Published online Jul 28, 2016. doi: 10.3748/wjg.v22.i28.6345
Peer-review started: April 4, 2016
First decision: May 12, 2016
Revised: June 11, 2016
Accepted: July 6, 2016
Article in press: July 6, 2016
Published online: July 28, 2016
Processing time: 115 Days and 12.2 Hours
The prognosis of patients with metastatic colorectal cancer (mCRC) remain poor despite the impressive improvement of treatments observed over the last 20 years that led to an increase in median overall survival from 6 mo, with the only best supportive care, to approximately 30 mo with the introduction of active chemotherapy drugs and targeted agents. The monoclonal antibodies (moAbs) cetuximab and panitumumab, directed against the epidermal growth factor receptor (EGFR), undoubtedly represent a major step forward in the treatment of mCRC, given the relevant efficacy in terms of progression-free survival, overall survival, response rate, and quality of life observed in several phase III clinical trials among different lines of treatment. However, the anti-EGFR moAbs were shown only to be effective in a subset of patients. For instance, KRAS and NRAS mutations have been identified as biomarkers of resistance to these drugs, improving the selection of patients who might derive a benefit from these treatments. Nevertheless, several other alterations might affect the response to these drugs, and unfortunately, even the responders eventually become resistant by developing secondary (or acquired) resistance in approximately 13-18 mo. Several studies highlighted that the landscape of responsible alterations of both primary and acquired resistance to anti-EGFR drugs biochemically converge into MEK-ERK and PIK3CA-AKT pathways. In this review, we describe the currently known mechanisms of primary and acquired resistance to anti-EGFR moAbs together with the various strategies evaluated to prevent, overcame or revert them.
Core tip: The treatment with anti-epidermal growth factor receptor (EGFR) monoclonal antibodies cetuximab and panitumumab in metastatic colorectal cancer is unfortunately burdened by the lack of clinical and molecular biomarkers that correlate with treatment response. Primary and acquired resistance have been shown to be the major culprits of the failure of anti-EGFR treatments. However, a deeper understanding of the molecular basis underlying both types of resistance has led to the proposal of several approaches designed to prevent, overcome or revert the drug resistance. Nevertheless, these approaches deserve further clinical investigation to allow us to use EGFR-targeted therapies more effectively in the correct population.
- Citation: Sforza V, Martinelli E, Ciardiello F, Gambardella V, Napolitano S, Martini G, della Corte C, Cardone C, Ferrara ML, Reginelli A, Liguori G, Belli G, Troiani T. Mechanisms of resistance to anti-epidermal growth factor receptor inhibitors in metastatic colorectal cancer. World J Gastroenterol 2016; 22(28): 6345-6361
- URL: https://www.wjgnet.com/1007-9327/full/v22/i28/6345.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i28.6345
Colorectal cancer (CRC) is considered the third most commonly diagnosed cancer in males and the second in females worldwide, with an estimated 1.4 million new cases in 2012. In the same year, CRC was responsible for 693900 deaths, making it the fourth leading cause of cancer-related death in men and the third in women[1]. Although the advances in screening and medical treatments have led a trend in reduction of both incidence and mortality, almost 20% of patients present metastases at the time of diagnosis, and approximately 35% of patients will subsequently develop a metastatic disease[2]. The prognosis of patients with metastatic colorectal cancer (mCRC) remains poor despite the impressive improvement observed over the last 20 years that led to an increase in median overall survival (OS) from 6 mo, with the only best supportive care (BSC), to approximately 30 mo with the introduction of active chemotherapy drugs, such as fluropyrimidines, oxaliplatin, irinotecan, TAS-102, and of targeted drugs, such as bevacizumab, cetuximab, panitumumab, aflibercept, and regorafenib[3,4]. However, despite these great advances, the efficacy of these treatments remains limited and substantially unpredictable. The reasons for this restricted success are firstly the development of various mechanisms of resistance to treatments and secondly the lack of clinical and molecular biomarkers that correlate with treatment response. This drug resistance is particularly relevant for drugs that target the epidermal growth factor receptor (EGFR).
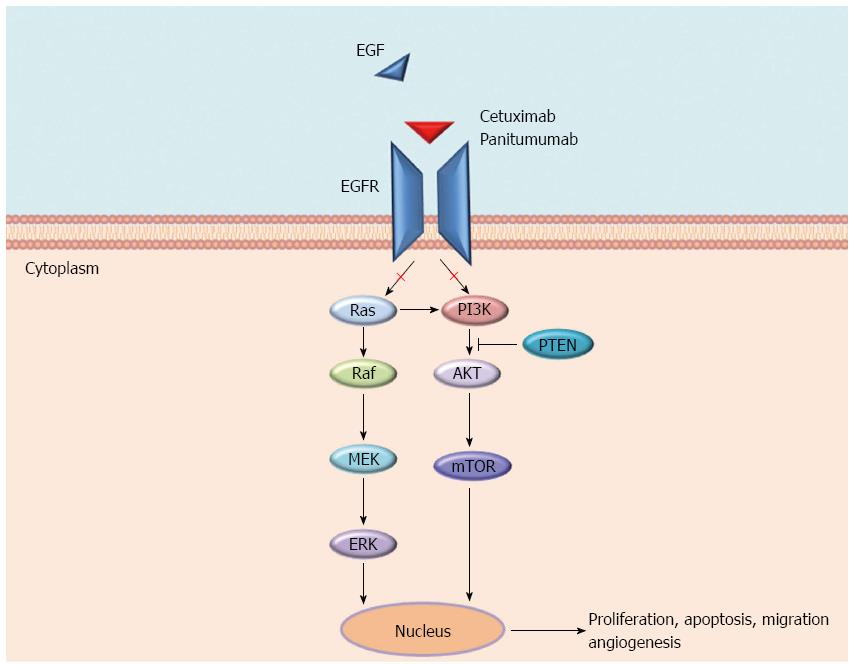
EGFR is a tyrosine kinases receptor (RTK) and a member of the ErbB family to which HER2/neu (ERBB2), HER3 (ErbB3), and HER4 (ErbB4) also belong. EGFR is expressed in various cancers and in 60% to 80% of CRC, where it plays a key role in tumour development and progression. Various ligands (EGF, TGFα, amphiregulin, and epiregulin) bind specific extracellular domains (ECDs) of EGFR, inducing homo- and hetero-dimerization with other ErbB members and the subsequent activation of several pathways, including RAS-RAF-MEK-ERK and PIK3CA-AKT cascades, the SRC family kinases, PLCγ-PKC, and STATs. These pathways are involved in several cellular processes including tumour growth, inhibition of apoptosis, angiogenesis, invasion, and metastasis[5,6] (Figure 1).
For this reason, EGFR has been proposed as a target for anticancer therapies, and to date, two monoclonal antibodies (moAbs) against the EGFR, cetuximab and panitumumab, have been registered for the treatment of mCRC patients. Cetuximab, is a human-mouse chimeric monoclonal antibody (IgG1 subtype), whereas panitumumab is a fully human anti-EGFR monoclonal antibody (IgG2κ subtype). These antibodies bind the ECD of EGFR, blocking ligand-induced EGFR tyrosine kinase activation and causing EGFR cellular internalization and degradation[7,8]. Furthermore, cetuximab activates antibody-dependent cellular cytotoxicity. Cetuximab and panitumumab provided similar OS benefits in KRAS exon 2 wild-type (WT), chemotherapy-refractory mCRC in the ASPECCT trial[9].
EGFR-targeted therapies, both as single agents or in combination with chemotherapy, undoubtedly represent a major step forward in the treatment of mCRC, given the relevant efficacy in terms of progression-free survival (PFS), OS, response rate (RR), as well as quality of life, observed in several phase III clinical trials among different lines of treatment[3]. However, not all patients will benefit from these treatments. Indeed, cetuximab and panitumumab when used as single agents in unselected patients with chemotherapy-refractory mCRC, achieved a RR of only 10%[10,11]. This low RR suggests that the majority of tumours harbour genetic alterations in proteins involved in EGFR pathway that impair the response to the anti-EGFR moAbs (intrinsic or primary resistance). Moreover, even the subset of patients who initially respond to these treatments will ultimately become refractory in approximately 3-18 mo by developing secondary (or acquired) resistance to anti-EGFR drugs[12]. These phenomenon might be explained if we consider that CRC, and in particular metastatic disease, is highly heterogeneous[13]. This heterogeneity implies that tumours from the same organ might have a completely different molecular landscape (inter-tumour heterogeneity) as well as different sensitivity to targeted agents, depending on which pathway is driving their growth. Furthermore, even in the same lesion, we might find clones with different sensitivity to drugs (intra-tumour heterogeneity) depending on the different molecular alterations harboured[14]. Unfortunately, to date, the molecular characteristics that allow the response to anti-EGFR moAbs are not yet completely understood, and the lack of predictive biomarkers do not permit the selection of patients who will potentially respond to these drugs. For instance, differently from other cancers, mutations in the EGFR or in downstream effectors of its signalling cascades (e.g., KRAS, NRAS, BRAF, PIK3CA and PTEN loss) are not predictive of the efficacy of targeted agents[15,16].
In the era of targeted medicine, translational and clinical research efforts are being spent to better understand the complex molecular landscape of tumours to increasingly tailor the treatments to the molecular characteristics of the specific patient. The aim of this review is to provide an overview of the molecular mechanisms that underlie both primary and acquired resistance to anti-EGFR drugs in mCRC and to discuss possible future ways to circumvent them.
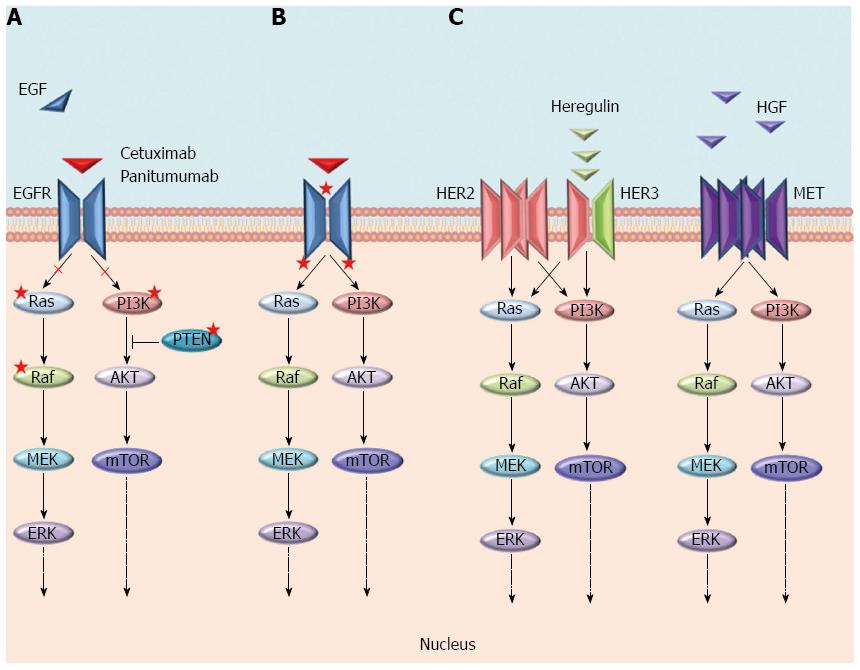
The mechanisms of resistance to anti-EGFR moAbs can be categorized as primary or acquired according to the time of onset in respect to the treatment with these drugs, and also, although without a strict boundary, by the molecular alterations underlying them (Figure 2). Generally, the most frequent mechanisms of resistance are a result of genomic alterations in downstream effectors (e.g., KRAS, NRAS, BRAF, and PIK3CA) of the EGFR signalling pathway, while the activation of other RTKs, such as MET or ERBB2 and their pathways, are more rare mechanisms[17-19]. In both cases, unless the EGFR continues to be pharmacologically blocked, an alternative signal transducer becomes activated, escaping the receptor inhibition. Notably, these genetic alterations have been identified as both mechanisms of primary and acquired resistance, and almost all of them biochemically converge on the activation of the MEK-ERK pathway[12]. The only exceptions are represented by rare mutations either in the ECD or in the tyrosine kinase domain of EGFR that have only been described as acquired mechanisms of resistance in patients treated with anti-EGFR moAbs[20-22]. Furthermore, different from primary resistance, acquired resistance is generally sustained by several genetic alterations that concomitantly emerge at treatment failure[22]. These aberrations may arise either as new genetic alterations, due to treatment-induced mutagenesis and tumour-intrinsic genomic instability (e.g., mutations in ECD or tyrosine kinase domain of EGFR), or through the positive selection pressure of anti-EGFR therapies on a resistant subpopulation of cells already present in the original tumour[13]. Because of the overlapping of resistance mechanisms, the next chapters are focused on the description of single molecular alterations and whether the resulting mechanisms of resistance can be categorized as primary, secondary or both.
RAS is a family of three small GTPases (KRAS, NRAS, and HRAS) that work as downstream effectors within the mitogen-activated protein kinase (MAPK) pathway, coupling EGFR with intracellular signalling cascades[23]. The KRAS gene has been found mutated in approximately 40% of CRCs, mostly in exon 2 codons 12 (70%-80%) and 13 (15%-20%), whereas only a small percentage has been found in codons 61 (5%) and 146 (5%). These point mutations impair the intrinsic ATPase activity of RAS and cause the accumulation of mutant proteins in the active conformation (GTP-bound). The latter leads to constitutive MAPK pathway activation, regardless of the EGFR inhibition, that results in mitogenic and antiapoptotic signalling[17]. The mutational status of KRAS is highly concordant between the primary tumour and the metastasis, suggesting it has a role in the early processes of carcinogenesis[24].
In the early clinical trials in which cetuximab and panitumumab were used as monotherapies to treat patients with chemorefractory mCRC, an objective response rate (ORR) of only 10% was achieved[10,11]; these findings motivated researchers to elucidate the factors that were negatively impacting the efficacy of these drugs. In particular, retrospective analysis of KRAS mutational status from tumour samples of several randomized trials were able to strongly support the hypothesis that the KRAS mutations in codons 12 and 13 (exon 2) were associated with the lack of patient response to EGFR moAbs[17,25-27]. All together, the evidence led the American and European health authorities in 2009 to restrict the use of panitumumab and cetuximab only to the approximately 60% of patients with KRAS exon 2 WT tumours[26,28-31].
Nevertheless, because not all KRAS WT patients benefit from treatment with EGFR-directed therapy, researchers have tried to identify additional biomarkers of resistance that could explain this heterogeneity in clinical response. In particular, the retrospective analysis of the PRIME trial assessed the efficacy and safety of panitumumab plus oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) compared with chemotherapy alone in first-line mCRC patients, according to RAS (KRAS or NRAS) mutation status. The results suggested that even mutations occurring in exon 3 (comprising codons 59 and 61), and exon 4 (which includes codons 117 and 146) of KRAS as well as mutations in exons 2, 3 and 4 of NRAS (all together called “expanded RAS mutations”) can also be predictive of resistance to anti-EGFR treatments. These data were subsequently confirmed by the analyses of other phase II and III clinical studies[32-36]. In these trials, the prevalence of the expanded RAS mutations in patients defined as WT at exon 2 of KRAS ranged from 15% to 27%. Thus, considering all KRAS and NRAS mutations, approximately 53% of patients with mCRC are considered to have mutations in RAS and, therefore, to be refractory to EGFR blockade[32-36]. In line with these findings, our group published the results from the phase II CAPRI trial, in which patients with KRAS exon 2 WT mCRC were treated with FOLFIRI plus cetuximab in first-line treatment and were then randomized at progression to receive FOLFOX alone or FOLFOX plus cetuximab. Our results confirmed the lack of benefit of cetuximab among the subset of patients harbouring KRAS or NRAS mutations[37]. Furthermore, in 2014, Sorich et al[16] published a meta-analysis of nine randomized controlled trials (RCTs) evaluating the role of EGFR antibodies in all lines of mCRC therapy. The meta-analysis revealed that treatment with anti-EGFR antibodies had superior efficacy in terms of PFS and OS for all RAS WT tumours compared with the expanded RAS mutant subgroup, and the efficacy was not significantly different between the expanded RAS mutant and KRAS exon 2 mutant subgroups. These results suggest that tumours with one of the new RAS mutations are more appropriately grouped with the tumours with a KRAS exon 2 mutation (forming the any RAS mutant group), rather than with tumours that do not have any RAS mutations[16]. These results demonstrated the prominent role of RAS mutations as biomarkers of primary resistance to anti-EGFR therapies. In response to the meta-analysis, the EMA and FDA have updated the prescribing indications for panitumumab and cetuximab, restricting their use to patients with RAS WT mCRC[38,39].
As said before, genetic alterations in RAS are even the most common molecular mechanisms that drive secondary resistance to anti-EGFR therapy in 50% to 80% of patients. These mutations may be present in a small fraction of cells within the tumour before treatment initiation and then may be selected by pressure from the anti-EGFR treatments or arise as a result of continued mutagenesis during the treatment[40,41]. Different from primary resistance, secondary resistance generally arises from more than one driver and arises at different frequencies. This pattern has been observed in both cell lines made resistant to cetuximab or panitumumab as well as in samples obtained from patients[42,43]. It is worth noting that the frequency of mutations at codon 61 of exon 3, in either KRAS or NRAS genes, is more prevalent in the acquired setting[22,42]. However, the KRAS gene has been found not only mutated but also amplified, although in a very small percentage of patients (0.7%), and this amplification has been observed as a mechanism in both primary and acquired resistance[43].
However, preclinical data and retrospective analysis from phase III trials highlighted that not all KRAS mutations have the same role in mediating EGFR-resistance, and some patients with KRAS mutated tumours occasionally respond to anti-EGFR treatments. In particular, patients harbouring the KRAS G13D mutation have been found to achieve a benefit from cetuximab in both first-line and advanced lines of treatment[44,45]. A recently published meta-analysis of eight RCTs have assessed whether the efficacy of anti-EGFR mAbs for mCRC differs between tumours harbouring the KRAS G13D mutation and KRAS mutations other than the KRAS G13D mutation. The authors did not find any significant difference in terms of PFS or in OS between KRAS G13D and other KRAS mutations[46]. Schirripa et al[47] conducted a phase II single-arm trial to provide prospective proof of the clinical benefit of cetuximab in KRAS G13D mutant mCRC patients. However, among 12 treated patients, no responses have been observed. Along the same lines, the Australasian Gastro-Intestinal Trials Group recently published the results of the phase II ICE CREAM study (Irinotecan Cetuximab Evaluation and Cetuximab Response Evaluation Among Patients with a G13D Mutation), in which patients with G13D-mutated chemotherapy-refractory mCRC, who had progressed within 6 mo of irinotecan therapy, were randomly assigned to receive cetuximab with or without irinotecan. Among the 51 patients recruited, there was no statistically significant improvement in disease control at 6 mo with either cetuximab monotherapy or cetuximab plus irinotecan. Furthermore, no responses were observed with single-agent cetuximab[48].
The intra-tumour heterogeneity noted that CRC is often formed of clones with different mutational profiles and that in many tumours only a fraction of neoplastic cells carries the mutant allele. Given these considerations, Normanno et al[49] have proposed a quantitative assessment of KRAS mutation load as a tool to discriminate whether a low content of KRAS mutant alleles in mCRC cells may affect the response anti-EGFR moAbs. Although they found that patients with low KRAS mutation content responded to EGFR-based therapy, this benefit did not translate in a PFS advantage; indeed, PFS was similar to patients with high KRAS tumours. The authors have suggested that it might be explained by either the expansion of a small fraction of cells carrying a resistance mutation or by the coexistence, in several low KRAS mutated tumours, of other mutations such as BRAF and PIK3CA. A quantitative assessment of mutational load might be useful to identify the priority target for therapeutic intervention; however, the complexity of tumour mutational profiles suggests that for many tumours combinations of target-based agents will likely to be necessary to control tumour growth[49].
Considering the key role of RAS mutations as mechanisms of resistance to anti-EGFR moAbs, many approaches have been investigated to target these mutations. One of the earlier approaches was the inhibition of RAS farnesylation that showed a potent antitumour activity in preclinical studies; however, this result was not confirmed in clinical experience. Several other approaches have been used to target RAS: blocking downstream effectors such as MEK and PIK3CA, identification of synthetic lethal (SL) interactions with mutant KRAS, or the use of small molecule inhibitors of KRAS[42,50-54]. Recently, a combination therapy co-targeting MEK and CDK4/6 with trametinib and palbociclib, respectively, was well tolerated and highly efficacious in KRAS-mutant CRC patient tumour-derived PDX[55,56]. However, a clinical evaluation of these agents is currently lacking. A biological strategy with Reovirus Serotype 3 - Dearing Strain (Reolysin®), a naturally occurring ubiquitous, non-enveloped human Reovirus, has been explored in KRAS-mutated mCRC because reovirus has been shown to replicate selectively in RAS mutant cells, causing cell lysis. A multicentre phase I study of Reolysin® in combination with FOLFIRI and bevacizumab in naïve patients with KRAS-mutated mCRC is ongoing[57]. To date, RAS remains the most elusive gene to target; thus, patients with RAS mutations are currently treated with chemotherapy with or without antiangiogenic drugs[3].
Mutation in other downstream effectors of the MAPK pathway beyond RAS may invalidate the effects of anti-EGFR moAbs, cetuximab and panitumumab. One example is BRAF, a serine/threonine protein kinase that is found mutated in approximately 10%-15% of CRCs[58]. The most common BRAF alteration is the point mutation V600E in the kinase domain, accounting for 80% of all BRAF mutations[59]. The BRAF V600E mutation is generally mutually exclusive with RAS mutations. However, recent data from the CAPRI trial demonstrated, in 12 out of 15 BRAF mutated tumour samples, the coexistence of BRAF mutations with other mutations, including TP53, KRAS, and PIK3CA exon 9 and exon 20[37,60].
The BRAF V600E mutation represents a biomarker of poor prognosis in the CRC population. This finding emerged from analyses of a large cohort of patients enrolled in several clinical trials that have consistently demonstrated the association of the BRAF V600E mutation with an increased colon cancer-specific mortality[61,62].
Although the prognostic role of BRAF mutation is well established, its role as a predictive biomarker to anti-EGFR treatments is not clearly understood because of both its low prevalence and the prominent negative prognostic role. Several studies have showed that patients harbouring a BRAF mutation do not achieve any benefit from anti-EGFR treatments in second-line or in later lines of therapy[62-64]. However, data from the first-line setting are less clear. For instance, in both CRYSTAL and OPUS studies, the addition of cetuximab to FOLFIRI and FOLFOX, respectively, slightly improved PFS and OS compared to chemotherapy alone[31,65]. On the contrary, recent studies in patients receiving first-line anti-EGFR moAbs in combination with chemotherapy did not show a statistically significant correlation between BRAF V600E mutation and response[32,34]. Also a recent meta-analysis of nine phase III trials revealed that the addition of an anti-EGFR moAbs to first-line doublet chemotherapy for patients with BRAF-mutant disease was not associated with improved OS, PFS or ORR[66]. In conclusion, the small percentage of BRAF mutated patients, together with a lack of prospective studies, do not allow one to establish with certainty the predictive role of BRAF mutation for treatment with cetuximab and panitumumab.
BRAF mutation has been recognized, by circulating tumour DNA (ctDNA) analysis, also as a mechanism of acquired resistance in patients who first responded to anti-EGFR therapy[22].
In a phase II trial, the BRAF inhibitor vemurafenib has been tested in previously treated patients with BRAF-mutated mCRC showing only a modest clinical activity (5% of RR) with respect to the impressive results (RR of 48% to 67%) obtained in BRAF-mutated melanoma[67]. In cell line models, it has been found that resistance to the BRAF-targeted approach seems to be caused by either persistent activation of the EGFR signalling pathway or the activation of other pathways such as PIK3CA/AKT[68,69]. These findings showed that the biology of BRAF activation is more heterogeneous in CRC than in other tumours and suggested a potential role for a combination approach. Indeed, the combination of dabrafenib and trametinib (BRAF/MEK inhibitor therapy) obtained better results than vemurafenib monotherapy in 43 patients with BRAF V600-mutant mCRC[70]. Furthermore, the first trials testing the double blockade with the BRAF inhibitor vemurafenib and either the EGFR inhibitor panitumumab or a PI3KCA/mTOR inhibitor demonstrated modest activity[71,72]. To date, several clinical trials are assessing the effects of BRAF inhibitors in combination with MEK, EGFR and PI3K inhibitors[73].
The use of ERK inhibitors to suppress MAPK activity is another potential strategy because it has been observed that MAPK is usually upregulated in patients resistant to RAF inhibitors[74].
It is worth noting that the TRIBE study, comparing FOLFOXIRI plus bevacizumab vs FOLFOXIRI in first-line mCRC patients, showed a relevant hazard ratio for progression of 0.55 in favour of the combination with bevacizumab (interaction test P = 0.320), in the subgroup of 28 patients with tumours harbouring BRAF mutations[75].
The PIK3CA/AKT/mTOR signalling pathway is associated with several RTKs, including EGFR[76]. Alterations in genes that encode for these proteins play an important role in the development of malignant tumours and could impair the response to EGFR-targeted moAbs. In particular, activating mutations of PIK3CA, mostly occurring in exons 9 and 20, have been found in 10%-20% of CRCs, and in preclinical models, they were found to be predictive of a lack of benefit from cetuximab treatment[77-79]. In a retrospective analysis of 110 mCRC patients treated with anti-EGFR moAbs, a statistically significant association between PIK3CA mutations and primary resistance to treatment with cetuximab or panitumumab was reported in the population of patients with KRAS WT tumours[80]. A large retrospective analysis of 1022 tumour samples of patients treated with cetuximab described two important findings: (1) only PIK3CA exon 20 mutations was predictive of a lack of response to cetuximab in the KRAS WT subpopulation; and (2) PIK3CA exon 9 mutations and KRAS mutations were associated, suggesting a secondary role of PIK3CA exon 9 mutations on cetuximab efficacy[17]. Thereafter, two meta-analyses also described a negative predictive role of only PIK3CA mutations in exon 20 in terms of ORR, PFS, and OS in WT KRAS mCRC patients treated with anti-EGFR therapies[81,82]. Nevertheless, is difficult to evaluate the precise role of PIK3CA mutations with respect to anti-EGFR resistance because they are mostly found concurrently with KRAS or BRAF mutations and because of their low incidence, especially the exon 20 mutations. PIK3CA mutations have also been identified as mechanisms of secondary resistance in samples from patients who experienced relapse after treatment with EGFR-targeted moAbs[21].
Several data have also suggested a role of PIK3CA mutations as a prognostic biomarker. Indeed, an increased colon cancer-specific mortality has been found in patients with PIK3CA-mutated tumours, compared with WT ones. However, only the coexistence of PIK3CA exon 9 and 20 mutations has been reported to be associated with a worse prognosis than WT tumours[83]. Furthermore, PIK3CA mutations seem to predict a worse prognosis only in KRAS WT patients compared with KRAS-mutated[84].
PIK3CA signalling pathway may also be pathologically activated by the loss of PTEN, found in 30% of CRCs[85]. Firstly, Frattini et al[86] found in a cohort of patients treated with cetuximab and irinotecan that of 11 patients with lower PTEN expression in tumour samples at immunohistochemistry (IHC), none of the patients responded to treatment when compared with patients with normal PTEN expression who achieved a partial response. PTEN loss was also associated with shorter OS in patients with KRAS WT tumours treated with a cetuximab-based regimen[64]. In a retrospective analysis of a cohort of patients treated with cetuximab plus irinotecan, Loupakis et al[87] showed that PTEN IHC results were not completely concordant between primary tumours and metastases and that the PTEN status of primary tumours was not significantly predictive of cetuximab activity. Conversely, when PTEN IHC was performed on metastases, 36% of the patients with PTEN-positive samples responded to therapy compared with patients who harboured a PTEN-negative status. They also showed that patients who harboured both a KRAS WT tumour and PTEN-positive metastasis were much more likely to benefit from treatment in terms of RR, PFS, and OS. However, in the NCIC CTG/AGITG CO.17 trial, where 572 patients with pretreated mCRC were randomly assigned to receive cetuximab or BSC, no statistical significance was found with respect of the loss of PTEN and the clinical outcome of patients treated with cetuximab[88].
PTEN loss has been identified only as a primary mechanism of resistance to cetuximab and panitumumab. However, to date, PIK3CA mutations and PTEN expression have not been validated as predictive markers for EGFR moAbs therapy in mCRC for several key reasons. Firstly, PIK3CA and PTEN alterations mostly co-occur with RAS and/or BRAF mutations; secondly, the expression of PTEN protein by IHC is burdened by conflicting interpretations; and lastly, only PTEN expression in metastases, but not in primary tumours, has been associated with outcome[89].
Targeted treatments against PIK3CA or its downstream effectors such as mTOR or AKT in patients harbouring PIK3CA mutations or PTEN loss, although interesting, did not show a meaningful clinical activity[90,91]. A greater therapeutic effect has been observed when these drugs have been combined with RTK inhibitors in preclinical models; however, this benefit has not been confirmed in phase I trials[92]. Clinical trials evaluating the combination of the mTOR inhibitor everolimus with panitumumab and irinotecan in first-line mCRC patients are ongoing[93]. The combination of a PIK3CA inhibitor and a MEK inhibitor in preclinical models was more effective than MEK and PI3K/mTOR inhibition, and several clinical trials are exploiting this combination[94-96]. Furthermore, low-dose aspirin seems to improve survival in patients with PIK3CA-mutant tumours by aspirin-mediated COX2 inhibition, but this observation requires further prospective evaluation[97].
HER2 is a member of the ErbB family and is a recognized target in breast cancer[6]. This receptor does not have any known ligand, and its activation is provided by the hetero-dimerization with other ligand-bound receptors of the same family. The preferred partner is HER3, and the heterodimer HER2-HER3 represents a powerful activator of intracellular signalling[98]. HER2 amplification allows the activation of MEK-ERK cascade regardless of the signalling of EGFR.
In two different studies, HER2 amplification has been highlighted as a predictor of lack of response to anti-EGFR antibodies[99,100]. In 2011, Bertotti et al[19] recognized HER2 amplification as a potential mechanism of primary resistance to cetuximab within a quadruple WT population (KRAS, NRAS, BRAF, and PIK3CA wild type) of immune-compromised mice (xenopatients) transplanted with fragments of CRC samples from patients. Firstly, the authors observed that HER2 was amplified only in a small percentage (2%-3%) of genetically unselected CRC patients. However, a greater frequency of HER2 amplification was observed in KRAS WT patients resistant to cetuximab (13.6%) and in up to 36% of xenopatients in the subset of quadruple WT, in which treatment with cetuximab was ineffective. The authors also envisioned a possible role of HER2 as a positive predictor of response to HER2-targeting agents in CRC. Hence, they performed a multiarm xenotrial demonstrating that the association of a dual EGFR/HER2 small molecule inhibitor (lapatinib) and cetuximab or pertuzumab, a monoclonal antibody directed against EGFR/HER2 heterodimer, was active in the subset of cetuximab-resistant, quadruple-negative, HER2-amplified metastatic CRC xenopatients and was a feasible combination for clinical trials[19]. Based on these findings, Siena and colleagues designed the HERACLES trial, a multicentre open-label phase II trial, assessing the RR of trastuzumab combined with either lapatinib (cohort A) or pertuzumab (cohort B), in KRAS exon 2 (codons 12 and 13) WT and HER2 amplified mCRC patients who were resistant to standard therapies, including anti-EGFRs. To date, the results from cohort A have been recently published. The authors reported a frequency of 5% (48 patients) of HER2-positive tumours among 914 patients screened for the trial. Of the 27 patients enrolled, eight (30%, 95%CI: 14-50) achieved an overall objective response, and the median duration of the responses was 38 wk. Median PFS was 21 wk (95%CI: 16-32), while median OS calculated post hoc was 46 wk (95%CI: 33-68). The authors reported that responses were significantly more common in tumours with high HER2 gene copy number. Additionally, the PFS was longer in this population. Finally, the combination was well tolerated, with most toxic effects being grade 1 or 2. To date, HER2 is the first druggable target in mCRC that has been shown to be a good predictor of response to targeted treatments[99].
Data published by Yonesaka et al[99] demonstrated the role of HER2 signalling in the context of acquired resistance to anti-EGFR moAbs. Moreover, they reported that hyper-activation of HER2 signalling was not only led by HER2 amplification but also through the overproduction of heregulin, an HER3 ligand. Using resistant clones from cetuximab-sensitive cell lines, as well as plasma and tissue samples from cetuximab-treated mCRC patients, the authors found that patients with acquired resistance to cetuximab had an increased percentage of HER2 amplification in post-treatment samples (71%) compared to the proportion present in pretreatment tumour cells (14%). In the same way, heregulin levels in plasma and tumour samples were significantly higher in patients who experienced a disease progression on anti-EGFR therapy. This indicates that enhanced HER2 signalling confers not only primary but also acquired resistance to anti-EGFR moAbs by leading to persistent activation of ERK signalling[99].
Additionally, HER3 has been described to have a role as a potential biomarker of resistance to anti-EGFR treatments. In a cohort of metastatic CRC patients treated in second- or third-line therapy with irinotecan and cetuximab, HER3 overexpression was associated with shorter PFS and OS[101]. Moreover, HER3 has been found mutated in approximately 11% of CRC patients, and these mutations, even if HER2 is not amplified, may limit the responsiveness to EGFR inhibitors[102].
MEHD7945A is a humanized IgG1 mAb with dual anti HER3/EGFR activity. In multiple xenograft models, MEHD7945A was demonstrated to be significantly superior with respect to the monospecific EGFR targeting agents[103]. In a phase I study, promising results have been achieved among patients with pretreated mCRC; however, no benefit for MEHD7945A + FOLFIRI vs cetuximab + FOLFIRI has been observed in a phase II randomized trial in KRAS WT mCRC patients refractory to oxaliplatin[104,105].
EGFR mutations in CRC represent a mechanism of resistance described only in the acquired setting and might occur in approximately 20% of patients treated with cetuximab and in only 1% of patients treated with panitumumab[106]. In particular, Montagut et al[20] discovered a point mutation in the ECD of EGFR (S492R) in a CRC cell line made resistant to cetuximab and also confirmed these data in a small percentage of patients who relapsed after cetuximab treatment. This mutation does not allow the binding of cetuximab to the receptor, but it still allows the binding of panitumumab; indeed, the authors reported the experience of a patient with this mutation who was treated with panitumumab and who achieved a response from treatment[20]. Recently, Arena et al[21] discovered several other mutations in the EGFR ECD. They analysed tumour samples (pre- and post-treatment with cetuximab) obtained from 37 mCRC patients with acquired resistance to cetuximab and found two novel mutations, R451C and K467T, in two patients. The authors also discovered several other novel EGFR variants (S464L, G465R and I491M) in CRC cell lines made resistant to cetuximab. These mutations are located in the cetuximab-binding region, with the exception of the R451C mutation. Functionally, all of these mutations prevent binding of cetuximab, and only R451C and K467T mutations are permissive for interaction with panitumumab[21].
Furthermore, Bettegowda et al[22] also described mutations in the EGFR kinase domain at codons 714 and 794 that were identified as circulating mutations by cell-free DNA analysis only in the setting of acquired resistance.
One possible strategy to overcome the resistance to anti-EGFR moAbs mediated by mutations in ECD of EGFR would be to create moAbs that bind to different epitopes located in this region. For instance, Sym004 is a mixture of two different mAbs directed against non-overlapping epitopes of domain III of the EGFR. The antibodies bind simultaneously to the ECD of EGFR, inducing internalization and degradation of the receptor. In preclinical studies, Sym004 demonstrated a stronger tumour suppression compared to cetuximab and panitumumab[22].
Dienstmann and colleagues reported the results from the phase I trial in which 42 patients with mCRC and acquired resistance to anti-EGFR inhibitors were enrolled to receive different doses (9 or 12 mg/kg weekly) of Sym004. All patients had a documented response to previous anti-EGFR mAb treatment followed by disease progression. Of the 39 patients evaluable for response, 17 (44%) had a different degree of tumour shrinkage, with an overall disease control rate (partial response and stable disease) of 67%; median PFS was 3.3 mo (95%CI: 2.6-4.9). Regarding toxicities, the most common drug-related adverse events of any grade were skin rash, dry skin, hypomagnesemia and pruritus[107,108]. Currently, Sym004 is under investigation in a phase II clinical trial as a monotherapy in selected patients with KRAS WT CRC progressing to previous cetuximab or panitumumab-based therapy within 6 mo from trial enrolment.
Another drug, MM-151, is a third-generation EGFR inhibitor consisting of a mixture of three fully human IgG1 antibodies that bind distinct, non-overlapping epitopes on EGFR. In preclinical models, MM-151 showed an enhanced anticancer effect by improving the EGFR pathway inhibition, as well as inducing a more profound downregulation of the receptor and stimulating the innate immune responses[109]. The results of the phase I trial with MM-151 alone vs combination with irinotecan demonstrated the safety of the drug, and in particular, demonstrated an unusually long-lasting disease control in the combination arm.
MET is a tyrosine kinase receptor involved in several cell processes, such as proliferation, apoptosis, invasion and angiogenesis. It is activated by its ligand, the hepatocyte growth factor (HGF). Several mechanisms may lead to an abnormal activation of MET, including MET amplification and/or increased expression of HGF[110].
In mCRC cancer, MET has been found amplified in approximately 2% of samples, where it has been associated with the development of distant metastases, and it has been significantly correlated with poor outcomes[111,112]. Furthermore, Bardelli et al[18] highlighted the role of MET amplification as a mechanism of both primary and acquired resistance to anti-EGFR moAbs in WT KRAS mCRC patients. They also showed an increased rate of amplification (12.5%) in a cetuximab-resistant xenopatients WT for RAS, BRAF, PIK3CA, and HER2[18]. However, only focal, high-grade amplification of the MET locus is associated with lack of response instead of modest gene copy number gains or polysomy of chromosome 7[113].
In preclinical models, Troiani et al[114] found that overexpression of TGF-alpha might contribute to cetuximab resistance in CRC cells through the induction of a EGFR-MET interaction; the treatment of these cells with a selective MET inhibitor restores cetuximab sensitivity, suggesting that the combined inhibition of both EGFR and MET pathways could represent a rational therapeutic strategy for preventing and/or overcoming cetuximab resistance in patients with mCRC.
In a randomized phase II clinical trial of chemo-refractory, KRAS WT, anti-EGFR-naive mCRC, the combination of an anti-HGF moAb and panitumumab led to higher RR and a trend for a better outcome in the population with MET-overexpressed[115]. A phase I trial assessing the role of cabozantinib, a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR2, plus panitumumab in chemo-refractory, KRAS WT patients is currently ongoing[116].
Treatment with anti-EGFR moAbs cetuximab and panitumumab might be limited by the presence of primary resistance or the emergence of multiple acquired mechanisms to escape from the EGFR blockade. To overcome these phenomena, several approaches have been proposed, and some of them have been anticipated above (Table 1).
| Target | Drug | Study | ClinicalTrials.gov | Phase |
| Identifier | ||||
| EGFR | Afatinib | Afatinib and cetuximab combo vs cetuximab alone in treatment of patients with refractory KRAS WT mCRC | NCT01919879 | Phase II |
| Cetuximab | ||||
| EGFR | Sym004 | Sym004 vs investigator's choice (best supportive care, capecitabine, 5-FU) in subjects with mCRC and acquired resistance to anti-EGFR moAbs | NCT02083653 | Phase II |
| EGFR, HER2, HER4 | Neratinib | Neratinib and cetuximab in KRAS/NRAS/BRAF/PIK3CA WT mCRC patients resistant to cetuximab | NCT01960023 | Phase I/II |
| Cetuximab | ||||
| EGFR | Cetuximab | Cetuximab plus irinotecan as rechallenge 3rd-line treatment of KRAS, NRAS and BRAF WT irinotecan-pretreated mCRC patients progressing after an initial response to a 1st-line cetuximab-containing therapy and a standard 2nd-line | NCT02296203 | Phase II |
| Irinotecan | ||||
| EGFR | Panitumumab | FOLFIRI plus panitumumab in extended RAS WT and BRAF WT mCRC with acquired resistance to prior cetuximab (or Panitumumab) plus irinotecan-based therapy and who failed at least one subsequent non-anti-EGFR containing regimen | NCT02508077 | Phase II |
| FOLFIRI | ||||
| ERBB2 | Pertuzumab | Pertuzumab and lapatinib in KRAS exon 2 WT and HER2-positive mCRC refractory to standard of care (including cetuximab or panitumumab) | - | Phase II |
| Trastuzumab | ||||
| MET | Cetuximab INC280 | INC280 in combination with cetuximab in c-MET positive mCRC and HNSCC Patients who have progressed after anti-EGFR moAbs therapy | NCT02205398 | Phase |
| Ib | ||||
| MET | Cabozantinib | Cabozantinib with panitumumab in subjects with KRAS WT refractory mCRC | Phase II | |
| Panitumumab | ||||
| BRAF/EGFR | Vemurafenib | Irinotecan and cetuximab with or without vemurafenib in BRAF mutant mCRC patients | NCT02164916 | Phase II |
| Cetuximab | ||||
| Irinotecan | ||||
| BRAF/PI3K/EGFR | LGX818 BYL719 | LGX818 and cetuximab or LGX818, BYL719, and cetuximab in BRAF mutant mCRC patients | NCT01719380 | Phase I/II |
| Cetuximab | ||||
| BRAF/MEK/EGFR | Trametinib | Trametinib and dabrafenib administered in combination with panitumumab in BRAF-V600E positive mCRC patients with secondary resistance to prior anti-EGFR therapy | NCT01750918 | Phase I/II |
| Dabrafenib | ||||
| Panitumumab | ||||
| PI3K-mTOR | Panitumumab | Second line therapy with panitumumab, irinotecan and everolimus in KRAS WT mCRC patients | NCT01139138 | Phase |
| Everolimus | Ib/II | |||
| Irinotecan | ||||
| PI3K/MEK | BKM120 | BKM120 plus MEK162 in adult patients with selected advanced solid tumors | NCT01363232 | Phase |
| MEK162 | Ib | |||
| Immune evasion | Pembrolizumab | Pembrolizumab plus cetuximab for KRAS-NRAS-BRAF WT mCRC patients | NCT02318901 | Phase I/II |
| Cetuximab |
To increase the efficacy of anti-EGFR mAbs, combinations of these drugs and vascular endothelial growth factor receptor (VEGFR) inhibitors were evaluated. However, the addition of cetuximab or panitumumab to bevacizumab and oxaliplatin-based chemotherapy in first-line KRAS WT mCRC patients, did not improve the outcomes[117,118].
Furthermore, although there were positive effects on both PFS and RR, the combination of cetuximab plus brivanib (a VEGFR multikinase inhibitor) increased toxicity and did not improve OS in patients with chemotherapy-refractory, KRAS WT mCRC[119].
One of the most promising approaches to circumvent or reverse resistance to anti-EGFR moAbs is to target more than one downstream effector of the EGFR pathway. As reported above, their use as single agents did not prove clinical efficacy because of the activation of alternative pathways as a mechanism to escape the blockade. Because the alterations that confer resistance to the anti-EGFR moAbs biochemically converge to activate the MEK-ERK and AKT pathways, selective inhibitors of MEK kinases seemed an attractive target. In preclinical models, MEK inhibitors suppressed KRAS mutated cells resistant to cetuximab[120]. Several studies showed that activation of the PIK3CA pathway is a major mechanism of resistance that impairs the efficacy of MEK inhibitors in KRAS mutated cancers. Hence, dual inhibition with MEK and PIK3CA inhibitors resulted in a strong inhibition of tumour cell growth[121,122]. Troiani et al[123] demonstrated that combined inhibition of both EGFR and MEK had a synergistic antiproliferative and apoptotic effect in cells and xenografts with either primary or acquired resistance to cetuximab, representing a rational therapeutic strategy for preventing and/or overcoming cetuximab resistance in patients with mCRC. The same group also suggested that combining classical EGFR inhibitors with multitarget agents may circumvent primary and acquired resistance to EGFR inhibitors. For example, the combination of cetuximab and regorafenib, an oral multi-kinase inhibitor, could be an active combination and deserves further testing in a clinical setting[124].
Re-challenging patients with an alternative anti-EGFR moAb after failure with a drug of the same family has been tested. However, panitumumab has been demonstrated to provide minimal benefit in patients with KRAS WT mCRC who have experienced progression to cetuximab as prior therapy[125,126]. The hypotheses that pre-existing sensitive subclones may emerge after treatment breaks with anti-EGFR moAb has led the design of several clinical trials prospectively evaluating the rechallenge with anti-EGFR moAbs in the third-line setting after a response to the first-line therapy. Only patients who are quadruple WT are being enrolled[127].
Interestingly, Ciardiello et al[128] recently presented the results from the second line of the CAPRI study and demonstrated that quadruple WT mCRC patients showed a significantly prolonged PFS as well as OS and RR from continuing cetuximab with a different chemotherapeutic agent beyond cancer progression after first-line chemotherapy plus the same anti-EGFR. These findings highlighted that molecularly selected mCRC patients have tumours that are highly dependent on EGFR signalling for their growth, despite the progression to the anti-EGFR drug[128]. These data are interesting and deserve further investigations.
Furthermore, as described above, new anti-EGFR such as Sym004 and MM-151 seem to be active but deserve further investigations[108,109]. Another new drug that has been proposed is GA201 (also known as RG7160, imgatuzumab), a humanized anti-EGFR IgG1 moAb that has showed an increased binding affinity for all FcgRIIIa variants expressed on immune effector cells, such as natural killer cells, leading to a significant improvement in terms of antibody-dependent cell mediated cytotoxicity-based cell killing. Encouraging results have been emerged from the phase I trial; however, a randomized phase II trial assessing the role of GA201 in combination with FOLFIRI vs cetuximab plus FOLFIRI in second-line mCRC patients showed no PFS benefit for the experimental arm in both KRAS WT and KRAS mutant patients[129,130].
A promising approach is represented by the immune check point blockade with the antibodies against CTLA-4 (ipilimumab) or PD-1 (pembrolizumab, nivolumab) designed to interrupt the immune evasion strategies adopted by cancer cells. Mismatch-repair status has been found to be a useful biomarker in predicting the clinical benefit of immune checkpoint blockade with pembrolizumab. Indeed, a higher response has been achieved by patients with Microsatellite Instability High (MSI-High) tumours[131]. Several trials mainly targeting the PD-1/PDL-1 immune checkpoint pathway are ongoing[132]. Furthermore, preclinical and clinical evidence has suggested that the immune system contributes substantially to the therapeutic effects of mAbs in vivo[133]. The combination of immune modulators or checkpoint inhibitors with cetuximab is under evaluation as a first-line therapy of KRAS WT mCRC[134].
Early detection of resistance cell clones to anti-EGFR moAbs is another possible approach. However, the classical tumour biopsy might not be representative of tumour heterogeneity and is also an invasive procedure that is often not feasible due to the inaccessibility of metastatic lesions or due to the refusal of patients to be re-biopsied. However, liquid biopsies, i.e., analysing ctDNA in blood samples, have been demonstrated to be useful tools for monitoring the emergence of drug resistance during the course of treatment. Indeed, different groups have demonstrated that analysis of ctDNA in plasma samples allowed detection of mutations predictive of EGFR moAbs resistance, approximately 10 mo before progression was assessed by radiological methods[22,41,135]. Nevertheless, larger and prospective trials are needed before this technique can enter in clinical routine.
CRC is still a leading cause of cancer-related mortality in the developed world. In recent years, remarkable advances in the genetic and biological understanding of cancer have led to the development of different targeted cancer therapies, such as the anti-EGFR moAbs cetuximab and panitumumab. However, the overall progress achieved with these drugs has been modest because they have been shown to be effective only in a subset of patients. Primary and acquired resistance have been shown to be the major culprits of the failure of anti-EGFR treatments. However, a deeper understanding of the molecular basis underlying both types of resistance has contributed to the proposal of several approaches to prevent, overcome or reverse drug resistance. Nevertheless, these approaches deserve further clinical investigation to allow us to use the EGFR-targeted therapies more effectively in the correct population.
Manuscript Source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Italy
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hu FL, Sinagra E, Valladares-Ayerbes M S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21366] [Article Influence: 2136.6] [Reference Citation Analysis (3)] |
| 2. | Schmoll HJ, Van Cutsem E, Stein A, Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde CJ, Balmana J, Regula J. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann Oncol. 2012;23:2479-2516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1107] [Article Influence: 85.2] [Reference Citation Analysis (1)] |
| 3. | Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25 Suppl 3:iii1-iii9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 658] [Cited by in RCA: 798] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 4. | Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 1009] [Article Influence: 100.9] [Reference Citation Analysis (0)] |
| 5. | Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4920] [Cited by in RCA: 5111] [Article Influence: 213.0] [Reference Citation Analysis (1)] |
| 6. | Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2397] [Cited by in RCA: 2487] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 7. | Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358:1160-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1515] [Cited by in RCA: 1567] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 8. | Martinelli E, De Palma R, Orditura M, De Vita F, Ciardiello F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin Exp Immunol. 2009;158:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 9. | Price TJ, Peeters M, Kim TW, Li J, Cascinu S, Ruff P, Suresh AS, Thomas A, Tjulandin S, Zhang K. Panitumumab versus cetuximab in patients with chemotherapy-refractory wild-type KRAS exon 2 metastatic colorectal cancer (ASPECCT): a randomised, multicentre, open-label, non-inferiority phase 3 study. Lancet Oncol. 2014;15:569-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 335] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 10. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3767] [Cited by in RCA: 3708] [Article Influence: 176.6] [Reference Citation Analysis (1)] |
| 11. | Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1472] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 12. | Misale S, Di Nicolantonio F, Sartore-Bianchi A, Siena S, Bardelli A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov. 2014;4:1269-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 399] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 13. | Burrell RA, Swanton C. Tumour heterogeneity and the evolution of polyclonal drug resistance. Mol Oncol. 2014;8:1095-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 329] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 14. | Burrell RA, McGranahan N, Bartek J, Swanton C. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1517] [Cited by in RCA: 1652] [Article Influence: 137.7] [Reference Citation Analysis (0)] |
| 15. | Chung KY, Shia J, Kemeny NE, Shah M, Schwartz GK, Tse A, Hamilton A, Pan D, Schrag D, Schwartz L. Cetuximab shows activity in colorectal cancer patients with tumors that do not express the epidermal growth factor receptor by immunohistochemistry. J Clin Oncol. 2005;23:1803-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 821] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 16. | Sorich MJ, Wiese MD, Rowland A, Kichenadasse G, McKinnon RA, Karapetis CS. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta-analysis of randomized, controlled trials. Ann Oncol. 2015;26:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 404] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 17. | De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1491] [Cited by in RCA: 1656] [Article Influence: 110.4] [Reference Citation Analysis (1)] |
| 18. | Bardelli A, Corso S, Bertotti A, Hobor S, Valtorta E, Siravegna G, Sartore-Bianchi A, Scala E, Cassingena A, Zecchin D. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 2013;3:658-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 546] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 19. | Bertotti A, Migliardi G, Galimi F, Sassi F, Torti D, Isella C, Corà D, Di Nicolantonio F, Buscarino M, Petti C. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011;1:508-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 735] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 20. | Montagut C, Dalmases A, Bellosillo B, Crespo M, Pairet S, Iglesias M, Salido M, Gallen M, Marsters S, Tsai SP. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med. 2012;18:221-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 391] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 21. | Arena S, Bellosillo B, Siravegna G, Martínez A, Cañadas I, Lazzari L, Ferruz N, Russo M, Misale S, González I. Emergence of Multiple EGFR Extracellular Mutations during Cetuximab Treatment in Colorectal Cancer. Clin Cancer Res. 2015;21:2157-2166. [PubMed] |
| 22. | Bettegowda C, Sausen M, Leary RJ, Kinde I, Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6:224ra24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2770] [Cited by in RCA: 3549] [Article Influence: 322.6] [Reference Citation Analysis (0)] |
| 23. | Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682-4689. [PubMed] |
| 24. | Santini D, Loupakis F, Vincenzi B, Floriani I, Stasi I, Canestrari E, Rulli E, Maltese PE, Andreoni F, Masi G. High concordance of KRAS status between primary colorectal tumors and related metastatic sites: implications for clinical practice. Oncologist. 2008;13:1270-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 186] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 25. | Lièvre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Côté JF, Tomasic G, Penna C, Ducreux M. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992-3995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1669] [Cited by in RCA: 1699] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 26. | Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2724] [Cited by in RCA: 2761] [Article Influence: 162.4] [Reference Citation Analysis (0)] |
| 27. | Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, Juan T, Sikorski R, Suggs S, Radinsky R. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:1626-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2504] [Cited by in RCA: 2403] [Article Influence: 141.4] [Reference Citation Analysis (0)] |
| 28. | European Medicines Agency. Committee for Medicinal Products for Human Use. May 2008 Plenary Meeting Monthly Report. Available from: http://www.emea.europa.eu/pdfs/human/press/pr/27923508en.pdf. |
| 29. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1393] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 30. | Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, André T, Chan E, Lordick F, Punt CJ. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:4706-4713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 709] [Cited by in RCA: 758] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 31. | Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1452] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 32. | Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1610] [Cited by in RCA: 1731] [Article Influence: 144.3] [Reference Citation Analysis (0)] |
| 33. | Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, Yu H, Oliner KS, Go WY. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. 2014;32:2240-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 495] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 34. | Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1330] [Article Influence: 120.9] [Reference Citation Analysis (0)] |
| 35. | Van Cutsem E, Lenz HJ, Köhne CH, Heinemann V, Tejpar S, Melezínek I, Beier F, Stroh C, Rougier P, van Krieken JH. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 632] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 36. | Bokemeyer C, Köhne CH, Ciardiello F, Lenz HJ, Heinemann V, Klinkhardt U, Beier F, Duecker K, van Krieken JH, Tejpar S. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer. 2015;51:1243-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 37. | Ciardiello F, Normanno N, Maiello E, Martinelli E, Troiani T, Pisconti S, Giuliani F, Barone C, Cartenì G, Rachiglio AM. Clinical activity of FOLFIRI plus cetuximab according to extended gene mutation status by next-generation sequencing: findings from the CAPRI-GOIM trial. Ann Oncol. 2014;25:1756-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 38. | European Medicines Agency. Committee for Medicinal Products for Human Use. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000558/WC500160158.pdf. |
| 39. | European Medicines Agency. Committee for Medicinal Products for Human Use. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000741/WC500148667.pdf. |
| 40. | Diaz LA, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, Allen B, Bozic I, Reiter JG, Nowak MA. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature. 2012;486:537-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1259] [Cited by in RCA: 1354] [Article Influence: 104.2] [Reference Citation Analysis (0)] |
| 41. | Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, Valtorta E, Schiavo R, Buscarino M, Siravegna G. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. 2012;486:532-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1313] [Cited by in RCA: 1473] [Article Influence: 113.3] [Reference Citation Analysis (0)] |
| 42. | Misale S, Arena S, Lamba S, Siravegna G, Lallo A, Hobor S, Russo M, Buscarino M, Lazzari L, Sartore-Bianchi A. Blockade of EGFR and MEK intercepts heterogeneous mechanisms of acquired resistance to anti-EGFR therapies in colorectal cancer. Sci Transl Med. 2014;6:224ra26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 220] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 43. | Valtorta E, Misale S, Sartore-Bianchi A, Nagtegaal ID, Paraf F, Lauricella C, Dimartino V, Hobor S, Jacobs B, Ercolani C. KRAS gene amplification in colorectal cancer and impact on response to EGFR-targeted therapy. Int J Cancer. 2013;133:1259-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 44. | De Roock W, Jonker DJ, Di Nicolantonio F, Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M, Piessevaux H. Association of KRAS p.G13D mutation with outcome in patients with chemotherapy-refractory metastatic colorectal cancer treated with cetuximab. JAMA. 2010;304:1812-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 584] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 45. | Tejpar S, Celik I, Schlichting M, Sartorius U, Bokemeyer C, Van Cutsem E. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J Clin Oncol. 2012;30:3570-3577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 287] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 46. | Rowland A, Dias MM, Wiese MD, Kichenadasse G, McKinnon RA, Karapetis CS, Sorich MJ. Meta-analysis comparing the efficacy of anti-EGFR monoclonal antibody therapy between KRAS G13D and other KRAS mutant metastatic colorectal cancer tumours. Eur J Cancer. 2016;55:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Schirripa M, Loupakis F, Lonardi S, Cremolini C, Bergamo F, Zagonel V, Falcone A. Phase II study of single-agent cetuximab in KRAS G13D mutant metastatic colorectal cancer. Ann Oncol. 2015;26:2503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Segelov E, Thavaneswaran S, Waring PM, Desai J, Robledo KP, Gebski VJ, Elez E, Nott LM, Karapetis CS, Lunke S. Response to Cetuximab With or Without Irinotecan in Patients With Refractory Metastatic Colorectal Cancer Harboring the KRAS G13D Mutation: Australasian Gastro-Intestinal Trials Group ICECREAM Study. J Clin Oncol. 2016;34:2258-2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 49. | Normanno N, Rachiglio AM, Lambiase M, Martinelli E, Fenizia F, Esposito C, Roma C, Troiani T, Rizzi D, Tatangelo F. Heterogeneity of KRAS, NRAS, BRAF and PIK3CA mutations in metastatic colorectal cancer and potential effects on therapy in the CAPRI GOIM trial. Ann Oncol. 2015;26:1710-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 50. | Kohl NE, Omer CA, Conner MW, Anthony NJ, Davide JP, deSolms SJ, Giuliani EA, Gomez RP, Graham SL, Hamilton K. Inhibition of farnesyltransferase induces regression of mammary and salivary carcinomas in ras transgenic mice. Nat Med. 1995;1:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 374] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 51. | Macdonald JS, McCoy S, Whitehead RP, Iqbal S, Wade JL, Giguere JK, Abbruzzese JL. A phase II study of farnesyl transferase inhibitor R115777 in pancreatic cancer: a Southwest oncology group (SWOG 9924) study. Invest New Drugs. 2005;23:485-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 112] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 52. | Migliardi G, Sassi F, Torti D, Galimi F, Zanella ER, Buscarino M, Ribero D, Muratore A, Massucco P, Pisacane A. Inhibition of MEK and PI3K/mTOR suppresses tumor growth but does not cause tumor regression in patient-derived xenografts of RAS-mutant colorectal carcinomas. Clin Cancer Res. 2012;18:2515-2525. [PubMed] |
| 53. | Costa-Cabral S, Brough R, Konde A, Aarts M, Campbell J, Marinari E, Riffell J, Bardelli A, Torrance C, Lord CJ. Correction: CDK1 Is a Synthetic Lethal Target for KRAS Mutant Tumours. PLoS One. 2016;11:e0154007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 54. | Ostrem JM, Peters U, Sos ML, Wells JA, Shokat KM. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature. 2013;503:548-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1819] [Article Influence: 151.6] [Reference Citation Analysis (0)] |
| 55. | Ziemke EK, Dosch JS, Maust JD, Shettigar A, Sen A, Welling TH, Hardiman KM, Sebolt-Leopold JS. Sensitivity of KRAS-Mutant Colorectal Cancers to Combination Therapy That Cotargets MEK and CDK4/6. Clin Cancer Res. 2016;22:405-414. [PubMed] |
| 56. | Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med. 2015;66:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 57. | ClinicalTrials. gov Identifier: NCT01274624. Available from: https://clinicaltrials.gov/. |
| 58. | Sridhar SS, Hedley D, Siu LL. Raf kinase as a target for anticancer therapeutics. Mol Cancer Ther. 2005;4:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 177] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 59. | Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7459] [Cited by in RCA: 7635] [Article Influence: 332.0] [Reference Citation Analysis (0)] |
| 60. | Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 938] [Cited by in RCA: 952] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 61. | Bokemeyer C, Van Cutsem E, Rougier P, Ciardiello F, Heeger S, Schlichting M, Celik I, Köhne CH. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur J Cancer. 2012;48:1466-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 433] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 62. | Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705-5712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1242] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 63. | Laurent-Puig P, Cayre A, Manceau G, Buc E, Bachet JB, Lecomte T, Rougier P, Lievre A, Landi B, Boige V. Analysis of PTEN, BRAF, and EGFR status in determining benefit from cetuximab therapy in wild-type KRAS metastatic colon cancer. J Clin Oncol. 2009;27:5924-5930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 525] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 64. | Seymour MT, Brown SR, Middleton G, Maughan T, Richman S, Gwyther S, Lowe C, Seligmann JF, Wadsley J, Maisey N. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): a prospectively stratified randomised trial. Lancet Oncol. 2013;14:749-759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 254] [Cited by in RCA: 311] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 65. | Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: the OPUS study. Ann Oncol. 2011;22:1535-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 586] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 66. | Pietrantonio F, Petrelli F, Coinu A, Di Bartolomeo M, Borgonovo K, Maggi C, Cabiddu M, Iacovelli R, Bossi I, Lonati V. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 2015;51:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 373] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 67. | Kopetz S, Desai J, Chan E, Hecht JR, O’Dwyer PJ, Maru D, Morris V, Janku F, Dasari A, Chung W. Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J Clin Oncol. 2015;33:4032-4038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 428] [Cited by in RCA: 551] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 68. | Prahallad A, Sun C, Huang S, Di Nicolantonio F, Salazar R, Zecchin D, Beijersbergen RL, Bardelli A, Bernards R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1560] [Article Influence: 120.0] [Reference Citation Analysis (0)] |
| 69. | Mao M, Tian F, Mariadason JM, Tsao CC, Lemos R, Dayyani F, Gopal YN, Jiang ZQ, Wistuba II, Tang XM. Resistance to BRAF inhibition in BRAF-mutant colon cancer can be overcome with PI3K inhibition or demethylating agents. Clin Cancer Res. 2013;19:657-667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 70. | Corcoran RB, Atreya CE, Falchook GS, Kwak EL, Ryan DP, Bendell JC, Hamid O, Messersmith WA, Daud A, Kurzrock R. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600-Mutant Colorectal Cancer. J Clin Oncol. 2015;33:4023-4031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 415] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 71. | Yaeger R, Cercek A, O’Reilly EM, Reidy DL, Kemeny N, Wolinsky T, Capanu M, Gollub MJ, Rosen N, Berger MF. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin Cancer Res. 2015;21:1313-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 231] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 72. | Kelway B, Simpson KH, Smith RJ, Halsall PJ. Effects of atropine and glycopyrrolate on cognitive function following anaesthesia and electroconvulsive therapy (ECT). Int Clin Psychopharmacol. 1986;1:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 456] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 73. | ClinicalTrials. gov Identifier: NCT01750918; NCT01719380. Available from: https://clinicaltrials.gov/. |
| 74. | Ahronian LG, Sennott EM, Van Allen EM, Wagle N, Kwak EL, Faris JE, Godfrey JT, Nishimura K, Lynch KD, Mermel CH. Clinical Acquired Resistance to RAF Inhibitor Combinations in BRAF-Mutant Colorectal Cancer through MAPK Pathway Alterations. Cancer Discov. 2015;5:358-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 258] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 75. | Cremolini C, Loupakis F, Lonardi S, Tomasello G, Ronzoni M, Zaniboni A, Tonini G, Valsuani C, Chiara S, Boni C. Subgroup analyses in RAS mutant, BRAF mutant and “all-wt” metastatic colorectal cancer patients treated with FOLFOXIRI plus bevacizumab (bev) or FOLFIRI plus bev in the TRIBE study. Ann Oncol. 2014;25:ii107-ii108. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 76. | De Roock W, De Vriendt V, Normanno N, Ciardiello F, Tejpar S. KRAS, BRAF, PIK3CA, and PTEN mutations: implications for targeted therapies in metastatic colorectal cancer. Lancet Oncol. 2011;12:594-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 466] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 77. | Huang CH, Mandelker D, Schmidt-Kittler O, Samuels Y, Velculescu VE, Kinzler KW, Vogelstein B, Gabelli SB, Amzel LM. The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3Kalpha mutations. Science. 2007;318:1744-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 462] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 78. | Karakas B, Bachman KE, Park BH. Mutation of the PIK3CA oncogene in human cancers. Br J Cancer. 2006;94:455-459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 388] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 79. | Jhawer M, Goel S, Wilson AJ, Montagna C, Ling YH, Byun DS, Nasser S, Arango D, Shin J, Klampfer L. PIK3CA mutation/PTEN expression status predicts response of colon cancer cells to the epidermal growth factor receptor inhibitor cetuximab. Cancer Res. 2008;68:1953-1961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 369] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 80. | Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, Di Nicolantonio F, Saletti P, De Dosso S, Mazzucchelli L. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 560] [Cited by in RCA: 595] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 81. | Mao C, Yang ZY, Hu XF, Chen Q, Tang JL. PIK3CA exon 20 mutations as a potential biomarker for resistance to anti-EGFR monoclonal antibodies in KRAS wild-type metastatic colorectal cancer: a systematic review and meta-analysis. Ann Oncol. 2012;23:1518-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 82. | Huang L, Liu Z, Deng D, Tan A, Liao M, Mo Z, Yang X. Anti-epidermal growth factor receptor monoclonal antibody-based therapy for metastatic colorectal cancer: a meta-analysis of the effect of PIK3CA mutations in KRAS wild-type patients. Arch Med Sci. 2014;10:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 83. | Liao X, Morikawa T, Lochhead P, Imamura Y, Kuchiba A, Yamauchi M, Nosho K, Qian ZR, Nishihara R, Meyerhardt JA. Prognostic role of PIK3CA mutation in colorectal cancer: cohort study and literature review. Clin Cancer Res. 2012;18:2257-2268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 214] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 84. | Ogino S, Nosho K, Kirkner GJ, Shima K, Irahara N, Kure S, Chan AT, Engelman JA, Kraft P, Cantley LC. PIK3CA mutation is associated with poor prognosis among patients with curatively resected colon cancer. J Clin Oncol. 2009;27:1477-1484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 275] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 85. | Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 684] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 86. | Frattini M, Saletti P, Romagnani E, Martin V, Molinari F, Ghisletta M, Camponovo A, Etienne LL, Cavalli F, Mazzucchelli L. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer. 2007;97:1139-1145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 413] [Cited by in RCA: 436] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 87. | Loupakis F, Pollina L, Stasi I, Ruzzo A, Scartozzi M, Santini D, Masi G, Graziano F, Cremolini C, Rulli E. PTEN expression and KRAS mutations on primary tumors and metastases in the prediction of benefit from cetuximab plus irinotecan for patients with metastatic colorectal cancer. J Clin Oncol. 2009;27:2622-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 331] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 88. | Karapetis CS, Jonker D, Daneshmand M, Hanson JE, O’Callaghan CJ, Marginean C, Zalcberg JR, Simes J, Moore MJ, Tebbutt NC. PIK3CA, BRAF, and PTEN status and benefit from cetuximab in the treatment of advanced colorectal cancer--results from NCIC CTG/AGITG CO.17. Clin Cancer Res. 2014;20:744-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 131] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 89. | Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6773] [Cited by in RCA: 6668] [Article Influence: 512.9] [Reference Citation Analysis (0)] |
| 90. | Di Nicolantonio F, Arena S, Tabernero J, Grosso S, Molinari F, Macarulla T, Russo M, Cancelliere C, Zecchin D, Mazzucchelli L. Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J Clin Invest. 2010;120:2858-2866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 301] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 91. | Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1146] [Cited by in RCA: 1349] [Article Influence: 122.6] [Reference Citation Analysis (0)] |
| 92. | Bendell JC, Jones SF, Hart L, Spigel DR, Lane CM, Earwood C, Infante JR, Barton J, Burris HA. A phase Ib study of linsitinib (OSI-906), a dual inhibitor of IGF-1R and IR tyrosine kinase, in combination with everolimus as treatment for patients with refractory metastatic colorectal cancer. Invest New Drugs. 2015;33:187-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | ClinicalTrials. gov Identifier: NCT01139138. Available from: https://clinicaltrials.gov/. |
| 94. | Zhang YJ, Tian XQ, Sun DF, Zhao SL, Xiong H, Fang JY. Combined inhibition of MEK and mTOR signaling inhibits initiation and progression of colorectal cancer. Cancer Invest. 2009;27:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 95. | Temraz S, Mukherji D, Shamseddine A. Dual Inhibition of MEK and PI3K Pathway in KRAS and BRAF Mutated Colorectal Cancers. Int J Mol Sci. 2015;16:22976-22988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 96. | ClinicalTrials. gov Identifier: NCT01363232; NCT01449058. Available from: https://clinicaltrials.gov/. |
| 97. | Liao X, Lochhead P, Nishihara R, Morikawa T, Kuchiba A, Yamauchi M, Imamura Y, Qian ZR, Baba Y, Shima K. Aspirin use, tumor PIK3CA mutation, and colorectal-cancer survival. N Engl J Med. 2012;367:1596-1606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 651] [Cited by in RCA: 668] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 98. | Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci USA. 2003;100:8933-8938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 763] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 99. | Yonesaka K, Zejnullahu K, Okamoto I, Satoh T, Cappuzzo F, Souglakos J, Ercan D, Rogers A, Roncalli M, Takeda M. Activation of ERBB2 signaling causes resistance to the EGFR-directed therapeutic antibody cetuximab. Sci Transl Med. 2011;3:99ra86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 522] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 100. | Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, Zagonel V, Leone F, Depetris I, Martinelli E. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 2016;17:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 741] [Article Influence: 82.3] [Reference Citation Analysis (0)] |
| 101. | Scartozzi M, Mandolesi A, Giampieri R, Bittoni A, Pierantoni C, Zaniboni A, Galizia E, Giustini L, Silva RR, Bisonni R. The role of HER-3 expression in the prediction of clinical outcome for advanced colorectal cancer patients receiving irinotecan and cetuximab. Oncologist. 2011;16:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 102. | Jaiswal BS, Kljavin NM, Stawiski EW, Chan E, Parikh C, Durinck S, Chaudhuri S, Pujara K, Guillory J, Edgar KA. Oncogenic ERBB3 mutations in human cancers. Cancer Cell. 2013;23:603-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 286] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 103. | Schaefer G, Haber L, Crocker LM, Shia S, Shao L, Dowbenko D, Totpal K, Wong A, Lee CV, Stawicki S. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell. 2011;20:472-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 260] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 104. | Cervantes-Ruiperez A, Juric D, Hidalgo M, Messersmith WA, Blumenschein GR, Baselga J, Perez DR, Dienstmann R, Calles A, Jimeno A. A phase I study of MEHD7945A (MEHD), a first-in-class HER3/EGFR dual-action antibody, in patients (pts) with refractory/recurrent epithelial tumors: expansion cohorts. J Clin Oncol. 2012;30 Suppl:abstract 2568. |
| 105. | Presented at AACR Annual Meeting, April 18-21, 2015, Philadelphia, PA. Available from: http://qfuse.com/client_downloads/AACR2015_DARECK_poster_15Apr15.pdf. |
| 106. | Price TJ, Newhall J, Peeters M. Prevalence and outcomes of patients (pts) with EGFR S492R ectodomain mutations in ASPECCT: Panitumumab (pmab) vs. cetuximab (cmab) in pts with chemorefractory wild-type KRAS exon 2 metastatic colorectal cancer (mCRC). J Clin Oncol. 2015;33 Suppl 3:abstract 740. |
| 107. | Pedersen MW, Jacobsen HJ, Koefoed K, Hey A, Pyke C, Haurum JS, Kragh M. Sym004: a novel synergistic anti-epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res. 2010;70:588-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 176] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 108. | Dienstmann R, Patnaik A, Garcia-Carbonero R, Cervantes A, Benavent M, Roselló S, Tops BB, van der Post RS, Argilés G, Skartved NJ. Safety and Activity of the First-in-Class Sym004 Anti-EGFR Antibody Mixture in Patients with Refractory Colorectal Cancer. Cancer Discov. 2015;5:598-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 109. | Kearns JD, Bukhalid R, Sevecka M, Tan G, Gerami-Moayed N, Werner SL, Kohli N, Burenkova O, Sloss CM, King AM. Enhanced Targeting of the EGFR Network with MM-151, an Oligoclonal Anti-EGFR Antibody Therapeutic. Mol Cancer Ther. 2015;14:1625-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 110. | Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 660] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 111. | Di Renzo MF, Olivero M, Giacomini A, Porte H, Chastre E, Mirossay L, Nordlinger B, Bretti S, Bottardi S, Giordano S. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res. 1995;1:147-154. [PubMed] |
| 112. | Liu Y, Yu XF, Zou J, Luo ZH. Prognostic value of c-Met in colorectal cancer: a meta-analysis. World J Gastroenterol. 2015;21:3706-3710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 113. | Cappuzzo F, Varella-Garcia M, Finocchiaro G, Skokan M, Gajapathy S, Carnaghi C, Rimassa L, Rossi E, Ligorio C, Di Tommaso L. Primary resistance to cetuximab therapy in EGFR FISH-positive colorectal cancer patients. Br J Cancer. 2008;99:83-89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 114. | Troiani T, Martinelli E, Napolitano S, Vitagliano D, Ciuffreda LP, Costantino S, Morgillo F, Capasso A, Sforza V, Nappi A. Increased TGF-α as a mechanism of acquired resistance to the anti-EGFR inhibitor cetuximab through EGFR-MET interaction and activation of MET signaling in colon cancer cells. Clin Cancer Res. 2013;19:6751-6765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 120] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 115. | Van Cutsem E, Eng C, Nowara E, Swieboda-Sadlej A, Tebbutt NC, Mitchell E, Davidenko I, Stephenson J, Elez E, Prenen H. Randomized phase Ib/II trial of rilotumumab or ganitumab with panitumumab versus panitumumab alone in patients with wild-type KRAS metastatic colorectal cancer. Clin Cancer Res. 2014;20:4240-4250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 116. | ClinicalTrials. gov Identifier: NCT02008383. Available from: https://clinicaltrials.gov/. |
| 117. | Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 999] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 118. | Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 636] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 119. | Siu LL, Shapiro JD, Jonker DJ, Karapetis CS, Zalcberg JR, Simes J, Couture F, Moore MJ, Price TJ, Siddiqui J. Phase III randomized, placebo-controlled study of cetuximab plus brivanib alaninate versus cetuximab plus placebo in patients with metastatic, chemotherapy-refractory, wild-type K-RAS colorectal carcinoma: the NCIC Clinical Trials Group and AGITG CO.20 Trial. J Clin Oncol. 2013;31:2477-2484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 120. | Yoon J, Koo KH, Choi KY. MEK1/2 inhibitors AS703026 and AZD6244 may be potential therapies for KRAS mutated colorectal cancer that is resistant to EGFR monoclonal antibody therapy. Cancer Res. 2011;71:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 121. | Mirzoeva OK, Das D, Heiser LM, Bhattacharya S, Siwak D, Gendelman R, Bayani N, Wang NJ, Neve RM, Guan Y. Basal subtype and MAPK/ERK kinase (MEK)-phosphoinositide 3-kinase feedback signaling determine susceptibility of breast cancer cells to MEK inhibition. Cancer Res. 2009;69:565-572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 309] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 122. | Wee S, Jagani Z, Xiang KX, Loo A, Dorsch M, Yao YM, Sellers WR, Lengauer C, Stegmeier F. PI3K pathway activation mediates resistance to MEK inhibitors in KRAS mutant cancers. Cancer Res. 2009;69:4286-4293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 360] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 123. | Troiani T, Napolitano S, Vitagliano D, Morgillo F, Capasso A, Sforza V, Nappi A, Ciardiello D, Ciardiello F, Martinelli E. Primary and acquired resistance of colorectal cancer cells to anti-EGFR antibodies converge on MEK/ERK pathway activation and can be overcome by combined MEK/EGFR inhibition. Clin Cancer Res. 2014;20:3775-3786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 124. | Napolitano S, Martini G, Rinaldi B, Martinelli E, Donniacuo M, Berrino L, Vitagliano D, Morgillo F, Barra G, De Palma R. Primary and Acquired Resistance of Colorectal Cancer to Anti-EGFR Monoclonal Antibody Can Be Overcome by Combined Treatment of Regorafenib with Cetuximab. Clin Cancer Res. 2015;21:2975-2983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 125. | Wadlow RC, Hezel AF, Abrams TA, Blaszkowsky LS, Fuchs CS, Kulke MH, Kwak EL, Meyerhardt JA, Ryan DP, Szymonifka J. Panitumumab in patients with KRAS wild-type colorectal cancer after progression on cetuximab. Oncologist. 2012;17:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 126. | Saif MW, Kaley K, Chu E, Copur MS. Safety and efficacy of panitumumab therapy after progression with cetuximab: experience at two institutions. Clin Colorectal Cancer. 2010;9:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 127. | ClinicalTrials. gov Identifier: NCT02296203; NCT02316496. Available from: https://clinicaltrials.gov/. |
| 128. | Ciardiello F, Normanno N, Martinelli E, Troiani T, Pisconti S, Cardone C, Nappi A, Bordonaro AR, Rachiglio M, Lambiase M. Cetuximab continuation after first progression in metastatic colorectal cancer (CAPRI-GOIM): a randomized phase II trial of FOLFOX plus cetuximab versus FOLFOX. Ann Oncol. 2016;27:1055-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 129. | Paz-Ares LG, Gomez-Roca C, Delord JP, Cervantes A, Markman B, Corral J, Soria JC, Bergé Y, Roda D, Russell-Yarde F. Phase I pharmacokinetic and pharmacodynamic dose-escalation study of RG7160 (GA201), the first glycoengineered monoclonal antibody against the epidermal growth factor receptor, in patients with advanced solid tumors. J Clin Oncol. 2011;29:3783-3790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 130. | Bridgewater JA, Cervantes A, Markman B, Siena S, Cubillo A, Carbonero RG, Sigal D, Aprile G, Cunningham D, Nadal C. GAIN-(C): Efficacy and safety analysis of imgatuzumab (GA201), a novel dual-acting monoclonal antibody (mAb) designed to enhance antibody-dependent cellular cytotoxicity (ADCC), in combination with FOLFIRI compared to cetuximab plus FOLFIRI in second-line KRAS exon 2 wild type (e2WT) or with FOLFIRI alone in mutated (e2MT) metastatic colorectal cancer (mCRC). J Clin Oncol. 2015;33 Suppl:abstr 669. |
| 131. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7239] [Article Influence: 723.9] [Reference Citation Analysis (0)] |
| 132. | ClinicalTrials. gov Identifier: NCT02298946; NCT01375842; NCT02335918; NCT01633970; NCT01772004; NCT02227667. Available from: https://clinicaltrials.gov/. |
| 133. | Perez EA, Thompson EA, Ballman KV, Anderson SK, Asmann YW, Kalari KR, Eckel-Passow JE, Dueck AC, Tenner KS, Jen J. Genomic analysis reveals that immune function genes are strongly linked to clinical outcome in the North Central Cancer Treatment Group n9831 Adjuvant Trastuzumab Trial. J Clin Oncol. 2015;33:701-708. [PubMed] |
| 134. | ClinicalTrials. gov Identifier: NCT01309126; NCT02318901. Available from: https://clinicaltrials.gov/. |
| 135. | Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, Parkinson C, Chin SF, Kingsbury Z, Wong AS. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1312] [Article Influence: 109.3] [Reference Citation Analysis (0)] |