Published online Jul 14, 2016. doi: 10.3748/wjg.v22.i26.6049
Peer-review started: February 29, 2016
First decision: March 31, 2016
Revised: April 1, 2016
Accepted: April 20, 2016
Article in press: April 20, 2016
Published online: July 14, 2016
Processing time: 128 Days and 21.6 Hours
AIM: To develop and validate a risk score for advanced colorectal adenoma (ACA) recurrence after endoscopic polypectomy.
METHODS: Out of 3360 patients who underwent colon polypectomy at University of Foggia between 2004 and 2008, data of 843 patients with 1155 ACAs was retrospectively reviewed. Surveillance intervals were scheduled by guidelines at 3 years and primary endpoint was considered 3-year ACA recurrence. Baseline clinical parameters and the main features of ACAs were entered into a Cox regression analysis and variables with P < 0.05 in the univariate analysis were then tested as candidate variables into a stepwise Cox regression model (conditional backward selection). The regression coefficients of the Cox regression model were multiplied by 2 and rounded in order to obtain easy to use point numbers facilitating the calculation of the score. To avoid overoptimistic results due to model fitting and evaluation in the same dataset, we performed an internal 10-fold cross-validation by means of bootstrap sampling.
RESULTS: Median lesion size was 16 mm (12-23) while median number of adenomas was 2.5 (1-3), whereof the number of ACAs was 1.5 (1-2). At 3 years after polypectomy, recurrence was observed in 229 ACAs (19.8%), of which 157 (13.5%) were metachronous neoplasms and 72 (6.2%) local recurrences. Multivariate analysis, after exclusion of the variable “type of resection” due to its collinearity with other predictive factors, confirmed lesion size, number of ACAs and grade of dysplasia as significantly associated to the primary outcome. The score was then built by multiplying the regression coefficients times 2 and the cut-off point 5 was selected by means of a Receiver Operating Characteristic curve analysis. In particular, 248 patients with 365 ACAs fell in the higher-risk group (score ≥ 5) where 3-year recurrence was detected in 174 ACAs (47.6%) whereas the remaining 595 patients with 690 ACAs were included in the low-risk group (score < 5) where 3-year recurrence rate was 7.9% (55/690 ACAs). Area under the curve of the model was 0.81 (0.72-0.86) with an overall classification error rate of 0.09. The model was finally validated by means of 10-fold cross validation.
CONCLUSION: Our study provides support for the use of a novel risk score as a clinical predictor of ACA recurrence after colon polypectomy.
Core tip: This is a retrospective study to develop and validate a novel risk score aimed at predicting advanced colorectal adenoma (ACA) recurrence after endoscopic polypectomy. The score based on lesion size, number of ACAs and grade of dysplasia, considering 5 as cut-off point, defined two different risk groups: high-risk group (score ≥ 5) with a 3-year recurrence rate of 47.6% and low-risk group (score < 5) with a 3-year recurrence rate of 7.9%. Further evidence, provided by large randomized controlled trials, is necessary in order to completely address this important issue.
- Citation: Facciorusso A, Di Maso M, Serviddio G, Vendemiale G, Muscatiello N. Development and validation of a risk score for advanced colorectal adenoma recurrence after endoscopic resection. World J Gastroenterol 2016; 22(26): 6049-6056
- URL: https://www.wjgnet.com/1007-9327/full/v22/i26/6049.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i26.6049
Colorectal cancer (CRC) is the second cause of cancer-related mortality in developed countries and the third most common malignancy worldwide[1]. However, CRC death rates have declined by approximately 3% per year during the past decade, which is most likely due to the improvement of screening programs and standard treatments[1].
Early interruption of adenoma-carcinoma sequence, by means of screening and surveillance programs, is widely recognized to prevent CRC occurrence and to have a significant influence on patient survival[2,3].
According to current guidelines, surveillance colonoscopy should be repeated at 5-10 years after endoscopic resection of a single (or two) lesions < 1 cm presenting tubular features or low-grade dysplasia at histology, while follow-up should be schedule at 3 years in cases of advanced colorectal adenomas (ACAs)[4], defined by at least one of the following: ≥ 1 cm in diameter, villous component and high-grade dysplasia (HGD), namely those features determining an higher risk of progression to carcinoma[5-7].
However, even in presence of ACAs, recurrence rates widely vary on the basis of several baseline clinical variables and ACA-related features such as size, number and histological characteristics[7] in addition to type of endoscopic resection (whether en bloc or piecemeal)[8].
Therefore, an accurate risk stratification model aimed at suggesting the appropriate post-polypectomy surveillance remains an unmet need in gastrointestinal endoscopy[6-10].
A number of recent studies have explored the impact of different risk factors on local recurrence after ACA resection but the interaction between these factors is still unclear[5-7,9,11].
Aim of this study is to develop and validate an easy-to-use numeric score point able to accurately predict ACA recurrence after colon polypectomy in order to guide the decision for a more correct and accurate follow-up schedule “tailored” to patient characteristics.
Between Jan 2004 and Dec 2008 about 3360 patients underwent colonoscopic polypectomy at University of Foggia and among them data of 843 patients diagnosed with ACA was retrieved. This timespan corresponded to the period when polypectomy was performed conventionally at our center, before introducing a novel technique using polidocanol injection described in a recent paper published by our group[12]. Institutional Review Board approbation for retrospective analysis of de-identified patients’ data was obtained.
Inclusion criteria to our study were: complete adenoma resection, retrieval of resected lesion for pathological analysis, no previous diagnosis of CRC or familiar hereditary polyposis syndromes, exclusion of inflammatory bowel disease, complete follow-up data.
All colonoscopies were performed by two board-certified gastroenterologists (M.d.M., N.M.) and written informed consent was obtained from all patients before the procedure.
All colonoscopies were performed under deep sedation using Propofol (Diprivan®, AstraZeneca, London, United Kingdom) monitored by a board-certified anesthesiologist with an Olympus CF-230 or CF-240 video colonoscope, following cleansing of the bowel using a polyethylene glycol-electrolyte solution (Selg-Esse®, Promefarm, Bergamo, Italy). Bowel preparation was split between the evening before and the morning of the procedure.
The interventional endoscopic techniques adopted at our center has been described elsewhere[10,12]. Briefly, a disposable injection needle (Innoflex®, Innovamedica, Milan, Italy) was inserted at one edge of the lesion for submucosal injection with 9 mL of saline with 1 mL of adrenaline 1:10.000 (Adrenalina, SALF, Bergamo, Italy). The volume of solution injected was dependent on the adenoma size. After the submucosal injection, the polyp was cut with a disposable electrosurgical snare (Rotable Snare®, Boston Scientific, Natickama, United States) placed over the elevated tissue and connected to the ERBE electrosurgical unit (VIO 300; ERBE, Tubingen, Germany) set to Endocut Q, Effect 3. No other ablative techniques in addition to snare resection were needed.
En bloc resection was performed whenever feasible, otherwise (in cases where the lesion was too large) piecemeal resection was undertaken. Complete resection was defined as no remaining adenomatous tissue after endoscopic mucosal resection.
ACAs were identified according to Paris classification as: polypoid pedunculated type (0-1p), sessile (0-1s), non-polypoid (0-IIa, 0-IIb and 0-IIc)[13,14].
All resected specimens were retrieved for histopathological analysis, and classified as tubular, tubulovillous, villous or serrated adenomas.
Patients were hospitalized for observation for 24 h, had the procedure in the day hospital or underwent ambulatorial colonoscopy, depending on the complexity of the procedure and comorbidity. In each case the monitoring protocol was the same.
All 843 recruited patients underwent follow-up colonoscopies at our Institution. Surveillance intervals were scheduled by guidelines at 3 years in the case of en bloc resection and after 3 mo in the case of piecemeal resection, since all the patients included in the study presented advanced adenomas[4].
Recurrence was assessed by the endoscopist during follow-up, including in this definition both local recurrence (in the same site of a previous polypectomy) and the occurrence of metachronous distant polyps[7,10].
As described elsewhere, adverse event rates (such as bleeding or perforation) were evaluated during the procedure and, in order to capture delayed bleeding, at 24 h, 7, 10 and 14 d by means of ambulatory visits and telephone calls[12].
Only cases of significant bleeding, those requiring interruption of the operation to perform hemostasis or thermal treatment using coagulation with snare tip or application of clips (Resolution Clip; Boston Scientific, Natick, United States), were reported[12].
Patients characteristics were summarized using conventional statistics, like median and interquartile range (IQR) for continuous variables and absolute frequencies and percentages for categorical data. Three-year recurrence was the main outcome measure. Baseline factors with a potential prognostic effect on recurrence were initially analyzed by means of uni/multivariate logistic regression test. The effect of continuous variables on recurrence rate was assessed for each variable by forming four groups at its quartiles. When the respective regression test was significant, a spline-based approach was applied to assess the functional form of the variable on recurrence[15]. Based on this graphical representation a clinically sensible and applicable dichotomization of the respective variable was applied.
Variables with P < 0.05 in the univariate analysis were entered as candidate variables into a stepwise regression model (conditional backward selection). The regression coefficients of the Cox regression model were multiplied by 2 and rounded in order to obtain easy to use point numbers facilitating the calculation of the score. A Receiver Operating Characteristic (ROC) curve analysis was conducted aimed at identifying the more accurate cut-off points for the risk score.
The performance of the model was evaluated with the area under the curve (AUC) and error rate. To avoid overoptimistic results due to overfitting, we tested the performance of our model by means of 10-fold cross validation. Ten-fold cross-validation refers to the process of dividing the original patient sample into 10 equal groups, then removing 1 group, used as validation sample, and reconstructing the model using the reduced sample set. The new model is then tested for predictive accuracy against the excluded fraction, the process is repeated 10 times (each time with a different excluded subset). Finally, ten-fold cross-validation is repeated 250 times by means of bootstrapping to reduce the effect of random splits, and an overall c-index and error rate is calculated[10].
The analysis was performed using R Statistical Software (Foundation for Statistical Computing, Vienna, Austria) and significance threshold was established at the 0.05 level (two-sided).
Baseline characteristics of the whole study population of 843 patients with 1155 ACAs who underwent colon polypectomy are reported in Table 1.
| Variable | All patients (n = 843) |
| Age (yr) | 58 (52-67) |
| Gender | |
| Male | 522 (61.9) |
| Female | 321 (38.1) |
| BMI | 25 (22-28) |
| ASA score | 2 (1-3) |
| Lesion size (mm) | 16 (12-23) |
| Number of adenomas | 2.5 (1-3) |
| Number of ACAs | 1.5 (1-2) |
| Morphology1 pedunculated (Paris 1p) | 473 (40.9) |
| Sessile (Paris 1s) | 458 (39.6) |
| Nonpolypoid (Paris 0-IIa, 0-IIb, 0-IIc) | 224 (19.5) |
| Location1 | |
| Right side of the colon | 431 (37.3) |
| Left side of the colon | 724 (62.7) |
| Type of resection1 | 937 (81.1) |
| En bloc piecemeal | 218 (18.9) |
| Histology1 | |
| Tubular | 436 (37.7) |
| Tubulo-villous | 632 (54.7) |
| Villous | 87 (7.6) |
| Hystologic grade of dysplasia1 | |
| Low grade | 878 (76.1) |
| High grade | 277 (23.9) |
Median age was 58 (IQR 52-67) and most patients were male (61.9%) with median Body Mass Index (BMI) and American Society of Anaesthesiology (ASA) score of 25 (22-28) and 2 (1-3), respectively.
Median lesion size detected was 16 mm (12-23) while median number of adenomas was 2.5 (1-3), whereof number of ACAs was 1.5 (1-2).
Polyps were pedunculated (Paris 0-1p) in 40.9%, sessile (Paris 0-1s) in 39.6% whereas non-polypoid lesions (Paris 0-IIa, 0-IIb and 0-IIc) accounted for 19.5% of the 1155 ACAs detected.
A little over one third of ACAs were located in the right colon (37.3%) with tubule-villous as the most frequent histology (54.7%).
Out of 1155 ACAs detected, en bloc resection was feasible in 937 (81.1%) cases.
Neither procedure-related deaths nor transmural burn syndromes were reported. Immediate bleeding was experienced by 51 patients (6%). All immediate bleeding events clinically presented with small amount of blood and none of the patients required hospitalization or transfusion.
Delayed bleeding rate was 19/843 (2.2%) and no clip application was needed to control delayed bleeding events. Free perforation was observed in 2 patients (0.2%), both successfully treated with surgery.
At 3 years after polypectomy, recurrence was observed in 229 ACAs (19.8%), of which 157 (13.5%) were metachronous neoplasms (150 ACAs and 7 adenocarcinomas) and 72 (6.2%) were local recurrences (70 ACAs and 2 adenocarcinomas).
Univariate logistic regression selected the number of ACAs, lesion size, morphology, type of resection, and grade of dysplasia as significant predictors of 3-year recurrence (Table 2). The same variables resulted significant predictors of both local recurrence and metachronous polyps occurrence when stratifying the regression analysis by recurrence pattern (data not shown).
| Variables | Odds ratio (95%CI) | P-value |
| Age (reference ≤ 55 yr) | 1.31 (0.88-1.44) | 0.14 |
| Gender (reference female) | 1.47 (0.87-1.88) | 0.09 |
| Size (reference ≤ 15 mm) | 2.84 (1.75-4.19) | < 0.001 |
| Number of ACAs (reference 1) | 2.69 (1.88-4.53) | < 0.001 |
| Morphology (reference pedunculated) | 0.01 | |
| Sessile | 1.96 (1.21-2.43) | |
| Nonpolypoid | 2.43 (1.14-3.26) | |
| Location (reference right side colon) | 1.18 (0.76-1.35) | 0.57 |
| Type of resection (reference en bloc) | 8.49 (3.87-11.47) | < 0.001 |
| Histology (reference Tubular) | 0.07 | |
| Tubulo-Villous | 1.49 (0.47-5.18) | |
| Villous | 1.73 (0.68-4.45) | |
| Grade of dysplasia (reference low-grade) | 3.25 (1.23-5.60) | < 0.001 |
The significant parameters “number of ACAs”, “lesion size”, “morphology”, and “grade of dysplasia” were then entered into multivariate regression analysis. The variable “type of resection” was preliminarily excluded due to its collinearity with other parameters (mainly ACA size and morphology).
After stepwise removal of the variable “ACA morphology”, which did not result significant in multivariate setting (P = 0.51), ACA size, number and grade of dysplasia remained significant predictors of 3-year recurrence. The calculated regression coefficients were multiplied times 2 and rounded in order to facilitate the calculation of the score.
As described in Table 3, patients were given 4 points in presence of HGD, whereas lesions > 15 mm and multiple ACAs determined 3 and 2 additional score points, respectively (Table 3).
| Variable | Odds ratio (95%CI) | Regression coefficient | Score points1 | P-value |
| Grade of dysplasia | ||||
| Low-grade | 1 | - | ||
| High-grade | 4.25 (2.11-7.5) | 1.93 | 4 | < 0.001 |
| Size | ||||
| ≤ 15 mm | 1 | - | ||
| > 15 mm | 3.96 (1.87-7.55) | 1.61 | 3 | < 0.001 |
| Number of ACAs | ||||
| 1 | 1 | - | ||
| > 1 | 3.22 (2.19-5.39) | 1.21 | 2 | < 0.001 |
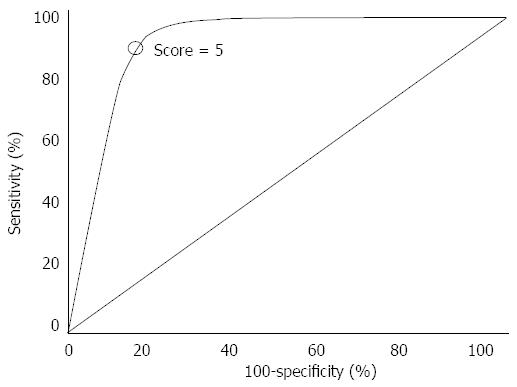
We then calculated the risk score for all the recruited patients and performed an ROC curve analysis in order to select the more accurate cut-off point able to stratify the study population according to the recurrence score (Figure 1). ROC analysis showed a score point of 5 as the value at higher specificity and sensitivity for 3-year ACA recurrence rate (Figure 1). In particular, 248 patients with 365 ACAs fell in the high-risk group (score ≥ 5) where 3-year recurrence was detected in 174 ACAs (47.6%) whereas the remaining 595 patients with 690 ACAs were included in the low-risk group (score < 5) where 3-year recurrence rate was 7.9% (55/690 ACAs). AUC of the model was 0.81 (0.72-0.86) with a classification error rate of 0.09.
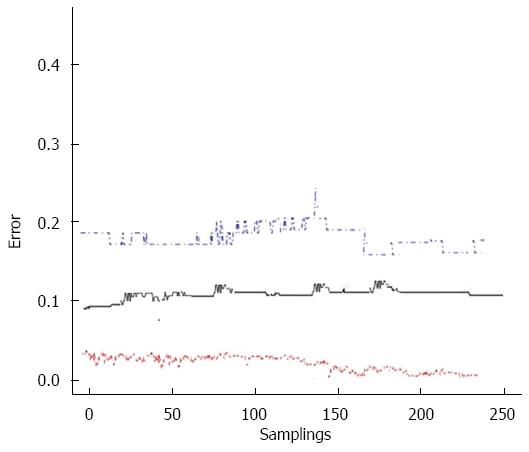
The model was tested by means of ten-fold cross validation. The original patient sample was partitioned into 10 equal groups, then 1 group was randomly removed each time and used as validation sample while reconstructing the model using the remaining sample set. Cross-validation thus consisted in testing each of these reduced sample sets for predictive accuracy against the excluded fractions. Finally, ten-fold cross-validation was repeated 250 times by means of bootstrapping to reduce the effect of random splits, and an overall AUC and error rate were calculated. This validation method resulted in an AUC of 0.79 (95%CI: 0.72-0.83) and in an overall error rate of 0.12 for our model (Figure 2).
Colorectal cancer represents a major health problem and screening colonoscopy with removal of detected adenomas has proven an effective strategy to decrease CRC incidence and mortality[3,4].
Since only 6% of patients with adenoma will develop CRC[4], a number of recent studies have focused on the definition of risk factors for recurrence in order to identify the proper time interval from index colonoscopy to the next examination[5,6,11].
A group of experts has recently updated the guidelines of colonoscopy surveillance after colon polypectomy[4], but tailoring the frequency and time intervals of follow-up remains an unmet need.
Advanced colorectal adenomas (those ≥ 1 cm and/or with villous component and/or HGD) are well-known to present higher risk of adenoma recurrence after polypectomy and to more frequently develop into adenocarcinoma[5,16,17]. However, ACAs represent a wide variety of lesions with very different recurrence rates after colon polypectomy or likelihood to degenerate[6,11].
In a recent paper, Seo et al[6] demonstrated in a large retrospective series of ACAs that the presence of 2 or more unfavorable features and piecemeal resection determine an higher risk of recurrence. Unfortunately, an accurate individualization of the risk was not possible since the exact weight of each feature and the interaction among them could not be captured by the conventional logistic regression applied by the authors. On the other hand, Martínez et al[7] showed in a pooled-data analysis that 7.4% of patients identified as at low risk by current guidelines finally developed ACA or invasive cancer, thus clearly claiming among the conclusions of their paper the need for a formal prediction model able to determine the combination of factors which would maximally distinguish the risk of recurrence.
Aim of this study was therefore to establish an objective and simple tool to define the recurrence risk for ACA patients and consequently to guide the decision process for the surveillance protocol.
We found that baseline ACA size > 15 mm, presence of multiple ACAs and high-grade dysplasia were associated to higher risk of adenoma recurrence at 3 years (Table 3).
These findings are in keeping with the published literature[6,11,18,19]. It should be noted that histology was not selected by the multivariate model, probably because tubular ACAs are likely to present in greater sizes since, by definition, adenomas > 10 mm are to be considered “advanced” regardless of histology, whereas villous adenomas are always considered advanced, regardless of size. Lesion size hence probably “masked” the impact of histological pattern on final outcomes.
On the basis of these results, we developed a risk score by using the regression coefficients of these variables in our multivariate regression model. Once selected the value 5 as an accurate cut-off value for the point score, our model identified two groups at different risk of ACA recurrence.
Taking a closer look at our data, the low-risk group (when the total score was < 5) showed a 3-year ACA recurrence rate of 7.9% (55/690 ACAs) whereas the higher-risk group (score ≥ 5) experienced recurrence at 3 years in 47.6% of detected lesions (174/365).
We think that modelling an objective risk score enabled us to overcome the final findings of previous reports[6,20,21], which concluded that the number of predictive ACA characteristics was more important than the type of characteristic in defining recurrence risk. On the other hand, our analysis identified two different risk classes based on an objective numeric score able to take into account either the number of ACA characteristics and the type of their features.
In our study, the incidence of adenocarcinoma after polypectomy was low (0.7%), consistently with previous reports[6,11], thus confirming the efficacy of current surveillance programs.
The findings of the current paper are of key clinical relevance for several reasons. First, our score is simple and easily applicable in a real-life clinical setting even in countries with limited healthcare resources. Second, the application of the score may be useful in better define the surveillance schedule and protect patients with low-risk features from an excessively strict follow-up. On the other hand, “tailoring” the surveillance schedule to single patient and even ACA characteristics may significantly decrease the recurrence rate in higher risk patients, actually not adequately followed-up with the current protocols.
Nevertheless, there are some weaknesses to our study. First, the retrospective nature of the report may have introduced some outcome biases as, for instance, patients who underwent piecemeal resection were evaluated at 3 mo after polypectomy unlike those treated with en bloc resection. However, we performed the analysis considering as the sole dependent variable 3-year recurrence rate and did not consider time-to-recurrence, which could have been affected by the different follow-up schedule. Second, as all the patients were followed-up according to current guidelines, it was not possible to assess recurrence rates at different time points, unlike other studies conducted in countries where the low medical cost of colonoscopy allowed more frequent examinations[6]. As a consequence, we may postulate that high-risk lesions could benefit from a more intensive follow-up schedule (i.e., before 3 years after colonoscopy) but definitive data in such regard is lacking. Third, the single-center experience reported in the study did not allow the external validation of the model in a different cohort. Nevertheless, an internal validation by means of 250 bootstrap samplings randomly drawn with replacement from the original population, was performed. This way, both the model building process and its performance were simultaneously validated in a broad range of random samples, thus obviating the lack of an external cohort, as recently confirmed by simulation studies[22].
In conclusion, in the current paper we propose an objective tool aimed at classifying advanced colorectal adenomas in two groups at different risk of recurrence, based on the number of ACAs, their size and the presence of high-grade dysplasia. A score point ≥ 5 (given by the combination of at least two of the aforementioned ACA features) determine a significantly higher recurrence risk at 3 years and probably calls for a stricter follow-up schedule. Further evidence, provided by large randomized controlled trials assessing recurrence rate at several time points, is necessary in order to completely address this important issue.
Early interruption of adenoma-carcinoma sequence, by means of screening and surveillance programs, is widely recognized to prevent colorectal cancer occurrence and to have a significant influence on patient survival. According to current guidelines, surveillance colonoscopy should be repeated at 5-10 years after endoscopic resection of a single (or two) lesions < 1 cm presenting tubular features or low-grade dysplasia at histology, while follow-up should be schedule at 3 years in cases of advanced colorectal adenomas (ACAs), defined by at least one of the following: ≥ 1 cm in diameter, villous component and high-grade dysplasia, namely those features determining an higher risk of progression to carcinoma. However, even in presence of ACAs, recurrence rates widely vary on the basis of several variables. Therefore, an objective and easy-to-use tool aimed at suggesting the appropriate post-polypectomy surveillance remains an unmet need in gastrointestinal endoscopy. In fact, a number of recent studies have explored the impact of different risk factors on local recurrence after ACA resection but the interaction between these factors is still unclear.
The authors propose an objective tool aimed at classifying advanced colorectal adenomas in two groups at different risk of recurrence, based on the number of ACAs, their size and the presence of high-grade dysplasia. Further evidence, provided by large randomized controlled trials assessing recurrence rate at several time points, is necessary in order to completely address this important issue.
The authors think that building an objective risk score allows to overcome the final findings of previous reports, which concluded that the number of predictive ACA characteristics was more important than the type of characteristic in defining recurrence risk. On the other hand, our analysis identified two different risk classes based on an objective numeric score able to take into account either the number of ACA characteristics and the type of their features. In this study, the incidence of adenocarcinoma after polypectomy was low (0.7%), consistently with previous reports, thus confirming the efficacy of current surveillance programs. The findings of the current paper are of key clinical relevance for several reasons. First, our score is simple and easily applicable in a real-life clinical setting. Second, the application of the score may be useful in better define the surveillance schedule and protect patients with low-risk features from an excessively strict follow-up. On the other hand, “tailoring” the surveillance schedule to single patient and even ACA characteristics may significantly decrease the recurrence rate in higher risk patients, actually not adequately followed-up with the current protocols.
This study provides support for the use of a novel risk score as predictor of 3-year ACA recurrence. A score point ≥ 5 implies an higher risk of recurrence.
ACA: Advanced colorectal adenomas, namely those ≥ 1 cm and/or villous component and/or with HGD, which are well-known to present higher risk of adenoma recurrence after polypectomy and to development into adenocarcinoma. Colon polypectomy: endoscopic removal of a mucosal lesion, aimed at interrupting the adenoma-carcinoma sequence.
This manuscript reported a development of a novel risk score tool for colorectal adenoma recurrence after endoscopic mucosal resection and investigated the validation with relatively large sample size. The scoring tool has a good performance for predicting the recurrence and is easily applicable at the bedside. This study design involves several limitations such as retrospective database-based study and regarding follow-up time, but the authors well discussed on these matters in the manuscript.
P- Reviewer: Luigiano C, Matsuda A S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9958] [Article Influence: 995.8] [Reference Citation Analysis (0)] |
| 2. | Morson BC. Evolution of cancer of the colon and rectum. Proc Inst Med Chic. 1974;30:145-148. [PubMed] |
| 3. | Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, Waye JD, Schapiro M, Bond JH, Panish JF. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1977-1981. [PubMed] |
| 4. | Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1423] [Cited by in RCA: 1457] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 5. | Martínez ME, Sampliner R, Marshall JR, Bhattacharyya AK, Reid ME, Alberts DS. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology. 2001;120:1077-1083. [PubMed] |
| 6. | Seo JY, Chun J, Lee C, Hong KS, Im JP, Kim SG, Jung HC, Kim JS. Novel risk stratification for recurrence after endoscopic resection of advanced colorectal adenoma. Gastrointest Endosc. 2015;81:655-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Martínez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, Zauber AG, Jiang R, Ahnen DJ, Bond JH. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 429] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 8. | Facciorusso A, Antonino M, Di Maso M, Muscatiello N. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J Gastrointest Endosc. 2014;6:555-563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 110] [Cited by in RCA: 119] [Article Influence: 10.8] [Reference Citation Analysis (2)] |
| 9. | Leung WK, Lau JY, Suen BY, Wong GL, Chow DK, Lai LH, To KF, Yim CK, Lee ES, Tsoi KK. Repeat-screening colonoscopy 5 years after normal baseline-screening colonoscopy in average-risk Chinese: a prospective study. Am J Gastroenterol. 2009;104:2028-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Facciorusso A, Di Maso M, Serviddio G, Vendemiale G, Spada C, Costamagna G, Muscatiello N. Factors Associated With Recurrence of Advanced Colorectal Adenoma After Endoscopic Resection. Clin Gastroenterol Hepatol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Chung SJ, Kim YS, Yang SY, Song JH, Kim D, Park MJ, Kim SG, Song IS, Kim JS. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut. 2011;60:1537-1543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 12. | Facciorusso A, Di Maso M, Antonino M, Del Prete V, Panella C, Barone M, Muscatiello N. Polidocanol injection decreases the bleeding rate after colon polypectomy: a propensity score analysis. Gastrointest Endosc. 2015;82:350-358.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Kudo Se, Lambert R, Allen JI, Fujii H, Fujii T, Kashida H, Matsuda T, Mori M, Saito H, Shimoda T, Tanaka S, Watanabe H, Sung JJ, Feld AD, Inadomi JM, O’Brien MJ, Lieberman DA, Ransohoff DF, Soetikno RM, Triadafilopoulos G, Zauber A, Teixeira CR, Rey JF, Jaramillo E, Rubio CA, Van Gossum A, Jung M, Vieth M, Jass JR, Hurlstone PD. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68:S3-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 364] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 14. | Facciorusso A, Antonino M, Di Maso M, Barone M, Muscatiello N. Non-polypoid colorectal neoplasms: Classification, therapy and follow-up. World J Gastroenterol. 2015;21:5149-5157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Heinzl H, Kaider A. Gaining more flexibility in Cox proportional hazards regression models with cubic spline functions. Comput Methods Programs Biomed. 1997;54:201-208. [PubMed] |
| 16. | Winawer SJ, Zauber AG, O’Brien MJ, Ho MN, Gottlieb L, Sternberg SS, Waye JD, Bond J, Schapiro M, Stewart ET. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med. 1993;328:901-906. [PubMed] |
| 17. | Lieberman DA, Weiss DG, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, Schnell TG, Chejfec G, Campbell DR, Kidao J. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133:1077-1085. [PubMed] |
| 18. | Atkin WS, Morson BC, Cuzick J. Long-term risk of colorectal cancer after excision of rectosigmoid adenomas. N Engl J Med. 1992;326:658-662. [PubMed] |
| 19. | Yang G, Zheng W, Sun QR, Shu XO, Li WD, Yu H, Shen GF, Shen YZ, Potter JD, Zheng S. Pathologic features of initial adenomas as predictors for metachronous adenomas of the rectum. J Natl Cancer Inst. 1998;90:1661-1665. [PubMed] |
| 20. | Buchner AM, Guarner-Argente C, Ginsberg GG. Outcomes of EMR of defiant colorectal lesions directed to an endoscopy referral center. Gastrointest Endosc. 2012;76:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 21. | Luigiano C, Consolo P, Scaffidi MG, Strangio G, Giacobbe G, Alibrandi A, Pallio S, Tortora A, Melita G, Familiari L. Endoscopic mucosal resection for large and giant sessile and flat colorectal polyps: a single-center experience with long-term follow-up. Endoscopy. 2009;41:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Steyerberg EW, Bleeker SE, Moll HA, Grobbee DE, Moons KG. Internal and external validation of predictive models: a simulation study of bias and precision in small samples. J Clin Epidemiol. 2003;56:441-447. [PubMed] |