Published online Jul 14, 2016. doi: 10.3748/wjg.v22.i26.6027
Peer-review started: March 26, 2016
First decision: May 12, 2016
Revised: June 2, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: July 14, 2016
Processing time: 102 Days and 20.9 Hours
AIM: To evaluate the role of microRNA (miR)-146a, -155 and -122 in the duodenal mucosa of pediatric patients with Crohn’s disease (CD) and the effect of transforming growth factor-β (TGF-β) on these miRs in duodenal epithelial and fibroblast cells.
METHODS: Formalin-fixed, paraffin-embedded biopsies derived from the macroscopically inflamed (CD inflamed: n = 10) and intact (CD intact: n = 10) duodenal mucosa of pediatric CD patients and control children (C: n = 10) were examined. Expression of miR-146a, -155 and -122 was determined by real-time polymerase-chain reaction (PCR). The expression of the above miRs was investigated in recombinant human TGF-β (1 nmol/L, 24 h) or vehicle treated small intestinal epithelial cells (CCL-241) and primary duodenal fibroblast cells derived from healthy children as well.
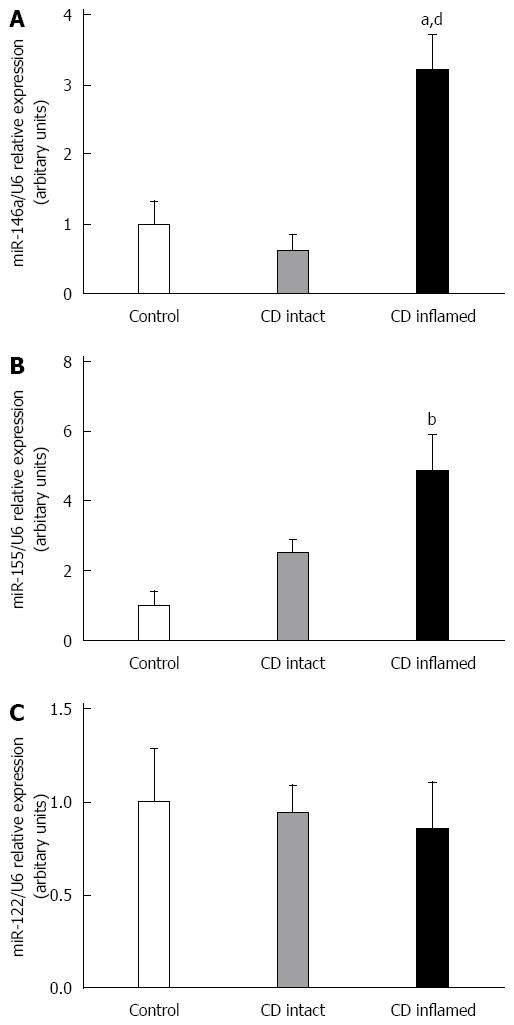
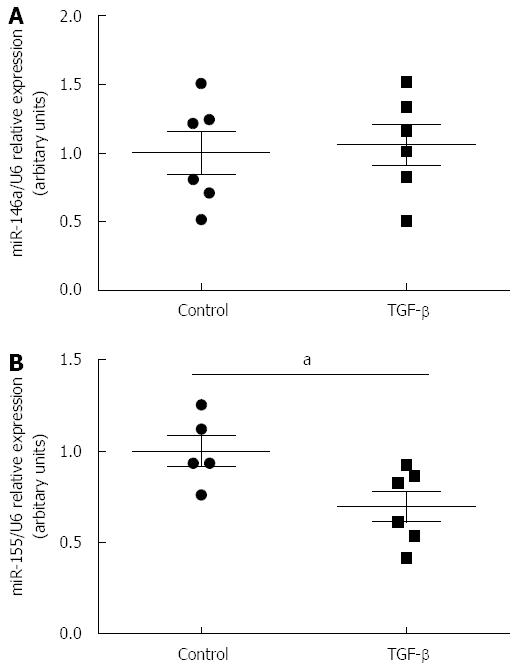
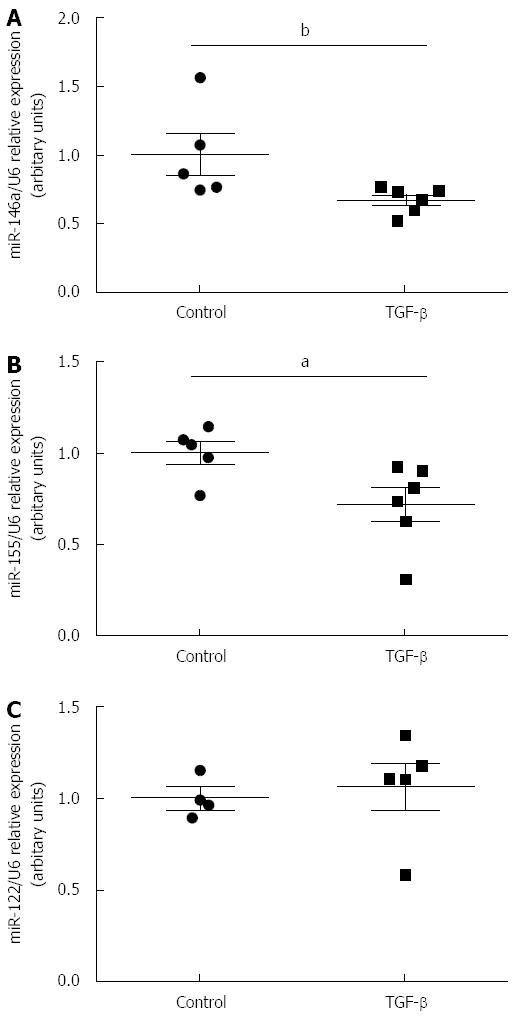
RESULTS: Expression of miR-146a was significantly higher in the inflamed duodenal mucosa compared to the intact duodenal mucosa of children with CD (CD inflamed: 3.21 ± 0.50 vs CD intact: 0.62 ± 0.26, P≤ 0.01) and to the control group (CD inflamed: 3.21 ± 0.50 vs C: 1.00 ± 0.33, P≤ 0.05). The expression of miR-155 was significantly increased in the inflamed region of the duodenum compared to the control group (CD inflamed: 4.87 ± 1.02 vs Control: 1.00 ± 0.40, P≤ 0.001). The expression of miR-122 was unchanged in the inflamed or intact mucosa of CD patients compared to controls. TGF-β treatment significantly decreased the expression of miR-155 in small intestinal epithelial cells (TGF-β: 0.7 ± 0.083 vs Control: 1 ± 0.09, P≤ 0.05) and also the expression of miR-146a (TGF-β: 0.67 ± 0.04 vs Control: 1 ± 0.15, P≤ 0.01) and miR-155 (TGF-β: 0.72 ± 0.09 vs Control: 1 ± 0.06, P≤ 0.05) in primary duodenal fibroblasts compared to corresponding vehicle treated controls. TGF-β treatment did not influence the expression of miR-122.
CONCLUSION: The elevated expression of miR-146a and -155 in the inflamed duodenal mucosa of CD patients suggests the role of these miRs in the pathomechanism of inflammatory bowel disease. Anti-inflammatory TGF-β plays an important role in the regulation of the expression of these miRs.
Core tip: Recent evidence suggests that besides the genetic basis, epigenetic factors including microRNAs (miRs) also act as potent inflammatory modulators during the pathogenesis of inflammatory bowel disease. MiR expression in the upper-gastrointestinal tract of pediatric patients with Crohn’s disease (CD) has not yet been analyzed. Moreover, the relation of transforming growth factor-β, playing a prominent role in the pathomechanism of CD, to miRs in this setting is also unknown. The description of precise miR patterns specific for the different segments of the gastrointestinal tract may contribute to the introduction of novel diagnostic markers and to the identification of potential therapeutic targets.
- Citation: Szűcs D, Béres NJ, Rokonay R, Boros K, Borka K, Kiss Z, Arató A, Szabó AJ, Vannay &, Sziksz E, Bereczki C, Veres G. Increased duodenal expression of miR-146a and -155 in pediatric Crohn’s disease. World J Gastroenterol 2016; 22(26): 6027-6035
- URL: https://www.wjgnet.com/1007-9327/full/v22/i26/6027.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i26.6027
Crohn’s disease (CD) is a chronic immune-mediated disorder, which is frequently characterized by the appearance of lesions in the upper gastrointestinal (GI) tract, primarily in pediatric patients[1,2]. In adults, upper GI lesions in the cardiac area with bamboo joint-like appearance[3] are regarded to be significant risk factors for the progression of the disease from the inflammatory to stenotic or penetration form of CD[4].
In the pathogenesis of CD, epigenetic factors including microRNAs (miRs) have come into focus as potent modulators of the progression of the disease. An increasing number of studies investigates the role of these short single-stranded RNAs in inflammatory bowel disease (IBD)[5-11]; however, the expression profile of miRs in the upper GI region of CD patients is completely unknown.
The aim of the present study was to investigate the mucosal expression of miR-146a, -155 and -122 in the upper GI tract of children with CD, and to examine the effect of the anti-inflammatory transforming growth factor (TGF)-β on the expression of the investigated miRs. These miRs affect a number of key biological functions involved in the pathomechanism of CD, including inflammatory response, intracellular signaling cascades and response to the presence of bacteria[5].
However, upper GI tract endoscopy is an important tool to diagnose CD in pediatric patients, recently there have been no specific markers to definitely distinguish it from other GI diseases with erosions, ulcerations [Helicobacter pylori (H. pylori) infection, peptic ulcer caused by an infection or medication, or eosinophil gastritis], and disorders with granulomas (sarcoidosis, Mycobacterium tuberculosis). Therefore, we suppose that our results may contribute to the identification of potential novel biomarkers or therapeutic targets of CD.
CD was diagnosed according to the Porto criteria[12,13]. The disease activity score was assessed regarding the Pediatric CD Activity Index (PCDAI). Control children were referred to the outpatient clinic due to recurrent abdominal pain and GI symptoms to exclude organic diseases. Esophago-gastro-duodenoscopy was part of their diagnostic procedure showing normal macroscopic appearance and histology. Duodenal biopsy samples were taken from different patients, macroscopically inflamed (CD inflamed: n = 10) and non-inflamed (CD intact: n = 10) regions of the duodenal mucosa from children with CD and controls (C: n = 10). Biopsies were immediately fixed in buffered formaldehyde and embedded into paraffin (PF). Clinical characteristics of patients with CD and controls are shown in Table 1. Written informed consent was obtained from the parents prior to the procedure, and the study was approved by the Semmelweis University Regional and Institutional Research Ethics Committee (TUKEB No.: 10408/2012).
| Control | CD intact | CD inflamed | |
| n | 10 | 10 | 10 |
| Age | 8.75 ± 2.36 | 12.4 ± 1.52 | 12.11 ± 1.63 |
| Gender | 2f/8m | 5f/5m | 2f/8m |
| BMI (kg/m2) | 20.07 ± 2.64 | 14.83 ± 0.8 | 18.64 ± 1.81 |
| PCDAI | 0 | 21.94 ± 4.37c | 16.11 ± 3.51b |
| Iron (μmol/L) | 15.83 ± 2.53 | 4.89 ± 0.84b | 8.33 ± 2.61a |
| TIBC (μmol/L) | 60.4 ± 2.71 | 48.5 ± 2.64 | 59.71 ± 8.70 |
| Albumin (g/L) | 47.33 ± 1.31 | 38.67 ± 1.62 | 48.4 ± 5.56 |
| Hemoglobin (g/L) | 132.8 ± 6.72 | 113.1 ± 4.02a | 124.4 ± 5.93 |
| Hematocrit (%) | 0.38 ± 0.02 | 0.344 ± 0.01 | 0.37 ± 0.02 |
| Platelet count (Giga/L) | 365.5 ± 30.29 | 517.1 ± 34.62a | 416.7 ± 63.77 |
Normal small intestinal epithelial cells (CCL-241, American Type Culture Collection, Manassas, VA, United States) were grown in HybriCare medium (American Type Culture Collection, Manassas, VA, United States) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, United States), 30 ng/mL epidermal growth factor (R&D Systems, Minneapolis, MN, United States), and 1% Penicillin and Streptomycin mixture (Sigma-Aldrich Co., St. Louis, MO, United States). Epithelial cells were grown under standard cell culture conditions (37 °C, humidified, 5% CO2/95% air environment).
Based on the previously described method, primary duodenal fibroblast cells were freshly isolated[14] from the duodenal mucosa of healthy children. Briefly the biopsies were washed in phosphate-buffered saline (PBS) and homogenized in 1 mg/mL collagenase content PBS (Sigma-Aldrich Co., St. Louis, MO, United States). Cells were grown in Dulbecco’s Modified Eagle Medium supplemented with 1% FBS (Invitrogen, Carlsbad, CA, United States) and 1% Penicillin and Streptomycin mixture. Cells were grown under standard cell culture conditions (37 °C, humidified, 5% CO2/95% air environment) until a confluent monolayer was obtained. During culturing, the unattached cells were removed after every 24 h culture period.
Epithelial and primary fibroblast cells were seeded into 6-well plates at a density of 5 × 105 cells/well and treated for 24 h with recombinant human TGF-β (R&D Systems, Minneapolis, MN, United States) at a concentration of 1 nmol/L or vehicle only for control cells.
Total RNA was isolated from formalin-fixed, paraffin-embedded biopsies using RNeasy Minikit (Qiagen, Düsseldorf, Germany) after removing paraffin from the samples according to the instructions of the manufacturer. All contaminants were efficiently washed away, DNase was used to remove DNA from the samples using on-column DNase treatment. Concentrated RNA was filtrated using RNeasy MinElute spin columns (Qiagen, Düsseldorf, Germany). RNA was eluted in 30 μL water.
Total RNA of the intestinal epithelial and fibroblast cells was isolated by the Quick-RNA MiniPrep isolation kit (Zymo Research, Irvine, CA, United States) according to the instructions of the manufacturer. RNA Lysis Buffer purified the RNA using Zymo-Spin™ Columns. All contaminants were washed away (RNA Prep and Wash Buffer) and DNase was used to remove the DNA from the samples. RNA was eluted in 30 μL water and used further immediately.
The total RNA was reversely transcribed by the TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems - ABI, Foster City, CA, United States). TaqMan MicroRNA Assay (Applied Biosystems - ABI, Foster City, CA, United States) was used to quantify individual microRNA levels with real-time Reverse transcription and real-time polymerase-chain-reaction (RT-PCR) on a LightCycler 480 instrument (Roche Diagnostics, Basel, Switzerland). Reactions were performed in triplicates. Relative expression of miRs was determined by the 2ΔCq method using U6 as an internal control.
Statistical analysis was performed by Graphpad statistical software package (Graphpad Software, La Jolla, CA, United States). Normality was tested by the Shapiro-Wilk test. Analysis was based on the Mann-Whitney U-test, Kruskal-Wallis-test, Analysis of variance (ANOVA) and Dunn’s Post-Hoc test, and P≤ 0.05 was considered as statistically significant. Data were presented as mean ± SE.
The expression of miR-146a was significantly higher in the inflamed duodenal mucosa of children with CD compared to the intact mucosa (CD inflamed: 3.21 ± 0.50 vs CD intact: 0.62 ± 0.26, P≤ 0.01) and controls (CD inflamed: 3.21 ± 0.50 vs Control: 1.00 ± 0.33, P≤ 0.05). There was no significant difference between the uninflamed group and the control one (CD intact vs Control: P = N.S.) (Figure 1A).
miR-155 showed significantly elevated expression in the inflamed region of the duodenal mucosa of CD patients compared to the control group (CD inflamed: 4.87 ± 1.02 vs Control: 1.00 ± 0.40, P≤ 0.001). There was no significant difference between the uninflamed group and the control one (CD intact: 2.50 ± 0.38 vs Control: 1.00 ± 0.40, P = N.S.) (Figure 1B).
There was no significant difference in the expression of miR-122 between the CD and control groups (CD inflamed: 0.86 ± 0.25, CD intact: 0.96 ± 0.14, Control: 1.00 ± 0.28, P = N.S.) (Figure 1C).
TGF-β had no effect on the expression of miR-146a (P = N.S., Figure 2A); however, it decreased significantly the expression of miR-155 in CCL-241 small intestinal epithelial cells (TGF-β: 0.7 ± 0.083 vs Control: 1 ± 0.09, P≤ 0.05) (Figure 2B). No miR-122 was detected in the small intestinal epithelial cells.
TGF-β significantly decreased the expression of miR-146a (TGF-β: 0.67 ± 0.04 vs Control: 1 ± 0.15, P≤ 0.01) (Figure 3A) and miR-155 (TGF-β: 0.72 ± 0.09 vs Control: 1 ± 0.06, P≤ 0.05) (Figure 3B) in duodenal fibroblasts compared to vehicle treated control cells. There was no difference in the expression of miR-122 when TGF-β was administered compared to the control group (P = N.S., Figure 3C).
In the present study, we demonstrated the elevated expression of miR-146a and -155 in the macroscopically inflamed duodenal mucosa of newly diagnosed, treatment-naive pediatric patients with CD compared to the control group for the first time. These results are in accordance with our previous observations demonstrating the increased expression of miR-146a and -155 in the inflamed colonic region of pediatric IBD patients. However, in contrary to our earlier observations related to the colon, we found unchanged expression of miR-122 in the duodenal mucosa of children with CD[5].
According to the criteria of the “Porto” IBD Working Group of the European Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), upper GI tract endoscopy is recommended to be performed to identify lesions in all children irrespective of presence or absence of upper GI symptoms[15]. Based on the Hungarian Pediatric IBD Registry Group (HUPIR), upper GI tract abnormalities were found in 64% and 40% of children with CD and UC, respectively[16]. Characteristic findings (ulcers, erosions, aphthous lesions, and granulomas) were noted in about one third of children with CD; moreover, upper GI tract endoscopy was advantageous in establishing the final diagnosis in 9% of children with CD (diagnostic yield)[16]. However, to the best of our knowledge, there is no specific marker to clearly diagnose CD in the upper GI tract and to distinguish it from H. pylori infection, eosinophilic enteropathy or drug-induced lesions. Moreover, H. pylori-negative chronic active gastritis appears frequently in adult patients with CD, which is hard to differentiate from CD lesions[17]. Therefore, the aim of our present study was to establish a duodenum-specific miR pattern in pediatric patients with CD, which could facilitate the deeper understanding of the pathomechanism of IBD, and it may serve as a diagnostic tool in the future.
Recently miR-146a, -155 and -122 have come into focus as potential regulators of the inflammatory response, intracellular signaling cascades, regulation of cytokine production and response to bacteria, all of which play an important role in IBD. The main findings of the previous studies regarding to the expression of miR-146a, -155 and -122 in the GI tract of adult and pediatric IBD patients also confirm it. Based on these results and our present findings, we can conclude that independently of the localization of the intestinal inflammatory regions (colon, rectum, or duodenum), the expression of miR-146a and -155 was elevated in patients with CD suggesting that these miRs are rather inflammation- than region-specific[5,8,18-26]. Both in vivo and in vitro studies confirm that miR-146a and -155 have opposite effects during inflammation. While miR-155 is a promoter of inflammation, miR-146a is a mediator of immune suppressive responses[27,28].
In contrary to miR-146a and -155, different expression pattern of miR-122 has been demonstrated throughout the GI tract. Previously we observed elevated expression of miR-122 in the intact colonic region of pediatric CD patients[5], but in the present study we found no difference in the duodenal miR-122 expression between CD patients and the control ones. Chen et al[29] have found that miR-122 may decrease intestinal injury by suppressing the NOD2-induced NF-κB signaling pathway. On the contrary, overexpression of miR-122 in enterocytes have resulted in the mRNA degradation of occludin leading to disturbed tight-junction integrity and increased intestinal permeability, which are important processes in the pathogenesis of IBD[30,31].
Inflammatory factors have been shown to modulate the expression of miRs, but the potential regulators of miR-146a, -155 and -122 in IBD are largely unknown. One of the known key modulators of inflammatory responses is anti-inflammatory TGF-β[32-34].
In case of IBD, increased expression of TGF-β was demonstrated in the inflamed intestinal regions. However, due to the reduced level of phosphorylated mothers against decapentaplegic homolog (SMAD)3 and diminished complex formation with SMAD4, the TGF-β-mediated signaling is insufficient in IBD patients[35].
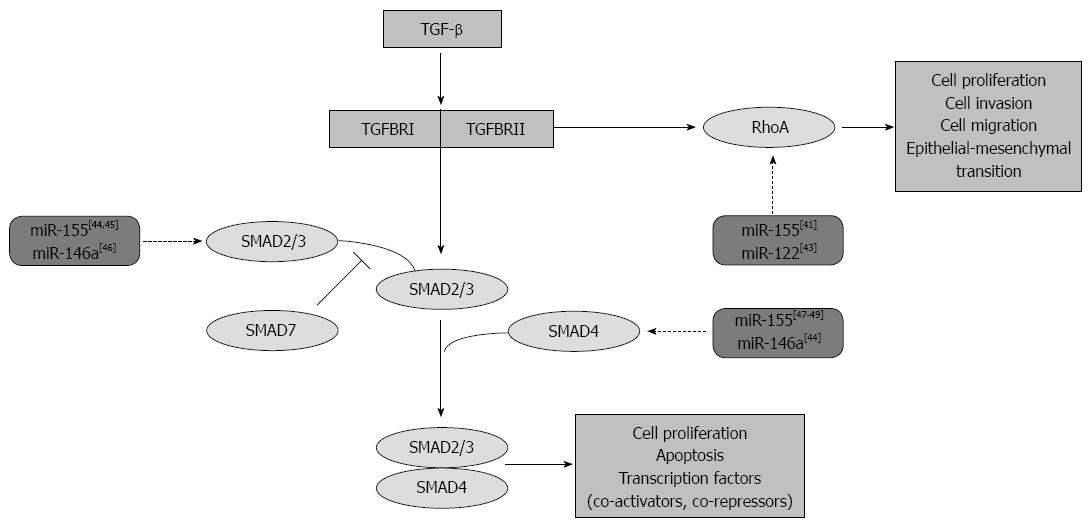
Moreover, elevated level of SMAD7, the known endogenous inhibitor of TGF-β signaling, contributes to the enhanced production of pro-inflammatory cytokines resulting in the maintenance of inflammation in IBD[36,37]. This disadvantageous effect of SMAD7 in IBD is proved by its inhibition in a human phase II study. In fact, the oral administration of SMAD7 targeting antisense oligonucleotides has led to clinical remission in 60% of adult CD patients[38]. Moreover, Rho proteins, which are also connected to the TGF-β signaling pathway are involved in the regulation of IBD. Previous studies have reported Ras homolog gene family member A (RhoA) protein activation in CD patients. RhoA is involved in inflammation through the activation of the NF-κB, IL-1β and the TNF-α pathway, regulation of cell adhesion, migration, phagocytosis, and proliferation[39,40] (Figure 4).
Based on these key effects of TGF-β in the pathomechanism of IBD, we also aimed to investigate the possible link between TGF-β and miR-146a, -155 and -122. Following TGF-β treatment, we observed downregulation of miR-155 in both small intestinal epithelial and primary duodenal fibroblast cells and reduced expression of miR-146a in duodenal fibroblasts. However, TGF-β had no effect on the expression of miR-122 either in epithelial or in fibroblast cells.
Previous studies have confirmed the effect of these miRs on the TGF-β-mediated signaling pathway[41-49] (Figure 4). In chondrocytes, fetal femur derived skeletal stem cells and gastric cancer cells miR-146a negatively regulates the expression of SMAD 2/3 and SMAD4, while in monocyte, cervical and prostate cancer cells miR-155 negatively modulates SMAD2/3, the key mediators of TGF-β-induced effects[46,47,49,50]. Moreover, the ectopic expression of miR-155 and -122 has significantly reduced the expression of RhoA indicating the role of these miRs in the TGF-β-induced epithelial-mesenchymal transition, cell migration and invasion, as well[41,43].
In conclusion, increased expression of miR-146a and -155 in the inflamed intestinal mucosa suggests their involvement in the pathomechanism of CD as inflammation-specific markers. Our results suggest that the expression of miR-146a and -155 is independent of and that of miR-122 is dependent on the localization of CD. Moreover, anti-inflammatory TGF-β is a negative regulator of miR-146a and -155 in small intestine epithelial and primary duodenal fibroblast cells. Our recent data have provided a baseline to explore the possible role of these miRs as diagnostic markers or their potential as therapeutic targets.
We are grateful to Maria Bernáth for her skillful technical assistance. The authors are grateful to Csilla Keresztes for the linguistic correction of the manuscript.
Crohn’s disease (CD) is a chronic immune-mediated disorder frequently characterized by lesions of the upper gastrointestinal (GI) tract, primarily in pediatric patients. Recently, epigenetic factors including microRNAs (miRs) have come into focus as potent modulators of the progression of the disease. An increasing number of studies investigate the role of these short single-stranded RNAs in inflammatory bowel disease (IBD); however, the expression profile of miRs in the upper GI region of pediatric CD patients is completely unknown.
In the previous study, the authors observed increased expression of miR-146a, -155 and -122 in the inflamed colonic region of pediatric IBD patients.
This is the first study investigating the mucosal expression of miR-146a, -155 and -122 in the upper GI tract of children with CD and examining the effect of anti-inflammatory transforming growth factor (TGF)-β on their expression of small intestinal epithelial and primary duodenal fibroblast cells.
Increased expression of miR-146a and -155 in the inflamed intestinal mucosa of pediatric patients with CD suggests their involvement in the pathomechanism of CD as inflammation-specific markers. Anti-inflammatory TGF-β is a negative regulator of miR-146a and -155 in the small intestinal epithelial and primary duodenal fibroblast cells. The recent data provide a baseline to explore the possible use of these miRs as diagnostic markers or their potential role as therapeutic targets.
MiRs are 19-24 nucleotide-long single-stranded RNAs involved in the regulation of gene expression at transcriptional and posttranscriptional level. MiRs play a determining regulatory role in innate and adaptive immune processes.
The authors demonstrated an interesting study based on their previous findings connected to the expression of miR-146a, -155 and -122 in the colonic mucosa of pediatric inflammatory bowel disease. It would be interesting to measure the effect of the pro-inflammatory tumor-necrosis-factor-α on the small intestine epithelial and primary duodenal fibroblast cells as a future plan.
Manuscript source: Invited manuscript
P- Reviewer: Decorti G S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Herfarth H, Rogler G. Inflammatory bowel disease. Endoscopy. 2005;37:42-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Oberhuber G, Hirsch M, Stolte M. High incidence of upper gastrointestinal tract involvement in Crohn’s disease. Virchows Arch. 1998;432:49-52. [PubMed] |
| 3. | Fujiya M, Sakatani A, Dokoshi T, Tanaka K, Ando K, Ueno N, Gotoh T, Kashima S, Tominaga M, Inaba Y. A Bamboo Joint-Like Appearance is a Characteristic Finding in the Upper Gastrointestinal Tract of Crohn’s Disease Patients: A Case-Control Study. Medicine (Baltimore). 2015;94:e1500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Magro F, Rodrigues-Pinto E, Coelho R, Andrade P, Santos-Antunes J, Lopes S, Camila-Dias C, Macedo G. Is it possible to change phenotype progression in Crohn’s disease in the era of immunomodulators? Predictive factors of phenotype progression. Am J Gastroenterol. 2014;109:1026-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | Béres NJ, Szabó D, Kocsis D, Szűcs D, Kiss Z, Müller KE, Lendvai G, Kiss A, Arató A, Sziksz E. Role of Altered Expression of miR-146a, miR-155, and miR-122 in Pediatric Patients with Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Iborra M, Bernuzzi F, Correale C, Vetrano S, Fiorino G, Beltrán B, Marabita F, Locati M, Spinelli A, Nos P. Identification of serum and tissue micro-RNA expression profiles in different stages of inflammatory bowel disease. Clin Exp Immunol. 2013;173:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Iborra M, Bernuzzi F, Invernizzi P, Danese S. MicroRNAs in autoimmunity and inflammatory bowel disease: crucial regulators in immune response. Autoimmun Rev. 2012;11:305-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 8. | Schaefer JS, Attumi T, Opekun AR, Abraham B, Hou J, Shelby H, Graham DY, Streckfus C, Klein JR. MicroRNA signatures differentiate Crohn’s disease from ulcerative colitis. BMC Immunol. 2015;16:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 145] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 9. | Ventham NT, Kennedy NA, Nimmo ER, Satsangi J. Beyond gene discovery in inflammatory bowel disease: the emerging role of epigenetics. Gastroenterology. 2013;145:293-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 10. | Chen WX, Ren LH, Shi RH. Implication of miRNAs for inflammatory bowel disease treatment: Systematic review. World J Gastrointest Pathophysiol. 2014;5:63-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 11. | Coskun M, Bjerrum JT, Seidelin JB, Nielsen OH. MicroRNAs in inflammatory bowel disease--pathogenesis, diagnostics and therapeutics. World J Gastroenterol. 2012;18:4629-4634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, Fell J, Ruemmele FM, Walters T, Sherlock M. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis. 2011;17:1314-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1216] [Cited by in RCA: 1144] [Article Influence: 81.7] [Reference Citation Analysis (0)] |
| 13. | Levine A, Koletzko S, Turner D, Escher JC, Cucchiara S, de Ridder L, Kolho KL, Veres G, Russell RK, Paerregaard A. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014;58:795-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 993] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 14. | Seymour ML, Binion DG, Compton SJ, Hollenberg MD, MacNaughton WK. Expression of proteinase-activated receptor 2 on human primary gastrointestinal myofibroblasts and stimulation of prostaglandin synthesis. Can J Physiol Pharmacol. 2005;83:605-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Ruemmele FM, Veres G, Kolho KL, Griffiths A, Levine A, Escher JC, Amil Dias J, Barabino A, Braegger CP, Bronsky J. Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn’s disease. J Crohns Colitis. 2014;8:1179-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 843] [Article Influence: 76.6] [Reference Citation Analysis (0)] |
| 16. | Kovacs M, Muller KE, Arato A, Lakatos PL, Kovacs JB, Varkonyi A, Solyom E, Polgar M, Nemes E, Guthy I. Diagnostic yield of upper endoscopy in paediatric patients with Crohn’s disease and ulcerative colitis. Subanalysis of the HUPIR registry. J Crohns Colitis. 2012;6:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | So H, Ye BD, Park YS, Kim J, Kim JS, Moon W, Lee KM, Kim YS, Keum B, Kim SE. Gastric lesions in patients with Crohn’s disease in Korea: a multicenter study. Intest Res. 2016;14:60-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Fasseu M, Tréton X, Guichard C, Pedruzzi E, Cazals-Hatem D, Richard C, Aparicio T, Daniel F, Soulé JC, Moreau R. Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS One. 2010;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 190] [Cited by in RCA: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 19. | Günaltay S, Nyhlin N, Kumawat AK, Tysk C, Bohr J, Hultgren O, Hultgren Hörnquist E. Differential expression of interleukin-1/Toll-like receptor signaling regulators in microscopic and ulcerative colitis. World J Gastroenterol. 2014;20:12249-12259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Kanaan Z, Rai SN, Eichenberger MR, Barnes C, Dworkin AM, Weller C, Cohen E, Roberts H, Keskey B, Petras RE. Differential microRNA expression tracks neoplastic progression in inflammatory bowel disease-associated colorectal cancer. Hum Mutat. 2012;33:551-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Lin CJ, Gong HY, Tseng HC, Wang WL, Wu JL. miR-122 targets an anti-apoptotic gene, Bcl-w, in human hepatocellular carcinoma cell lines. Biochem Biophys Res Commun. 2008;375:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 22. | Min M, Peng L, Yang Y, Guo M, Wang W, Sun G. MicroRNA-155 is involved in the pathogenesis of ulcerative colitis by targeting FOXO3a. Inflamm Bowel Dis. 2014;20:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Pathak S, Grillo AR, Scarpa M, Brun P, D’Incà R, Nai L, Banerjee A, Cavallo D, Barzon L, Palù G. MiR-155 modulates the inflammatory phenotype of intestinal myofibroblasts by targeting SOCS1 in ulcerative colitis. Exp Mol Med. 2015;47:e164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 24. | Svrcek M, El-Murr N, Wanherdrick K, Dumont S, Beaugerie L, Cosnes J, Colombel JF, Tiret E, Fléjou JF, Lesuffleur T. Overexpression of microRNAs-155 and 21 targeting mismatch repair proteins in inflammatory bowel diseases. Carcinogenesis. 2013;34:828-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Takagi T, Naito Y, Mizushima K, Hirata I, Yagi N, Tomatsuri N, Ando T, Oyamada Y, Isozaki Y, Hongo H. Increased expression of microRNA in the inflamed colonic mucosa of patients with active ulcerative colitis. J Gastroenterol Hepatol. 2010;25 Suppl 1:S129-S133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 26. | Zahm AM, Hand NJ, Tsoucas DM, Le Guen CL, Baldassano RN, Friedman JR. Rectal microRNAs are perturbed in pediatric inflammatory bowel disease of the colon. J Crohns Colitis. 2014;8:1108-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Sun W, Sheng Y, Chen J, Xu D, Gu Y. Down-Regulation of miR-146a Expression Induces Allergic Conjunctivitis in Mice by Increasing TSLP Level. Med Sci Monit. 2015;21:2000-2007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, Seabra MC, Round JL, Ward DM, O’Connell RM. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 465] [Cited by in RCA: 596] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 29. | Chen Y, Wang C, Liu Y, Tang L, Zheng M, Xu C, Song J, Meng X. miR-122 targets NOD2 to decrease intestinal epithelial cell injury in Crohn’s disease. Biochem Biophys Res Commun. 2013;438:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 30. | Ye D, Guo S, Al-Sadi R, Ma TY. MicroRNA regulation of intestinal epithelial tight junction permeability. Gastroenterology. 2011;141:1323-1333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 250] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 31. | Landy J, Ronde E, English N, Clark SK, Hart AL, Knight SC, Ciclitira PJ, Al-Hassi HO. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J Gastroenterol. 2016;22:3117-3126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 294] [Cited by in RCA: 369] [Article Influence: 41.0] [Reference Citation Analysis (4)] |
| 32. | Biancheri P, Giuffrida P, Docena GH, MacDonald TT, Corazza GR, Di Sabatino A. The role of transforming growth factor (TGF)-β in modulating the immune response and fibrogenesis in the gut. Cytokine Growth Factor Rev. 2014;25:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 33. | Li C, Kuemmerle JF. Mechanisms that mediate the development of fibrosis in patients with Crohn’s disease. Inflamm Bowel Dis. 2014;20:1250-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Principi M, Giorgio F, Losurdo G, Neve V, Contaldo A, Di Leo A, Ierardi E. Fibrogenesis and fibrosis in inflammatory bowel diseases: Good and bad side of same coin? World J Gastrointest Pathophysiol. 2013;4:100-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 35. | Feagins LA. Role of transforming growth factor-β in inflammatory bowel disease and colitis-associated colon cancer. Inflamm Bowel Dis. 2010;16:1963-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Monteleone G, Kumberova A, Croft NM, McKenzie C, Steer HW, MacDonald TT. Blocking Smad7 restores TGF-beta1 signaling in chronic inflammatory bowel disease. J Clin Invest. 2001;108:601-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 458] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 37. | Monteleone G, Caruso R, Pallone F. Role of Smad7 in inflammatory bowel diseases. World J Gastroenterol. 2012;18:5664-5668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Monteleone G, Di Sabatino A, Ardizzone S, Pallone F, Usiskin K, Zhan X, Rossiter G, Neurath MF. Impact of patient characteristics on the clinical efficacy of mongersen (GED-0301), an oral Smad7 antisense oligonucleotide, in active Crohn’s disease. Aliment Pharmacol Ther. 2016;43:717-724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Ippolito C, Colucci R, Segnani C, Errede M, Girolamo F, Virgintino D, Dolfi A, Tirotta E, Buccianti P, Di Candio G. Fibrotic and Vascular Remodelling of Colonic Wall in Patients with Active Ulcerative Colitis. J Crohns Colitis. 2016; Epub ahead of print. [PubMed] |
| 40. | Segain JP, Raingeard de la Blétière D, Sauzeau V, Bourreille A, Hilaret G, Cario-Toumaniantz C, Pacaud P, Galmiche JP, Loirand G. Rho kinase blockade prevents inflammation via nuclear factor kappa B inhibition: evidence in Crohn’s disease and experimental colitis. Gastroenterology. 2003;124:1180-1187. [PubMed] |
| 41. | Kong W, Yang H, He L, Zhao JJ, Coppola D, Dalton WS, Cheng JQ. MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol. 2008;28:6773-6784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 548] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 42. | Rai D, Kim SW, McKeller MR, Dahia PL, Aguiar RC. Targeting of SMAD5 links microRNA-155 to the TGF-beta pathway and lymphomagenesis. Proc Natl Acad Sci USA. 2010;107:3111-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 43. | Wang SC, Lin XL, Li J, Zhang TT, Wang HY, Shi JW, Yang S, Zhao WT, Xie RY, Wei F. MicroRNA-122 triggers mesenchymal-epithelial transition and suppresses hepatocellular carcinoma cell motility and invasion by targeting RhoA. PLoS One. 2014;9:e101330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 44. | Louafi F, Martinez-Nunez RT, Sanchez-Elsner T. MicroRNA-155 targets SMAD2 and modulates the response of macrophages to transforming growth factor-{beta}. J Biol Chem. 2010;285:41328-41336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 175] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 45. | Li CL, Nie H, Wang M, Su LP, Li JF, Yu YY, Yan M, Qu QL, Zhu ZG, Liu BY. microRNA-155 is downregulated in gastric cancer cells and involved in cell metastasis. Oncol Rep. 2012;27:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Cheung KS, Sposito N, Stumpf PS, Wilson DI, Sanchez-Elsner T, Oreffo RO. MicroRNA-146a regulates human foetal femur derived skeletal stem cell differentiation by down-regulating SMAD2 and SMAD3. PLoS One. 2014;9:e98063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Li J, Huang J, Dai L, Yu D, Chen Q, Zhang X, Dai K. miR-146a, an IL-1β responsive miRNA, induces vascular endothelial growth factor and chondrocyte apoptosis by targeting Smad4. Arthritis Res Ther. 2012;14:R75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 48. | Liu Z, Lu CL, Cui LP, Hu YL, Yu Q, Jiang Y, Ma T, Jiao DK, Wang D, Jia CY. MicroRNA-146a modulates TGF-β1-induced phenotypic differentiation in human dermal fibroblasts by targeting SMAD4. Arch Dermatol Res. 2012;304:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Xiao B, Zhu ED, Li N, Lu DS, Li W, Li BS, Zhao YL, Mao XH, Guo G, Yu PW. Increased miR-146a in gastric cancer directly targets SMAD4 and is involved in modulating cell proliferation and apoptosis. Oncol Rep. 2012;27:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Ji H, Li Y, Jiang F, Wang X, Zhang J, Shen J, Yang X. Inhibition of transforming growth factor beta/SMAD signal by MiR-155 is involved in arsenic trioxide-induced anti-angiogenesis in prostate cancer. Cancer Sci. 2014;105:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |