Published online Jul 28, 2016. doi: 10.3748/wjg.v22.i28.6484
Peer-review started: April 7, 2016
First decision: May 12, 2016
Revised: May 24, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: July 28, 2016
Processing time: 111 Days and 21.4 Hours
Hepatitis due to hepatitis B virus (HBV) reactivation can be severe and potentially fatal, but is preventable. HBV reactivation is most commonly reported in patients receiving cancer chemotherapy, especially rituximab-containing therapy for hematological malignancies and those receiving stem cell transplantation. All patients with hematological malignancies receiving anticancer therapy should be screened for active or resolved HBV infection by blood tests for hepatitis B surface antigen (HBsAg) and antibody to hepatitis B core antigen (anti-HBc). Patients found to be positive for HBsAg should be given prophylactic antiviral therapy to prevent HBV reactivation. For patients with resolved HBV infection, no standard strategy has yet been established to prevent HBV reactivation. There are usually two options. One is pre-emptive therapy guided by serial HBV DNA monitoring, whereby antiviral therapy is given as soon as HBV DNA becomes detectable. However, there is little evidence regarding the optimal interval and period of monitoring. An alternative approach is prophylactic antiviral therapy, especially for patients receiving high-risk therapy such as rituximab, newer generation of anti-CD20 monoclonal antibody, obinutuzumab or hematopoietic stem cell transplantation. This strategy may effectively prevent HBV reactivation and avoid the inconvenience of repeated HBV DNA monitoring. Entecavir or tenofovir are preferred over lamivudine as prophylactic therapy. Although there is no well-defined guideline on the optimal duration of prophylactic therapy, there is growing evidence to recommend continuing prophylactic antiviral therapy for at least 12 mo after cessation of chemotherapy, and even longer for those who receive rituximab or who had high serum HBV DNA levels before the start of immunosuppressive therapy. Many novel agents have recently become available for the treatment of hematological malignancies, and these agents may be associated with HBV reactivation. Although there is currently limited evidence to guide the optimal preventive measures, we recommend antiviral prophylaxis in HBsAg-positive patients receiving novel treatments, especially the Bruton tyrosine kinase inhibitors and the phosphatidylinositol 3-kinase inhibitors, which are B-cell receptor signaling modulators and reduce proliferation of malignant B-cells. Further studies are needed to clarify the risk of HBV reactivation with these agents and the best prophylactic strategy in the era of targeted therapy for hematological malignancies.
Core tip: Hepatitis due to hepatitis B virus (HBV) reactivation can be severe and potentially fatal. All patients with hematological malignancies receiving anticancer therapy should be screened for hepatitis B surface antigen (HBsAg) and antibody to hepatitis B core antigen. Patients found to be positive for HBsAg should be given prophylactic antiviral therapy. For patients with resolved HBV infection, either pre-emptive therapy guided by serial HBV DNA monitoring or prophylactic antiviral therapy, especially for patients receiving high-risk therapy are reasonable options. Further studies are needed to find out the best prophylactic strategy in the era of targeted therapy for hematological malignancies.
- Citation: Law MF, Ho R, Cheung CK, Tam LH, Ma K, So KC, Ip B, So J, Lai J, Ng J, Tam TH. Prevention and management of hepatitis B virus reactivation in patients with hematological malignancies treated with anticancer therapy. World J Gastroenterol 2016; 22(28): 6484-6500
- URL: https://www.wjgnet.com/1007-9327/full/v22/i28/6484.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i28.6484
Patients infected with hepatitis B virus (HBV) are at risk of reactivation of the virus if they receive chemotherapy, even if they have chronic or resolved HBV infection. HBV reactivation is most commonly reported in patients receiving cancer chemotherapy for haematological malignancies or haemopoietic stem cell transplantation (HSCT) recipients, although it has also been reported in patients receiving treatment for solid tumours like breast cancer.
The first few reports of hepatitis B reactivation were in patients with lymphoma[1], but the highest risk of HBV reactivation is seen with the potent anti-CD20 monoclonal antibody, rituximab, which came into use in the last two decades and results in profound B-cell depletion.
The newer generation of anti-CD20 monoclonal antibody, obinutuzumab, will induce a more profound B-cell depletion than rituximab does, and the risk of HBV reactivation is expected to be high. Similarly, the newer targeted therapies, such as the Bruton’s tyrosine kinase (BTK) inhibitors and phosphatidylinositol 3-kinase (PI3K) inhibitors, which have recently proven to be successful in a number of hematological malignancies[2-12], may also potentially cause HBV reactivation because they block B-cell antigen receptor signalling and reduce malignant proliferation of B-cells. The increasing use of proteasome inhibitors in multiple myeloma and hypomethylating agents in elderly patients with acute myeloid leukemia should arouse caution on the risk of HBV reactivation in these hematological malignancies.
The initiating factor of HBV reactivation is thought to be loss of immune control over viral replication. During chemotherapy, when the immune system is suppressed, HBV replicates dramatically and the viral load increases causing widespread infection of hepatocytes. When chemotherapy is stopped and immune function is restored, liver cells containing HBV may produce a strong immune-mediated reaction causing liver damage[13].
The clinical manifestations of HBV reactivation range from asymptomatic self-limiting anicteric hepatitis to potentially fatal, severe liver failure. Deranged liver function due to HBV reactivation may also lead to a delay or interruption of the chemotherapy regimen, which is likely to increase the risk of morbidity and mortality associated with the underlying malignancy.
Most of the data of HBV reactivation in cancer patients come from lymphoma patients receiving chemotherapy and rituximab. This article describes the risk and clinical course of HBV reactivation in patients with lymphoma, as well as those with other hematological malignancies such as multiple myeloma or acute leukemia, or receiving the novel agents used in hematological malignancies. We will also discuss the choice of antiviral agent for the prevention of HBV reactivation and duration of antiviral prophylactic therapy and management of HBV reactivation.
Approximately 350 million people worldwide are chronically infected with HBV. All patients with HBV infection develop anti-HBc and the antibody persists after clearance of HBsAg. Therefore, anti-HBc is a good marker of current as well as past infection with HBV. Anti-HBs can be present due to previous infection or successful hepatitis B vaccination. The presence of anti-HBc without HBsAg or anti-HBs indicates resolved HBV infection, but patients with this marker are still at risk of HBV reactivation and a fatal outcome. Although HBV DNA is rarely found in peripheral blood after the HBV infection has resolved, trace amounts are often found within the liver and can be activated when the immune response is suppressed.
HBV reactivation is diagnosed by a marked increase or de novo appearance of HBV DNA in serum[14]. Reactivation of HBV replication is defined as a marked increase in HBV replication [≥ 2log increase (100 fold) from baseline levels or a new appearance of HBV DNA to a level of ≥ 100 IU/mL] in a person with previously stable or undetectable levels of HBV DNA[15]. Newer and more sensitive HBV DNA assays have increased the number of patients who can be diagnosed with HBV reactivation[16].
HBV reactivation can be described as exacerbation of chronic hepatitis B or reactivation of past hepatitis B infection. The latter can be further defined as reverse HBsAg seroconversion (reappearance of HBsAg), or appearance of HBV DNA in serum in the absence of HBsAg.
The course of HBV reactivation has three phases[15,17]. During the first phase, there is an increase in levels of HBV DNA in the serum of an HBsAg-positive person or a reappearance of HBsAg or HBV DNA in serum in a person who was previously HBsAg-negative or had undetectable serum HBV DNA, respectively. Symptoms of hepatitis are usually absent and levels of liver enzymes, e.g., alanine aminotransferase (ALT), are not elevated.
During the second phase, serum HBV DNA levels continue to increase, and there is an increase in ALT levels, with or without symptoms of acute hepatitis. Hepatic injury may progress in some patients, causing liver failure and even death. These changes in the second phase may occur between chemotherapy administrations or after cessation of chemotherapy, and may result from reconstitution of the host immune response[18].
In the third phase, hepatic injury resolves either spontaneously or as a result of withholding immunosuppressive therapy or initiation of antiviral therapy.
HBV reactivation can occur at any time during or after chemotherapy. It typically occurs after the second or third courses of chemotherapy for patients undergoing treatment for lymphoma. In patients receiving rituximab for the treatment of non-Hodgkin’s lymphoma, HBV reactivation may occur after a median of six doses and up to 12 mo after the last dose of rituximab[19].
HBsAg-negative/anti-HBc-positive patients who receive allogeneic HSCT are at risk of developing HBV reactivation for several years after transplantation because of the long delay in reconstituting the recipient’s immune response to HBV[20].
HBV reactivation is most commonly reported in patients with lymphoma, but it is unclear whether lymphoma itself increases the risk of HBV reactivation because there are no studies comparing the risk in patients with other diseases receiving similar chemotherapeutic regimens. The frequent association between lymphoma and HBV reactivation might be related to the intensity of the chemotherapy regimen, resulting in marked immunosuppression. Alternatively, it might also be related to a higher prevalence of HBV infection among lymphoma patients[21-25].
Deng et al[26] recently showed that in HBsAg-positive diffuse large B-cell lymphoma (DLBCL) patients, the majority (96%) of patients’ amino acid sequences of heavy- and light-chain complementarity-determining region 3 exhibited a high homology to antibodies specific for HBsAg, and 90% of IgHV and IgLV genes were mutated. This suggests that HBV-associated DLBCL might arise from HBV antigen-selected B-cells.
Although most early reports of HBV reactivation were in patients with lymphoma, more data on HBV reactivation have recently emerged in patients with other hematological diseases like multiple myeloma. Multiple myeloma is the second most common hematological malignancy. HBV reactivation has been reported in patients who are HBsAg-positive and in those who are HBsAg-negative/anti-HBc-positive[27-30]. Moreover, severe immune dysfunction associated with advanced myeloma may also predispose myeloma patients to virus reactivation[31].
Mya et al[27] investigated the incidence of hepatitis B reactivation in 273 patients with multiple myeloma undergoing high-dose therapy followed by autologous stem cell transplant (HDT-ASCT) and treatment with novel agents. Patients were screened for the presence of HBsAg and anti-HBc. The prevalence of HBV infection was 5.5%, including three cases of HBV reactivation despite lamivudine prophylaxis. Of the three patients with HBV reactivation, two developed reactivation 3 to 5 mo after HDT-ASCT while receiving thalidomide maintenance, and one reactivated 3 years after HDT-ASCT followed by bortezomib salvage therapy.
Another study by Li et al[30] analyzed 139 myeloma patients. HBsAg-positive patients underwent prophylactic therapy before starting immunosuppressive therapy, and the incidence of HBV reactivation was 22.1%. This high incidence of HBV reactivation is believed to be due to the use of bortezomib and/or treatment with ASCT.
The risk of HBV reactivation is significant in patients with acute myeloid leukemia (AML) receiving chemotherapy, with an incidence similar to that in patients with lymphoma. A recent study by Chen et al[32] analysed 490 AML patients and found that the incidence of HBV reactivation and HBV-related hepatitis were 9.5 and 8.3 per 100 person-years, respectively, in AML patients who are also chronic hepatitis B carriers. This is similar to the incidence of HBV reactivation in lymphoma patients. Prophylaxis with anti-HBV agents significantly decreased the risk of hepatitis B reactivation among HBV carriers (13% vs 61%, P < 0.001). Since fulminant hepatitis B is a catastrophic event for AML patients infected with HBV[33-35], periodic assessment of liver function and HBV serological status or prophylactic antiviral therapy is important during chemotherapy. Further prospective studies of patients with AML would be useful to assess the true incidence of HBV flare-ups and the best prophylactic strategy.
HBV reactivation is common in the setting of HSCT as a result of profound immunosuppression, the use of multiple immunosuppressive agents for allogeneic transplantations and substitution of the preexisting immune system with one that has not been exposed to HBV in the past[14].
The risk is greatest among patients undergoing allogeneic HSCT because of the requirement for high-dose conditioning chemotherapy, and the profound immunosuppression and prolonged use of immunosuppressive agents to prevent the development of graft-vs-host-disease after HSCT. Patients undergoing autologous HSCT receive high-dose chemotherapy like high-dose melphalan in multiple myeloma or BEAM [BCNU (carmustine), etoposide, cytarabine, melphalan] in relapsed or refractory lymphoma. The risk of HBV reactivation in autologous HSCT is similar to that in patients undergoing intensive chemotherapy.
HBV reactivation is not uncommon in HBsAg-negative/anti-HBc positive patients undergoing HSCT, with a rate of HBsAg seroconversion (reappearance of HBsAg in a person who was HBsAg-negative, anti-HBc-positive before HSCT) of 20% according to a retrospective study[20]. The cumulative probability of HBsAg seroconversion was 42.9% at 4 years after HSCT.
Male sex is a consistent host factor shown to be associated with an increased risk of HBV reactivation[1,36]. In one study of 78 HBsAg-positive patients with various types of cancer, 29% of the male patients had HBV reactivation compared with 10% of the female patients[36].
HBsAg-positive patients have a higher risk of HBV reactivation than patients who are HBsAg-negative-anti-HBc-positive. HBsAg positivity is associated with a 5- to 8-fold increase in the risk for HBV reactivation[37]. Moreover, those with detectable or high levels of serum HBV DNA prior to start of immunosuppressive therapy have a higher risk of HBV reactivation than do those with undetectable or low levels of HBV DNA[38-40].
HBV reactivation in HBsAg-negative patients is also increasingly being encountered nowadays because of the use of B-cell-depleting agents[16,41,42]. Most HBsAg-negative-anti-HBc-positive patients have undetectable serum HBV DNA levels, but the risk of HBV reactivation is higher in the minority with detectable serum HBV DNA at baseline. Moreover, in HBsAg-negative-anti-HBc-positive patients, those who have an undetectable or low titre of anti-HBs level at the onset of immunosuppressive therapy or have a loss of anti-HBs during immunosuppressive therapy have an increased risk of HBV reactivation[43-47].
Steroids are a common component of treatment for hematological malignancies, especially lymphoid diseases, and are combined with chemotherapy in most regimens used to treat lymphoma and multiple myeloma. It was shown that long-term treatment with prednisolone increases HBsAg levels and HBV DNA in hepatocytes, with rebound immune T-cell function and subsequent hepatocyte destruction upon withdrawal of the corticosteroid[48]. High-dose steroid (> 20 mg/d of prednisolone) and long duration of therapy (> 4 wk) increases the risk of HBV reactivation.
Anthracyclines, such as doxorubicin or daunorubicin, are commonly used to treat hematological malignancies such as lymphoma and acute leukemia. There is a significant risk of HBV reactivation in patients receiving doxorubicin as part of the chemotherapeutic regimens[49,50].
B-cell depleting agents, like anti-CD20 monoclonal antibodies, are used in various hematological malignancies, including lymphoma and chronic lymphocytic leukemia (CLL). These monoclonal antibodies (including rituximab, ofatumumab) act against B-lymphocyte antigen CD20, resulting in profound depletion of the B-cells involved in priming specific cytotoxic T-cells[51]. Rituximab was the first monoclonal antibody approved by the Food and Drug Administration (FDA), and is the most widely used treatment for CD20-positive B-cell lymphoma patients. Rituximab is a chimeric type 1 antibody that kills CLL cells primarily by means of complement-dependent and antibody-dependent cellular cytotoxicity after binding to CD20. The use of rituximab is associated with a more than 5-fold increase in the risk of HBV reactivation[19]. In 2013, the United States FDA issued a black box warning concerning the risk of HBV reactivation in patients receiving anti-CD20 monoclonal antibodies[52].
The profound B-cell depletion induced by rituximab interferes with the generation of anti-HBs, which can neutralize circulating HBsAg. Rituximab worsens the impairment of antigen-presenting B-cells that is seen with chronic HBV, leading to insufficient induction of CD4 T-cell activation and proliferation and a T-cell hyporesponsive state[53].
Alemtuzumab is an anti-CD52 monoclonal antibody that is useful in refractory chronic lymphocytic leukemia patients and certain types of T-cell lymphoma. HBV reactivation has also been reported to occur with alemtuzumab therapy[54-57].
Tyrosine kinase inhibitors (e.g., imatinib, dasatinib, nilotinib) are currently the standard treatment for all phases of chronic myeloid leukemia. There have been some reports of HBV reactivation in patients who have been given these agents[58-64]. The exact mechanism for HBV reactivation is not clear with tyrosine kinase inhibitors, but it may be related to immune restoration. Clinicians need to give prophylactic antiviral therapy or regularly monitor HBV DNA and liver enzymes of HBV-infected patients during imatinib treatment.
The use of hypomethylating agents including decitabine and azacitidine has increased in recent years, especially in the elderly acute myeloid leukemia (AML) patients. AML is common in elderly patients and the median age of onset is 67 years[65,66]. To date, there has been no reported case of HBV reactivation in patients using hypomethylating agents, possibly because the degree of myelosuppression with these agents is not as severe as it is with cytotoxic chemotherapy for AML. There is no recommended prophylactic strategy for HBV reactivation in these patients using hypomethylating agents. We would recommend consideration of antiviral prophylaxis for HBsAg positive patients using hypomethylating agents for the treatment of AML, since myelosuppression is a side effect of these agents. Table 1 summarizes the drug classes and corresponding risks of HBV reactivation[49,67].
| Drug class | Drug | Risk of HBV reactivation for HBsAg-positive patients | Risk of HBV reactivation for HBsAg-negative/anti-HBc-positive patients |
| Monoclonal antibody | Rituximab | High (30%-60%) | High (> 10%) |
| Ofatumumab | |||
| Obinutuzumab | |||
| Anthracycline chemotherapy | Doxorubicin | High (15%-30%) | High (> 10%) |
| Epirubicin | |||
| Daunorubicin | |||
| Corticosteroids | High dose, e.g., Prednisolone ≥ 20 mg for ≥ 4 wk | High (> 10%) | Not available |
| Moderate dose, e.g., Prednisolone < 20 mg for ≥ 4 wk | Moderate (1%-10%) | Moderate (1%-10%) | |
| Low dose, e.g., Prednisolone < 1 wk | Low (< 1%) | Low (< 1%) | |
| Tyrosine kinase inhibitors | Imatinib, nilotinib | Moderate (1%-10%) | Moderate (1%-10%) |
| Traditional immunosuppressive agents | Methotrexate, azathioprine, 6-mercaptopurine | Low (< 1%) | Low (< 1%) |
Adult T-cell leukemia/lymphoma (ATLL) is a rare but aggressive subtype of T-cell lymphoma with a higher prevalence in Japan and South America. Mogamulizumab is a humanized monoclonal antibody targeting the C-C chemokine receptor 4 that was developed and introduced into the management of ATLL. However, there have been reports of HBV reactivation in Japanese patients treated with this agent, affecting both HBsAg-positive and HBsAg-negative/anti-HBc patients[68-70]. One of these cases of HBV reactivation with mogamulizumab in an ATLL patient was fatal[68]. Further prospective studies are needed to estimate the risk and incidence of HBV reactivation in patients using this agent and to establish regular HBV DNA monitoring-guided preemptive antiviral therapy for such patients.
Brentuximab vedotin is an anti-CD30 drug-conjugated antibody used in the treatment of relapsed or refractory Hodgkin lymphoma and CD30 positive T-cell lymphoma[71-73]. CD30 can be found on malignant lymphoid cells and activated T-cells. CD30 has an important role in developing memory and effector CD4+ T-cells, but its effects on B-cells are controversial. Following binding to CD30, brentuximab vedotin will be internalized rapidly and cause cell cycle arrest and apoptosis. There has been a case report from China of HBV reactivation in one patient given brentuximab vedotin[74].
Obinutuzumab is a newer generation of anti-CD20 monoclonal antibody. Similar to rituximab, it is a humanized, glycol-engineered type 2 antibody also targeted against CD20[75]. Based on the risk of HBV reactivation with rituximab, the FDA has mandated a warning about risk of HBV reactivation with obinutuzumab, although no specific events of reactivation have been reported with this agent. Obinutuzumab showed higher efficacy than rituximab, by inducing direct cell death and enhanced antibody-dependent cellular cytotoxicity (with less complement-dependent cytotoxicity). It can cause more profound suppression of CD20 than rituximab. A comparison of obinutuzumab with rituximab showed better complete response rate and longer progression-free survival with obinutuzumab than with rituximab, when both were given in combination with chlorambucil in the treatment of CLL[76]. The use of this agent would be expected to increase in the treatment of CLL and other lymphoproliferative diseases.
There are many new targeted agents for hematological malignancies, including the BTK ibrutinib and the PI3K delta inhibitor idelalisib, which are both B-cell receptor signaling modulators. They have been approved by FDA for the treatment of CLL and certain low-grade non-Hodgkin’s lymphomas (NHL). These oral compounds are currently in various clinical trials in patients with different subtypes of aggressive and low-grade B-cell NHLs and they will be used more clinically in the future.
There is already a case report concerning the HBV reactivation in a CLL patient receiving ibrutinib[77]. This patient with relapsed CLL had previously resolved hepatitis B infection and was negative for HBsAg and positive for anti-HBc Ab. HBV reactivation occurred five months after starting ibrutinib treatment. The patient recovered from HBV reactivation after receiving entecavir therapy. Ibrutinib acts by blocking B-cell antigen receptor signalling, thereby reducing malignant proliferation of B-cells and inducing cell death[4]. Ibrutinib irreversibly binds IL-2-inducible kinase and inhibits activation of Th2 cells after T-cell receptor stimulation. This inhibition is specific to Th2-polarized CD4 T-cells, because redundant resting lymphocyte kinase remains functional, thus providing a compensatory platform for activation of Th1 and CD8 T-cells[78].
Physicians should be vigilant about the possibility of HBV reactivation when using these agents. We recommend screening patients for HBV antigens before using the BTK or PI3K blockers, and prescribing prophylactic antiviral therapy in HBsAg-positive patients. Further studies are required to assess the risk of HBV reactivation in patients receiving small molecules in the treatment of lymphoma and leukemia.
Myelofibrosis is a myeloproliferative disease characterized by excessive production of reticulin and collagen fibers, hepatosplenomegaly, anemia and a tendency to transform to acute leukemia. About 50% of patients present the JAK2V617F mutation, resulting in a constitutively activated Janus-activated kinase (JAK) signal and increased production of cytokines. Ruxolitinib is a novel inhibitor JAK1 and JAK2 that has been approved for the treatment of patients with myelofibrosis. Clinical trials have shown that ruxolitinib can effectively reduce the size of the spleen and liver and improve myelofibrosis-related symptoms[79,80]. However, there are also reports of HBV reactivation in patients with myelofibrosis following ruxolitinib treatment[81,82].
The approval of the proteasome inhibitor bortezomib for newly diagnosed and relapsed or refractory multiple myeloma patients marked a revolutionary change in the drug treatment of multiple myeloma[83-85]. Bortezomib is also the backbone of induction therapy for transplant-eligible patients prior to stem cell harvest. It inhibits T-cell function[86,87], which could promote viral replication in patients with multiple myeloma. Bortezomib has been shown to be associated with HBV reactivation in both HBsAg positive patients and HBsAg negative/anti-HBc positive patients with or without stem cell transplant[29,30,88]. HBV reactivation has been reported 1 to 3 years after stem cell transplantation in some patients and it may be due to immune dysfunction in patients with multiple myeloma and factors associated with bortezomib-containing chemotherapy and autologous stem cell transplant.
Multiple myeloma cells uniformly overexpress CD38. Daratumumab, a CD38-targeting, human IgG1κ monoclonal antibody, was shown to have a favorable safety profile and encouraging efficacy in heavily pretreated patients with refractory myeloma[89]. Daratumumab can potentially increase the risk of HBV reactivation because CD38 is expressed in B-cell hematological malignancies. There is no reported case so far and further studies are needed to assess the risk of HBV reactivation with this monoclonal antibody.
There has been a report of HBV reactivation occurring after six cycles of pomalidomide/doxorubicin/dexamethasone treatment in a myeloma patient with an initially unknown HBV carrier status[90]. This had not been observed after previous administration of highly immunosuppressive high-dose melphalan prior to autologous stem cell transplantation, which may indicate that the immunomodulatory agent (pomalidomide) was the cause of the HBV reactivation. More data are needed to determine whether immunomodulators affect the risk of HBV reactivation.
The key to prevention of HBV reactivation is the identification of patients with HBV infection before initiation of chemotherapy. Patients at risk of HBV reactivation are easily identified by testing HBsAg and anti-HBc. The European Association for the Study of the Liver and Asian-Pacific Association for the Study of the Liver recommend universal HBV screening prior to initiation of immunosuppressive therapy while the American Association for the Study of Liver Diseases, American Gastroenterological Association and American Society of Clinical Oncology recommend screening patients with risk factors[49,91-94]. We recommend screening all patients with hematological malignancies for active or prior HBV infection by testing for HBsAg and anti-HBc in serum.
The risk of HBV reactivation is high in HBsAg-positive patients receiving chemotherapy. The risk is even higher in patients with high HBV replication at baseline with high serum HBV DNA level, receiving rituximab-containing chemotherapy or a stem cell transplant. A systematic review of 14 studies showed that, in HBsAg-positive patients who were not given prophylaxis with the antiviral agent lamivudine during chemotherapy, the rate of HBV reactivation, liver failure, and death were 32%, 13%, and 7%, respectively[95]. Furthermore, lamivudine prophylaxis could decrease HBV reactivation and HBV hepatitis by 80% to 100% and eliminate HBV liver failure, as well as reducing the delay or interruption of chemotherapy due to HBV reactivation[96].
Therefore, once a patient is identified as being seropositive for HBsAg, the patient will benefit from receiving prophylactic antiviral treatment for hepatitis B before or concomitantly with starting chemotherapy, irrespective of the HBV DNA level. The number needed to treat to prevent one reactivation was 3 patients[97]. Antiviral therapy started after HBV reactivation may not be able to prevent hepatitis or hepatitis flares[16], because antiviral therapy usually takes a few weeks to months to reduce viral loads and control the disease, during which there is ongoing inflammation and necrosis of the liver[14]. Moreover, randomized trials have shown that prophylactic antiviral therapy is more effective than a pre-emptive strategy in HBsAg-positive patients[98,99]. Another major limitation of pre-emptive antiviral therapy is its reliance on close monitoring of HBV DNA levels.
Patients with resolved HBV infection may have occult HBV infection with persistent detectable viremia despite the absence of detectable circulating HBsAg[14]. The HBV reactivation rate ranged from 8.9% to 41.5% in HBsAg-negative/anti-HBc-positive patients receiving rituximab-containing chemotherapy in different studies with different definitions of HBV reactivation, using either HBsAg seroconversion or detectable HBV DNA[16,41,42,45,99]. A meta-analysis of 15 studies (6 prospective and 9 retrospective) involving 578 patients gave a pooled risk estimation of HBV reactivation (ALT more than 3 times the upper limit of normal and either HBsAg seroconversion or an increase in serum HBV DNA) of 6.3% in patients exposed to rituximab[100]. Most of the reported cases of HBV reactivation in patients with resolved HBV infection were self-limited, but fulminant hepatitis and hepatitis-induced mortality were sometimes reported[99,101-105].
For patients with resolved HBV infection, no standard strategy has yet been established to prevent HBV reactivation. There are usually two options. One is pre-emptive therapy guided by serial HBV DNA monitoring, whereby the antiviral drug is given as soon as HBV DNA becomes detectable. Pre-emptive antiviral therapy may be a reasonable strategy, but there is limited evidence regarding the optimal interval and period of monitoring[16,106]. An alternative approach is prophylactic antiviral therapy, especially for high-risk chemotherapy such as rituximab-containing chemotherapy. This strategy may effectively prevent HBV reactivation and avoid the inconvenience of repeated HBV DNA monitoring[107]. Moreover, antiviral therapy may be less effective once high titers of viremia are attained, and the same medication could be more effective if given earlier, when viremia is minimal.
Prophylactic antiviral therapy is more effective than pre-emptive antiviral therapy in preventing HBV reactivation in high-risk patients, such as those receiving rituximab or undergoing HSCT. Whether pre-emptive antiviral therapy is more cost-effective in clinical settings associated with moderate risk of HBV reactivation (for example, HBsAg-negative-anti-HBc-positive patients receiving chemotherapy for lymphoma that does not include anti-CD20 therapy) is unclear. A major limitation of pre-emptive antiviral therapy is its reliance on close monitoring of HBV DNA levels and prompt initiation of antiviral therapy when HBV reactivation is detected. Table 2 summarizes the prospective studies in HBsAg negative/anti-HBc positive patients with hematological malignancies who received pre-emptive or prophylactic treatment.
| Author/year | Disease | Study design | Sample size | Definition of HBV reactivation | Rate of HBV reactivation | Risk factor(s) identified for HBV reactivation | Death from HBV reactivation |
| Yeo et al[99] 2009 | Diffuse large B-cell lymphoma | All patients were observed every 2-3 wk during anticancer therapy and every 6-8 wk for 9 mo after anticancer therapy | 46 | HBsAg seroconversion (the reappearance of HBsAg) with an increase in HBV DNA levels when compared with baseline HBV DNA levels, in the absence of acute infection with HAV, HCV, or other systemic infections | 25% in patients receiving R-CHOP | Absence of anti-HBs, use of rituximab and male sex | 20% died of HBV reactivation |
| Start lamivudine upon HBV reactivation | |||||||
| Huang et al[107] 2013 | B-cell lymphoma | Entecavir prophylaxis (continued until 3 mo after completing chemotherapy) vs preemptive treatment | 80 | Elevation of HBV viral load to 2000 IU/mL with two consecutive determinations (2 wk apart) | Incidence was 4.3% in entecavir group and 25.9% in the control group at 1.5 yr | Lack of entecavir prophylaxis | Nil |
| Seto et al[45] 2014 | CD20 positive B-cell lymphoma | HBV DNA monitoring every 4 wk | 63 | HBV DNA ≥ 10 IU/mL | 2-yr cumulative rate 41.5% | Anti-HBs < 10 mIU/mL | Nil |
| Start entecavir upon detection of HBV reactivation | |||||||
| Hsu et al[16] 2014 | CD20 positive B-cell lymphoma | HBV DNA monitoring every 4 wk | 150 | > 10-fold increase in HBV DNA, compared with previous nadir levels | Incidence was 10.4% | Absence of anti-HBs | Nil |
| Start entecavir upon detection of HBV reactivation | |||||||
| Kusumoto et al[46] 2015 | CD20 positive B-cell lymphoma | HBV DNA monitoring every 4 wk | 269 | HBV DNA ≥ 11 IU/mL | Incidence was 8.3% at 1.5 yr | Anti-HBs < 10 mIU/mL and baseline HBV DNA below level of quantification | Nil |
| Start entecavir upon detection of HBV reactivation |
More reports of resolved HBV infection in other hematological malignancies like multiple myeloma have been published in recent few years. In a recently published study of 230 HBsAg-negative/anti-HBc-positive multiple myeloma patients, 12 (5.2%) displayed HBV reactivation, which was defined as the reappearance of HBsAg with or without de novo detection of HBV DNA in the blood[29]. The cumulative rate of HBV reactivation was 5% at 2 years and 8% at 5 years[29]. Absence of anti-HBs and high-dose therapy and then autologous stem cell transplant were found to be risk factors associated with HBV reactivation. The cumulative incidence rate of HBV reactivation rate at 2 years was significantly higher in patients who were anti-HBs negative than anti-HBs positive at baseline (9% vs 3%, P = 0.033) patients, and was also significantly higher for patients who had received high-dose therapy and then autologous stem cell transplant than for patients who had not (7% vs 0%, P = 0.025). These high-risk patients may benefit from regular monitoring of HBV DNA levels.
Future prospective studies are needed on cost-effective strategies for the optimal timing and duration for the regular monitoring of HBV DNA and indication of prophylactic antiviral therapy in multiple myeloma patients for avoiding HBV-related liver complications and mortality.
Another recent study included 315 HBsAg-negative, anti-HBc positive patients who received autologous or allogenic stem cell transplantation. The primary endpoint was the incidence of HBV reactivation. With a median follow-up duration was 21.4 mo, antiviral prophylaxis was not given to 219 patients, and 96 received prophylaxis. The median duration of prophylaxis was 7.0 mo. HBV reactivated in 12 patients (4 in the group receiving prophylaxis and 8 in the non-prophylactic group). The median time to reactivation was 20.5 mo after starting chemotherapy[108]. All patients who developed HBV reactivation were treated with antiviral agents. The risk of reactivation was increased significantly in patients who had an allogeneic HSCT and those with loss of anti-HBs. This study showed that short-term antiviral prophylaxis may not be able to decrease the risk of HBV reactivation. This suggests that prophylaxis should be used for longer than 24 mo or careful monitoring of HBV DNA should be combined with on-demand antiviral treatment to prevent hepatitis flares in post-transplant patients.
HBV reactivation in patients with isolated anti-HBs is extremely rare. In general, individuals who are seropositive only for anti-HBs (HBsAg negative and anti-HBc negative) are usually considered as having a history of HBV vaccination and are at no risk for HBV reactivation. Routine monitoring is usually not recommended. However, HBV reactivation has been reported to occur in patients who were seropositive for anti-HBs alone[109,110].
A patient with follicular lymphoma without record of hepatitis B vaccination had only anti-HBs but no anti-HBc before chemotherapy. He later developed high viremia with an HBV escape mutant, which was difficult to detect by HBsAg assays[109]. The escape mutants of HBV carry mutations in the major antigenic region of HBsAg. They are able to grow in the presence of anti-HBs. Anti-HBc may appear very late.
Another patient with diffuse large B-cell lymphoma without record of vaccination against HBV was negative for HBsAg and anti-HBc, and positive for anti-HBs (127 IU/mL) before chemotherapy. She had HBV reactivation after finishing rituximab-containing chemotherapy. She died of liver failure although antiviral treatment was started after detection of HBV reactivation[110].
Therefore, clinicians should be aware of this possible complication when they give chemotherapy to hematology patients who are HBsAg negative/anti-HBc negative/anti-HBs positive. When the patient has unexplained deranged liver function, HBV DNA should be checked to look for HBV reactivation in this setting.
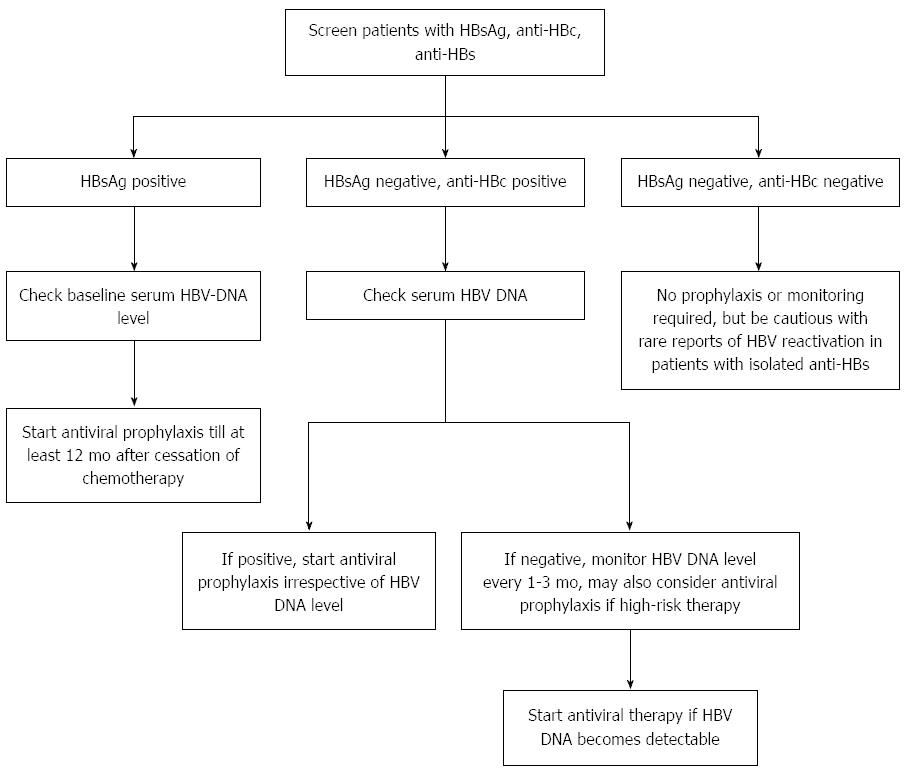
Figure 1 is a suggested algorithm for testing of HBV and management of patients with hematological malignancies receiving anticancer therapy. Table 3 summarizes the recent international guidelines on the management of patients with HBV infection receiving chemotherapy.
| Association guidelines | HBV screening | Screening tests | HBsAg positive patients | HBsAg negative, anti-HBc positive patients | Antiviral drug recommended | Duration of antiviral therapy |
| EASL 2012[91] | All candidates for chemo- and immunosuppressive therapy | HBsAg, anti-HBc, HBV DNA if serology positive | Prophylactic antiviral therapy, test for HBV-DNA level | Test for HBV DNA; provide prophylactic antiviral therapy if detectable HBV DNA; monitor ALT and HBV DNA levels closely if no detectable HBV DNA | Lamivudine if HBV DNA < 2000 IU/mL and the treatment duration is finite and short, Entecavir or tenofovir if HBV DNA is high, and/or lengthy and repeated cycles of immunosuppression | 12 mo after cessation of therapy |
| APASLD 2012[92] | All patients prior to receiving immunosuppression or chemotherapy | HBsAg, anti-HBc | Prophylactic antiviral therapy | Monitor HBV-DNA | Lamivudine or entecavir or tenofovir | Continue until 6 mo after end of chemotherapy |
| AGA 2015[49] | High risk of HBV reactivation (> 10%) and moderate risk of HBV reactivation (1%-10%) | HBsAg, anti-HBc , HBV DNA if serology positive | Prophylactic antiviral therapy | Antiviral prophylaxis over monitoring for patients if the chemotherapy is associated with high or moderate risk of HBV reactivation | Drug with high barrier to resistance is favored over lamivudine | 6 mo after discontinuation of therapy and at least 12 mo for B-cell depleting agents |
| Routine screening not recommended for low risk of HBV reactivation (< 1%) | ||||||
| AASLD 2009[93], no update in the version in 2016[126] | Anyone at high risk of HBV infection | HBsAg and anti-HBc | Prophylactic antiviral therapy | No recommendation | Lamivudine or telbivudine if the anticipated treatment duration is < 12 mo and baseline HBV DNA is not detectable | Maintain for 6 mo after completion of chemotherapy |
| Tenofovir or entecavir if anticipated treatment duration > 12 mo | ||||||
| ASCO 2015[94] | Risk-adapted HBV screening strategy | HBsAg, anti-HBc , HBV DNA if serology positive | Prophylactic antiviral therapy | Consider | Entecavir, tenofovir | Minimum of 6 mo after stopping therapy, longer than 12 mo for patients receiving anti-CD20 monoclonal antibodies |
| Antiviral prophylaxis if the systemic cancer therapy is associated with high risk of HBV reactivation |
Lamivudine was the first oral antiviral agent used to treat chronic HBV infection, and has been studied in randomized controlled trials for its efficacy to prevent HBV reactivation in patients receiving immunosuppressive therapies[85,98]. Lamivudine is generally safe and well tolerated, but it is also associated with a high incidence of viral resistance after prolonged use for more than 6 mo due to the emergence of YMDD mutations[93]. Lamivudine has been shown to have a low barrier to resistance with rates of antiviral resistance of 14%-32% after 1 year of treatment and 60%-70% after 5 years of treatment in clinical trials in patients with chronic hepatitis B[93]. Emergence of drug resistance mutations may outweigh the benefits of preventative antiviral therapy and can lead to hepatitis flares, liver failure and death.
Antiviral agents with high genetic barriers to resistance (i.e., entecavir or tenofovir) are preferred to lamivudine in the treatment of chronic HBV infection[79]. A prospective randomized study by Huang et al[111] recruited 121 patients seropositive for the HBsAg with untreated DLBCL (61 received entecavir prophylaxis and 60 received lamivudine prophylaxis). It showed that the rate of various endpoints were significantly lower in the entecavir than the lamivudine group: for HBV-related hepatitis (0% vs 13.3%, P = 0.003), HBV reactivation (6.6% vs 30%, P = 0.001), and chemotherapy disruption (1.6% vs 18.3%, P = 0.002)[111].
A recent retrospective study in 213 patients (entecavir group, 70 patients; lamivudine group, 143 patients) found a lower incidence of HBV reactivation in the entecavir group than the lamivudine group (0% vs 7.0%, P = 0.02). No HBV reactivation was noted in the patients with a baseline HBV DNA level < 2000 IU/mL. A baseline HBV DNA level > 2000 IU/mL, HBeAg, and lamivudine were significantly associated with HBV reactivation[40]. Another recent study also showed that, compared with lamivudine, entecavir has more potent antiviral efficacy and may be a better choice for prophylaxis of HBV reactivation in HBsAg-positive allogenic HSCT recipients[112].
There are also recent data suggesting that tenofovir may help to prevent HBV reactivation. During routine clinical practice, none of the 25 patients who received prophylactic treatment with tenofovir had HBV flares and all achieved undetectable serum HBV DNA during a mean follow-up period of 17.2 mo. One patient even had HBsAg seroconversion[113].
Entecavir will be a better option in the case of significant renal impairment as tenofovir has a small risk of inducing proximal tubular dysfunction and renal insufficiency[114-116]. On the other hand, tenofovir is preferred to entecavir if patients have received lamivudine therapy previously because of a high rate of resistance (genotypic resistance of 51% and virologic breakthrough of 43% within 5 years)[117]. Prophylaxis with telbivudine or adefovir is not recommended because of development of drug resistance and there are limited data from clinical trials using these agents[49].
A recent study showed that concurrent telbivudine treatment with initial chemotherapy can reduce HBV reactivation in HBsAg-positive lymphoma patients, and the efficacy is independent of the baseline HBV viral loads[118]. This study in 60 HBsAg-positive patients showed that the rate of HBV reactivation was 11.7% (7/60), and the median time to HBV reactivation was 228 d (range 113-699 d). The fulminant hepatitis rate was 6.6%. The rates of undetectable HBV DNA and ALT normalization were 61.7% and 83.3%, respectively[118].
There are few data available in the literature to guide physicians on when antiviral therapy can be stopped. For patients receiving B cell-depleting agents, the duration of antiviral therapy should be extended to at least 12 mo after cessation of the B-cell depleting agent, because there is profound suppression of B-cell function[14,19,107]. The duration of nucleoside analog therapy also depends on the patient’s baseline serum HBV DNA level and the degree of fibrosis/cirrhosis present. A previous study showed that high levels of serum HBV DNA (≥ 4log10 copies/mL) before chemotherapy was the most important predictor of HBV reactivation after withdrawal of prophylactic antiviral therapy[119].
There is growing evidence that HBsAg-positive patients with lymphoma can develop hepatitis B flares due to HBV reactivation more than 6 mo after cessation of chemotherapy[21,120]. It is reasonable to consider extending the duration of prophylactic antiviral therapy to at least one year after completion of chemotherapy.
Kim et al[121] also reported the importance of HBV DNA levels and consolidation period as predictors of HBV reactivation after withdrawal of prophylactic antiviral therapy. Their study enrolled 95 HBsAg-positive patients who were analyzed for sustained off-treatment virological response, defined as HBV DNA levels below 2000 IU/mL for at least 12 mo after the end of therapy. The baseline HBV DNA level was shown to be an independent factor associated with sustained off-treatment virological response. The rate of HBV reactivation was 72.1% and 23.5% for those with HBV DNA < 2000 IU/mL and ≥ 2000 IU/mL, respectively (P < 0.001). Consolidation treatment duration showed association with sustained off-treatment virological response only for those with low baseline HBV DNA levels. The sustained off-treatment virological response rates were 54.5%, 71.4%, 73.9%, and 100% for consolidation treatment durations of < 3, 3-6, 6-12, and ≥ 12 mo, respectively, among those with baseline HBV DNA < 2000 IU/mL[121].
Liu et al[122] recently analyzed 107 newly diagnosed DLBCL patients with HBV infection who received chemotherapy. The median time from the cessation of antitumor therapy to the withdrawal of prophylactic antiviral therapy was 6.1 mo. Ten of the 46 patients in the HBsAg-positive group (21.7%) experienced delayed HBV reactivation, whereas none in the HBsAg-negative/anti-HBc-positive group exhibited delayed HBV reactivation.
HBV reactivation can occur many years after HSCT, so antiviral prophylaxis is required for a longer duration. The optimal duration of therapy is not well defined in HSCT patients with occult infection. HBV reactivation can occur months or even years (up to 6 years) after transplantation[20,123].
Any adverse change in liver function in a patient undergoing chemotherapy needs to be thoroughly investigated, to distinguish HBV reactivation from other potential causes, including infections by other hepatitis virus (A, C, D, E) or opportunistic infections (e.g., cytomegalovirus, herpes viruses) and hepatotoxic drugs. Once HBV reactivation is detected, patients should receive antiviral therapy with either entecavir or tenofovir[49]. Severe flares can be accompanied by an increase in IgM hepatitis B core antibody, which may be misdiagnosed as acute HBV infection.
Hepatitis B flare-ups are generally rare when patients are receiving anti-HBV prophylaxis with potent antivirals. When patients are receiving lamivudine as a prophylaxis, they may develop resistance and may benefit from rescue therapy such as entecavir or tenofovir. We need to watch out for resistance when using low genetic barrier drug such as lamivudine. A recent study also showed that some patients could recover virologically and biochemically after combination regimens with lamivudine plus adefovir or entecavir plus adefovir, respectively[124].
The goal of treatment is to prevent the development of severe hepatitis, hepatic failure and even mortality. Close monitoring of liver enzymes, bilirubin levels and the clotting profile is essential. Occasionally, patients progress to hepatic failure despite nucleoside therapy[16], especially if they already have jaundice or a marked increase in liver enzymes. Although there have been a few reports of successful liver transplantation in patients who develop liver failure, most of those with hematological malignancy will die because their underlying disease will preclude them from being candidates for liver transplantation[125].
Hepatitis due to HBV reactivation is a common and important complication in patients receiving cancer chemotherapy, especially for hematological malignancies. Although potentially fatal, HBV reactivation is preventable through screening with blood tests and administration of prophylactic antiviral therapy for patients with moderate or high risk of HBV reactivation.
We recommend undertaking screening tests for HBsAg, anti-HBc and anti-HBs in all hematology patients about to start anticancer therapy. Risk stratification based on their serologic status and the types of therapies would be the next step. For HBsAg-positive patients, prophylactic antiviral therapy is essential. There are two options for HBsAg-negative/anti-HBc-positive patients. One is pre-emptive therapy guided by serial HBV DNA monitoring, whereby the antiviral drug is as soon as HBV DNA becomes detectable. The other approach is routine prophylactic antiviral therapy. We recommend prophylactic therapy in patients receiving high-risk therapies like anti-CD20 monoclonal antibodies or HSCT recipients.
Entecavir or tenofovir are preferred over lamivudine as prophylactic therapy. We recommend continuing preventative antiviral therapy for at least 12 mo after the completion of chemotherapy and even longer for those who receive rituximab or who had high serum HBV DNA levels before the start of chemotherapy. Screening for HBV before the start of chemotherapy is the key to preventing HBV reactivation.
In the era of targeted therapy for hematological malignancies, further studies are needed to estimate the risk of HBV infection and the optimal prophylactic strategy in patients receiving new targeted therapies. Although there is currently limited evidence to guide the optimal preventive measures, we recommend consideration of antiviral prophylaxis in HBsAg-positive patients to minimize the risk of HBV reactivation, especially with the BTK and PI3K inhibitors, which are B-cell receptor signaling modulators and reduce proliferation of malignant B-cells.
Manuscript Source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: China
Peer-Review Report Classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Matsui T, Sinn DH S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Report of a prospective study. Gastroenterology. 1991;100:182-188. [PubMed] |
| 2. | Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, Grant B, Sharman JP, Coleman M, Wierda WG. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1706] [Cited by in RCA: 1850] [Article Influence: 154.2] [Reference Citation Analysis (0)] |
| 3. | Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, Jurczak W, Advani RH, Romaguera JE, Williams ME. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1178] [Cited by in RCA: 1314] [Article Influence: 109.5] [Reference Citation Analysis (0)] |
| 4. | Cameron F, Sanford M. Ibrutinib: first global approval. Drugs. 2014;74:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, Bairey O, Hillmen P, Bartlett NL, Li J. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015;373:2425-2437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1157] [Cited by in RCA: 1224] [Article Influence: 122.4] [Reference Citation Analysis (0)] |
| 6. | Treon SP, Tripsas CK, Meid K, Warren D, Varma G, Green R, Argyropoulos KV, Yang G, Cao Y, Xu L. Ibrutinib in previously treated Waldenström’s macroglobulinemia. N Engl J Med. 2015;372:1430-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 734] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 7. | Gopal AK, Kahl BS, de Vos S, Wagner-Johnston ND, Schuster SJ, Jurczak WJ, Flinn IW, Flowers CR, Martin P, Viardot A. PI3Kδ inhibition by idelalisib in patients with relapsed indolent lymphoma. N Engl J Med. 2014;370:1008-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 874] [Article Influence: 79.5] [Reference Citation Analysis (0)] |
| 8. | Furman RR, Cheng S, Lu P, Setty M, Perez AR, Guo A, Racchumi J, Xu G, Wu H, Ma J. Ibrutinib resistance in chronic lymphocytic leukemia. N Engl J Med. 2014;370:2352-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1351] [Article Influence: 122.8] [Reference Citation Analysis (0)] |
| 9. | Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, Coutre S, Tam CS, Mulligan SP, Jaeger U. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1287] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 10. | Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, Offner F, Caballero D, Joao C, Witzens-Harig M. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387:770-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 362] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 11. | Chanan-Khan A, Cramer P, Demirkan F, Fraser G, Silva RS, Grosicki S, Pristupa A, Janssens A, Mayer J, Bartlett NL. Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17:200-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 347] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 12. | Byrd JC, Harrington B, O’Brien S, Jones JA, Schuh A, Devereux S, Chaves J, Wierda WG, Awan FT, Brown JR. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016;374:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 670] [Cited by in RCA: 717] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 13. | Faggioli P, De Paschale M, Tocci A, Luoni M, Fava S, De Paoli A, Tosi A, Cassi E. Acute hepatic toxicity during cyclic chemotherapy in non Hodgkin’s lymphoma. Haematologica. 1997;82:38-42. [PubMed] |
| 14. | Di Bisceglie AM, Lok AS, Martin P, Terrault N, Perrillo RP, Hoofnagle JH. Recent US Food and Drug Administration warnings on hepatitis B reactivation with immune-suppressing and anticancer drugs: just the tip of the iceberg? Hepatology. 2015;61:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 15. | Hwang JP, Lok AS. Management of patients with hepatitis B who require immunosuppressive therapy. Nat Rev Gastroenterol Hepatol. 2014;11:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 16. | Hsu C, Tsou HH, Lin SJ, Wang MC, Yao M, Hwang WL, Kao WY, Chiu CF, Lin SF, Lin J. Chemotherapy-induced hepatitis B reactivation in lymphoma patients with resolved HBV infection: a prospective study. Hepatology. 2014;59:2092-2100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 17. | Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49:S156-S165. [PubMed] |
| 18. | Lalazar G, Rund D, Shouval D. Screening, prevention and treatment of viral hepatitis B reactivation in patients with haematological malignancies. Br J Haematol. 2007;136:699-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Evens AM, Jovanovic BD, Su YC, Raisch DW, Ganger D, Belknap SM, Dai MS, Chiu BC, Fintel B, Cheng Y. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol. 2011;22:1170-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 271] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 20. | Hammond SP, Borchelt AM, Ukomadu C, Ho VT, Baden LR, Marty FM. Hepatitis B virus reactivation following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15:1049-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Law MF, Lai HK, Chan HN, Ha CY, Ng C, Yeung YM, Yip SF. The impact of hepatitis B virus (HBV) infection on clinical outcomes of patients with diffuse large B-cell lymphoma. Eur J Cancer Care (Engl). 2015;24:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol. 2010;11:827-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Lim ST, Fei G, Quek R, Lim LC, Lee LH, Yap SP, Loong S, Tao M. The relationship of hepatitis B virus infection and non-Hodgkin’s lymphoma and its impact on clinical characteristics and prognosis. Eur J Haematol. 2007;79:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Park SC, Jeong SH, Kim J, Han CJ, Kim YC, Choi KS, Cho JH, Lee M, Jung HH, Ki SS. High prevalence of hepatitis B virus infection in patients with B-cell non-Hodgkin’s lymphoma in Korea. J Med Virol. 2008;80:960-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Wang F, Xu RH, Han B, Shi YX, Luo HY, Jiang WQ, Lin TY, Huang HQ, Xia ZJ, Guan ZZ. High incidence of hepatitis B virus infection in B-cell subtype non-Hodgkin lymphoma compared with other cancers. Cancer. 2007;109:1360-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Deng L, Song Y, Young KH, Hu S, Ding N, Song W, Li X, Shi Y, Huang H, Liu W. Hepatitis B virus-associated diffuse large B-cell lymphoma: unique clinical features, poor outcome, and hepatitis B surface antigen-driven origin. Oncotarget. 2015;6:25061-25073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Mya DH, Han ST, Linn YC, Hwang WY, Goh YT, Tan DC. Risk of hepatitis B reactivation and the role of novel agents and stem-cell transplantation in multiple myeloma patients with hepatitis B virus (HBV) infection. Ann Oncol. 2012;23:421-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Huang B, Li J, Zhou Z, Zheng D, Liu J, Chen M. High prevalence of hepatitis B virus infection in multiple myeloma. Leuk Lymphoma. 2012;53:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Lee JY, Lim SH, Lee MY, Kim H, Sinn DH, Gwak GY, Choi MS, Lee JH, Jung CW, Jang JH. Hepatitis B reactivation in multiple myeloma patients with resolved hepatitis B undergoing chemotherapy. Liver Int. 2015;35:2363-2369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Li J, Huang B, Li Y, Zheng D, Zhou Z, Liu J. Hepatitis B virus reactivation in patients with multiple myeloma receiving bortezomib-containing regimens followed by autologous stem cell transplant. Leuk Lymphoma. 2015;56:1710-1717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Feyler S, Selby PJ, Cook G. Regulating the regulators in cancer-immunosuppression in multiple myeloma (MM). Blood Rev. 2013;27:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Chen CY, Huang SY, Cheng A, Chou WC, Yao M, Tang JL, Tsay W, Sheng WH, Tien HF. High Risk of Hepatitis B Reactivation among Patients with Acute Myeloid Leukemia. PLoS One. 2015;10:e0126037. [PubMed] |
| 33. | Kojima H, Abei M, Takei N, Mukai Y, Hasegawa Y, Iijima T, Nagasawa T. Fatal reactivation of hepatitis B virus following cytotoxic chemotherapy for acute myelogenous leukemia: fibrosing cholestatic hepatitis. Eur J Haematol. 2002;69:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Ishiga K, Kawatani T, Suou T, Tajima F, Omura H, Idobe Y, Kawasaki H. Fulminant hepatitis type B after chemotherapy in a serologically negative hepatitis B virus carrier with acute myelogenous leukemia. Int J Hematol. 2001;73:115-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Yujiri T, Tanaka M, Taguchi A, Tanaka Y, Nakamura Y, Tanizawa Y. Reactivation of hepatitis B virus in a hepatitis B surface antigen-negative patient with acute promyelocytic leukemia treated with arsenic trioxide. Ann Hematol. 2014;93:351-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, Hui P, Leung NW, Zee B, Johnson PJ. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 37. | Shouval D, Shibolet O. Immunosuppression and HBV reactivation. Semin Liver Dis. 2013;33:167-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 38. | Yeo W, Zee B, Zhong S, Chan PK, Wong WL, Ho WM, Lam KC, Johnson PJ. Comprehensive analysis of risk factors associating with Hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer. 2004;90:1306-1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 246] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 39. | Lau GK, Leung YH, Fong DY, Au WY, Kwong YL, Lie A, Hou JL, Wen YM, Nanj A, Liang R. High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood. 2002;99:2324-2330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 171] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 40. | Chen WC, Cheng JS, Chiang PH, Tsay FW, Chan HH, Chang HW, Yu HC, Tsai WL, Lai KH, Hsu PI. A Comparison of Entecavir and Lamivudine for the Prophylaxis of Hepatitis B Virus Reactivation in Solid Tumor Patients Undergoing Systemic Cytotoxic Chemotherapy. PLoS One. 2015;10:e0131545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Matsue K, Kimura S, Takanashi Y, Iwama K, Fujiwara H, Yamakura M, Takeuchi M. Reactivation of hepatitis B virus after rituximab-containing treatment in patients with CD20-positive B-cell lymphoma. Cancer. 2010;116:4769-4776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 42. | Kim SJ, Hsu C, Song YQ, Tay K, Hong XN, Cao J, Kim JS, Eom HS, Lee JH, Zhu J. Hepatitis B virus reactivation in B-cell lymphoma patients treated with rituximab: analysis from the Asia Lymphoma Study Group. Eur J Cancer. 2013;49:3486-3496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | Ferraro D, Pizzillo P, Di Marco V, Vultaggio A, Iannitto E, Venezia G, Craxì A, Di Stefano R. Evaluating the risk of hepatitis B reactivation in patients with haematological malignancies: is the serum hepatitis B virus profile reliable? Liver Int. 2009;29:1171-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Onozawa M, Hashino S, Izumiyama K, Kahata K, Chuma M, Mori A, Kondo T, Toyoshima N, Ota S, Kobayashi S. Progressive disappearance of anti-hepatitis B surface antigen antibody and reverse seroconversion after allogeneic hematopoietic stem cell transplantation in patients with previous hepatitis B virus infection. Transplantation. 2005;79:616-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 45. | Seto WK, Chan TS, Hwang YY, Wong DK, Fung J, Liu KS, Gill H, Lam YF, Lie AK, Lai CL. Hepatitis B reactivation in patients with previous hepatitis B virus exposure undergoing rituximab-containing chemotherapy for lymphoma: a prospective study. J Clin Oncol. 2014;32:3736-3743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 46. | Kusumoto S, Tanaka Y, Suzuki R, Watanabe T, Nakata M, Takasaki H, Fukushima N, Fukushima T, Moriuchi Y, Itoh K. Monitoring of Hepatitis B Virus (HBV) DNA and Risk of HBV Reactivation in B-Cell Lymphoma: A Prospective Observational Study. Clin Infect Dis. 2015;61:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 47. | Cho Y, Yu SJ, Cho EJ, Lee JH, Kim TM, Heo DS, Kim YJ, Yoon JH. High titers of anti-HBs prevent rituximab-related viral reactivation in resolved hepatitis B patient with non-Hodgkin’s lymphoma. J Med Virol. 2016;88:1010-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 48. | Hanson RG, Peters MG, Hoofnagle JH. Effects of immunosuppressive therapy with prednisolone on B and T lymphocyte function in patients with chronic type B hepatitis. Hepatology. 1986;6:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:221-244.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 390] [Article Influence: 39.0] [Reference Citation Analysis (1)] |
| 50. | Ling WH, Soe PP, Pang AS, Lee SC. Hepatitis B virus reactivation risk varies with different chemotherapy regimens commonly used in solid tumours. Br J Cancer. 2013;108:1931-1935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Lazdina U, Alheim M, Nyström J, Hultgren C, Borisova G, Sominskaya I, Pumpens P, Peterson DL, Milich DR, Sällberg M. Priming of cytotoxic T cell responses to exogenous hepatitis B virus core antigen is B cell dependent. J Gen Virol. 2003;84:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 52. | Mitka M. FDA: Increased HBV reactivation risk with ofatumumab or rituximab. JAMA. 2013;310:1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 53. | Xu X, Shang Q, Chen X, Nie W, Zou Z, Huang A, Meng M, Jin L, Xu R, Zhang JY. Reversal of B-cell hyperactivation and functional impairment is associated with HBsAg seroconversion in chronic hepatitis B patients. Cell Mol Immunol. 2015;12:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 54. | Kim SJ, Moon JH, Kim H, Kim JS, Hwang YY, Intragumtornchai T, Issaragrisil S, Kwak JY, Lee JJ, Won JH. Non-bacterial infections in Asian patients treated with alemtuzumab: a retrospective study of the Asian Lymphoma Study Group. Leuk Lymphoma. 2012;53:1515-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Iannitto E, Minardi V, Calvaruso G, Mulè A, Ammatuna E, Di Trapani R, Ferraro D, Abbadessa V, Craxí A, Di Stefano R. Hepatitis B virus reactivation and alemtuzumab therapy. Eur J Haematol. 2005;74:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 56. | Cortelezzi A, Viganò M, Zilioli VR, Fantini NN, Pasquini MC, Deliliers GL, Colombo M, Lampertico P. Adefovir added to lamivudine for hepatitis B recurrent infection in refractory B-cell chronic lymphocytic leukemia on prolonged therapy with Campath-1H. J Clin Virol. 2006;35:467-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Moses SE, Lim ZY, Sudhanva M, Devereux S, Ho AY, Pagliuca A, Zuckerman M, Mufti GJ. Lamivudine prophylaxis and treatment of hepatitis B Virus-exposed recipients receiving reduced intensity conditioning hematopoietic stem cell transplants with alemtuzumab. J Med Virol. 2006;78:1560-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Kang BW, Lee SJ, Moon JH, Kim SN, Chae YS, Kim JG, Hwang YJ, Sohn SK. Chronic myeloid leukemia patient manifesting fatal hepatitis B virus reactivation during treatment with imatinib rescued by liver transplantation: case report and literature review. Int J Hematol. 2009;90:383-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 59. | Ando T, Kojima K, Isoda H, Eguchi Y, Honda T, Ishigami M, Kimura S. Reactivation of resolved infection with the hepatitis B virus immune escape mutant G145R during dasatinib treatment for chronic myeloid leukemia. Int J Hematol. 2015;102:379-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 60. | Wang YD, Cui GH, Li M, Gowrea B, Xia J, Hu Y. Hepatitis B virus reactivation in a chronic myeloid leukemia patient treated with imatinib mesylate. Chin Med J (Engl). 2012;125:2636-2637. [PubMed] |
| 61. | Thia TJ, Tan HH, Chuah TH, Chow WC, Lui HF. Imatinib mesylate-related fatal acute hepatic failure in a patient with chronic myeloid leukaemia and chronic hepatitis B infection. Singapore Med J. 2008;49:e86-e89. [PubMed] |
| 62. | Ikeda K, Shiga Y, Takahashi A, Kai T, Kimura H, Takeyama K, Noji H, Ogawa K, Nakamura A, Ohira H. Fatal hepatitis B virus reactivation in a chronic myeloid leukemia patient during imatinib mesylate treatment. Leuk Lymphoma. 2006;47:155-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Lai GM, Yan SL, Chang CS, Tsai CY. Hepatitis B reactivation in chronic myeloid leukemia patients receiving tyrosine kinase inhibitor. World J Gastroenterol. 2013;19:1318-1321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 64. | Lakhani S, Davidson L, Priebat DA, Sherker AH. Reactivation of chronic hepatitis B infection related to imatinib mesylate therapy. Hepatol Int. 2008;2:498-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Fenaux P, Mufti GJ, Hellström-Lindberg E, Santini V, Gattermann N, Germing U, Sanz G, List AF, Gore S, Seymour JF. Azacitidine prolongs overall survival compared with conventional care regimens in elderly patients with low bone marrow blast count acute myeloid leukemia. J Clin Oncol. 2010;28:562-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 716] [Cited by in RCA: 765] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 66. | Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, Gau JP, Chou WC, Buckstein R, Cermak J. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 924] [Article Influence: 71.1] [Reference Citation Analysis (0)] |
| 67. | Reddy KR, Beavers KL, Hammond SP, Lim JK, Falck-Ytter YT; American Gastroenterological Association Institute. American Gastroenterological Association Institute guideline on the prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. 2015;148:215-219; quiz 216-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 451] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 68. | Ifuku H, Kusumoto S, Tanaka Y, Totani H, Ishida T, Okada M, Murakami S, Mizokami M, Ueda R, Iida S. Fatal reactivation of hepatitis B virus infection in a patient with adult T-cell leukemia-lymphoma receiving the anti-CC chemokine receptor 4 antibody mogamulizumab. Hepatol Res. 2015;45:1363-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 69. | Nakano N, Kusumoto S, Tanaka Y, Ishida T, Takeuchi S, Takatsuka Y, Akinaga S, Mizokami M, Ueda R, Utsunomiya A. Reactivation of hepatitis B virus in a patient with adult T-cell leukemia-lymphoma receiving the anti-CC chemokine receptor 4 antibody mogamulizumab. Hepatol Res. 2014;44:354-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 70. | Totani H, Kusumoto S, Ishida T, Masuda A, Yoshida T, Ito A, Ri M, Komatsu H, Murakami S, Mizokami M. Reactivation of hepatitis B virus (HBV) infection in adult T-cell leukemia-lymphoma patients with resolved HBV infection following systemic chemotherapy. Int J Hematol. 2015;101:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 71. | Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, Forero-Torres A. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363:1812-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 984] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 72. | Gopal AK, Chen R, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Connors JM, Engert A, Larsen EK, Chi X. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 73. | Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, Chen AI, Stiff P, Gianni AM, Carella A. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin’s lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385:1853-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 527] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 74. | Yang H, Cao Z, Wang Z, Liu M, Zhou H, Yang Q. [Hepatitis B virus reactivation induced by Brentuximab vedotin in the treatment of Hodgkin lymphoma: a case report and literature review]. Zhonghua Xue Ye Xue Zazhi. 2014;35:949-950. [PubMed] |
| 75. | Owen C, Stewart DA. Obinutuzumab for the treatment of lymphoproliferative disorders. Expert Opin Biol Ther. 2012;12:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 76. | Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, Chagorova T, de la Serna J, Dilhuydy MS, Illmer T. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1138] [Article Influence: 103.5] [Reference Citation Analysis (0)] |
| 77. | de Jésus Ngoma P, Kabamba B, Dahlqvist G, Sempoux C, Lanthier N, Shindano T, Van Den Neste E, Horsmans Y. Occult HBV reactivation induced by ibrutinib treatment: a case report. Acta Gastroenterol Belg. 2015;78:424-426. [PubMed] |
| 78. | Dubovsky JA, Beckwith KA, Natarajan G, Woyach JA, Jaglowski S, Zhong Y, Hessler JD, Liu TM, Chang BY, Larkin KM. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122:2539-2549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 656] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 79. | Verstovsek S, Mesa RA, Gotlib J, Levy RS, Gupta V, DiPersio JF, Catalano JV, Deininger M, Miller C, Silver RT. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366:799-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1559] [Cited by in RCA: 1635] [Article Influence: 125.8] [Reference Citation Analysis (0)] |
| 80. | Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med. 2012;366:787-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1274] [Cited by in RCA: 1418] [Article Influence: 109.1] [Reference Citation Analysis (0)] |
| 81. | Shen CH, Hwang CE, Chen YY, Chen CC. Hepatitis B virus reactivation associated with ruxolitinib. Ann Hematol. 2014;93:1075-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Caocci G, Murgia F, Podda L, Solinas A, Atzeni S, La Nasa G. Reactivation of hepatitis B virus infection following ruxolitinib treatment in a patient with myelofibrosis. Leukemia. 2014;28:225-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 83. | Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352:2487-2498. [PubMed] |
| 84. | San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1476] [Cited by in RCA: 1440] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 85. | Cavo M, Tacchetti P, Patriarca F, Petrucci MT, Pantani L, Galli M, Di Raimondo F, Crippa C, Zamagni E, Palumbo A. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: a randomised phase 3 study. Lancet. 2010;376:2075-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 662] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 86. | Basler M, Lauer C, Beck U, Groettrup M. The proteasome inhibitor bortezomib enhances the susceptibility to viral infection. J Immunol. 2009;183:6145-6150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 87. | Vogelbacher R, Meister S, Gückel E, Starke C, Wittmann S, Stief A, Voll R, Daniel C, Hugo C. Bortezomib and sirolimus inhibit the chronic active antibody-mediated rejection in experimental renal transplantation in the rat. Nephrol Dial Transplant. 2010;25:3764-3773. [PubMed] |
| 88. | Yoshida T, Kusumoto S, Inagaki A, Mori F, Ito A, Ri M, Ishida T, Komatsu H, Iida S, Sugauchi F. Reactivation of hepatitis B virus in HBsAg-negative patients with multiple myeloma: two case reports. Int J Hematol. 2010;91:844-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 89. | Lonial S, Dimopoulos M, Palumbo A, White D, Grosicki S, Spicka I, Walter-Croneck A, Moreau P, Mateos MV, Magen H. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2015;373:621-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 976] [Cited by in RCA: 1056] [Article Influence: 105.6] [Reference Citation Analysis (0)] |
| 90. | Danhof S, Schreder M, Strifler S, Einsele H, Knop S. Long-term disease control by pomalidomide-/dexamethasone-based therapy in a patient with advanced multiple myeloma: a case report and review of the literature. Case Rep Oncol. 2015;8:189-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 91. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [PubMed] |
| 92. | Liaw YF, Kao JH, Piratvisuth T, Chan HL, Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 792] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 93. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2170] [Article Influence: 135.6] [Reference Citation Analysis (0)] |
| 94. | Hwang JP, Somerfield MR, Alston-Johnson DE, Cryer DR, Feld JJ, Kramer BS, Sabichi AL, Wong SL, Artz AS. Hepatitis B Virus Screening for Patients With Cancer Before Therapy: American Society of Clinical Oncology Provisional Clinical Opinion Update. J Clin Oncol. 2015;33:2212-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 95. | Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, Csako G. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148:519-528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 354] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 96. | Martyak LA, Taqavi E, Saab S. Lamivudine prophylaxis is effective in reducing hepatitis B reactivation and reactivation-related mortality in chemotherapy patients: a meta-analysis. Liver Int. 2008;28:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 97. | Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, Cheung M, Zhang HY, Lie A, Ngan R. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125:1742-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 331] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 98. | Hsu C, Hsiung CA, Su IJ, Hwang WS, Wang MC, Lin SF, Lin TH, Hsiao HH, Young JH, Chang MC. A revisit of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in non-Hodgkin’s lymphoma: a randomized trial. Hepatology. 2008;47:844-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 243] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 99. | Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, Chan HL, Hui EP, Lei KI, Mok TS. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009;27:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 500] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 100. | Mozessohn L, Chan KK, Feld JJ, Hicks LK. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive patients receiving rituximab for lymphoma: a meta-analysis. J Viral Hepat. 2015;22:842-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 101. | Yamagata M, Murohisa T, Tsuchida K, Okamoto Y, Tsunoda S, Nakamura M, Kusano K, Majima Y, Kuniyoshi T, Iijima M. Fulminant B hepatitis in a surface antigen and hepatitis B DNA-negative patient with diffuse large B-cell lymphoma after CHOP chemotherapy plus rituximab. Leuk Lymphoma. 2007;48:431-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Perceau G, Diris N, Estines O, Derancourt C, Lévy S, Bernard P. Late lethal hepatitis B virus reactivation after rituximab treatment of low-grade cutaneous B-cell lymphoma. Br J Dermatol. 2006;155:1053-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 69] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 103. | Hui CK, Cheung WW, Zhang HY, Au WY, Yueng YH, Leung AY, Leung N, Luk JM, Lie AK, Kwong YL. Kinetics and risk of de novo hepatitis B infection in HBsAg-negative patients undergoing cytotoxic chemotherapy. Gastroenterology. 2006;131:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 362] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 104. | Ji D, Cao J, Hong X, Li J, Wang J, Chen F, Wang C, Zou S. Low incidence of hepatitis B virus reactivation during chemotherapy among diffuse large B-cell lymphoma patients who are HBsAg-negative/ HBcAb-positive: a multicenter retrospective study. Eur J Haematol. 2010;85:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Koo YX, Tay M, Teh YE, Teng D, Tan DS, Tan IB, Tai DW, Quek R, Tao M, Lim ST. Risk of hepatitis B virus (HBV) reactivation in hepatitis B surface antigen negative/hepatitis B core antibody positive patients receiving rituximab-containing combination chemotherapy without routine antiviral prophylaxis. Ann Hematol. 2011;90:1219-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 106. | Fukushima N, Mizuta T, Tanaka M, Yokoo M, Ide M, Hisatomi T, Kuwahara N, Tomimasu R, Tsuneyoshi N, Funai N. Retrospective and prospective studies of hepatitis B virus reactivation in malignant lymphoma with occult HBV carrier. Ann Oncol. 2009;20:2013-2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 107. | Huang YH, Hsiao LT, Hong YC, Chiou TJ, Yu YB, Gau JP, Liu CY, Yang MH, Tzeng CH, Lee PC. Randomized controlled trial of entecavir prophylaxis for rituximab-associated hepatitis B virus reactivation in patients with lymphoma and resolved hepatitis B. J Clin Oncol. 2013;31:2765-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 265] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 108. | Yoo JJ, Cho EJ, Cho YY, Lee M, Lee DH, Cho Y, Lee JH, Yu SJ, Yoon SS, Yoon JH. Efficacy of antiviral prophylaxis in HBsAg-negative, anti-HBc positive patients undergoing hematopoietic stem cell transplantation. Liver Int. 2015;35:2530-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 109. | Awerkiew S, Däumer M, Reiser M, Wend UC, Pfister H, Kaiser R, Willems WR, Gerlich WH. Reactivation of an occult hepatitis B virus escape mutant in an anti-HBs positive, anti-HBc negative lymphoma patient. J Clin Virol. 2007;38:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 110. | Ferreira R, Carvalheiro J, Torres J, Fernandes A, Giestas S, Mendes S, Agostinho C, Campos MJ. Fatal hepatitis B reactivation treated with entecavir in an isolated anti-HBs positive lymphoma patient: a case report and literature review. Saudi J Gastroenterol. 2012;18:277-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 111. | Huang H, Li X, Zhu J, Ye S, Zhang H, Wang W, Wu X, Peng J, Xu B, Lin Y. Entecavir vs lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA. 2014;312:2521-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 112. | Shang J, Wang H, Sun J, Fan Z, Huang F, Zhang Y, Jiang Q, Dai M, Xu N, Lin R. A comparison of lamivudine vs entecavir for prophylaxis of hepatitis B virus reactivation in allogeneic hematopoietic stem cell transplantation recipients: a single-institutional experience. Bone Marrow Transplant. 2016;51:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 113. | Koskinas JS, Deutsch M, Adamidi S, Skondra M, Tampaki M, Alexopoulou A, Manolakopoulos S, Pectasides D. The role of tenofovir in preventing and treating hepatitis B virus (HBV) reactivation in immunosuppressed patients. A real life experience from a tertiary center. Eur J Intern Med. 2014;25:768-771. [PubMed] |
| 114. | Gara N, Zhao X, Collins MT, Chong WH, Kleiner DE, Jake Liang T, Ghany MG, Hoofnagle JH. Renal tubular dysfunction during long-term adefovir or tenofovir therapy in chronic hepatitis B. Aliment Pharmacol Ther. 2012;35:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 115. | Pipili C, Cholongitas E, Papatheodoridis G. Review article: nucleos(t)ide analogues in patients with chronic hepatitis B virus infection and chronic kidney disease. Aliment Pharmacol Ther. 2014;39:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 116. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1364] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 117. | Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, Wichroski MJ, Xu D, Yang J, Wilber RB. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology. 2009;49:1503-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 615] [Cited by in RCA: 633] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 118. | Li W, Huang L, Guo H, Wei X, Liang Z. Antiviral efficacy analysis of telbivudine concurrent with the first cycle of chemotherapy in HBsAg-positive lymphoma patients. J Clin Virol. 2014;61:199-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 119. | Hui CK, Cheung WW, Au WY, Lie AK, Zhang HY, Yueng YH, Wong BC, Leung N, Kwong YL, Liang R. Hepatitis B reactivation after withdrawal of pre-emptive lamivudine in patients with haematological malignancy on completion of cytotoxic chemotherapy. Gut. 2005;54:1597-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 120. | Dai MS, Chao TY, Kao WY, Shyu RY, Liu TM. Delayed hepatitis B virus reactivation after cessation of preemptive lamivudine in lymphoma patients treated with rituximab plus CHOP. Ann Hematol. 2004;83:769-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 121. | Kim HJ, Sinn DH, Kim NJ, Kim JH, Kim E, Gwak GY, Paik YH, Choi MS, Lee JH, Koh KC. Stopping Preemptive Antiviral Therapy for Hepatitis B Virus Can Be Considered for Patients with Favorable Predictors. Dig Dis Sci. 2015;60:3794-3800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 122. | Liu WP, Wang XP, Zheng W, Ping LY, Zhang C, Wang GQ, Song YQ, Zhu J. Hepatitis B virus reactivation after withdrawal of prophylactic antiviral therapy in patients with diffuse large B cell lymphoma. Leuk Lymphoma. 2016;57:1355-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 123. | Mikulska M, Nicolini L, Signori A, Rivoli G, Del Bono V, Raiola AM, Di Grazia C, Dominietto A, Varaldo R, Ghiso A. Hepatitis B reactivation in HBsAg-negative/HBcAb-positive allogeneic haematopoietic stem cell transplant recipients: risk factors and outcome. Clin Microbiol Infect. 2014;20:O694-O701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 124. | An J, Shim JH, Kim SO, Choi J, Kim SW, Lee D, Kim KM, Lim YS, Lee HC, Chung YH. Comprehensive outcomes of on- and off-antiviral prophylaxis in hepatitis B patients undergoing cancer chemotherapy: A competing risks analysis. J Med Virol. 2016;88:1576-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 125. | Noterdaeme T, Longrée L, Bataille C, Deroover A, Lamproye A, Delwaide J, Beguin Y, Honoré P, Detry O. Liver transplantation for acute hepatic failure due to chemotherapy-induced HBV reactivation in lymphoma patients. World J Gastroenterol. 2011;17:3069-3072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 126. | Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1582] [Article Influence: 175.8] [Reference Citation Analysis (2)] |