Published online Jul 14, 2016. doi: 10.3748/wjg.v22.i26.6036
Peer-review started: January 18, 2016
First decision: March 7, 2016
Revised: April 16, 2016
Accepted: May 23, 2016
Article in press: May 23, 2016
Published online: July 14, 2016
Processing time: 174 Days and 16.8 Hours
AIM: To evaluate the efficacy of umbilical cord-derived mesenchymal stem cells (UC-MSCs) transplantation in the treatment of liver fibrosis.
METHODS: Cultured human UC-MSCs were isolated and transfused into rats with liver fibrosis induced by dimethylnitrosamine (DMN). The effects of UC-MSCs transfusion on liver fibrosis were then evaluated by histopathology; serum interleukin (IL)-4 and IL-10 levels were also measured. Furthermore, Kupffer cells (KCs) in fibrotic livers were isolated and cultured to analyze their phenotype. Moreover, UC-MSCs were co-cultured with KCs in vitro to assess the effects of UC-MSCs on KCs’ phenotype, and IL-4 and IL-10 levels were measured in cell culture supernatants. Finally, UC-MSCs and KCs were cultured in the presence of IL-4 antibodies to block the effects of this cytokine, followed by phenotypical analysis of KCs.
RESULTS: UC-MSCs transfused into rats were recruited by the injured liver and alleviated liver fibrosis, increasing serum IL-4 and IL-10 levels. Interestingly, UC-MSCs promoted mobilization of KCs not only in fibrotic livers, but also in vitro. Co-culture of UC-MSCs with KCs resulted in increased production of IL-4 and IL-10. The addition of IL-4 antibodies into the co-culture system resulted in decreased KC mobilization.
CONCLUSION: UC-MSCs could increase IL-4 and promote mobilization of KCs both in vitro and in vivo, subsequently alleviating the liver fibrosis induced by DMN.
Core tip: Dysregulation of the M1/M2 macrophages phenotypic balance governs the pathogenesis of liver fibrosis. Flow cytometry, immunohistochemistry and liver function tests showed that umbilical cord-derived mesenchymal stem cells (UC-MSCs) could promote the mobilization of M1 Kupffer cells (KCs) into the M2 phenotype in vivo and in vitro thereby ameliorating liver inflammation and liver fibrosis. Thus, UC-MSC transfusion yielded promising results with regard to reversal of liver injury and alleviated liver fibrosis by promoting KC mobilization and hepatocyte differentiation. The application of UC-MSCs might provide a new tool for cell therapy of liver fibrosis.
- Citation: Chai NL, Zhang XB, Chen SW, Fan KX, Linghu EQ. Umbilical cord-derived mesenchymal stem cells alleviate liver fibrosis in rats. World J Gastroenterol 2016; 22(26): 6036-6048
- URL: https://www.wjgnet.com/1007-9327/full/v22/i26/6036.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i26.6036
Liver fibrosis is attributed to the excess deposition of collagen. It is usually caused by chronic liver injury, which triggers hepatocyte apoptosis, inflammatory cell recruitment, endothelial barrier damage, increased levels of transforming growth factor β1 (TGF-β1) and activated myofibroblast, which are responsible for scar tissue formation[1]. Inflammation might be the most critical factor in the initiation and maintenance of liver fibrogenesis[1]. When the liver is injured, the damaged epithelial and endothelial cells release inflammatory mediators, and the peripheral blood inflammatory cells are recruited to the affected liver, releasing fibrosis-related mediators such as TGF-β1 and tumor necrosis factor-α (TNF-α), inducing the activation of hepatic stellate cells and as well as deposition of collagen. Anti-smooth muscle α-actin (α-SMA) is a marker of activated hepatic stellate cells (HSCs),and HSCs play key roles in the pathogenesis of liver fibrosis. It is acknowledged that liver fibrosis can be effectively reversed[1], and the promotion of the repair process is considered a therapeutic strategy for liver fibrosis.
Currently, stem cell therapy is considered a promising treatment for various liver diseases, with most studies yielding positive results[2]. Mesenchymal stem cells (MSCs) are the most commonly used stem cells in transplantation. They are multipotent, non-hematopoietic progenitor cells that can differentiate into multiple lineages and have been applied in tissue regeneration and repair. Their hypo-immunogenicity and potential immunomodulatory capacity ensure that the MSCs have clinical value[2]. Increasing evidence suggests that MSCs contribute to the direct production of new hepatocytes[3,4]. Among MSCs, the umbilical cord-derived MSCs (UC-MSCs) possess an excellent proliferative potential, and their low immunogenicity and ease of preparation make them a good choice for use in future clinical studies[5]. Previous studies have shown that UC-MSCs are a well-tolerated therapy. They have the potential to improve the liver function and reduce ascites and mortality, especially in hepatitis B virus patients with decompensated liver cirrhosis[6] and liver failure[7]. Although the effects of UC-MSCs on liver fibrosis had been confirmed in many studies, the detailed mechanism remains unclear.
TGF-β1 is a potent fibrogenic cytokine, playing an important role in the activation of fibrogenic myofibroblasts. In fibrosis, its major source is the Kupffer cells (KCs; liver resident macrophages)[8]. Many clinical and experimental data have indicated that the activation of KCs is the key step in the initiation of liver injury[9-11]. Macrophages are divided into two major cell subpopulations: classically activated proinflammatory M1 macrophages and alternatively activated anti-inflammatory or wound repair M2 macrophages. The M1 type is induced by interferon γ (IFNγ), TLR-4 ligands and bacterial infection, while the M2 type is mostly induced by Interleukin-4 (IL-4), IL-10 or TGF-β[12]. Several studies[13-15] have demonstrated that when the liver is injured, these two functionally distinct macrophage types will be recruited to it. During the injury phase, pro-fibrogenic macrophages (M1) promote myofibroblast proliferation and apoptosis. In contrast, during the injury repair phase, the M2 macrophages predominate and mediate matrix degradation[16]. Some papers have confirmed that M2 macrophages are present during the injury repair phase when the levels of pro-fibrogenic and inflammatory mediators are decreasing[13]. Therefore, the disequilibrium between M1 and M2 macrophages appears to be the major pathogenesis that induces liver fibrosis. Strategies for restraining M1 macrophage mobilization or encouraging the M2 macrophage phenotype might prevent liver injury and thus alleviate liver fibrosis.
The goal of our study was to evaluate the efficacy of UC-MSCs transplantation to treat liver fibrosis in rats. Furthermore, because activation of KCs is the key step in the initiation of liver injury, we were also interested in the influence of UC-MSCs transplantation on the mobilization of KCs and its mechanism.
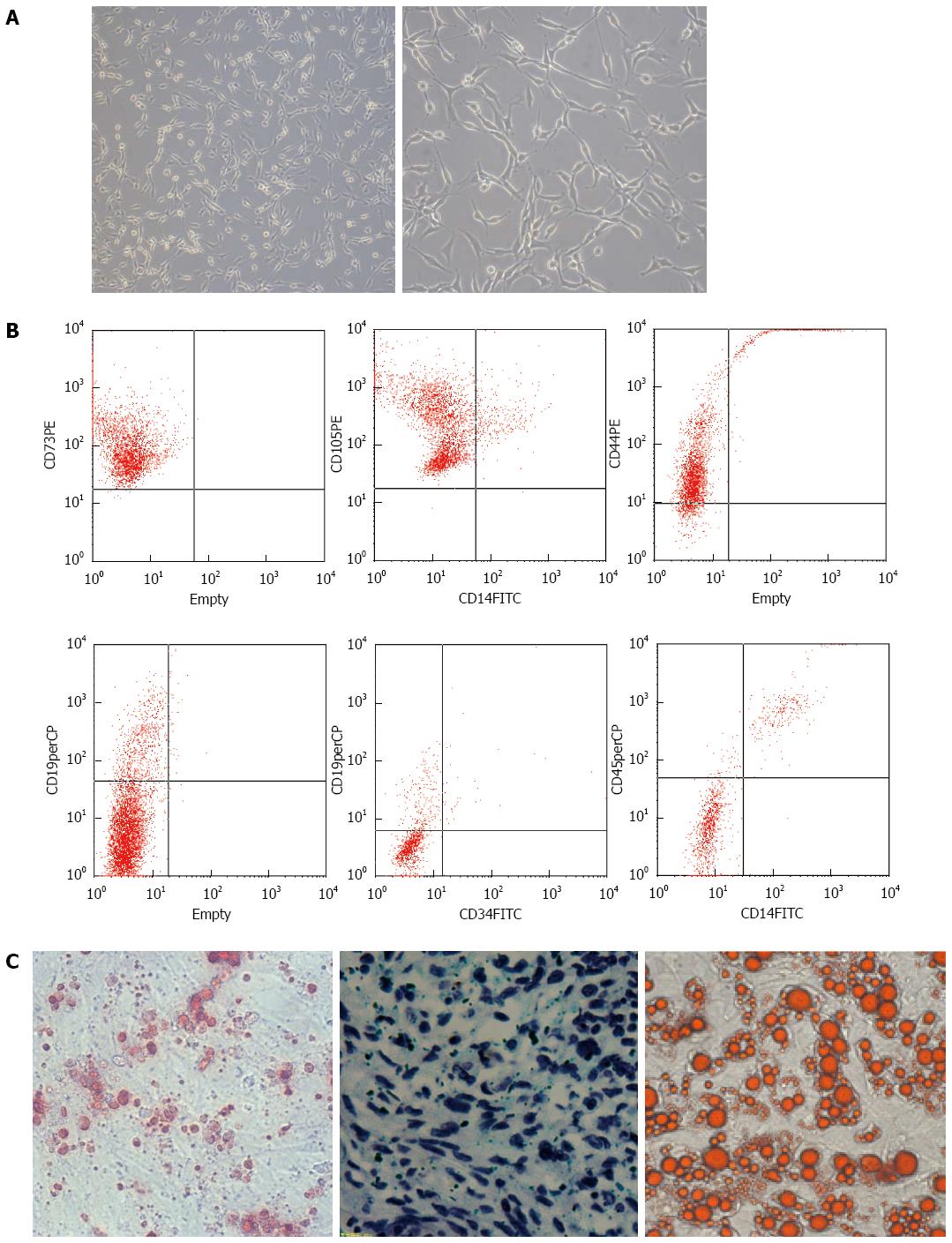
Research protocols that involved human participants were reviewed and approved by the Local Ethics Committee of Chinese PLA Medical Academy (Beijing, China). Written informed consent was provided by each participant in advance. Human umbilical cord samples were obtained from umbilical veins immediately after cesarean section, with the mother’s consent, at the Chinese PLA Medical Academy of the PLA general hospital. We mixed the umbilical cord samples with HetaSep solution (Stemcell Technologies, Vancouver, BC, Canada) at a ratio of 5:1 and incubated the mixture at room temperature to deplete erythrocytes. We then collected the supernatant carefully and used Ficoll density-gradient centrifugation at 398 ×g for 20 min to obtain the mononuclear cells. We washed the cells once or twice in phosphate-buffered saline (PBS) and seeded them into plates at a density of 2 × 105 to 2 × 106 cells/cm2. We incubated these cells in a humidified atmosphere containing 5% CO2 at 37 °C. The cells were then resuspended in 10 mL MSC medium (Alpha Modified Eagles Medium, Invitrogen, Carlsbad, CA, United States), supplemented with 10% fetal bovine serum (FBS; Invitrogen), 10% horse serum (Invitrogen), and 5 mg/mL streptomycin and 5 IU/mL penicillin (Sigma, St. Louis, MO, United States). We collected the mixture in a 15 mL Falcon tube and centrifuged it at 238 ×g for seven minutes at 4 °C. The cells were then counted and added to a 75 cm2 flask containing 15 mL of MSC medium. The adherent cells formed colonies and grew rapidly, exhibiting a spindle-shaped morphology. When the UC- MSCs reached 85% confluence, they were passaged, and cells at the fourth passage were used for transfusion into rats. Before transfusion, UC-MSCs were subjected to quality control, including the detection of CD14, CD19, CD34, CD44, CD45, CD73 and CD105 by flow cytometry analysis, and bacteriological testing.
Standard osteogenic, adipogenic and chondrogenic assays were used to assess their differentiation potential. Osteogenic differentiation of confluent UC-MSCs monolayers obtained as described above was induced using 100 nmol/L dexamethasone, 0.05 mmol/L L-ascorbic acid-2-phosphate and 10 mmol/L β-glycerophosphate (all from Sigma). Alizarin red staining using a Sigma kit was performed to observe calcium deposition in the cultures. Adipogenic differentiation was induced in DMEM/10% FBS supplemented with 0.5 mmol/L isobutylmethylxanthine (Sigma), 60 mol/L indomethacin (ICN, Basingstoke, United Kingdom) and 0.5 mmol/L hydrocortisone (Sigma). Accumulation of lipid vacuoles was visualized using 0.5% Oil Red-O. For chondrogenic differentiation, cells (2.5 × 105) were placed in serum-free medium consisting of high-glucose DMEM, 100 μg/mL sodium pyruvate, 40 μg/mL proline, 50 μg/mL L-ascorbic acid-2-phosphate, 1 mg/mL BSA, 1 × insulin-transferrin-selenium plus, 100 nmol/L dexamethasone (all from Sigma) and 10 ng/mL transforming growth factor-β3 (TGFβ3; R&D Systems, Abingdon, United Kingdom). Cell culture media were replaced every other day. Micromasses were harvested at week 3, and frozen sections (5-μm thick) were prepared.
Low- and high-passage MSCs were analyzed by flow cytometry. Briefly, 2 × 105 to 2 × 106 cells were plated into each well of a round-bottom 96-well plate. After washing with 0.1 mol/L PBS containing 1% bovine serum albumin (Sigma) and 0.1% azide (Sigma), cells were resuspended in 30 μL of primary antibodies against CD14, CD19, CD34, CD44, CD45, CD73, and CD105 (Santa Cruz Biotechnologies, Dallas, Texas, United States) for one hour at 4 °C, respectively. The cells were then rinsed twice and incubated in secondary antibody conjugated to AlexaFluor488 (Invitrogen) for one hour at 4 °C. Then, cells were rinsed twice, fixed using 4% paraformaldehyde for ten minutes on ice, and stored at 4 °C until analysis on a flow cytometer (BD Biosciences, San Diego, CA, United States).
All of the Sprague-Dawley (SD) rats chosen were male and weighed approximately 160 g. The rats were caged and raised under 12-h light-dark cycle in the specific pathogen free (SPF)-grade animal room of the experimental animal center of PLA General Hospital. Standard rat chow and water were provided for the rats. Our study was carried out according to the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health strictly. The Local Ethics Committee of the Chinese PLA Medical Academy approved the protocol (Permit Number: 2012-32).
To establish the animal model of hepatic fibrosis, 48 male SD rats were randomly divided into control (n = 12) and dimethylnitrosamine (DMN) -treatment groups (dimethyl nitrosamine, Tianjin Chemical Reagent Research Institute, Tianjin, China) (n = 36). In the DMN-treatment group, all of the rats received intraperitoneal injection with DMN and saline mixture at a dose of 10 mL/kg for three consecutive days per week for three weeks. The DMN group was further subdivided into three groups (n = 12): Model group, DMN only; Transplantation group, 5 × 106 UC-MSCs administered in 0.1 mL of normal saline by tail vein injection seven days after the first DMN treatment; Control group, DMN treatment + PBS administration by tail vein. After 3 weeks, the rats were euthanized and serum samples collected for biochemical tests. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) activities were detected using kits from Sigma. The right lobes of the livers were excised and soaked in 10% neutral formaldehyde for histology.
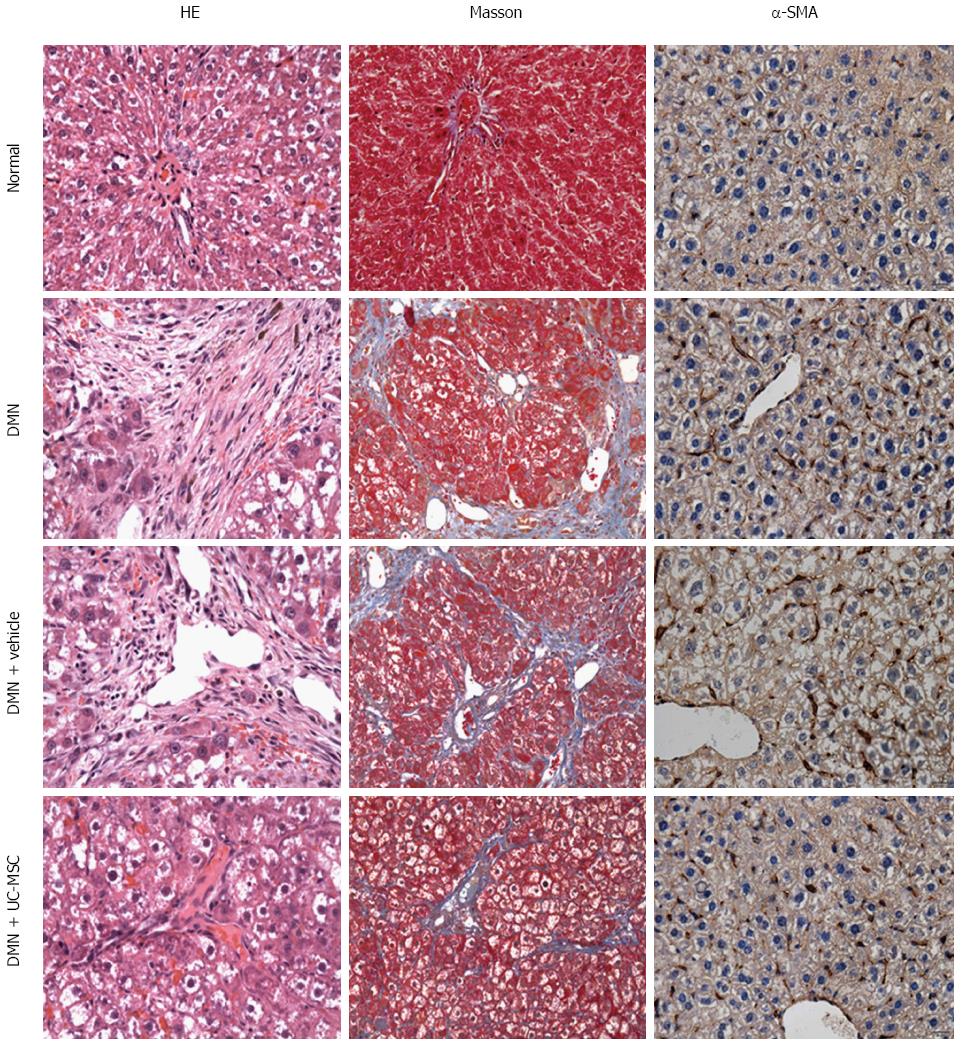
Hematoxylin and eosin (HE) and Masson’s trichrome staining were performed to assess semi-quantitatively the pathogenesis of liver inflammation and fibrosis. An expert pathologist was arranged to assess the condition of liver inflammatory cell infiltration. This was a single-blind test and was based on the microscopic characteristics of the nucleus. The liver inflammation assessment focused on the number of polymorphonuclear leukocytes per 100 hepatocytes. Liver fibrosis was also assessed by an expert pathologist who graded it into five stages: Stage 0, normal connective tissue (no fibrosis); Stage 1, fibrous portal expansion; Stage 2, periportal fibrosis with short septa extending into lobules or rare porto-portal septa (intact architecture); Stage 3, fibrous septa reaching adjacent portal tracts and the terminal hepatic venule (architecture distortion, but no obvious cirrhosis); Stage 4, diffuse nodular formation (cirrhosis).
Micrographs were acquired using a Nikon Micro-phot-FXA microscope equipped with a Nikon Digital Camera DXM1200F. Digital images of HE and Masson staining were analyzed using the Image-Pro Plus software.
Paraffin-embedded liver sections were deparaffinized, using xylene and alcohol. After hydration, slides were treated with a primary polyclonal antibody raised against α-SMA (1:200, Abcam, Cambridge, MA, United States), followed by biotinylated secondary antibody. Detection was carried out with streptavidin peroxidase, and the integrated optical intensity of α-SMA was semi-quantified using the Image-Pro Plus software.
IL-4 and IL-10 levels in serum and cell culture supernatants were measured using ELISA kits (R&D Systems), according to the manufacturer’s instructions. Samples were assessed in duplicate, and the absorbance was read at 450 nm on a Thermo Fisher microplate reader (Massachusetts, America). Cytokine concentrations were calculated using standard curves generated by the plate-reader’s software.
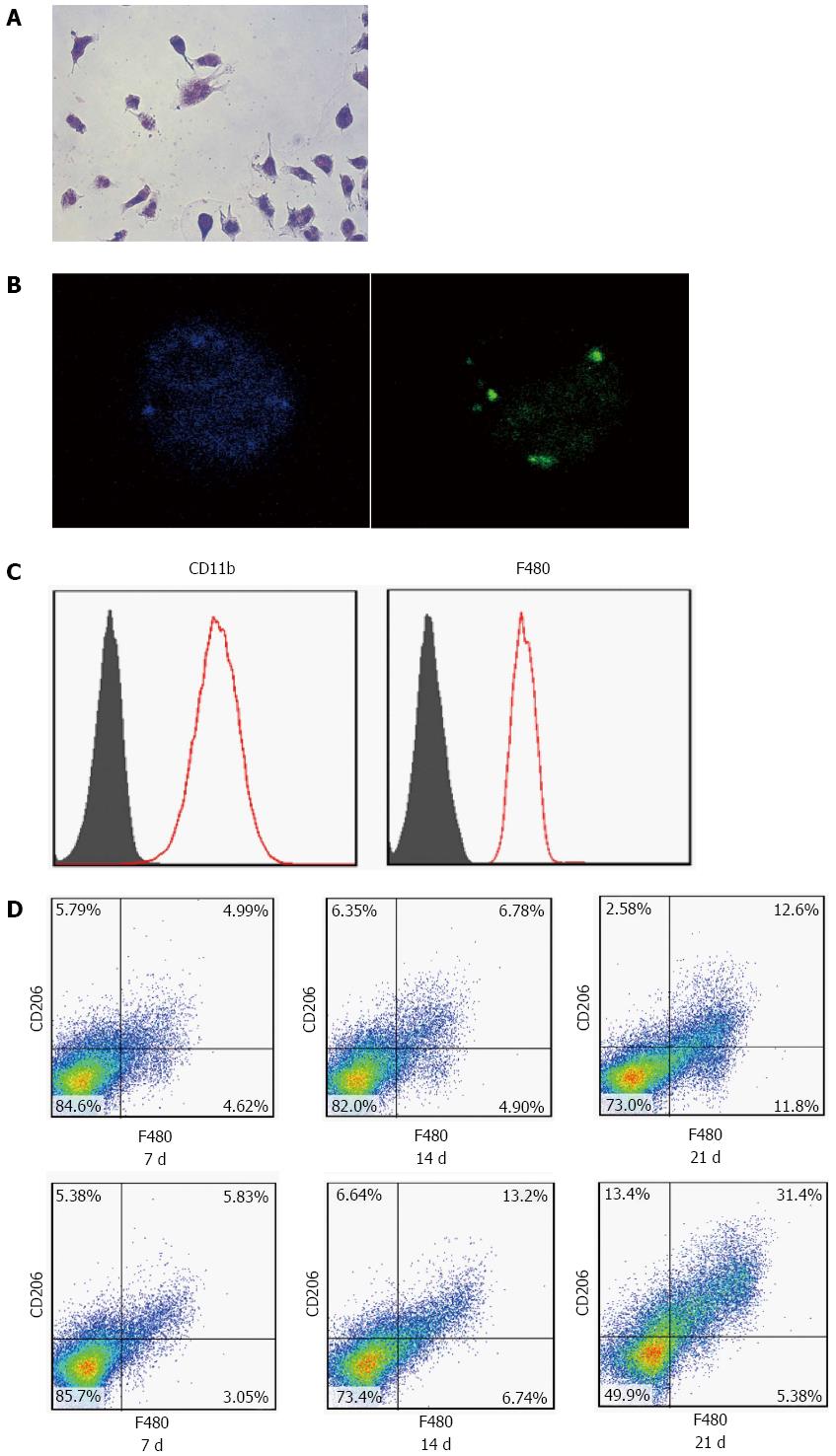
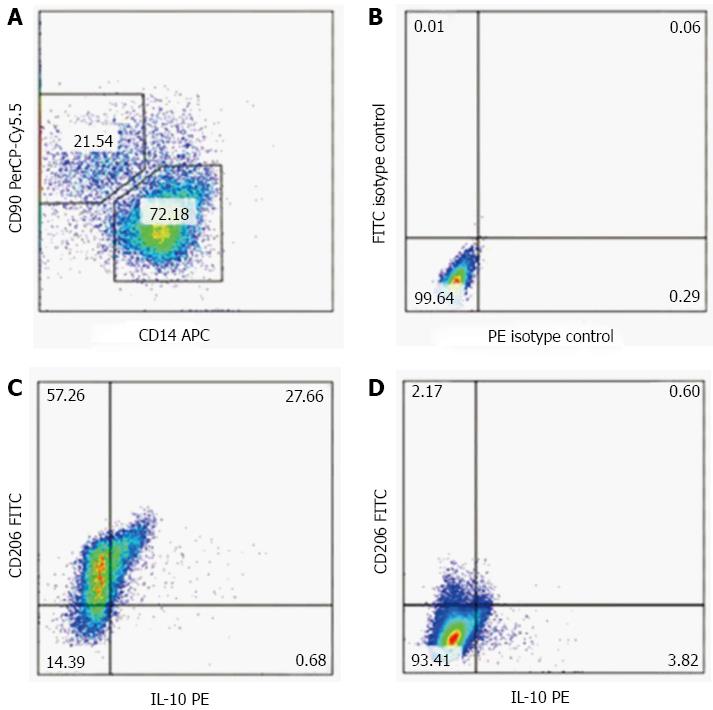
Liver macrophages were isolated from DMN-treated rats through the collagenase perfusion method. Livers were minced and immersed in Hanks balanced salt solution at 37 °C for 30 min. Hanks solution consisted of 24 μg/mL Liberase (Roche Diagnostics, GmbH, Penzberg, Germany) and 1.6 U/mL DNase I (Roche Diagnostics). After that, the liver pieces were homogenized and strained through a 40 μm filter. The filtrate was then plated in RPMI containing 2% fetal calf serum on regular non-tissue medium-treated Petri dishes overnight. Non-adherent cells were washed off, and the adherent cells used for quantitation after staining with F480 monoclonal (eBioscience, San Diego, CA, United States), CD11b monoclonal (Millipore, Billerica, MA, United States) and CD206 polyclonal (eBioscience) antibodies, respectively. The purity of the macrophages was estimated by flow cytometry using F480 monoclonal antibody, and purity of > 80% was obtained. A phagocytosis test with FITC-labeled ovalbumin was used to identify liver macrophages. M2 macrophages were identified as F480+/CD206+.
To observe the effect of IL-4 on macrophage mobilization, an overdose (final concentration 250 μg/mL) of an IL-4 monoclonal antibody (Santa Cruz) was incubated with cultured macrophages to block IL-4.
Before carrying out co-culture of UC-MSCs and macrophages, macrophages isolated from liver tissues were activated with lipopolysaccharide (final concentration of 1 μg/mL) for 18 h. Then, 1 × 105 to 3 × 105 UC-MSCs were seeded in a 24-well plate as a supporting layer, and 1 × 105 to 3 × 105 macrophages were added to the plates.
All statistical analyses were performed using the SPSS Version 16 software. Differences were considered statistically significant at P < 0.05. Values are presented as the mean ± SD. For semi-quantitative analysis of histological staging, non-parametric tests (Wilcoxon test) were used; other statistical analyses were performed using an unpaired Student’s t-test.
MSCs were long spindle-shaped cells (Figure 1A) with potent proliferation activity. MSCs derived from the umbilical cord did not express CD11b, CD19, CD34, CD45 and HLA-DR (0.61%), which was confirmed by flow cytometry. However, high numbers of cells expressing CD44 (99.99%), CD73 (99.98%), CD90 (99.99%) and CD105 (99.97%) were observed (Figure 1B). In addition, MSCs had osteogenic, adipogenic, and chondrogenic capabilities (Figure 1C). These findings suggested that these cells were MSCs.
DMN is a potent hepatotoxin that causes centrilobular necrosis and nephrotoxic damage following peritoneal injection in rats. After treatment, rats were necropsied, and the right lobe of the liver was extracted from each animal for histology. When the liver tissue was processed with Masson’s trichrome staining, periportal fibrosis was observed. The fibrous septa extended into the adjacent portal tracts and the terminal hepatic venule. In these DMN-treated rats, neutrophil infiltration and massive vacuolar degeneration were observed, which indicated the successful establishment of an animal model of hepatic fibrosis (Figure 2).
Liver fibrosis is initiated by inflammation, reflected by hepatocyte injury. Serum transaminase (ALT and AST) activities are known markers of such injury. In the DMN-treated rat model, ALT and AST levels were significantly increased compared with control rats (P < 0.001). Interestingly, these enzymes were significantly reduced after UC-MSCs transfusion, compared with rats in the DMN + Vehicle group (P = 0.008). Furthermore, liver fibrosis and cholestasis were significantly reduced by transfusion of UC-MSCs in the DMN-treated rats as compared with the DMN + Vehicle group, which was characterized with shorter septa extending into lobules or portal-portal septa (Figure 2). Densitometry measurements showed that the amount of collagen increased by more than 80-fold in DMN rats compared with normal rats (P < 0.001); after UC-MSC transfusion, this increase was reduced by about 45% compared with control rats in the DMN model (P = 0.021). These results indicated that UC-MSCs could ameliorate the hepatic fibrosis induced by DMN.
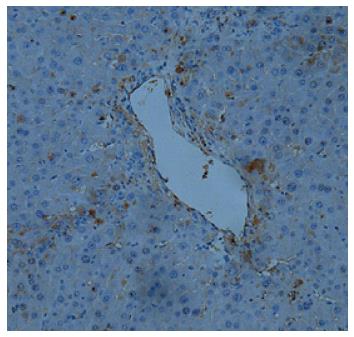
It has been reported that US-MSCs can differentiate into hepatocytes, the main cell type in the liver. We hypothesized that UC-MSCs transfused into rats could be recruited by the injured liver, differentiate into hepatocytes, and promote recovery from liver injury. To test this hypothesis, UC-MSCs were labeled with green fluorescent protein (GFP), and immunohistochemical detection was performed to assess the distribution of UC-MSCs in the liver of DMN-treated animals. As shown in Figure 3, UC-MSCs were located in the injured liver together with infiltrated inflammatory cells, indicating that UC-MSCs transfused into rats can be recruited into the injured liver.
KCs are resident hepatic macrophages that play important roles in liver physiology by secreting inflammatory factors in response to stimulation or toxic compounds. Classical (M1) and alternative (M2) macrophages are the extreme states of macrophage phenotypes. Although M1 macrophages will trigger inflammation, such inflammation can be counterbalanced by M2 macrophages. The alternatively polarized M2 macrophages facilitate the resolution of inflammation and tissue repair.
To observe the effects of UC-MSCs on KC mobilization, UC-MSCs were transfused on day 7 into rats with liver fibrosis induced by DMN. At days 7, 14 and 21, three rats were sacrificed, respectively, and KCs were isolated, identified by positive phagocytosis test, and by CD11b and F480 expression as assessed by flow cytometry (Figure 4A-C). Furthermore, after transfusion with UC-MSCs, CD206+ KCs (M2) increased significantly in a time-dependent manner; the largest number of M2 cells appeared at day 21 (Figure 4D). Given that M2 cells are CD206+ and M1 cells are CD206-, these data further confirmed that UC-MSCs transfusion could promote M1 to M2 transformation in liver fibrosis induced by DMN.
We confirmed that UC-MSC transfusion promotes the mobilization of KC in vivo. To assess the effect of UC-MSCs on KC mobilization in vitro, we isolated KCs from rats treated with DMN for three consecutive weeks, and co-cultured them with UC-MSCs. KCs isolated from the same rats and cultured alone were used as the control group. Less than 4% of CD206+ M2 cells appeared in the KCs cultured alone; meanwhile, more than 80% M2 cells appeared in KCs co-cultured with UC-MSCs (Figure 5A-D). Given the anti-fibrogenic effect of M2 cells and the pro-fibrogenic effect of M1 cells, our data showed that UC-MSCs promoted KC mobilization not only in vivo, but also in vitro.
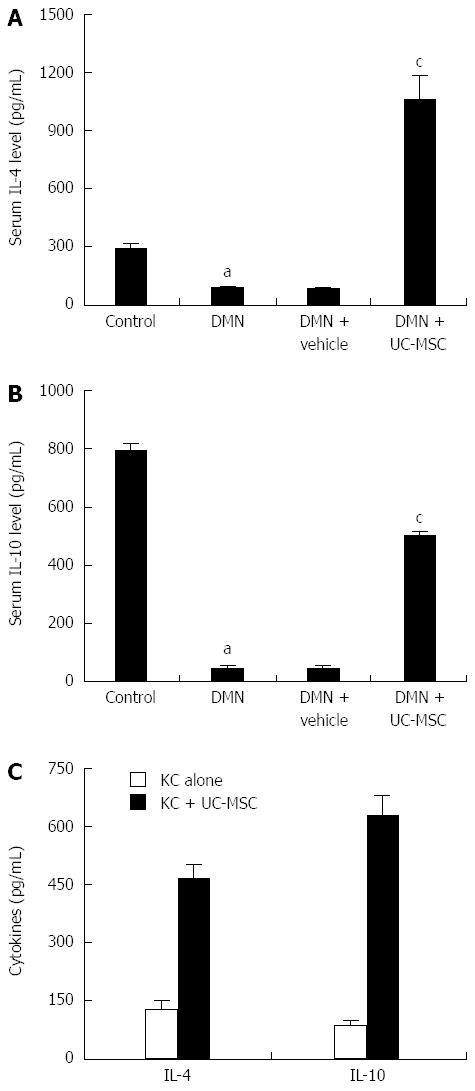
After transfusion of UC-MSCs into DMN rats, serum IL-4 and IL-10 levels were assessed by ELISA. Interestingly, IL-4 and IL-10 levels declined significantly after DMN treatment, and this effect was reversed by UC-MSCs transfusion. Serum IL-4 and IL-10 levels were significantly elevated in DMN-treated rats transfused with UC-MSCs compared with animals administered with DMN alone (Figure 6A and B).
In vitro, we co-cultured UC-MSCs with KCs isolated from DMN rats, and cell culture supernatants were collected for ELISA: IL-4 and IL-10 levels in KCs plus UC-MSCs were, respectively, more than 3- and 4-fold higher than the values obtained for KCs cultured alone (Figure 6C).
The consistent results between in vivo and in vitro data mean that UC-MSCs promote IL-4 and IL-10 production in KCs, which subsequently resulted in KC mobilization.
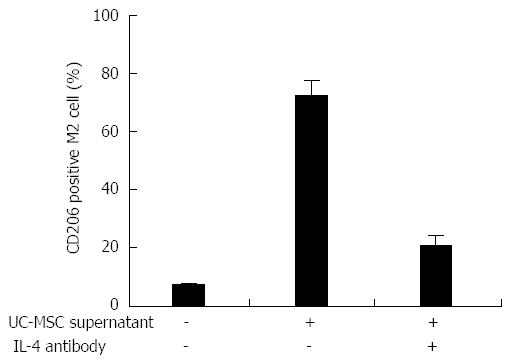
We detected IL-4 production in cell culture medium of UC-MSCs by ELISA (data not shown), and confirmed that UC-MSCs promote KC mobilization, accompanied with elevated IL-4 production; therefore, we hypothesized that UC-MSCs secrete IL-4, which participates in KC mobilization. To test this, KCs were isolated from DMN-treated rats, and flow cytometry analysis showed that about 6% KC were CD206+ M2 cells; when UC-MSCs were added into the culture medium, the proportion of M2 cells increased to more than 70%. Interestingly, anti-IL-4 antibody treatment blocked the UC-MSCs effect and less than 30% of KCs transformed into M2 (Figure 7).
As a heterogeneous population of cells, MSCs have the potential for multilineage differentiation. They can be isolated from various tissues, such as blood, muscle, adipose tissue, trabecular bone and even skin. Bone marrow mesenchymal stem cells (BMSCs) can differentiate into a variety of liver cells, under appropriate culture conditions[17-19]. Recent studies have demonstrated that BMSCs are useful to treat liver fibrosis and do not suffer from allograft rejection. BMSCs infusion is beneficial not only to ameliorate liver fibrosis, but also to reverse fulminant hepatic failure, which has been confirmed in rat models[20]. Many clinical studies have indicated that BMSCs are safe and effective in clinical studies. They can alleviate end-stage liver disease, and improve symptoms and liver function[21-23]. However, the invasive nature of bone marrow aspiration might limit their clinical application. Paradoxically, some studies indicated that BMSCs have the potential to promote fibrosis[24-26]. Thus, the application of BMSCs as a therapy for liver fibrosis remains controversial.
UC-MSCs are of particular interest because of their relatively easy accessibility and abundant source, making them a good substitute for BMSCs in future clinical studies. Indeed, UC-MSCs are reported to have greater proliferative capacity, lower immunological reactivity and lower risk of graft-vs-host disease compared with BMSCs[5]. Rosland et al[27] reported that the rate of BMSCs spontaneous malignant transformation during culture is 45.8% (11 of 24), concluding that spontaneous malignant transformation might represent a biohazard in long-term ex vivo expansion of BMSCs. Similar properties of BMSCs from both human and murine origins have been reported in other studies[28-30]. Interestingly, Tang et al[31] showed that human UC-MSCs propagating in continuous culture ultimately enter senescence and are not susceptible to spontaneous malignant transformation, suggesting the biosafety of expanding human UC-MSCs in vitro for use in regenerative medicine.
Recently, it has been reported that transfusion of UC-MSCs could improve significantly the symptoms of primary biliary cirrhosis, with few adverse effects[32]. These findings suggested that UC-MSCs carry a great promise for the treatment of chronic liver disease.
MSCs have a high differentiation potential both in vitro and in vivo[17,33]. Barry et al[33] were the first to describe the hepatic potential of MSCs, and MSC transfusion could be a useful strategy for cellular therapy in liver fibrosis. Oh et al[34] found that two liver-specific proteins (α-feto protein and albumin) are expressed in rat bone marrow cell culture. Other studies also reported that MSCs can express albumin in vitro[3,4]. In the present study, green fluorescent protein (GFP) labeled UC-MSCs were transfused into rats with liver fibrosis induced by DMN. Abundant GFP positive cells were observed in DMN-induced livers 1 wk after UC-MSCs transfusion, mostly around areas with inflammatory cell infiltration. We inferred that the UC-MSCs located in the injured liver had differentiated into hepatocytes, subsequently ameliorating the DMN-induced liver injury in rats, which further supported the concept of cell therapy for the treatment of liver injury.
The therapeutic effects of stem cell transplantation on liver fibrosis and cirrhosis have been widely investigated in mice and humans; however, the underlying mechanisms remain obscure. Depending on the cytokine composition in the tissue environment, macrophages differentiate into distinct subclasses. Classically activated macrophages (M1) differentiate in presence of Th1 cytokines (e.g., IFN-γ), or bacterial products such as lipopolysaccharide. M1 can trigger proinflammatory responses, which are needed to kill intracellular pathogens[35]. The alternatively activated macrophages (M2) are induced by Th2 cytokines, such as IL-4 and IL-13. They are associated with Th2-type immune responses, for example, in helminth parasite infections[35], and play an important role in protecting the organism against tissue damage[36] during inflammation. It has been reported that mobilization of KCs from the M1 phenotype to the M2 phenotype might promote recovery from liver injury[16]. However, we still know little about the mechanisms underlying the acquisition of the M2 phenotype.
Our data showed that UC-MSCs may promote M1 macrophage mobilization in liver fibrosis induced by DMN not only in vivo, but also in vitro, indicating that the alleviation of liver fibrosis after UC-MSCs transfusion is partly attributed to an increase in the conversion of M1 macrophages into M2 macrophages.
A recent study by Wan et al[37] reported that polarized M2 macrophages promote M1 macrophage apoptosis via IL-4, uncovering a novel mechanism for M1/M2 balance regulation that relies on M2-induced M1 macrophage apoptosis.
The cytokines produced by different types of macrophages are very important for the development and function of both innate and adaptive immune responses. IL-10 is secreted by M2 macrophages[38], and its anti-inflammatory effect has been reported in various models of acute and chronic liver injury[39,40]. Furthermore, Suh et al[41] demonstrated that bone marrow cells can alleviate inflammation and fibrosis through the expression of IL-10. In addition, previous data[37] identified IL-10 as the mediator of the apoptosis of M1 KCs induced by their M2 counterparts, by showing that anti-IL-10 antibodies blunt the pro-apoptotic effects of IL-4 in conditioned media. Herein, we demonstrated increased M1 macrophage mobilization and improvement of liver fibrosis following UC-MSCs transfusion; elevated IL10 production in the plasma and liver were also found. Therefore, it is plausible that UC-MSCs transfusion improves liver fibrosis via the following mechanism: UC-MSCs promote M1 macrophage conversion into M2 macrophages, which secrete IL-10 and subsequently increase M1 macrophage apoptosis.
IL-4 is one of the markers of M2 macrophages. Milner et al[42] found that IL-4 production leads to substantial M2 macrophage accumulation in the liver. Recent evidence has suggested an association between M2 macrophage activity and restriction of fibrosis[36,43]. This likely explains the observation that IL-4 receptor-deficient mice cannot exhibit an intact alternative activation in KCs and will increase liver inflammation, fibrosis and death during acute schistosomiasis by Schistosoma mansoni[36]. By contrast, IL-4-activated M2 macrophages improved both steatohepatitis and fibrosis during experimental and human nonalcoholic fatty liver disease[44,45].
In this study, increased M2 macrophages in the liver treated with DMN were observed. In addition, reduced liver inflammation and liver fibrosis occurred following UC-MSCs transfusion. Finally, elevated IL-4 levels in serum and liver were also noted. These findings may help further understand how UC-MSCs mediate the repair process during liver damage, which could be associated with increased IL-4 production, accompanied by subsequent M1 macrophage mobilization into M2 counterparts.
IL-4 is also regarded as a proinflammatory cytokine, with direct cytotoxic effects on hepatocytes, as shown in a previous study by Guillot et al[46], in which lethal hepatitis was induced by transduction with recombinant adenoviruses coding IL-4 (AdrIL-4). The mortality of lethal hepatitis induced by AdrIL-4 transduction was dose-dependent in that study. The observed hepatotoxicity and lack of macrophage activation with AdrIL-4 transduction differ from what we observed in our study. Excessive elevation of IL-4 levels in the liver could explain the difference. Milner et al[42] considered that the hepatotoxicity of AdrIL-4 might be attributed to the adenovirus vector itself.
This study is the first to assess the therapeutic value of UC-MSCs in an animal model of liver fibrosis. We found that UC-MSCs transfusion by tail vein presents satisfactory results with regard to improved liver injury and alleviated liver fibrosis. Our results suggested that the therapeutic effects of UC-MSCs on liver fibrosis rely on the activation of hepatic macrophages (KCs), which provides a partial explanation of the mechanisms of UC-MSCs-mediated therapeutic benefit in liver disease. We also found that the therapeutic effects of UC-MSCs were, at least in part, caused by their upregulation of IL-10 and IL-4 in a well-known rat model of liver fibrosis. The application of UC-MSCs might provide a powerful new tool for cell therapy of liver fibrosis.
Umbilical cord-derived mesenchymal stem cells (UC-MSCs) possess an excellent proliferative potential. Their low immunogenicity and ease of preparation provide these cells with a sound basis for their application in future clinical studies.
Currently, stem cell therapy is considered a promising treatment for various liver diseases. Increasing evidence suggests that MSCs contribute to the direct production of new hepatocytes. Among MSCs, UC-MSCs possess an excellent proliferative potential. Previous studies have shown that UC-MSCs are a well-tolerated therapy. They have the potential to improve liver function and reduce ascites and mortality, especially in hepatitis B virus patients with decompensated liver cirrhosis and liver failure.
This study was the first to assess the therapeutic value of UC-MSCs in an animal model of liver fibrosis. UC-MSCs transfusion could improve liver injury and alleviated liver fibrosis. The therapeutic effects of UC-MSCs on liver fibrosis rely on the activation of hepatic macrophages (Kupffer cells, KCs), which probably delineate partly the mechanisms of UC-MSCs-mediated therapeutic benefit in liver diseases. The authors also found that the therapeutic effects of UC-MSCs were, at least in part, caused their upregulation of IL-10 and IL-4, in a well-known rat model of liver fibrosis.
The application of UC-MSCs might provide a powerful new tool for cell therapy of liver fibrosis.
Transforming growth factor β1 (TGF-β1) is a potent fibrogenic cytokine, playing an important role in the activation of fibrogenic myofibroblasts. In fibrosis, its major source is the KCs (liver resident macrophages). Increased levels of TGF-β1 are responsible for scar tissue formation. Anti-smooth muscle α-actin is a marker of activated hepatic stellate cells, and HSCs play key roles in the pathogenesis of liver fibrosis.
This paper adds to the existing understanding of the use of stem cells to treat liver fibrosis.
P- Reviewer: Jamall IS S- Editor: Yu J L- Editor: Stewart G E- Editor: Wang CH
| 1. | Fallowfield JA. Therapeutic targets in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G709-G715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Pai M, Spalding D, Xi F, Habib N. Autologous bone marrow stem cells in the treatment of chronic liver disease. Int J Hepatol. 2012;2012:307165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Ali G, Masoud MS. Bone marrow cells ameliorate liver fibrosis and express albumin after transplantation in CCl4-induced fibrotic liver. Saudi J Gastroenterol. 2012;18:263-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275-1284. [PubMed] |
| 5. | Hwang WY, Samuel M, Tan D, Koh LP, Lim W, Linn YC. A meta-analysis of unrelated donor umbilical cord blood transplantation versus unrelated donor bone marrow transplantation in adult and pediatric patients. Biol Blood Marrow Transplant. 2007;13:444-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J, Chen L, Lv S, Li Y, Yu S. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27 Suppl 2:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 7. | Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, Zhang A, Shi J, Chen L, Lv S. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 273] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1554] [Article Influence: 86.3] [Reference Citation Analysis (1)] |
| 9. | Sakaguchi S, Takahashi S, Sasaki T, Kumagai T, Nagata K. Progression of alcoholic and non-alcoholic steatohepatitis: common metabolic aspects of innate immune system and oxidative stress. Drug Metab Pharmacokinet. 2011;26:30-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Louvet A, Teixeira-Clerc F, Chobert MN, Deveaux V, Pavoine C, Zimmer A, Pecker F, Mallat A, Lotersztajn S. Cannabinoid CB2 receptors protect against alcoholic liver disease by regulating Kupffer cell polarization in mice. Hepatology. 2011;54:1217-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 205] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 11. | Ramadori G, Moriconi F, Malik I, Dudas J. Physiology and pathophysiology of liver inflammation, damage and repair. J Physiol Pharmacol. 2008;59 Suppl 1:107-117. [PubMed] |
| 12. | Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2093] [Cited by in RCA: 2329] [Article Influence: 137.0] [Reference Citation Analysis (0)] |
| 13. | Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1126] [Cited by in RCA: 1188] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 14. | Mitchell C, Couton D, Couty JP, Anson M, Crain AM, Bizet V, Rénia L, Pol S, Mallet V, Gilgenkrantz H. Dual role of CCR2 in the constitution and the resolution of liver fibrosis in mice. Am J Pathol. 2009;174:1766-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Xidakis C, Ljumovic D, Manousou P, Notas G, Valatas V, Kolios G, Kouroumalis E. Production of pro- and anti-fibrotic agents by rat Kupffer cells; the effect of octreotide. Dig Dis Sci. 2005;50:935-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3290] [Cited by in RCA: 3108] [Article Influence: 207.2] [Reference Citation Analysis (0)] |
| 17. | Newsome PN, Johannessen I, Boyle S, Dalakas E, McAulay KA, Samuel K, Rae F, Forrester L, Turner ML, Hayes PC. Human cord blood-derived cells can differentiate into hepatocytes in the mouse liver with no evidence of cellular fusion. Gastroenterology. 2003;124:1891-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 200] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Houlihan DD, Newsome PN. Critical review of clinical trials of bone marrow stem cells in liver disease. Gastroenterology. 2008;135:438-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Zhao Q, Ren H, Zhu D, Han Z. Stem/progenitor cells in liver injury repair and regeneration. Biol Cell. 2009;101:557-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Parekkadan B, van Poll D, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2:e941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 407] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 21. | Gaia S, Smedile A, Omedè P, Olivero A, Sanavio F, Balzola F, Ottobrelli A, Abate ML, Marzano A, Rizzetto M. Feasibility and safety of G-CSF administration to induce bone marrow-derived cells mobilization in patients with end stage liver disease. J Hepatol. 2006;45:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 23. | Zheng L, Chu J, Shi Y, Zhou X, Tan L, Li Q, Cui L, Han Z, Han Y, Fan D. Bone marrow-derived stem cells ameliorate hepatic fibrosis by down-regulating interleukin-17. Cell Biosci. 2013;3:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Li C, Kong Y, Wang H, Wang S, Yu H, Liu X, Yang L, Jiang X, Li L, Li L. Homing of bone marrow mesenchymal stem cells mediated by sphingosine 1-phosphate contributes to liver fibrosis. J Hepatol. 2009;50:1174-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 25. | Yang L, Chang N, Liu X, Han Z, Zhu T, Li C, Yang L, Li L. Bone marrow-derived mesenchymal stem cells differentiate to hepatic myofibroblasts by transforming growth factor-β1 via sphingosine kinase/sphingosine 1-phosphate (S1P)/S1P receptor axis. Am J Pathol. 2012;181:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Baertschiger RM, Serre-Beinier V, Morel P, Bosco D, Peyrou M, Clément S, Sgroi A, Kaelin A, Buhler LH, Gonelle-Gispert C. Fibrogenic potential of human multipotent mesenchymal stromal cells in injured liver. PLoS One. 2009;4:e6657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lønning PE. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331-5339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 755] [Reference Citation Analysis (0)] |
| 28. | Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 430] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 29. | Li H, Fan X, Kovi RC, Jo Y, Moquin B, Konz R, Stoicov C, Kurt-Jones E, Grossman SR, Lyle S. Spontaneous expression of embryonic factors and p53 point mutations in aged mesenchymal stem cells: a model of age-related tumorigenesis in mice. Cancer Res. 2007;67:10889-10898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 30. | Popov BV, Petrov NS, Mikhaĭlov VM, Tomilin AN, Alekseenko LL, Grinchuk TM, Zaĭchik AM. [Spontaneous transformation and immortalization of mesenchymal stem cells in vitro]. Tsitologiia. 2009;51:91-102. [PubMed] |
| 31. | Tang Q, Chen Q, Lai X, Liu S, Chen Y, Zheng Z, Xie Q, Maldonado M, Cai Z, Qin S. Malignant transformation potentials of human umbilical cord mesenchymal stem cells both spontaneously and via 3-methycholanthrene induction. PLoS One. 2013;8:e81844. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Wang L, Li J, Liu H, Li Y, Fu J, Sun Y, Xu R, Lin H, Wang S, Lv S. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28 Suppl 1:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 33. | Barry FP, Murphy JM. Mesenchymal stem cells: clinical applications and biological characterization. Int J Biochem Cell Biol. 2004;36:568-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1203] [Cited by in RCA: 1173] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 34. | Oh SH, Miyazaki M, Kouchi H, Inoue Y, Sakaguchi M, Tsuji T, Shima N, Higashio K, Namba M. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem Biophys Res Commun. 2000;279:500-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 154] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 35. | Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4403] [Cited by in RCA: 4593] [Article Influence: 208.8] [Reference Citation Analysis (0)] |
| 36. | Herbert DR, Hölscher C, Mohrs M, Arendse B, Schwegmann A, Radwanska M, Leeto M, Kirsch R, Hall P, Mossmann H. Alternative macrophage activation is essential for survival during schistosomiasis and downmodulates T helper 1 responses and immunopathology. Immunity. 2004;20:623-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 596] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 37. | Wan J, Benkdane M, Teixeira-Clerc F, Bonnafous S, Louvet A, Lafdil F, Pecker F, Tran A, Gual P, Mallat A. M2 Kupffer cells promote M1 Kupffer cell apoptosis: a protective mechanism against alcoholic and nonalcoholic fatty liver disease. Hepatology. 2014;59:130-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 436] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 38. | Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3696] [Cited by in RCA: 4711] [Article Influence: 362.4] [Reference Citation Analysis (1)] |
| 39. | Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4053] [Cited by in RCA: 3856] [Article Influence: 275.4] [Reference Citation Analysis (0)] |
| 40. | Hill DB, D’Souza NB, Lee EY, Burikhanov R, Deaciuc IV, de Villiers WJ. A role for interleukin-10 in alcohol-induced liver sensitization to bacterial lipopolysaccharide. Alcohol Clin Exp Res. 2002;26:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Suh YG, Kim JK, Byun JS, Yi HS, Lee YS, Eun HS, Kim SY, Han KH, Lee KS, Duester G. CD11b(+) Gr1(+) bone marrow cells ameliorate liver fibrosis by producing interleukin-10 in mice. Hepatology. 2012;56:1902-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 42. | Milner JD, Orekov T, Ward JM, Cheng L, Torres-Velez F, Junttila I, Sun G, Buller M, Morris SC, Finkelman FD. Sustained IL-4 exposure leads to a novel pathway for hemophagocytosis, inflammation, and tissue macrophage accumulation. Blood. 2010;116:2476-2483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, El Kasmi KC, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA. Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog. 2009;5:e1000371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 625] [Cited by in RCA: 654] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 44. | Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell Metab. 2008;7:496-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 726] [Cited by in RCA: 706] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 45. | Baffy G. Kupffer cells in non-alcoholic fatty liver disease: the emerging view. J Hepatol. 2009;51:212-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 371] [Cited by in RCA: 374] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 46. | Guillot C, Coathalem H, Chetritt J, David A, Lowenstein P, Gilbert E, Tesson L, van Rooijen N, Cuturi MC, Soulillou JP. Lethal hepatitis after gene transfer of IL-4 in the liver is independent of immune responses and dependent on apoptosis of hepatocytes: a rodent model of IL-4-induced hepatitis. J Immunol. 2001;166:5225-5235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |