Published online Jul 14, 2016. doi: 10.3748/wjg.v22.i26.6076
Peer-review started: December 4, 2015
First decision: January 13, 2016
Revised: January 27, 2016
Accepted: February 20, 2016
Article in press: February 22, 2016
Published online: July 14, 2016
Processing time: 217 Days and 16.9 Hours
AIM: To provide appropriate treatment, it is crucial to share the clinical status of pancreas head cancer among multidisciplinary treatment members.
METHODS: A retrospective analysis of the medical records of 113 patients who underwent surgery for pancreas head cancer from January 2008 to December 2012 was performed. We developed preoperative defining system of pancreatic head cancer by describing “resectability - tumor location - vascular relationship - adjacent organ involvement - preoperative CA19-9 (initial bilirubin level) - vascular anomaly”. The oncologic correlations with this reporting system were evaluated.
RESULTS: Among 113 patients, there were 75 patients (66.4%) with resectable, 34 patients (30.1%) with borderline resectable, and 4 patients (3.5%) with locally advanced pancreatic cancer. Mean disease-free survival was 24.8 mo (95%CI: 19.6-30.1) with a 5-year disease-free survival rate of 13.5%. Pretreatment tumor size ≥ 2.4 cm [Exp(B) = 3.608, 95%CI: 1.512-8.609, P = 0.044] and radiologic vascular invasion [Exp(B) = 5.553, 95%CI: 2.269-14.589, P = 0.002] were independent predictive factors for neoadjuvant treatment. Borderline resectability [Exp(B) = 0.222, P = 0.008], pancreatic head cancer involving the pancreatic neck [Exp(B) = 9.461, P = 0.001] and arterial invasion [Exp(B) = 6.208, P = 0.010], and adjusted CA19-9 ≥ 50 [Exp(B) = 1.972 P = 0.019] were identified as prognostic clinical factors to predict tumor recurrence.
CONCLUSION: The suggested preoperative defining system can help with designing treatment plans and also predict oncologic outcomes.
Core tip: Owing to the anatomical complexity of the pancreas head cancer, it is not always easy to share the exact disease status among multidisciplinary treatment members. So, we made a preoperative defining system, which contained the important clinical variables (resectability, tumor location, vascular relationship, adjacent organ involvement, preoperative CA19-9, vascular anomaly) to decide the treatment plan for pancreas head cancer. Through internal validation, we proved that this system could be useful not only to clarify the disease characteristics but also to predict oncologic outcomes of pancreas head cancer.
- Citation: Yang SJ, Hwang HK, Kang CM, Lee WJ. Preoperative defining system for pancreatic head cancer considering surgical resection. World J Gastroenterol 2016; 22(26): 6076-6082
- URL: https://www.wjgnet.com/1007-9327/full/v22/i26/6076.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i26.6076
Pancreatic cancer is one of the lethal malignant diseases arising from the gastrointestinal tract. It is well-known that only margin-negative resection of the tumor can lead to long-term survival[1]. However, most patients treated with curative pancreatectomy develop tumor recurrences, especially in the liver. Therefore, effective adjuvant systemic chemotherapy should be mandatory[2].
In general, resectable pancreatic cancer is defined as a clinical tumor condition confined to the pancreas without radiologic evidence suggesting invasion of the major vascular system or systemic metastases. However, there is some controversy about the definition of borderline resectable pancreatic cancer[3]. Currently, there are two systems for defining borderline resectable pancreatic cancer[4,5]. Whatever definition is chosen, surgeons should plan the treatment modality and design the operative approach for curative resection based on preoperative radiologic assessment. In addition, they need to explain the patients’ chance of survival and prognosis based on clinically available information. However, there is no generalized preoperative defining system effectively showing the extent of pancreatic cancer and tumor biology.
It would be very helpful if a well-designed preoperative defining system of pancreatic cancer could: (1) estimate the clinical stage of cancer (tumor extension); (2) give practical information for designing the extent of surgery and choosing the treatment modality; (3) play a role as an effective communication tool among multidisciplinary team members; and (4) help predict oncologic outcomes.
In this study, we propose a preoperative defining system in patients with pancreatic head cancer considering curative resection. Our system may be useful in improving communication and developing strategies for treating pancreatic cancer.
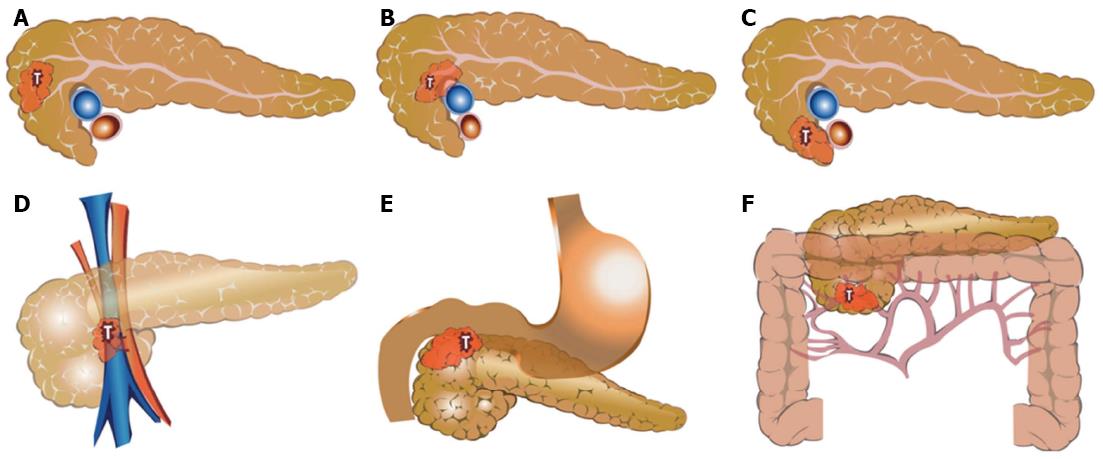
Based on radiological interpretation of preoperative images, resected pancreatic head cancers are intended to be described as the following structures: Pancreatic head cancer; “Resectability (tumor size, cm) - Tumor location-vascular relationship (length of involved segment (cm)/Circumferential involvement (%) - Adjacent organ involvement - Preoperative CA19-9 (initial bilirubin level) - Vascular anomaly”.
Radiological resectability follows the National Comprehensive Cancer Network (NCCN) guideline[5]. If there is no vascular involvement, no adjacent organ invasion, and no clinical metastasis, no descriptions were added to this defining system for explaining this negative information. Important abbreviations are listed in Table 1.
| Symbol | Description | Comments |
| Resectability | ||
| R | Resectable | |
| BR | Borderline resectable | |
| LA | Locally advanced | |
| Location | ||
| H | Pancreatic head | Hu: uncinate process, |
| Hn: neck portion | ||
| Vascular structure | ||
| CA | Celiac axis | |
| CHA | Common hepatic artery | |
| GDA | Gastroduodenal artery | OGA: origin of gastroduodenal artery |
| aRHA | Aberrant right hepatic artery | |
| Rt/Lt | Right/left | |
| SMA | Superior mesenteric artery | |
| SMV | Superior mesenteric vein | |
| SMV-SV-PV | Superior mesenteric vein-splenic vein-portal vein |
Organs such as the duodenum and bile duct that will be removed by standard pancreaticoduodenectomy were not described even in cases of cancer invasion. However, if the tumor invaded the pylorus or antrum, it was added as a factor of adjacent organ invasion. Figure 1 shows the examples for application of the new defining system in patients with pancreatic head cancer.
From January 2008 to December 2012, medical records and preoperative image studies of patients with resected pancreatic cancer were retrospectively reviewed. The clinic-pathological variables were checked. In particular, preoperative clinical information such as resectability, radiologic tumor size, tumor location, preoperative serum CA19-9, preoperative serum bilirubin, and adjusted CA19-9 (initial serum level of CA19-9 divided by initial serum level of bilirubin)[6] were also evaluated. The new defining system was applied to check if it could describe the tumor extent at the initial diagnosis.
Individual clinical components existing in the new preoperative defining system, such as radiological resectability, tumor size, tumor location, vascular involvement, adjacent organ invasion, preoperative initial serum CA19-9 level, and initial serum bilirubin were correlated with treatment strategy and oncologic outcomes.
All statistical analyses were performed using IBM® SPSS Statistics version 20. Continuous variables were indicated as mean ± SD and categorical variables as frequency and percentage (%). Student’s t-tests and χ2 tests were used. Logistic regression analysis was applied for multivariate analysis. Kaplan-Meier and Cox-proportional hazard models were applied for disease-free survival as univariate and multivariate analysis. P values < 0.05 were considered as statistically significant.
During the study period, 119 patients underwent potentially curative resection of pancreatic head cancer. All were confirmed as ductal adenocarcinoma by pathologic examination. Among them, six patients without available preoperative image studies were excluded, totally 113 patients were enrolled. The new preoperative defining system was applied to describe the extent of the tumor and some clinical information for all patients. Resectable pancreatic cancer (R) was noted in 75 patients (66.4%), borderline resectable pancreatic cancer (BR) in 34 (30.1%), and locally advanced pancreatic cancer (LA) in four patients (3.5%). The mean radiologic tumor size was measured as 2.4 ± 0.8 cm in the maximum diameter. Seventy-three tumors (64.6%) were located in the pancreatic head, 35 (31%) in the uncinate process, and five (4.4%) in the pancreatic head and neck area. Forty patients (35.4%) were found to have tumors involving major vascular structures. Mean initial serum level of CA19-9 was found to be 825.7 ± 2037.8 (U/mL), and initial serum bilirubin was 4.6 ± 5.0 (mg/dL). Adjusted CA19-9 was calculated as 401.9 ± 872.8 (U/mL).
It was found that the new preoperative defining system can help in decision-making about treatment strategies and surgical extent in pancreatic cancer management.
Thirty-nine patients (34.5%) underwent combined venous vascular resection. SMV/PV wedge resection was performed in 15 patients, and 24 patients underwent segmental resection of the PV system. Among the clinical factors used in the new preoperative defining system, radiologic tumor size, vascular components were associated with combined venous vascular resection (P < 0.05, Table 2). However, in multivariate analysis, only radiologic tumor size ≥ 2.4 cm [Exp(B) = 2.288, 95%CI: 1.029-5.087, P = 0.042] was noted to be independent clinical factor to predict combined venous vascular resection.
| Combined venous vascular resection | P value | ||
| No | Yes | ||
| Resectability | |||
| R | 54 | 21 | |
| BR | 17 | 17 | |
| LA | 2 | 2 | 0.054 |
| Radiologic tumor size (cm) | 2.3 ± 0.7 | 2.6 ± 0.8 | 0.044 |
| Tumor location | |||
| Head | 49 | 24 | |
| Uncinate | 22 | 13 | |
| Including neck | 2 | 3 | 0.485 |
| Radiologic vascular component | |||
| No | 52 | 21 | |
| Yes | 21 | 19 | 0.002 |
| Initial preoperative serum CA19-9 | 846.7 ± 2193.9 | 805.3 ± 1761.9 | 0.919 |
| Initial serum total bilirubin | 4.3 ± 5.0 | 4.9 ± 4.6 | 0.528 |
It was also found that resectability, radiologic tumor size, tumor location, and radiologic vascular component were related to neoadjuvant treatment before surgical resection (P < 0.05, Table 3). In multivariate analysis, radiologic tumor size ≥ 2.4 cm [Exp(B) = 3.608, 95%CI: 1.512-8.609, P = 0.004], and radiologic vascular component [Exp(B) = 5.553, 95%CI: 2.269-14.589, P < 0.001] were found to be independent predictive factors for preoperative neoadjuvant treatment in this study population.
| Neoadjuvant treatment | P value | ||
| No | Yes | ||
| Resectability | |||
| R | 52 | 23 | |
| BR | 10 | 24 | |
| LA | 1 | 3 | < 0.001 |
| Radiologic tumor size (cm) | 2.1 ± 5.7 | 2.7 ± 0.9 | < 0.001 |
| Tumor location | |||
| Head | 46 | 27 | |
| Uncinate | 15 | 20 | |
| Including neck | 2 | 3 | 0.049 |
| Radiologic vascular component | |||
| No | 52 | 21 | |
| Yes | 11 | 29 | < 0.001 |
| Initial preoperative serum CA19-9 | 600.8 ± 1640.6 | 1109.0 ± 2437.1 | 0.189 |
| Initial serum total bilirubin | 3.9 ± 4.9 | 5.5 ± 4.9 | 0.087 |
It was also noted that the proposed new defining system can be useful in predicting oncologic outcome even before confirming pathologic characteristics of the resected pancreatic cancer.
Mean disease-free survival was 24.8 mo (95%CI: 19.6-30.1) with a 5-year disease-free survival rate of 13.5%. Interestingly, when putting clinical variables used in the preoperative defining system into a Cox hazard regression model, it was found that anatomic resectability, especially borderline resectable pancreatic cancer [Exp(B) = 0.222]; radiologic tumor size ≥ 2.4cm [Exp(B) = 1.696], tumor location, especially pancreatic head cancer involving the pancreatic neck portion [Exp(B) = 9.461]; radiologic venous vascular component [Exp(B) = 2.788]; arterial component [Exp(B) = 6.208]; initial total bilirubin ≥ 4.6 [Exp(B) = 0.588]; and adjusted CA19-9 ≥ 50 [Exp(B) = 1.972] were identified as prognostic clinical factors to predict tumor recurrence (Table 4).
| Clinical variables | Exp(B) | 95%CI | P value |
| Resectability | 0.019 | ||
| BR | 0.222 | 0.073-0.676 | 0.008 |
| LA | 0.557 | 0.105-2.955 | 0.492 |
| Radiologic tumor size (cm) ≥ 2.4 | 1.696 | 0.993-2.897 | 0.053 |
| Tumor location | 0.007 | ||
| Hu | 0.952 | 0.557-1.629 | 0.858 |
| Hn | 9.461 | 2.634-33.976 | 0.001 |
| Vascular relationship with | 0.064 | ||
| Venous system | 2.788 | 0.952-8.165 | 0.061 |
| Arterial system | 6.208 | 1.562-24.669 | 0.010 |
| Both | 2.200 | 0.484-10.006 | 0.307 |
| CA19-9 ≥ 825 | 1.709 | 0.777-3.761 | 0.183 |
| Total bilirubin ≥ 4.6 | 0.588 | 0.339-1.022 | 0.060 |
| Adjusted CA19-9 ≥ 50 | 1.972 | 1.118-3.480 | 0.019 |
TNM staging system is widely accepted, and it is aimed at predicting survival. Some kinds of cancer cannot be simplified down to a TNM stage because of unique anatomical characteristics. One of these is hilar cholangiocarcinoma (HCCA) and another is pancreas head cancer. For hilar cholangiocarcinoma, there is already a presurgical staging system that considers surrounding anatomical structures[7]. The Jarnagin-Blumgart (J-B) classification has been used for deciding treatment plans and developing a prognosis of HCCA[8]. We need a more appropriate defining system for pancreas head cancer rather than the TNM stage, something similar to the J-B classification for HCCA.
The presented new preoperative defining system can suggest the treatment strategy, extent of surgery, and even tumor biology in resected pancreatic head cancer. It was found that all resected pancreatic head cancers could be described according to the new preoperative defining system based on a preoperative CT scan. In addition, in multivariate analysis, radiologic tumor size ≥ 2.4 cm [Exp(B) = 3.608, P = 0.004], and radiologic vascular component [Exp(B) = 5.553, P < 0.001] were independent predictive factors for preoperative neoadjuvant treatment. In particular, larger tumor size (tumor size ≥ 2.4 cm) was associated with combined venous vascular resection [Exp(B) = 2.288, P = 0.042], suggesting that clinical components used for the currently proposed new defining system can provide important clinical clues about treatment strategy and the extent of surgery in treating pancreatic cancer.
Most importantly, the current system can predict patients’ outcomes without requiring confirmation of the clinical stage of the cancer. Considering that most prognostic factors are based on pathologic characteristics[9-13], such as lymph node metastasis, lymph node ratio, perineural invasion, lymphovascular invasion, and cell differentiation, the proposing preoperative defining system showed that even clinical characteristics, such as anatomic resectability (P = 0.019), tumor location (P = 0.007), and adjusted CA19-9 (P = 0.019), which can be estimated before surgical intervention, were identified as good prognostic markers for predicting tumor recurrence (Table 4).
Adjusted CA19-9 is defined as the value of initial CA19-9 level divided by serum total bilirubin. This concept was developed because the actual serum level of CA19-9 is not reliable in patients with jaundice. We already demonstrated that adjusted CA19-9 was a prognostic clinical marker in resected pancreatic cancer[6], which was shown again in the present study. Further clinical investigation based on a large population is necessary to define the oncologic significance of preoperative adjusted CA19-9.
When applying this new system in cases of neoadjuvant treatment, it would be easy and more subjective to detect the radiologic responsiveness after neoadjuvant treatment in borderline resectable pancreatic cancer. If a radiologist described the radiologic changes according to the new defining system, the surgeon could be well aware of the current tumor status compared with the pre-neoadjuvant treatment status, which is one of many advantages of new system. For example, it can be described in this way: BR2cm-Hu-SMV2.5cm/30%-219 (8) → Neo-BR2cm-Hu-SMV2.0cm/10%-58 (2).
In spite of oncologic significance of lymph node metastasis, clinical N-stage (cN-stage) was not considered in this system because the accuracy of radiologic estimation of lymph node metastasis is not high[14,15]. In addition, preoperative cholangitis, pancreatitis, and interventional approaches due to obstructive cholangio-pancreatopathy can induce secondary lymph node enlargement. In fact, lymph node metastasis is one of the important prognostic factors in resected pancreatic cancer; however, several important randomized controlled studies have proven that the extent of lymph node dissection could not contribute to increasing oncologic outcome[16-18]. Therefore, cN-stage will not influence either prognosis or the clinical treatment strategy when the tumor is regarded as a resectable pancreatic cancer.
Instead, clinical information on the possibility of pylorus involvement or right colonic mesentery would be more useful in designing surgical extent. Recently, techniques for pyloric-ring resected pancreaticoduodenectomy[19], subtotal stomach-preserving pancreaticoduodenectomy[20], and combined resection of ascending colon are clinically available. In addition, descriptions of associated vascular anomaly, especially an aberrant right hepatic artery, will be another good guide for performing safe pancreaticoduodenectomy, because this artery is at risk for accidental injury during dissection of the hepatoduodenal ligament[21]. To design an optimal operation, it is mandatory to have exact anatomical delineation preoperatively. This proposed preoperative defining system can give compact and critical anatomical information to the surgical team.
There are several important flaws in our study. First, the new defining system is only based on retrospective data of operated patients. Therefore, this could not be reflective of all patients seen for consideration of surgery. It is needed to validate this defining system with all pancreas head cancer surgery candidates prospectively. Second, it seems to be complicated and difficult to describe. Third, this system cannot estimate actual lymph node status. However, in the era of the multidisciplinary team approach for treating pancreatic cancer, this defining system can be useful for improving communication among team members, planning the extent of surgery, developing the treatment strategy, and defining tumor biology. This system needs to be validated on different sets of patient data to confirm its clinical feasibility, reproducibility, and oncologic meanings.
In this multidisciplinary treatment era, it is most important to share the exact disease status among multidisciplinary team members for making appropriate treatment pathway. When it comes to pancreas head cancer, the anatomical complexity surrounding tumor can make it difficult not only to communicate with each members of team, but also to decide treatment plan. In this study, the authors suggested a preoperative defining system which contained the important anatomical and laboratory findings associated with pancreas head cancer. Then they evaluated the efficacy of this system for designing treatment plan and predict oncologic outcomes.
The National Comprehensive Cancer Network (NCCN) categorized the pancreas head cancer cases into resectable, borderline resectable or unresectable diseases. But this classification solely depends on vascular relationship in the preoperative radiologic evaluation. Several studies reported that tumor characteristics, adjusted preoperative CA19-9, vascular anomalies also should be considered preoperatively.
With the suggested defining system, they authors can estimate necessary of the neoadjuvant therapy or the combined vascular resection for pancreas head cancer. Moreover, the contents of this system are strongly related to the tumor recurrence.
This study demonstrates the new defining system for pancreas head cancer and will help the multidisciplinary board to communicate with each other about the individual disease status in a comprehensive way.
Borderline resectable pancreas cancer means there is a possibility of incomplete resection because of adjacent vital vessel invasion such as superior mesenteric vein, gastroduodenal artery, hepatic artery and superior mesenteric artery.
The authors recommended a good preoperative description system for pancreatic cancer patients. This proposal with possible implication in neoadjuvant treatment is very remarkable.
P- Reviewer: Cuadrado-Garcia A, Pantalone D S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Bakkevold KE, Arnesjø B, Dahl O, Kambestad B. Adjuvant combination chemotherapy (AMF) following radical resection of carcinoma of the pancreas and papilla of Vater--results of a controlled, prospective, randomised multicentre study. Eur J Cancer. 1993;29A:698-703. [PubMed] |
| 3. | Lopez NE, Prendergast C, Lowy AM. Borderline resectable pancreatic cancer: definitions and management. World J Gastroenterol. 2014;20:10740-10751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 4. | Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, Lee JE, Pisters PW, Evans DB, Wolff RA. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 650] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 5. | Al-Hawary MM, Francis IR, Chari ST, Fishman EK, Hough DM, Lu DS, Macari M, Megibow AJ, Miller FH, Mortele KJ. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the american pancreatic association. Gastroenterology. 2014;146:291-304.e1. [PubMed] |
| 6. | Kang CM, Kim JY, Choi GH, Kim KS, Choi JS, Lee WJ, Kim BR. The use of adjusted preoperative CA 19-9 to predict the recurrence of resectable pancreatic cancer. J Surg Res. 2007;140:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Burke EC, Jarnagin WR, Hochwald SN, Pisters PW, Fong Y, Blumgart LH. Hilar Cholangiocarcinoma: patterns of spread, the importance of hepatic resection for curative operation, and a presurgical clinical staging system. Ann Surg. 1998;228:385-394. [PubMed] |
| 8. | Ding G, Yang Y, Cao L, Chen W, Wu Z, Jiang G. A modified Jarnagin-Blumgart classification better predicts survival for resectable hilar cholangiocarcinoma. World J Surg Oncol. 2015;13:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Delcore R, Rodriguez FJ, Forster J, Hermreck AS, Thomas JH. Significance of lymph node metastases in patients with pancreatic cancer undergoing curative resection. Am J Surg. 1996;172:463-48; discussion 463-48;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Riediger H, Keck T, Wellner U, zur Hausen A, Adam U, Hopt UT, Makowiec F. The lymph node ratio is the strongest prognostic factor after resection of pancreatic cancer. J Gastrointest Surg. 2009;13:1337-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 286] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 11. | Nagakawa T, Mori K, Nakano T, Kadoya M, Kobayashi H, Akiyama T, Kayahara M, Ohta T, Ueno K, Higashino Y. Perineural invasion of carcinoma of the pancreas and biliary tract. Br J Surg. 1993;80:619-621. [PubMed] |
| 12. | Garcea G, Dennison AR, Ong SL, Pattenden CJ, Neal CP, Sutton CD, Mann CD, Berry DP. Tumour characteristics predictive of survival following resection for ductal adenocarcinoma of the head of pancreas. Eur J Surg Oncol. 2007;33:892-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Crippa S, Partelli S, Zamboni G, Barugola G, Capelli P, Inama M, Bassi C, Pederzoli P, Falconi M. Poorly differentiated resectable pancreatic cancer: is upfront resection worthwhile? Surgery. 2012;152:S112-S119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Imai H, Doi R, Kanazawa H, Kamo N, Koizumi M, Masui T, Iwanaga Y, Kawaguchi Y, Takada Y, Isoda H. Preoperative assessment of para-aortic lymph node metastasis in patients with pancreatic cancer. Int J Clin Oncol. 2010;15:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Kim YC, Park MS, Cha SW, Chung YE, Lim JS, Kim KS, Kim MJ, Kim KW. Comparison of CT and MRI for presurgical characterization of paraaortic lymph nodes in patients with pancreatico-biliary carcinoma. World J Gastroenterol. 2008;14:2208-2212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Jang JY, Kang MJ, Heo JS, Choi SH, Choi DW, Park SJ, Han SS, Yoon DS, Yu HC, Kang KJ. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg. 2014;259:656-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 17. | Nimura Y, Nagino M, Takao S, Takada T, Miyazaki K, Kawarada Y, Miyagawa S, Yamaguchi A, Ishiyama S, Takeda Y. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas: long-term results of a Japanese multicenter randomized controlled trial. J Hepatobiliary Pancreat Sci. 2012;19:230-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 18. | Michalski CW, Kleeff J, Wente MN, Diener MK, Büchler MW, Friess H. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg. 2007;94:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 208] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Kawai M, Tani M, Hirono S, Okada K, Miyazawa M, Yamaue H. Pylorus-resecting pancreaticoduodenectomy offers long-term outcomes similar to those of pylorus-preserving pancreaticoduodenectomy: results of a prospective study. World J Surg. 2014;38:1476-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Wu W, Hong X, Fu L, Liu S, You L, Zhou L, Zhao Y. The effect of pylorus removal on delayed gastric emptying after pancreaticoduodenectomy: a meta-analysis of 2,599 patients. PLoS One. 2014;9:e108380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Shukla PJ, Barreto SG, Kulkarni A, Nagarajan G, Fingerhut A. Vascular anomalies encountered during pancreatoduodenectomy: do they influence outcomes? Ann Surg Oncol. 2010;17:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |