Published online Jul 14, 2016. doi: 10.3748/wjg.v22.i26.6083
Peer-review started: February 23, 2016
First decision: March 21, 2016
Revised: April 27, 2016
Accepted: May 21, 2016
Article in press: May 23, 2016
Published online: July 14, 2016
Processing time: 145 Days and 20.5 Hours
AIM: To clarify the biological feature contributing to gastric cancer with diffuse bone metastases at diagnosis.
METHODS: The participants visited the Department of Clinical Oncology, Akita University Hospital, from January 2014 to August 2015. The selection criterion for gastric cancer with diffuse bone metastases at diagnosis includes over 29 hot spots of bone scintigraphy. Circulating tumor cell were collected from 20 mL of peripheral venous blood drawn using a CellSearch kit and a CellTracks AutoPrep system by SRL, a clinical laboratory. The endpoints of this study were correlations between circulating tumor cells (CTC) count and therapeutic outcomes.
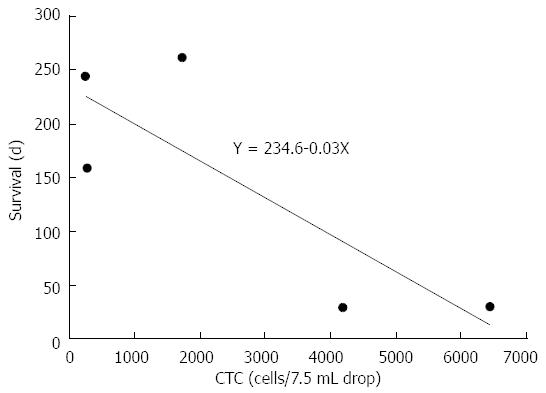
RESULTS: Among 39 patients with gastric cancer, 5 patients met the criterion. The incidence of this subtype was 12.8%. CTC counts ranged from 235 to 6440 cells/7.5 mL of peripheral blood (median of 1724). These values were much higher than common gastric cancers (2 cells). In chemo-sensitive cases, CTC counts decreased within 14 d (median) from 275, 235 and 1724 to 2, 7 and 66, respectively. On the other hand, CTC counts increased after treatment failure or insensitive case from 2, 7 and 6440 to 787, 513 and 7885, respectively. The correlation between CTC count and survival time showed a trend, but did not reach significance (Y = 234.6 - 0.03X, P = 0.085).
CONCLUSION: High CTC count is a biological hallmark of this subtype, and can be used as a direct and definitive indicator of therapeutic outcome.
Core tip: It has been reported in many times that a specific subtype of gastric cancer characterized with diffuse bone metastases at diagnosis, rapid progression and poorer prognosis apparently exists in almost one of ten gastric cancers. However, the basic and biological features of this subtype are not specified until today. In this study, we identified high number of circulating tumor cell of this subtype, and considered that circulating tumor cells (CTC) is responsible for the clinical features described above. CTC is not only a biological hallmark of this subtype, but also informative as a predictive or prognostic biomarkers.
- Citation: Shimazu K, Fukuda K, Yoshida T, Inoue M, Shibata H. High circulating tumor cell concentrations in a specific subtype of gastric cancer with diffuse bone metastasis at diagnosis. World J Gastroenterol 2016; 22(26): 6083-6088
- URL: https://www.wjgnet.com/1007-9327/full/v22/i26/6083.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i26.6083
Gastric cancer with associated diffuse bone metastases at diagnosis has rarely been reported[1]. This condition has been referred to in the literature as “diffuse bone metastasis with hematologic disorders from gastric cancer” or “gastric cancer, initially presenting as disseminated intravascular coagulation (DIC)”[2,3]. This condition is frequently accompanied by DIC, and the comorbidity rate with bone metastases is 82%-86%[2,3]. Similarly, the frequency of bone metastases and gastric cancer with DIC is reported to be 87%[1]. Rhee reported 21 patients with DIC at diagnosis among 1216 advanced gastric cancer patients, in whom 18 patients had bone metastases simultaneously (18/1216 = 1.5%)[3]. Although they are rare, they have outstanding features besides diffuse bone metastases and DIC. Additional clinical features of this subtype that differ from typical gastric cancers include a lower incidence of visceral metastases, more aggressive disease course, and poorer prognosis. For examples, Etoh reported 15 cases over the course of 20 years[1] and Toyoshima described 5 of the 42 reported cases (11.9%)[4]. Among the latter cases, who all had bone marrow metastases, the CTC counts ranged from 30 to 18015 cells/7.5 mL. The reported median survival time (MST) of this disease ranges from 8 to 22 wk[1,3,5], which is much shorter than that of more common stage IV gastric cancers, for which MSTs of 11.1 and 13.8 mo have been reported in patients treated with cisplatin plus 5-fluorouracil (5-FU) or capecitabine and trastuzumab, respectively[6]. Accordingly, medical oncologists consider this disease to be a distinct gastric cancer subtype[1-3], and we should know the clinicopathological characters of this subtype. The high frequency of bone metastasis with this specific subtype is in contrast to the less than 10% frequency with more common gastric cancers[7,8]. Bone metastasis may occur once cancer cells have infiltrated the vasculature and entered the blood stream. Such circulating tumor cells (CTCs) are often detected in the peripheral blood of patients with lung, breast, and prostate cancer, which are diseases with much higher incidences of bone metastases (36%-73%)[9]. High CTC counts have been reported for prostate and breast cancers (84 ± 885 and 75 ± 333 cells/7.5 mL in the peripheral blood, respectively)[10]. There are also reports of a CTC count > 50 cells/7.5 mL in 14% and 10% of prostate and breast cancer patients, respectively[10]. However, the median CTC count for patients with common forms of gastric cancer are much lower, at around 2 cells/7.5 mL, with a CTC count of > 50 cells/7.5 mL only seen in approximately 8% of gastric cancer cases[10-12]. The characteristics of diffuse bone metastases within this subtype might reflect an increased number of CTCs. Therefore, we examined the CTC count of this specific subtype. We have previously reported CTC counts for two patients with this gastric cancer subtype[13]. In this study, we report an additional three cases with this subtype and substantiate the clinical importance of CTCs.
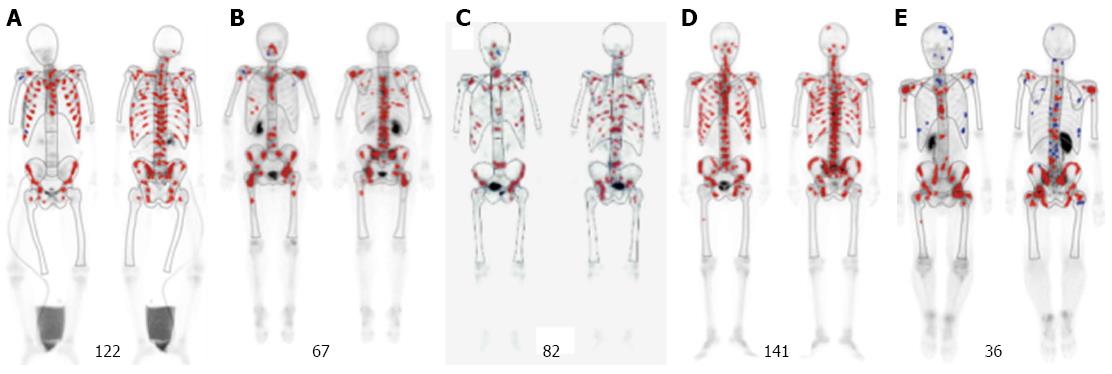
The patients, who visited and were diagnosed as gastric cancer at the Department of Clinical Oncology, Akita University Hospital, from January 2014 to August 2015, were analyzed. The selection criterion for identifying patients with this cancer subtype are as follows: (1) histopathologically confirmed gastric cancer; (2) apparent symptoms of diffuse bone metastases at onset; (3) diffuse bone metastases detected by bone scintigraphy (BS); and (4) a hot spot number over 29; This is derived from the data that the reported mean ± SD of BS hot spot number for gastric cancer was 16 ± 13[14] (Figure 1). This study was approved by the Akita University School of Medicine Ethics Committee (#828). Informed consent and an agreement to publish were obtained from all patients.
CTCs were isolated as previously described[15]. In brief, CTCs were isolated from 20 mL of peripheral venous blood drawn using a CellSearch kit and a CellTracks AutoPrep system (Janssen Diagnostics, LLC, New Jersey, United States). This procedure was outsourced to SRL, a clinical laboratory (Tokyo, Japan).
The primary endpoint of this study was CTC count and its change after chemotherapy. The secondary endpoints were correlations between CTC number and the therapeutic response, and between CTC number and survival. Evaluation of the therapeutic response was performed using response evaluation criteria in solid tumors (RECIST, version 1.1).
The values are shown as mean ± SD. Simple regression analysis was performed using StatMate III, version 3.14 (ATMS, Tokyo, Japan). This statistical method was reviewed by Professor Katsuyuki Murata from Department of Environmental Health Sciences, Graduate School of Medicine, Akita University.
During this period, 39 patients with gastric cancer (28 males and 11 females) visited our department. Five patients met the criterion, and were diagnosed as this subtype. The incidence of this subtype was 12.8%. They included four males and one female, who aged 24-78 years (median, 50 years) (Table 1). Patients were histopathologically diagnosed with adenocarcinomas, signet ring cell carcinomas, or mixed cancers. Distant metastases other than bone metastases are reported in Table 1. DIC was observed in two cases.
CTC counts before chemotherapy ranged from 235 to 6440 cells/7.5 mL of peripheral blood (median of 1724; Table 2), which is considered to be a characteristic of this gastric cancer subtype. These values were considerably higher than is typically found with more common gastric cancers, which have a reported median value of 2 cells/7.5 mL)[11].
| Case | Tumor markers | CTC count | Date1 (the X d) | Effects | Survival (d)2 | |
| pre (-) post3 | pre (-) post3 | |||||
| 1 | CEA | 288 (↓) 160 | 275 (↓) 2 | 16 | non CR/non PD | 160 |
| CA19-9 | 158 (↑) 690 | |||||
| 2 | CEA | 120 (↓) 83 | 235 (↓) 7 | 11 | (+) PR | 246 |
| CA19-9 | 5201 (↑) 6543 | |||||
| 3 | CEA | 1.4 (→) 1.3 | 6440 (↑) 7885 | 14 | (-) PD | 30 |
| CA19-9 | 7.6 (→) 8.3 | |||||
| 4 | CEA | 15 (↓) 1.1 | 1724 (↓) 66 | 14 | (+) PR | Alive > 120 |
| CA19-9 | 4.2 (→) 4.1 | |||||
| 5 | CEA | 2.4 (↑) 12 | 4197 -ND | ND | (+) PD | 31 |
| CA19-9 | 205 (↑) 653 | |||||
The therapeutic course for each case is presented in Table 2. Cases 1 and 2 were described in detail elsewhere[13]. With three cases (cases 1, 2, and 4), tumors were sensitive to chemotherapy administered immediately after the CTC examination. Tumor response was evaluated by computed tomography (CT) imaging performed nearly 3 mo after the initiation of chemotherapy. The patient CTC count was reassessed at a median of 14 d after chemotherapy (range, 11-16 d). A change in the CTC count may be an earlier indicator of the therapeutic outcome than changes visible upon imaging. Earlier detection could be critical because the progression of this gastric cancer subtype tends to be very rapid. If a tumor is insensitive to treatment, an alternative chemotherapeutic agent may be substituted after only one cycle, eliminating the need to continue an ineffective systemically administered treatment for several months, as is necessary to detect imaging changes. Moreover, this cancer subtype often lacks measurable targets except lymph node metastases, making imaging evaluation difficult. Furthermore, concurrent measurement of the serum tumor markers carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) produced considerably different results in two cases (cases 1 and 2; Table 2). For these cases, it was not possible to predict the therapeutic responses based on changes in these markers. Nevertheless, the CTC count can be used as a direct and definitive indicator of therapeutic outcome in this gastric cancer subtype. Alternatively, CTC counts were increased in cases 1 and 2 upon treatment failure. For those cases, CTC counts increased to 787 and 513 cells/7.5 mL peripheral blood, respectively. An additional patient (case 3) who was insensitive to the initial treatment showed an increase in the CTC count from 6440 to 7885 cells/7.5 mL peripheral blood. A second course of chemotherapy was not initiated because of a worsened general condition.
The peripheral blood CTC count also can predict patient survival. For case 5, chemotherapy was only administered for 3 d because of the patient’s rapidly worsening condition. With the exception of case 4 (still alive for > 180 d), the survival times for the other 4 cases appeared to correlate with their initial CTC count (Figure 2). The initial CTC counts were high for the two short-term survivors (cases 3 and 5) who lived until 30 d after their initial diagnosis (6440 and 4197 cells/7.5 mL peripheral blood, respectively). The long-term survivors who lived for more than 160 d (cases 1, 2, and 4) had considerably lower initial CTC counts (235, 275, and 1724 cells/7.5 mL peripheral blood, respectively). Although case 4 is still alive (over 263 d), the relationship between CTC count and survival time showed a negative trend but did not reach significance (Y = 234.6 - 0.03X, P = 0.085; Figure 2). Concerning case 4, the CTC was additionally examined 2 times during this period. Those are suppressed, and they were 33 and 60 cells/7.5 mL, respectively. That indicates the first line chemotherapy S1 plus cis-platinum is still effective for case 4. These observations suggest that the initial CTC, count is a useful prognostic biomarker for patients with this disease.
Our results indicate that, unlike typical gastric cancer, the subtype of gastric cancer that presents with diffuse bone metastases can be characterized as having a high CTC count. Therefore, we propose this subtype of gastric cancer as circulating gastric cancer (cGC). The incidence of cGC is roughly estimated to range from 1.5% to 11.9% of gastric cancer in the literature[3,4]. However, if CTC count is characteristics of this subtype, we can estimate the incidence more precisely. One of the reasons why cGC metastasizes to bone with high frequency is due to the blood stream from stomach, we considered. The blood stream from almost digestive tracts other than upper stomach and lower rectum flows into portal vein. However, a part of the blood stream from the proximal region of stomach flows into connecting vein between left gastric vein and esophageal vein, and leads to valveless Batson venous plexus, which forms venous plexus penetrating spines via azygous and hemiazygous veins[16]. This situation is similar to the blood stream from prostate, breast and lung. These high CTC counts may have contributed to the diffuse bone metastases observed with this subtype. Bone marrow is considered to be a common and easily accessed homing organ for tumor cells that escape epithelial tumors[17]. As the other factor for CTC to metastasize to bone, it is thought there are niches in the bone marrow where CTCs can easily reside, and the bone marrow is considered to be a reservoir for disseminated tumor cells[4,18]. It has also been suggested that cancer cells metastasize to the bone through a multistep process[18]. Ongoing research efforts may define the molecular basis of this subtype in the near future. There may be specific genetic mutations present in CTCs that confer the specific phenotypes that enable bone metastasis. In addition, these mutations may represent the characteristic features of this subtype that facilitate the rapid progression of circulating cells to the bone. Thus, CTC analysis might establish the molecular pathogenesis of this specific cancer subtype. Within this subtype, in all practicality, the CTC count may serves as a definitive biomarker for evaluating therapeutic effects, as has been previously suggested[19,20]. The CTC count has the further advantage of allowing the evaluation of chemotherapeutic benefit earlier than imaging measures. Furthermore, CTC measurement may assist in predicting patient survival. In spite of rareness of the incidence of cGC as low as 10%, further and larger study should be warranted in near future.
The authors would like to thank Enago (http://www.enago.jp) for the English language review.
The incidence of subtype of gastric cancer that presents with diffuse bone metastases at onset is roughly estimated as 10% or less of gastric cancer. However, the biological natures of this disease are not identified, and also this situation is not clearly defined.
Recently to examine cancer patients, liquid biopsy is very accessible and becomes a reliable way in which the authors can get DNA and RNA of cancer cells or even capture themselves from blood drawn. By this easy way, the authors can get any biological information of the cancer cells’ situation at real time.
Concerning this subtype of gastric cancer, no one argues the high number or importance of circulation tumor cell (CTC) for diagnosis. The authors also claim that CTC of this subtype is very useful as predictive and prognosis biomarkers.
The CTC count of this subtype should be measured prior to administration of chemotherapeutic agents. Then it should be reexamined just after one cycle of chemotherapy, and compared them to evaluate the sensitivity of the drugs used. That could result in a better outcome to the patient.
CTC is a living cancer cell in the patient’s blood flow. It can be captured by immunomagnetic beads coated suitable antibodies.
Shimazu et al described their clinical experience with 5 cases of a rare type of gastric cancer characterized by diffuse bone metastases at diagnosis, rapid progression and poor prognosis. They identified high number of CTC in this type of cancer, and considered that CTC is responsible for the clinical features. This is also an extension of their previous report on 2 cases included in the study.
Manuscript source: Invited manuscript
P- Reviewer: Saydam O, Su CC S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Etoh T, Baba H, Taketomi A, Nakashima H, Kohnoe S, Seo Y, Fukuda T, Tomoda H. Diffuse bone metastasis with hematologic disorders from gastric cancer: clinicopathological features and prognosis. Oncol Rep. 1999;6:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Takashima A, Shirao K, Hirashima Y, Takahari D, Okita NT, Nakajima TE, Kato K, Hamaguchi T, Yamada Y, Shimada Y. Sequential chemotherapy with methotrexate and 5-fluorouracil for chemotherapy-naive advanced gastric cancer with disseminated intravascular coagulation at initial diagnosis. J Cancer Res Clin Oncol. 2010;136:243-248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Rhee J, Han SW, Oh DY, Im SA, Kim TY, Bang YJ. Clinicopathologic features and clinical outcomes of gastric cancer that initially presents with disseminated intravascular coagulation: a retrospective study. J Gastroenterol Hepatol. 2010;25:1537-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Toyoshima K, Hayashi A, Kashiwagi M, Hayashi N, Iwatsuki M, Ishimoto T, Baba Y, Baba H, Ohta Y. Analysis of circulating tumor cells derived from advanced gastric cancer. Int J Cancer. 2015;137:991-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Etoh T, Baba H, Taketomi A, Nakashima H, Kohnoe S, Seo Y, Saito T, Tomoda H. Sequential methothrextate and 5-fruororacil therapy for diffuse bone metastasis from gastric cancer. Anticancer Res. 1998;18:2085-2088. [PubMed] |
| 6. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5332] [Article Influence: 355.5] [Reference Citation Analysis (3)] |
| 7. | Yoshikawa K, Kitaoka H. Bone metastasis of gastric cancer. Jpn J Surg. 1983;13:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Silvestris N, Pantano F, Ibrahim T, Gamucci T, De Vita F, Di Palma T, Pedrazzoli P, Barni S, Bernardo A, Febbraro A. Natural history of malignant bone disease in gastric cancer: final results of a multicenter bone metastasis survey. PLoS One. 2013;8:e74402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 9. | Galasko C. The anatomy and pathways of skeletal metastases. Bone metastases. Boston: GK Hall 1981; 49-63. |
| 10. | Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, Tibbe AG, Uhr JW, Terstappen LW. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897-6904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1810] [Cited by in RCA: 1955] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 11. | Hiraiwa K, Takeuchi H, Hasegawa H, Saikawa Y, Suda K, Ando T, Kumagai K, Irino T, Yoshikawa T, Matsuda S. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann Surg Oncol. 2008;15:3092-3100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Matsusaka S, Chìn K, Ogura M, Suenaga M, Shinozaki E, Mishima Y, Terui Y, Mizunuma N, Hatake K. Circulating tumor cells as a surrogate marker for determining response to chemotherapy in patients with advanced gastric cancer. Cancer Sci. 2010;101:1067-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Inoue M, Otsuka K, Shibata H. Circulating tumor cell count as a biomarker of a specific gastric cancer subgroup characterized by bone metastasis and/or disseminated intravascular coagulation - an early indicator of chemotherapeutic response. Oncol Lett. 2016;11:1294-1298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Ohmori K, Matsui H, Yasuda T, Kanamori M, Yudoh K, Seto H, Tsuji H. Evaluation of the prognosis of cancer patients with metastatic bone tumors based on serial bone scintigrams. Jpn J Clin Oncol. 1997;27:263-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Otsuka K, Imai H, Soeda H, Komine K, Ishioka C, Shibata H. Practical utility of circulating tumour cells as biomarkers in cancer chemotherapy for advanced colorectal cancer. Anticancer Res. 2013;33:625-629. [PubMed] |
| 16. | Maccauro G, Spinelli MS, Mauro S, Perisano C, Graci C, Rosa MA. Physiopathology of spine metastasis. Int J Surg Oncol. 2011;2011:107969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Alix-Panabières C, Riethdorf S, Pantel K. Circulating tumor cells and bone marrow micrometastasis. Clin Cancer Res. 2008;14:5013-5021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Patel LR, Camacho DF, Shiozawa Y, Pienta KJ, Taichman RS. Mechanisms of cancer cell metastasis to the bone: a multistep process. Future Oncol. 2011;7:1285-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LW. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3360] [Cited by in RCA: 3388] [Article Influence: 161.3] [Reference Citation Analysis (0)] |
| 20. | Moreno JG, O’Hara SM, Gross S, Doyle G, Fritsche H, Gomella LG, Terstappen LW. Changes in circulating carcinoma cells in patients with metastatic prostate cancer correlate with disease status. Urology. 2001;58:386-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |