, Dupont S. Molecular pathways: YAP and TAZ take center stage in organ growth and tumorigenesis. Clin Cancer Res. 2013;19:4925-4930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 100. | Fitamant J, Kottakis F, Benhamouche S, Tian HS, Chuvin N, Parachoniak CA, Nagle JM, Perera RM, Lapouge M, Deshpande V. YAP Inhibition Restores Hepatocyte Differentiation in Advanced HCC, Leading to Tumor Regression. Cell Rep. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 101. | Perra A, Kowalik MA, Ghiso E, Ledda-Columbano GM, Di Tommaso L, Angioni MM, Raschioni C, Testore E, Roncalli M, Giordano S. YAP activation is an early event and a potential therapeutic target in liver cancer development. J Hepatol. 2014;61:1088-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 102. | Han SX, Bai E, Jin GH, He CC, Guo XJ, Wang LJ, Li M, Ying X, Zhu Q. Expression and clinical significance of YAP, TAZ, and AREG in hepatocellular carcinoma. J Immunol Res. 2014;2014:261365. [PubMed] |
| 103. | Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, Lowe SW, Poon RT, Luk JM. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576-4585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 421] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 104. | Shimomura T, Miyamura N, Hata S, Miura R, Hirayama J, Nishina H. The PDZ-binding motif of Yes-associated protein is required for its co-activation of TEAD-mediated CTGF transcription and oncogenic cell transforming activity. Biochem Biophys Res Commun. 2014;443:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 105. | Wang J, Ma L, Weng W, Qiao Y, Zhang Y, He J, Wang H, Xiao W, Li L, Chu Q. Mutual interaction between YAP and CREB promotes tumorigenesis in liver cancer. Hepatology. 2013;58:1011-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 106. | Zhang T, Zhang J, You X, Liu Q, Du Y, Gao Y, Shan C, Kong G, Wang Y, Yang X. Hepatitis B virus X protein modulates oncogene Yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology. 2012;56:2051-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 107. | Li X, Tao J, Cigliano A, Sini M, Calderaro J, Azoulay D, Wang C, Liu Y, Jiang L, Evert K. Co-activation of PIK3CA and Yap promotes development of hepatocellular and cholangiocellular tumors in mouse and human liver. Oncotarget. 2015;6:10102-10115. [PubMed] |
| 108. | Li L, Wang J, Zhang Y, Zhang Y, Ma L, Weng W, Qiao Y, Xiao W, Wang H, Yu W. MEK1 promotes YAP and their interaction is critical for tumorigenesis in liver cancer. FEBS Lett. 2013;587:3921-3927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 109. | Yi C, Shen Z, Stemmer-Rachamimov A, Dawany N, Troutman S, Showe LC, Liu Q, Shimono A, Sudol M, Holmgren L. The p130 isoform of angiomotin is required for Yap-mediated hepatic epithelial cell proliferation and tumorigenesis. Sci Signal. 2013;6:ra77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 110. | Mao B, Hu F, Cheng J, Wang P, Xu M, Yuan F, Meng S, Wang Y, Yuan Z, Bi W. SIRT1 regulates YAP2-mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene. 2014;33:1468-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 111. | Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, Mahadevan N, Fitamant J, Bardeesy N, Camargo FD, Guan KL. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14:1322-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 392] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 112. | Xu MZ, Chan SW, Liu AM, Wong KF, Fan ST, Chen J, Poon RT, Zender L, Lowe SW, Hong W. AXL receptor kinase is a mediator of YAP-dependent oncogenic functions in hepatocellular carcinoma. Oncogene. 2011;30:1229-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 113. | Wierzbicki PM, Adrych K, Kartanowicz D, Stanislawowski M, Kowalczyk A, Godlewski J, Skwierz-Bogdanska I, Celinski K, Gach T, Kulig J. Underexpression of LATS1 TSG in colorectal cancer is associated with promoter hypermethylation. World J Gastroenterol. 2013;19:4363-4373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 114. | Serrano I, McDonald PC, Lock F, Muller WJ, Dedhar S. Inactivation of the Hippo tumour suppressor pathway by integrin-linked kinase. Nat Commun. 2013;4:2976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 115. | Konsavage WM, Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/β-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem. 2012;287:11730-11739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 116. | Avruch J, Zhou D, Bardeesy N. YAP oncogene overexpression supercharges colon cancer proliferation. Cell Cycle. 2012;11:1090-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 117. | Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 625] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 118. | Touil Y, Igoudjil W, Corvaisier M, Dessein AF, Vandomme J, Monté D, Stechly L, Skrypek N, Langlois C, Grard G. Colon cancer cells escape 5FU chemotherapy-induced cell death by entering stemness and quiescence associated with the c-Yes/YAP axis. Clin Cancer Res. 2014;20:837-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 119. | Wang L, Shi S, Guo Z, Zhang X, Han S, Yang A, Wen W, Zhu Q. Overexpression of YAP and TAZ is an independent predictor of prognosis in colorectal cancer and related to the proliferation and metastasis of colon cancer cells. PLoS One. 2013;8:e65539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 120. | Yuen HF, McCrudden CM, Huang YH, Tham JM, Zhang X, Zeng Q, Zhang SD, Hong W. TAZ expression as a prognostic indicator in colorectal cancer. PLoS One. 2013;8:e54211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 121. | Zhao H, Huang A, Li P, Quan Y, Feng B, Chen X, Mao Z, Zhu Z, Zheng M. E2A suppresses invasion and migration by targeting YAP in colorectal cancer cells. J Transl Med. 2013;11:317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 122. | Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G, Gupta S, Vietsch EE, Laughlin SZ, Wadhwa M. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014;7:ra42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 123. | Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 559] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 124. | Dong A, Gupta A, Pai RK, Tun M, Lowe AW. The human adenocarcinoma-associated gene, AGR2, induces expression of amphiregulin through Hippo pathway co-activator YAP1 activation. J Biol Chem. 2011;286:18301-18310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 125. | Zhu ZM, Xu YF, Su QJ, Du JD, Tan XL, Tu YL, Tan JW, Jiao HB. Prognostic significance of microRNA-141 expression and its tumor suppressor function in human pancreatic ductal adenocarcinoma. Mol Cell Biochem. 2014;388:39-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 126. | Zhang ZW, Men T, Feng RC, Li YC, Zhou D, Teng CB. miR-375 inhibits proliferation of mouse pancreatic progenitor cells by targeting YAP1. Cell Physiol Biochem. 2013;32:1808-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |