Published online Aug 7, 2016. doi: 10.3748/wjg.v22.i29.6736
Peer-review started: April 15, 2016
First decision: May 12, 2016
Revised: May 20, 2016
Accepted: June 29, 2016
Article in press: June 29, 2016
Published online: August 7, 2016
Processing time: 105 Days and 22.9 Hours
AIM: To identify clinicopathological factors predictive of lymph node metastasis (LNM) in intramucosal poorly differentiated early gastric cancer (EGC), and further to expand the possibility of using endoscopic submucosal dissection (ESD) for the treatment of intramucosal poorly differentiated EGC.
METHODS: Data for 81 surgically treated patients with intramucosal poorly differentiated EGC were collected, and the association between the clinicopathological factors and the presence of LNM was retrospectively analyzed by univariate and multivariate logistic regression analyses. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Several clinicopathologic factors were investigated to identify predictive factors for lymph nodes metastasis, including gender, age, family history of gastric cancer, number of tumors, tumor location, ulceration, tumor size, macroscopic type, lymphatic vessel involvement, and signet-ring-cell component.
RESULTS: Tumor size (OR = 7.273, 95%CI: 1.246-29.918, P = 0.042), lymphatic vessel involvement (OR = 42.219, 95%CI: 1.923-97.052, P = 0.018) and signet-ring-cell component (OR = 17.513, 95%CI: 1.647-77.469, P = 0.034) that were significantly associated with LNM by univariate analysis, were found to be significant and independent risk factors for LNM by multivariate analysis. However, gender, age, family history of gastric cancer, number, location, ulceration and macroscopic type of tumor were found not to be associated with LNM. Of these 81 patients diagnosed with intramucosal poorly differentiated EGC, 7 (8.6%) had LNM. The LNM rates were 9.1%, 22.2% and 57.1%, respectively, in cases with one, two and three of the risk factors. There was no LNM in 54 patients without the three risk clinicopathological factors.
CONCLUSION: Tumor size, lymphatic vessel involvement and signet-ring-cell component are independently associated with the presence of LNM in intramucosal poorly differentiated EGC. Thus, these three risk factors may be used as a simple criterion to expand the possibility of using ESD for the treatment of intramucosal poorly differentiated EGC.
Core tip: Endoscopic submucosal dissection (ESD) has recently been practiced on a differentiated type of early gastric cancer (EGC). However, there is no clear evidence for endoscopic treatment of intramucosal poorly differentiated EGC. We carried out this retrospectively study to determine the clinicopathological factors that are predictive of lymph node metastasis in intramucosal poorly differentiated EGC, and to guide the individual application of ESD in a suitable subgroup of patients with intramucosal poorly differentiated EGC.
- Citation: Li H, Huo ZB, Chen SB, Li H, Wu DC, Zhai TS, Xiao QH, Wang SX, Zhang LL. Feasibility study on expanded indication for endoscopic submucosal dissection of intramucosal poorly differentiated early gastric cancer. World J Gastroenterol 2016; 22(29): 6736-6741
- URL: https://www.wjgnet.com/1007-9327/full/v22/i29/6736.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i29.6736
The minimalization of therapeutic invasiveness to preserve quality of life is a major topic in the management of early gastric cancer (EGC). Endoscopic submucosal dissection (ESD) has been widely accepted as an alternative treatment to surgery for EGC[1-3]. This minimally invasive technique could be used in EGC management as well as avoiding risk of lymph node metastasis (LNM)[4-9]. For the reason of higher LNM risk in undifferentiated EGC, ESD application has been limited to well or moderately differentiated EGC with a diameter smaller than 2 cm and confined to the mucosa without ulceration[10,11]. Thus, gastrectomy with lymphadenectomy is now considered an indispensable treatment for patients with undifferentiated EGC. Undifferentiated gastric cancer was divided into signet ring cell carcinoma, poorly differentiated adenocarcinoma, and mucinous adenocarcinoma[12]. Nevertheless, about 96.6% of poorly differentiated EGC cases which are limited to the mucosa, were found not to have LNM[13], indicating that gastrectomy with lymphadenectomy may be an overtreatment among them.
Thus, we performed this current retrospective study to identify the clinicopathological factors which can be predictive in diagnosis of LNM in poorly differentiated EGC, and to guide the individual application of ESD in a suitable subgroup of patients with intramucosal poorly differentiated EGC.
The patients were enrolled from the Department of Oncology, Affiliated Xingtai People’s Hospital of Hebei Medical University, Xingtai, China between January 1987 and December 2007, and all these patients had undergone a radical check for identification of EGC.
The patients who met the following inclusion criteria were enrolled: (1) Patients who underwent lymph node dissection beyond limited (D1) dissection; (2) Patients who were diagnosed with intramucosal poorly differentiated EGC by pathological analysis of lymph nodes and resected specimens according to the Japanese Classification of Gastric Carcinoma (JCGC)[12]; and (3) Records can be retrieved in database.
Eighty-one patients (23 females and 58 males; mean age: 48 years; age range: 29 to 79 years) were identified to meet the inclusion criteria and were included for the following analysis.
The study protocol was approved by the Ethics Committee of Hebei Medical University.
All the lymph nodes from each case were dissected with great care from the en bloc specimens, and a well-trained surgeon was appointed to classify the dissected lymph nodes after she or he reviewed the excised specimens carefully based on the JCGC[12]. Afterward, the lymph nodes were sectioned and then stained with eosin and hematoxylin, followed by pathological examination for lymphatic vessel involvement (LVI) and metastasis using immunohistochemistry with D2-40.
Clinicopathological parameters from the JCGC[12] were included in this current study, which consisted of gender (female and male), age (< 60 years and ≥ 60 years), family history of gastric cancer, tumor number (single or multitude), tumor location (in lower, middle, or upper location of the stomach), ulceration, tumor size (maximum diameter ≥ 2 cm or < 2 cm), macroscopic type [protruded (type I), superficially elevated (type IIa), flat (type IIb), superficially depressed (type IIc), or excavated (type III)], lymphatic vessel involvement, signet-ring-cell component (intermingled components of signet-ring-cell cancer cells within a cancerous lesion). The association between LNM and various clinicopathological factors was examined as described below.
All data were analyzed using SPSS18.0 (Chicago, IL, United States). The differences between patients with and without LNM in the clinicopathological parameters were determined by the χ2 test. Independent risk factors for LNM were determined using multivariate stepwise logistic regression analysis. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. P < 0.05 was considered statistically significant.
The association between LNM and various clinicopathological characteristics was determined by χ2 test (Table 1). Tumor diameter ≥ 2.0 cm, LVI, and signet-ring-cell cancer cell intermingled components were significantly associated with a high LNM rate (P < 0.05 for all).
| Factor | Number of cases with lymph node metastasis | P value |
| Sex | ||
| Male (n = 58) | 4 (6.9) | 0.421 |
| Female (n = 23) | 3 (13.0) | |
| Age (yr) | ||
| < 60 (n = 39) | 2 (5.1) | 0.319 |
| ≥ 60 (n = 42) | 5 (11.9) | |
| Family history | ||
| Positive (n = 15) | 3 (20.0) | 0.126 |
| Negative (n = 66) | 4 (6.1) | |
| Number of tumors | ||
| Single (n = 77) | 6 (7.8) | 0.305 |
| Multitude (n = 4) | 1 (25.0) | |
| Location | ||
| Upper (n = 6) | 1 (16.7) | 0.247 |
| Middle (n = 16) | 3 (18.8) | |
| Lower (n = 59) | 3 (5.1) | |
| Ulceration | ||
| Negative (n = 70) | 5 (71.4) | 0.284 |
| Positive (n = 11) | 2 (18.2) | |
| Tumor size in diameter | ||
| < 2 cm (n = 59) | 2 (3.4) | 0.015 |
| ≥ 2 cm (n = 22) | 5 ( 22.7) | |
| Macroscopic type | ||
| I (n = 4) | 1 (25.0) | 0.524 |
| II (n = 43) | 4 (9.3) | |
| III (n = 34) | 2 (5.9) | |
| Lymphatic vessel involvement | ||
| Negative (n = 67) | 1 (1.5) | < 0.001 |
| Positive (n = 14) | 6 (42.9) | |
| Signet-ring-cell component1 | ||
| Absence (n = 74) | 4 (5.4) | 0.006 |
| Presence (n = 7) | 3 (42.9) |
On the other side, gender, age, family history of gastric cancer, tumor number, ulceration, location and type showed no significant association with LNM.
Multivariate analysis results showed that the factors which were significantly associated with a high LNM rate from univariate analysis were also significant for LNM (P < 0.05 for both) and are independent risk factors for LNM (Table 2).
| Characteristic | OR | 95%CI | P value |
| Tumor size | 7.273 | 1.246-29.918 | 0.042 |
| < 2 cm | |||
| ≥ 2 cm | |||
| Lymphatic vessel involvement | 42.219 | 1.923-97.052 | 0.018 |
| Negative | |||
| Positive | |||
| Signet-ring-cell component | 17.513 | 1.647-77.469 | 0.034 |
| Absence | |||
| Presence |
Of the 81 cases, LNM was diagnosed by histology in 7 (8.6%) patients. The LNM rates were 9.1%, 22.2% and 57.1% in intramucosal poorly-differentiated EGC for patients with one, two or three risk factors, respectively. LNM was not found in other 54 patients without one or more of three risk factors (Table 3).
| Number of risk factors | Lymph metastasis rate |
| None | 0% (0/54) |
| One | 9.1% (1/11) |
| Two | 22.2% (2/9) |
| Three | 57.1% (4/7) |
As a result of advances in diagnostic technology, including both the radiologic and endoscopic modalities, the detection rate of EGC has increased. Since EGC is associated with a favorable prognosis, many efforts and studies have been made to minimize resection invasiveness. Endoscopic mucosal resection (EMR) and ESD are included in the treatment of EGC. Compared with EMR, ESD has an advantage of allowing en bloc resection by dissection at submucosal location, which leads to accurate pathologic assessment of specimens[14-16]. ESD can maintain gastric function and keep a high life quality[17-22]. Nevertheless, currently the application of ESD is confined to differentiated EGC. One reason for choosing ESD is whether the presence of LNM or not can be precisely predicted. Thus, we tried to broaden the application of ESD to poorly differentiated EGC using retrospective examination of intramucosal poorly differentiated EGC to confirm how LNM can be predicted.
The multivariate and univariate analysis results indicated that a tumor ≥ 2.0 cm, LVI, and intermingled components of signet-ring-cell cancer cells were factors to predict LNM for patients with intramucosal poorly differentiated EGC. Current study results together with results from previous reports about undifferentiated EGC demonstrate that there is a significant correlation of the presence of LVI, large tumor and submucosal invasion with high LNM rate[23-30].
We tried to determine a subgroup of patients with intramucosal poorly differentiated EGC among whom we can rule out the risk of LNM, i.e., candidates who can be cured by ESD. Interestingly, we have not found LNM in patients without one or more of the three risk factors. This may be due to that ESD is sufficient in treating these cases, and no additional surgery is needed.
We further studied the association between the LNM rate and the number of three risk factors (tumor ≥ 2.0 cm, presence of LVI, and intermingled components of signet-ring-cell cancer cells) so that we can have a simple criterion to confirm what is an ideal treatment strategy for intramucosal poorly differentiated EGC. In the current study, the LNM rates were 9.1%, 22.2%, and 57.1% in cases with one, two or three risk factors, respectively. Therefore, for these patients gastrectomy with lymphadenectomy may be a better choice.
The present study has some limitations. First, this is a single-center retrospective study. Second, the small sample size was small. Thus, the results may not be sufficient to come to a definitive conclusion.
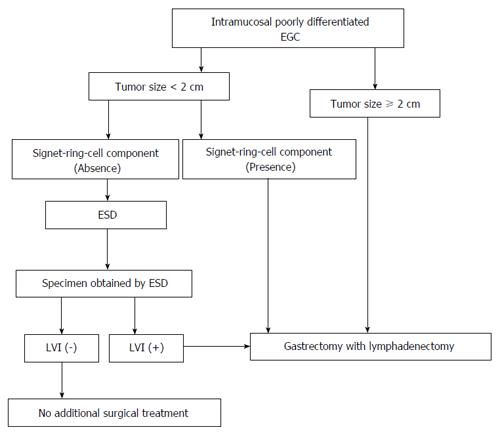
As the study results suggest, we would like to propose a new treatment for patients with intramucosal poorly differentiated EGC (Figure 1). For patients without any of the risk factors, ESD without lymphadenectomy is sufficient. When LVI is confirmed in specimens, gastrectomy with lymphadenectomy may be a better choice for these patients.
Gastrectomy with lymphadenectomy is a standard treatment for patients with poorly differentiated early gastric cancer (EGC) with lymph node metastasis (LNM). Nevertheless, about 96.6% of cases are confined to mucosa, otherwise many (approximately 80%) patients with submucosal extension were demonstrated not to have LNM, and for these patients, gastrectomy with lymphadenectomy might be an overtreatment. The authors tried to determine a subgroup of patients with intramucosal poorly differentiated EGC among whom we can rule out the risk of LNM so that these patients can be treated by endoscopic submucosal dissection (ESD), and this may be a breakthrough treatment for poorly differentiated EGC.
Some previous studies have tried to determine the risk factors which can predict LNM in EGC. However, only few reports have studied the possible applicability of ESD.
In poorly differentiated EGC, lymphatic vessel involvement, depth of invasion and tumor size were demonstrated to be independent risk factors for LNM. Additionally, the study developed a simple criterion which can help increase the usage of ESD to treat intramucosal poorly differentiated EGC.
The results of predictive factors of LNM suggest that ESD is an optional choice for treatment of intramucosal poorly differentiated EGC.
Compared with EMR, ESD has an advantage of allowing en bloc resection by dissection at submucosal location, which leads to accurate pathologic assessment of specimens. ESD can maintain gastric function and keep a high life quality and is an optional technique for minimally invasive treatment.
In the present study, authors identified predictive factors for LNM in intramucosal poorly differentiated EGC, and expanded usage of ESD for intramucosal poorly differentiated EGC treatment. Results indicated that tumor size, lymphatic vessel involvement and signet-ring-cell component that were significantly associated with LNM by univariate analysis, were found to be significant and independent risk factors for LNM by multivariate analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chandrakesan P, McHenry L S- Editor: Gong ZM L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Kang KJ, Kim KM, Min BH, Lee JH, Kim JJ. Endoscopic submucosal dissection of early gastric cancer. Gut Liver. 2011;5:418-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Mukasa M, Takedatsu H, Matsuo K, Sumie H, Yoshida H, Hinosaka A, Watanabe Y, Tsuruta O, Torimura T. Clinical characteristics and management of gastric tube cancer with endoscopic submucosal dissection. World J Gastroenterol. 2015;21:919-925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Hoteya S, Yahagi N, Iizuka T, Kikuchi D, Mitani T, Matsui A, Ogawa O, Yamashita S, Furuhata T, Yamada A. Endoscopic submucosal dissection for nonampullary large superficial adenocarcinoma/adenoma of the duodenum: feasibility and long-term outcomes. Endosc Int Open. 2013;1:2-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | Koeda K, Nishizuka S, Wakabayashi G. Minimally invasive surgery for gastric cancer: the future standard of care. World J Surg. 2011;35:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Nozaki I, Kubo Y, Kurita A, Tanada M, Yokoyama N, Takiyama W, Takashima S. Long-term outcome after laparoscopic wedge resection for early gastric cancer. Surg Endosc. 2008;22:2665-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Yoshida K, Yamaguchi K, Okumura N, Osada S, Takahashi T, Tanaka Y, Tanabe K, Suzuki T. The roles of surgical oncologists in the new era: minimally invasive surgery for early gastric cancer and adjuvant surgery for metastatic gastric cancer. Pathobiology. 2011;78:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Fatourou E, Roukos DH. Endoscopic submucosal dissection: can it safely expand indications for a minimally invasive approach to patients with early gastric cancer? Surg Endosc. 2010;24:1793-174; author reply 1795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Hyung WJ, Cheong JH, Kim J, Chen J, Choi SH, Noh SH. Application of minimally invasive treatment for early gastric cancer. J Surg Oncol. 2004;85:181-185; discussion 186. [PubMed] |
| 9. | Son T, Kwon IG, Hyung WJ. Minimally invasive surgery for gastric cancer treatment: current status and future perspectives. Gut Liver. 2014;8:229-236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1134] [Cited by in RCA: 1149] [Article Influence: 47.9] [Reference Citation Analysis (4)] |
| 11. | Abe N, Watanabe T, Suzuki K, Machida H, Toda H, Nakaya Y, Masaki T, Mori T, Sugiyama M, Atomi Y. Risk factors predictive of lymph node metastasis in depressed early gastric cancer. Am J Surg. 2002;183:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition -. Gastric Cancer. 1998;1:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 1942] [Article Influence: 71.9] [Reference Citation Analysis (0)] |
| 13. | Park YD, Chung YJ, Chung HY, Yu W, Bae HI, Jeon SW, Cho CM, Tak WY, Kweon YO. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy. 2008;40:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 14. | Kakushima N, Fujishiro M, Kodashima S, Muraki Y, Tateishi A, Yahagi N, Omata M. Technical feasibility of endoscopic submucosal dissection for gastric neoplasms in the elderly Japanese population. J Gastroenterol Hepatol. 2007;22:311-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 299] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 16. | Yokoi C, Gotoda T, Hamanaka H, Oda I. Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc. 2006;64:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, Kim JM, Kim YI, Ryu KW, Kong SH. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014;14:87-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 18. | Kitagawa Y, Kitano S, Kubota T, Kumai K, Otani Y, Saikawa Y, Yoshida M, Kitajima M. Minimally invasive surgery for gastric cancer--toward a confluence of two major streams: a review. Gastric Cancer. 2005;8:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Makuuchi H, Kise Y, Shimada H, Chino O, Tanaka H. Endoscopic mucosal resection for early gastric cancer. Semin Surg Oncol. 1999;17:108-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Ishikawa S, Togashi A, Inoue M, Honda S, Nozawa F, Toyama E, Miyanari N, Tabira Y, Baba H. Indications for EMR/ESD in cases of early gastric cancer: relationship between histological type, depth of wall invasion, and lymph node metastasis. Gastric Cancer. 2007;10:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 110] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Oda I, Saito D, Tada M, Iishi H, Tanabe S, Oyama T, Doi T, Otani Y, Fujisaki J, Ajioka Y. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 22. | Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 527] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 23. | Kim YY, Jeon SW, Kim J, Park JC, Cho KB, Park KS, Kim E, Chung YJ, Kwon JG, Jung JT. Endoscopic submucosal dissection for early gastric cancer with undifferentiated histology: could we extend the criteria beyond? Surg Endosc. 2013;27:4656-4662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Kim KJ, Park SJ, Moon W, Park MI. Analysis of factors related to lymph node metastasis in undifferentiated early gastric cancer. Turk J Gastroenterol. 2011;22:139-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Li C, Kim S, Lai JF, Oh SJ, Hyung WJ, Choi WH, Choi SH, Zhu ZG, Noh SH. Risk factors for lymph node metastasis in undifferentiated early gastric cancer. Ann Surg Oncol. 2008;15:764-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Lee H, Lee JH. Expanding indications of endoscopic submucosal dissection for early gastric cancer: hope or hype? Gut Liver. 2015;9:135-136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Ichikura T, Uefuji K, Tomimatsu S, Okusa Y, Yahara T, Tamakuma S. Surgical strategy for patients with gastric carcinoma with submucosal invasion. A multivariate analysis. Cancer. 1995;76:935-940. [PubMed] |
| 28. | Lee H, Yun WK, Min BH, Lee JH, Rhee PL, Kim KM, Rhee JC, Kim JJ. A feasibility study on the expanded indication for endoscopic submucosal dissection of early gastric cancer. Surg Endosc. 2011;25:1985-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Kunisaki C, Takahashi M, Nagahori Y, Fukushima T, Makino H, Takagawa R, Kosaka T, Ono HA, Akiyama H, Moriwaki Y. Risk factors for lymph node metastasis in histologically poorly differentiated type early gastric cancer. Endoscopy. 2009;41:498-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Komatsu S, Ichikawa D, Miyamae M, Shimizu H, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Kishimoto M, Otsuji E. Histological mixed-type as an independent prognostic factor in stage I gastric carcinoma. World J Gastroenterol. 2015;21:549-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |