Published online Jul 14, 2016. doi: 10.3748/wjg.v22.i26.5917
Peer-review started: March 28, 2016
First decision: May 27, 2016
Revised: June 5, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: July 14, 2016
Processing time: 101 Days and 18.9 Hours
Endoscopic mucosal resection (EMR) is problematic with regard to en bloc and curable resection rates. Advancements in endoscopic techniques have enabled novel endoscopic approaches such as endoscopic submucosal dissection (ESD), which has overcome some EMR problems, and has become the standard treatment for gastrointestinal tumors. However, ESD is technically difficult. Procedure time is longer and complications such as intraoperative perforation and bleeding occur more frequently than in EMR. Recently various traction methods have been introduced to facilitate ESD procedures, such as clip with line, external forceps, clip and snare, internal traction, double scope, and magnetic anchor. Each method must be used appropriately according to the anatomical characteristics. In this review we discuss recently proposed traction methods for ESD based on the characteristics of various anatomical sites.
Core tip: Endoscopic submucosal dissection (ESD) is technically one of the most difficult endoscopic procedures. Recently, traction methods have been introduced to facilitate ESD procedures, various types of which have been proposed. Each traction method must be used appropriately according to anatomical characteristics. We discuss recently proposed traction methods for ESD based on the characteristics of various anatomical sites.
- Citation: Tsuji K, Yoshida N, Nakanishi H, Takemura K, Yamada S, Doyama H. Recent traction methods for endoscopic submucosal dissection. World J Gastroenterol 2016; 22(26): 5917-5926
- URL: https://www.wjgnet.com/1007-9327/full/v22/i26/5917.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i26.5917
Endoscopic mucosal resection (EMR) has been performed for the treatment of superficial gastrointestinal tumors since the 1980s[1,2]. However, EMR is problematic with regard to the rates of en bloc and curable resection[1]. Advancements in endoscopic techniques has led to novel endoscopic approaches such as endoscopic submucosal dissection (ESD)[3-5], which has overcome some of the problems associated with EMR and has been applied to previously unresectable lesions, such as large tumors and tumors with ulcer scarring. Although endoscopic treatment is generally less invasive than open surgery, the technique of ESD is complicated and difficult. Because of its technical difficulty, the procedure time is longer and complications such as perforation and bleeding occur more frequently than in EMR[6,7]. In addition, technical difficulties have prevented its widespread use, and ESD remains unpopular in Western countries[8,9].
Recently, new concepts have been devised to facilitate the ESD procedure, one of which is the traction method[10]. New devices and techniques have been reported for this ESD approach. Traction methods are essentially used to facilitate visualization of the submucosal layer, thus enabling accurate identification of the cutting line and submucosal vessels. Traction is thus a promising approach to help reduce the procedure time and complications, and may lead to more widespread adoption of ESD.
ESD was developed primarily as a treatment for gastric tumor, but is now also used for pharyngeal, esophageal, and colorectal tumors. Types of traction methods include clip with line, external forceps, clip and snare, internal traction, double scope, and magnetic anchor[10]. Each traction technique has its own characteristics and must be used appropriately in accordance with individual anatomical considerations. Although Imaeda et al[10] reviewed the advantages and disadvantages of the traction method, they did not discuss its use based on anatomical features. Here, we describe in brief the various traction methods available (Table 1), followed by a discussion of recent traction techniques for ESD based on the characteristics of different anatomical sites.
| Traction direction | Control of traction | Complexity of procedure | Required device | |
| Clip with line method | Only pull | Possible | Simple | Requires no special device |
| External forceps method | Push and Pull | Possible | Simple | Requires no special device |
| Clip and snare methods using prelooping technique | Push and Pull | Possible | Simple | Requires no special device |
| Internal traction method | Any direction | Impossible | Complex | Requires special device |
| Double scope method | Any direction | Possible | Complex | Need for space and another endoscope |
The clip-with-line method was reported by Oyama et al[11,12] and Jeon et al[13], and is carried out as follows. A 3-0 silk line is tied to the arm part of the clip. After circumferential cutting, a clip applicator device is inserted into the accessory channel of the endoscope, and the clip with line is mounted on the tip of the applicator. The scope is inserted again, and the clip with line is attached to the edge of the lesion. The lesion is then pulled toward the oral side using the line. Although this technique is simple, traction is directed solely through pulling (Figure 1).
Imaeda et al[14,15] reported the efficacy of an ESD procedure using an external grasping forceps, performed as follows. After circumferential cutting, an external grasping forceps is grasped by a second grasping forceps inserted through the accessory channel. The external forceps is delivered with the help of the second grasping forceps, taking care to avoid injuring the mucosa, especially at the esophagocardial junction. After the external grasping forceps is anchored at the edge of the lesion, the second forceps is released. The direction of traction is controlled not only by pulling but also by pushing, using the external grasping forceps (Figure 2).
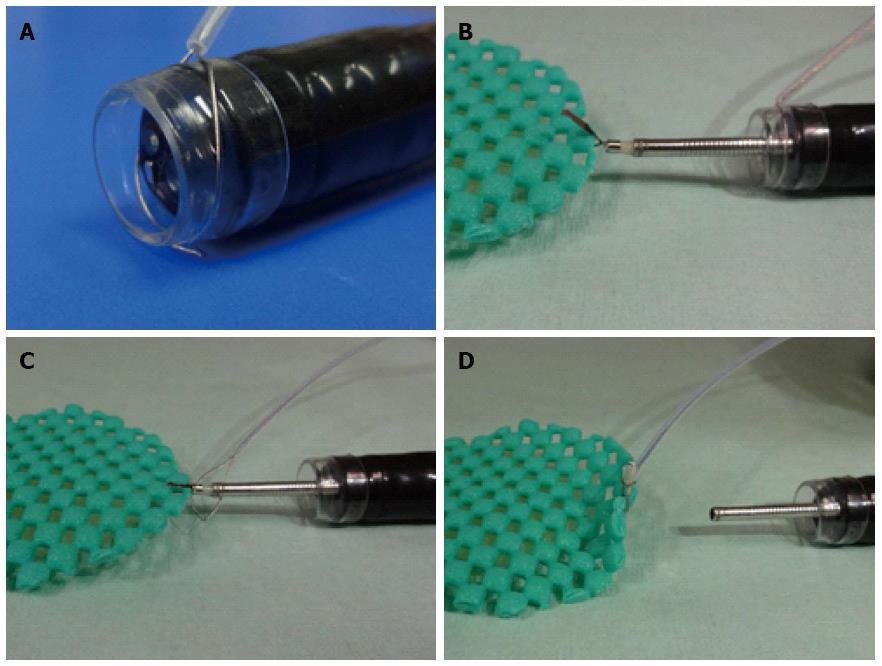
The clip-and-snare method (CSM), which uses a hemoclip and snare, has been reported by Yasuda et al[16] and Baldaque-Silva et al[17]. The traction of this method involves pulling and pushing the lesion by a hemoclip grasped with the snare. However, delivery of the snare is sometimes difficult[18]. Moreover, Yoshida et al[18] and Ota et al[19] reported use of the CSM using a prelooping technique (CSM-PLT), which improved delivery of the snare (Figure 3). CSM-PLT is carried out as follows. After circumferential cutting, the endoscope is withdrawn once to preloop a snare over it. The scope and snare are reinserted. A clip is inserted through the working channel of the endoscope and is used to grasp the mucosal flap. The prelooping snare is loosened and moved along the forceps up to the clip. The snare is then tightened to grasp the clip. Finally, the clip is released from its deployment device. Traction is maintained by the snare and clip independent of the scope. This method can be applied to any site.
Internal traction can employ several methods, such as the medical ring, the clip band technique, and clip modifications[20-22]. Although some differences exist among methods, internal traction is generally carried out as follows. First, a rubber band, medical ring, or nylon line is connected to the clip after circumferential cutting. Next, the clip is inserted into the working channel of the endoscope and attached to the edge of the resected lesion, then the second clip with the band is attached to the opposite mucosa. Dissection is facilitated by continuous traction exerted by this system (Figure 4).
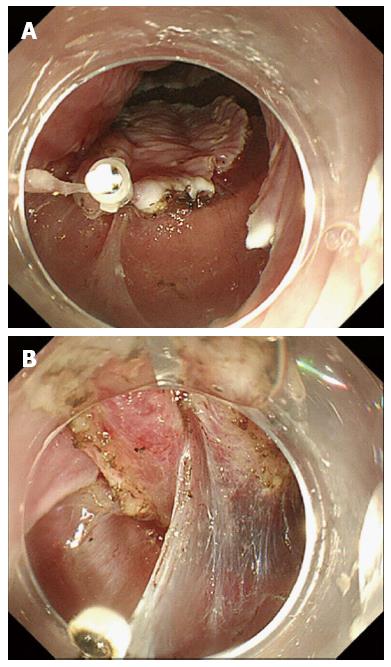
The double-scope method was reported by Morita et al[23], Higuchi et al[24], and Fujii et al[25], and is carried out as follows. Circumferential cutting is performed by the main endoscope, which is then left in the stomach after the lesion is grasped with the loop. A small-caliber endoscope is inserted along the main scope. A grasping forceps is inserted through the channel of the small-caliber endoscope and the lesion is grasped by a grasping forceps. The traction can be adjusted by the small-caliber endoscope in any direction (Figure 5).
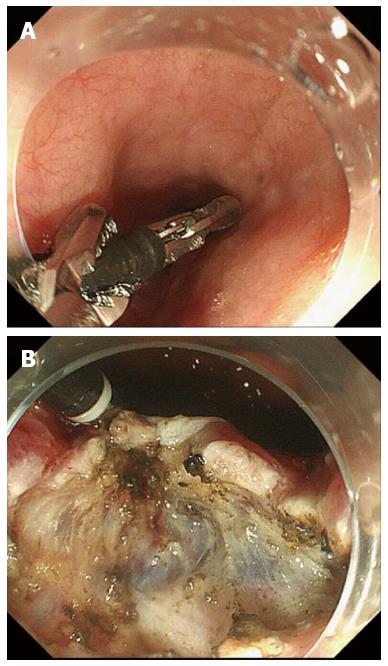
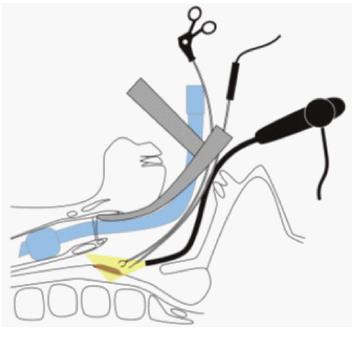
Recent advances in endoscopic devices, including magnifying endoscopy and narrow-band imaging, have enabled improvement in the early diagnosis of superficial head and neck carcinoma[26-28], and the use of ESD for such superficial lesions has been reported[29,30]. Although the anatomical structure of the head and neck is generally complex, approaching the lesion using a traction device is relatively easy because of the oral proximity. Iizuka et al[31] reported ESD using Fraenkel laryngeal forceps, whose length is approximately 23 cm (Figure 6A). This method is useful to facilitate visualization of the submucosal layer because it can be adjusted in any direction as needed (Figure 6B). However, disadvantages of this method are interference between the endoscope and forceps, the need for an assistant to operate the Fraenkel laryngeal forceps, and, crucially, potential damage to the specimen. The epithelium of the pharynx is frail and easily exfoliated; histological examination of the lateral margin is unclear in some cases[32]. Iizuka et al[31] proposed that the rear side of the specimen, instead of the edge, should be grasped by the Fraenkel laryngeal forceps to prevent specimen damage. Proficiency in the use of Fraenkel laryngeal forceps may be required to achieve proper traction.
Recently, endoscopic laryngopharyngeal surgery (ELPS), which was developed by modifying the ESD procedure, was reported (Figure 7)[33,34]. ELPS is similar to ESD but less invasive[35]. This procedure is performed by a head and neck surgeon with both hands under the guidance of a gastrointestinal scope. In a retrospective analysis, Tateya et al[35] reported that the operation time (35 min) of ELPS was shorter than that of ESD (50-65.3 min). Although ELPS is becoming a major option in the treatment of superficial head and neck carcinoma, it requires an experienced head and neck surgeon and is expensive. A prospective, randomized controlled trial is warranted to compare the functional and oncological results of ELPS and ESD.
The esophagus is a tube running from the pharynx to the stomach. Although its anatomical structure is not complex, the esophageal lumen is narrower than that of other organs, so most ESD procedures for esophageal tumor are performed only from the frontal view. Traction devices in the esophagus are required not to interfere with the endoscope in this narrow lumen. An approach such as the double-scope method is not suitable in terms of endoscope interference, while an internal traction method presents difficulty in setting two clips connected by a rubber or nylon ring because the space is limited.
The clip-with-line method was reported by Oyama et al[11,12], Ota et al[36], Jeon et al[13], and Tsao et al[37]. This technique is simple and does not require special devices and equipment[11,12]. Although the counteraction of this method is adjusted only by pulling, it is sufficient for esophageal tumor because ESD for such a tumor is performed only from the frontal view. A prospective study was performed to confirm the safety and efficacy of this technique by Koike et al[38], who demonstrated that the dissection time (19.8 min) of the traction method was shorter than that of conventional ESD (31.8 min), without complications. Given the established safety and shorter dissection time[38], the clip-with-line method for esophageal ESD is a viable option in the treatment of superficial esophageal tumor.
Another promising newly developed technique for esophageal ESD, reported by Ohata et al[39], Hirota et al[40], and Motohashi et al[41], is the modified external forceps method. The reported method uses an overtube equipped with a side channel or an Impact Shooter (TOP Co., Tokyo, Japan) mounted on the scope. The grasping forceps is inserted into the side channel to grasp the edge of the lesion in order to provide traction. Although this method is more difficult than the clip-with-line method in terms of interference, the advantage is that traction can be adjusted not only by pulling but also by rotation. The feasibility and potential efficacy of this method have been demonstrated[40,41], although the sample size was small. Further studies are warranted, including a prospective study.
The stomach is a J-shaped organ that is distensible and may take on various shapes, and is divided into five regions: cardia, fundus, body, antrum, and pylorus. Given the idiosyncrasies of each area, the difficulty of ESD varies depending on region and/or lesion.
The clip-with-line method is useful for stomach lesions. Jeon et al[13], He et al[42], Yoshida et al[43], and Suzuki et al[44] reported that this technique is effective and safe for ESD to treat gastric neoplasms. Okamoto et al[45] reported a similar method. Like ESD for esophageal tumor, the advantage of this technique is its simplicity. This method reduced procedure time without increasing adverse events in a matched case-control study[43,44]. Yoshida et al[43] reported that the procedure time (43 ± 24 min) in the traction group was shorter compared with that for conventional ESD (52 ± 30 min), consistent with the findings of Suzuki et al[44] (procedure time 82.2 ± 79.5 min for traction vs 118.2 ± 71.6 min for conventional ESD). A prospective, randomized controlled study with the aim of confirming the efficacy of this technique in Japan is now under way, registered in the UMIN Clinical Trial Registry as UMIN000018266. However, this technique is hampered by the fact that the direction of traction is limited. This disadvantage also applies to the pulley method, which is similar to the clip-with-line approach[46]. Oyama et al[11] reported that the clip with line is especially useful when the cancer exists in the greater curvature of the gastric body.
The external forceps method is an option for the treatment of gastric tumor. In a retrospective analysis, Imaeda et al[14,15] reported the efficacy and safety of ESD using an external grasping forceps for gastric neoplasia. The direction of traction was controlled not only by pulling but also by pushing using these forceps. However, disadvantages include injury to the patient and difficulty in carrying out the procedure for lesions in the cardia, lesser curvature, or posterior wall of the upper gastric body[15].
Yasuda et al[16] and Baldaque-Silva et al[17] reported on their experience with the CSM. Traction using a snare, which is a conventional endoscopic device, enables the lesion to be pulled and pushed. As the snare is more flexible than forceps, ensuing damage is less than occurs using external forceps. Although this technique is a potential improvement on external grasping forceps, delivery of the snare is sometimes difficult and risks gastric tissue injury, especially when lesions are located in the upper body[18]. CSM-PLT, which improved the delivery of the snare, was reported by Yoshida et al[18]. Using a prelooping technique enabled delivery of the snare to any location, and required no special device. CSM-PLT was considered effective and safe for gastric ESD. Yoshida et al[47] demonstrated that the procedure time (38.5 min) for the CSM-PLT group was shorter than that for the control group (59.5 min) in a retrospective matched-pair comparison (P < 0.023).
The internal traction method for gastric tumor was reported by Matsumoto et al[20], Parra-Blanco et al[21], and Chen et al[22]. However, this method also has problems. First, control of the traction direction is sometimes difficult because the traction is automatic. If the clips are incorrectly placed, traction might be applied in an incorrect direction. Parra-Blanco et al[21] recommend pushing the clip’s sheath distantly to the lesion to apply the second clip to the most adequate area. Second, this method might not be applicable for lesions in the pylorus or the cardia, where space is limited[22]. Third, this technique requires special devices and equipment.
The double-scope method is also an option for the treatment of gastric tumor[23-25]. This technique uses a small-caliber endoscope in addition to the main scope. The traction is adjusted in any direction by the small-caliber endoscope. Maneuvering the endoscope, changing the angle, and inserting the endoscope to apply the traction are easily accomplished. However, this method has two disadvantages. First, the two endoscopes tend to interfere with each other. Second, this method is not simple. Morita et al[23] and Fujii et al[25] reported that the technique required two light sources and instruments, as well as substantial space in the endoscopy room. Although Higuchi et al[24] reported using a single light source that could be transferred between endoscopes, this technique required time and effort.
Other less reported approaches include the percutaneous traction method, the magnetic anchor method, and the robot-assisted method. von Delius et al[48] reported a percutaneously assisted ESD using a PEG-minitrocar. However, this method is limited to the area of lesions and is more invasive than other traction methods. The magnetic anchor and robot-assisted methods have the potential to facilitate and change the procedure itself[49-51]. However, these systems are not yet practicable in clinical practice. Further research is required for continued improvement.
The colon is long, the lumen is angulated, and the intestinal wall is thin. Colorectal ESD is consequently limited to a few high-expertise centers, thus hampering its broader application to Western countries[52]. Moreover, colorectal ESD is not widely performed even by Eastern endoscopists because of its technical difficulty, longer procedure time, and increased risk of related complications[53,54]. Traction methods have been attempted to facilitate the procedure of colorectal ESD. Although it is easy to apply any traction methods to the rectum, it is difficult to do so in the deep colon because of difficulty in reinserting the endoscope and adjusting the traction[10]. It is therefore important to distinguish between the rectum and deep colon.
The double-scope method for colorectal tumors was reported by Uraoka et al[55]. This approach requires a second endoscopist to operate the thin endoscope for traction, and is limited to the rectum and distal sigmoid colon because of difficulty in inserting the thin endoscope[55]. ESD for rectal cancers using an external forceps was reported by Imaeda et al[56]. ESD using an external forceps was possible only for rectal tumors because of difficulty in inserting and controlling the forceps[56], making it too difficult to apply to the deep colon.
Internal traction methods such as rubber strips, S-O clips, loop-attached rubber bands, and latex bands are also promising for the treatment of colorectal tumors[57-62]. Although control of the traction direction is difficult and a special device is required, internal traction can be advantageous for deep colon procedures. First, internal traction methods do not require reinsertion of the endoscope, as clips connected by a rubber ring or nylon are used. Clips such as the S-O clip can be passed through the instrument channel of the endoscope. Second, this system is independent, and is thus not limited by endoscopic movement. A prospective study was performed to confirm the safety and efficacy of this method by Ritsuno et al[60], who demonstrated that the procedure time for the S-O clip-assisted ESD was significantly shorter than that for conventional ESD (37.4 ± 32.6 min vs 67.1 ± 44.1 min, P = 0.03). Saito et al[63] reported on a similar concept, a sinker-assisted endoscopic submucosal dissection. However, this technique required reinsertion of the scope to set up the sinker, and is therefore difficult to apply to the deep colon.
Recently, two novel traction methods that do not require special devices or equipment and enable access to the deep colon have been reported. Yamasaki et al[64,65] reported their modified clip-with-line method, which does not require withdrawal and reinsertion of the endoscope. However, it has two disadvantages. First, it is difficult to adjust the traction. The counteraction is adjusted solely by pulling, whereby it is difficult to add further traction to tighten the line. Second, the procedural success rate of traction-assisted clip and line was 87%[65]. Yamasaki et al[65] recommended pulling gently on the line within the proximal colon because the clip detached from three lesions, all of which were in the proximal colon.
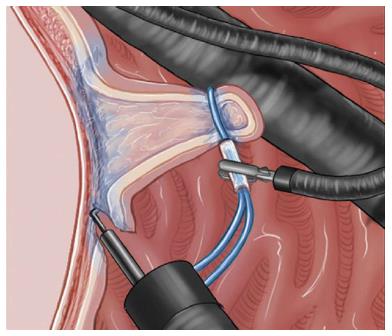
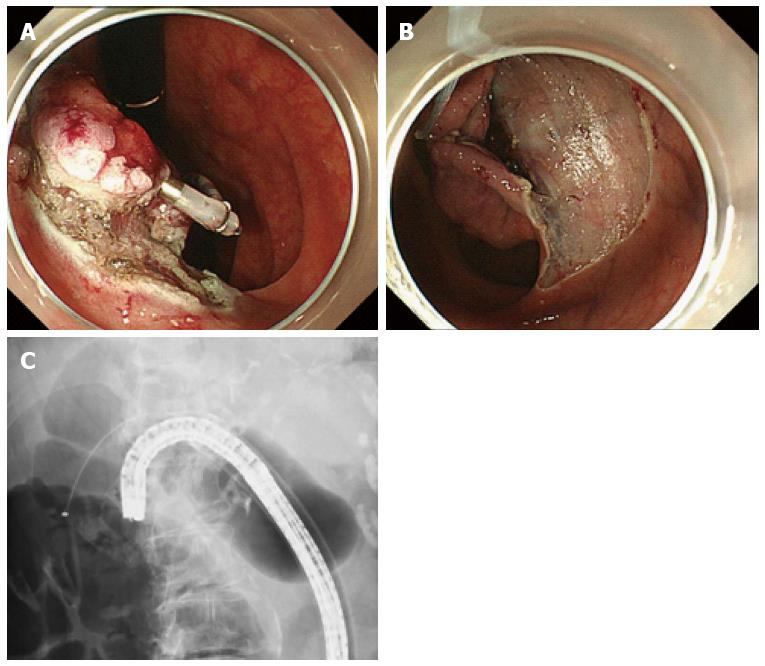
The second method, reported by Yamada et al[66] and Ota et al[19], uses the CSM-PLT approach to deep colon endoscopy. Although this method requires reinsertion of the endoscope, it can applied to any colon using the prelooping technique and a balloon overtube (ST-CB1; Olympus, Tokyo). Traction is maintained by the snare and the clip independent of the scope[19,66]. This method requires no special equipment, and is superior to the aforementioned method in two aspects. First, the clip-and-snare method enabled pushing and pulling movements for traction (Figure 8). Second, it has more flexibility. We were able to perform the ESD not only from the frontal but also the retroflex view using the clip-and-snare method. Moreover, this method enables widespread access to the submucosa through the placement of multiple clips on different edges of a lesion. This flexibility is important when ESD is performed for colorectal lesions because the clinical situation can change from moment to moment[19]. Yamada et al[66] demonstrated in a retrospective study that the procedure time for CSM-PLT was significantly shorter than that for a control group (45.6 min vs 70.1 min, P = 0.047). However, the number of patients was small. Further prospective studies are warranted to confirm the efficacy and safety of this method.
Various traction methods have been reported for ESD based on specific characteristics of each anatomical site. Each method has advantages and disadvantages, as delineated in Tables 1 and 2. Appropriate application of traction methods combined with technical proficiency will improve the outcomes of ESD procedures. Further advancements should be assessed through prospective, randomized controlled studies.
| Head and neck | Esophagus | Stomach | Colon and rectum | |
| Clip with line method | No report, but theoretically possible | Very useful | Useful, especially in the greater curvature of gastric body | Modified method is useful in any colon |
| External forceps method | Useful | Useful | Difficult in the cardia and the lesser curvature of upper gastric body | Rectum only |
| Clip and snare methods using prelooping technique | No report, but theoretically possible | No report, but theoretically possible | Useful | Useful, but requires overtube in deep colon |
| Internal traction method | No report | No report | Difficult in the pylorus and the cardia | Useful |
| Double scope method | No report, but theoretically possible | No report | Useful | Rectum only |
Manuscript source: Invited manuscript
P- Reviewer: Guo XY, Lorenzo-Zuniga V S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Uedo N, Takeuchi Y, Ishihara R. Endoscopic management of early gastric cancer: endoscopic mucosal resection or endoscopic submucosal dissection: data from a Japanese high-volume center and literature review. Ann Gastroenterol. 2012;25:281-290. [PubMed] |
| 2. | Tamura S, Nakajo K, Yokoyama Y, Ohkawauchi K, Yamada T, Higashidani Y, Miyamoto T, Ueta H, Onishi S. Evaluation of endoscopic mucosal resection for laterally spreading rectal tumors. Endoscopy. 2004;36:306-312. [PubMed] |
| 3. | Ribeiro-Mourão F, Pimentel-Nunes P, Dinis-Ribeiro M. Endoscopic submucosal dissection for gastric lesions: results of an European inquiry. Endoscopy. 2010;42:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Draganov PV, Coman RM, Gotoda T. Training for complex endoscopic procedures: how to incorporate endoscopic submucosal dissection skills in the West? Expert Rev Gastroenterol Hepatol. 2014;8:119-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, Hosokawa K, Shimoda T, Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225-229. [PubMed] |
| 6. | Ohkuwa M, Hosokawa K, Boku N, Ohtu A, Tajiri H, Yoshida S. New endoscopic treatment for intramucosal gastric tumors using an insulated-tip diathermic knife. Endoscopy. 2001;33:221-226. [PubMed] |
| 7. | Gotoda T, Yamamoto H, Soetikno RM. Endoscopic submucosal dissection of early gastric cancer. J Gastroenterol. 2006;41:929-942. [PubMed] |
| 8. | Rösch T, Sarbia M, Schumacher B, Deinert K, Frimberger E, Toermer T, Stolte M, Neuhaus H. Attempted endoscopic en bloc resection of mucosal and submucosal tumors using insulated-tip knives: a pilot series. Endoscopy. 2004;36:788-801. [PubMed] |
| 9. | Teoh AY, Chiu PW, Wong SK, Sung JJ, Lau JY, Ng EK. Difficulties and outcomes in starting endoscopic submucosal dissection. Surg Endosc. 2010;24:1049-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Imaeda H, Hosoe N, Kashiwagi K, Ohmori T, Yahagi N, Kanai T, Ogata H. Advanced endoscopic submucosal dissection with traction. World J Gastrointest Endosc. 2014;6:286-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Oyama T, Kikuchi Y, Shimaya S, Tomori A, Hotta K, Miyata Y, Yamada S. Endoscopic mucosal resection using a hooking knife (hooking EMR). Stomach Intestine. 2002;37:1155-1161. |
| 12. | Oyama T. Counter traction makes endoscopic submucosal dissection easier. Clin Endosc. 2012;45:375-378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Jeon WJ, You IY, Chae HB, Park SM, Youn SJ. A new technique for gastric endoscopic submucosal dissection: peroral traction-assisted endoscopic submucosal dissection. Gastrointest Endosc. 2009;69:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Imaeda H, Iwao Y, Ogata H, Ichikawa H, Mori M, Hosoe N, Masaoka T, Nakashita M, Suzuki H, Inoue N. A new technique for endoscopic submucosal dissection for early gastric cancer using an external grasping forceps. Endoscopy. 2006;38:1007-1010. [PubMed] |
| 15. | Imaeda H, Hosoe N, Ida Y, Kashiwagi K, Morohoshi Y, Suganuma K, Nagakubo S, Komatsu K, Suzuki H, Saito Y. Novel technique of endoscopic submucosal dissection using an external grasping forceps for superficial gastric neoplasia. Dig Endosc. 2009;21:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Yasuda M, Naito Y, Kokura S, Yoshida N, Yoshikawa T. Newly-developed ESD (CSL-ESD) for early gastric cancer using convenient and low-cost lifting method (lifting method using clips and snares) for lesions is clinically useful. Gastrointest Endosc. 2012;75:AB244. |
| 17. | Baldaque-Silva F, Vilas-Boas F, Velosa M, Macedo G. Endoscopic submucosal dissection of gastric lesions using the “yo-yo technique”. Endoscopy. 2013;45:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Yoshida N, Doyama H, Ota R, Tsuji K. The clip-and-snare method with a pre-looping technique during gastric endoscopic submucosal dissection. Endoscopy. 2014;46 Suppl 1 UCTN:E611-E612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Ota R, Doyama H, Tsuji K, Yamada S. Deep colonic endoscopic submucosal dissection using a modified clip and snare method incorporating a pre-looping technique. BMJ Case Rep. 2015;2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Matsumoto K, Nagahara A, Sakamoto N, Suyama M, Konuma H, Morimoto T, Sagawa E, Ueyama H, Takahashi T, Beppu K. A new traction device for facilitating endoscopic submucosal dissection (ESD) for early gastric cancer: the “medical ring”. Endoscopy. 2011;43 Suppl 2 UCTN:E67-E68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Parra-Blanco A, Nicolas D, Arnau MR, Gimeno-Garcia AZ, Rodrigo L, Quintero E. Gastric endoscopic submucosal dissection assisted by a new traction method: the clip-band technique. A feasibility study in a porcine model (with video). Gastrointest Endosc. 2011;74:1137-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Chen PJ, Chu HC, Chang WK, Hsieh TY, Chao YC. Endoscopic submucosal dissection with internal traction for early gastric cancer (with video). Gastrointest Endosc. 2008;67:128-132. [PubMed] |
| 23. | Morita Y, Masuda M, Tanaka S, Fujiwara M, Wakahara C, Toyonaga A new T, Azuma T. A new approach to treating difficult cases of early gastric cancer: development of a double scope-ESD using transnasal endoscope with a “Split Barrel” [in Japanese with English abstract]. Endoscopia Digestiva. 2010;22:846-851. |
| 24. | Higuchi K, Tanabe S, Azuma M, Sasaki T, Katada C, Ishido K, Naruke A, Mikami T, Koizumi W. Double-endoscope endoscopic submucosal dissection for the treatment of early gastric cancer accompanied by an ulcer scar (with video). Gastrointest Endosc. 2013;78:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Fujii L, Onkendi EO, Bingener-Casey J, Levy MJ, Gostout CJ. Dual-scope endoscopic deep dissection of proximal gastric tumors (with video). Gastrointest Endosc. 2013;78:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Muto M, Nakane M, Katada C, Sano Y, Ohtsu A, Esumi H, Ebihara S, Yoshida S. Squamous cell carcinoma in situ at oropharyngeal and hypopharyngeal mucosal sites. Cancer. 2004;101:1375-1381. [PubMed] |
| 27. | Muto M, Katada C, Sano Y, Yoshida S. Narrow band imaging: a new diagnostic approach to visualize angiogenesis in superficial neoplasia. Clin Gastroenterol Hepatol. 2005;3:S16-S20. [PubMed] |
| 28. | Muto M, Minashi K, Yano T, Saito Y, Oda I, Nonaka S, Omori T, Sugiura H, Goda K, Kaise M. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 523] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 29. | Muto M, Satake H, Yano T, Minashi K, Hayashi R, Fujii S, Ochiai A, Ohtsu A, Morita S, Horimatsu T. Long-term outcome of transoral organ-preserving pharyngeal endoscopic resection for superficial pharyngeal cancer. Gastrointest Endosc. 2011;74:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 30. | Emura F, Baron TH, Gralnek IM. The pharynx: examination of an area too often ignored during upper endoscopy. Gastrointest Endosc. 2013;78:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Iizuka T, Kikuchi D, Hoteya S, Takeda H, Kaise M. A new technique for pharyngeal endoscopic submucosal dissection: peroral countertraction (with video). Gastrointest Endosc. 2012;76:1034-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Iizuka T, Kikuchi D, Hoteya S, Nakamura M, Yamashita S, Mitani T, Takeda H, Yahagi N. Clinical advantage of endoscopic submucosal dissection over endoscopic mucosal resection for early mesopharyngeal and hypopharyngeal cancers. Endoscopy. 2011;43:839-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Satou Y, Omori T, Tagawa M. [Treatment of superficial carcinoma in the hypopharynx]. Nihon Jibiinkoka Gakkai Kaiho. 2006;109:581-586. [PubMed] |
| 34. | Tateya I, Muto M, Morita S, Miyamoto S, Hayashi T, Funakoshi M, Aoyama I, Higuchi H, Hirano S, Kitamura M. Endoscopic laryngo-pharyngeal surgery for superficial laryngo-pharyngeal cancer. Surg Endosc. 2016;30:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 35. | Tateya I, Shiotani A, Satou Y, Tomifuji M, Morita S, Muto M, Ito J. Transoral surgery for laryngo-pharyngeal cancer - The paradigm shift of the head and cancer treatment. Auris Nasus Larynx. 2016;43:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 36. | Ota M, Nakamura T, Hayashi K, Ohki T, Narumiya K, Sato T, Shirai Y, Kudo K, Yamamoto M. Usefulness of clip traction in the early phase of esophageal endoscopic submucosal dissection. Dig Endosc. 2012;24:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 37. | Tsao SK, Toyonaga T, Morita Y, Fujita T, Hayakumo T, Azuma T. Modified fishing-line traction system in endoscopic submucosal dissection of large esophageal tumors. Endoscopy. 2011;43 Suppl 2 UCTN:E119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Koike Y, Hirasawa D, Fujita N, Maeda Y, Ohira T, Harada Y, Suzuki K, Yamagata T, Tanaka M. Usefulness of the thread-traction method in esophageal endoscopic submucosal dissection: randomized controlled trial. Dig Endosc. 2015;27:303-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 39. | Ohata K, Fu K, Shouzushima M, Hamanaka J, Ono A, Ito T, Tsuji Y, Chiba H, Matsuhashi N. A novel traction system for esophageal endoscopic submucosal dissection. Endoscopy. 2012;44 Suppl 2 UCTN:E410-E411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Hirota M, Kato M, Yamasaki M, Kawai N, Miyazaki Y, Yamada T, Takahashi T, Takehara T, Mori M, Doki Y. A novel endoscopic submucosal dissection technique with robust and adjustable tissue traction. Endoscopy. 2014;46:499-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Motohashi O, Nishimura K, Nakayama N, Takagi S, Yanagida N. Endoscopic submucosal dissection (two-point fixed ESD) for early esophageal cancer. Dig Endosc. 2009;21:176-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 42. | He Y, Fu K, Leung J, Du Y, Wang J, Jin P, Yu Y, Yu D, Wang X, Sheng J. Traction with dental floss and endoscopic clip improves trainee success in performing gastric endoscopic submucosal dissection (ESD): a live porcine study (with video). Surg Endosc. 2016;30:3138-3144. [PubMed] |
| 43. | Yoshida M, Takizawa K, Ono H, Igarashi K, Sugimoto S, Kawata N, Tanaka M, Kakushima N, Ito S, Imai K. Efficacy of endoscopic submucosal dissection with dental floss clip traction for gastric epithelial neoplasia: a pilot study (with video). Surg Endosc. 2016;30:3100-3106. [PubMed] |
| 44. | Suzuki S, Gotoda T, Kobayashi Y, Kono S, Iwatsuka K, Yagi-Kuwata N, Kusano C, Fukuzawa M, Moriyasu F. Usefulness of a traction method using dental floss and a hemoclip for gastric endoscopic submucosal dissection: a propensity score matching analysis (with videos). Gastrointest Endosc. 2016;83:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 45. | Okamoto K, Okamura S, Muguruma N, Kitamura S, Kimura T, Imoto Y, Miyamoto H, Okahisa T, Takayama T. Endoscopic submucosal dissection for early gastric cancer using a cross-counter technique. Surg Endosc. 2012;26:3676-3681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Li CH, Chen PJ, Chu HC, Huang TY, Shih YL, Chang WK, Hsieh TY. Endoscopic submucosal dissection with the pulley method for early-stage gastric cancer (with video). Gastrointest Endosc. 2011;73:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Yoshida N, Doyama , H , Ota R, Takeda Y, Nakanishi H, Tominaga H, Tsuji S, Takemura K. Effectiveness of clip-and-snare method using pre-looping technique for gastric endoscopic submucosal dissection. World J Gastrointest Endsc. 2016;8:451-457. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | von Delius S, Karagianni A, von Weyhern CH, Feussner H, Schuster T, Schmid RM, Frimberger E. Percutaneously assisted endoscopic surgery using a new PEG-minitrocar for advanced endoscopic submucosal dissection (with videos). Gastrointest Endosc. 2008;68:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Gotoda T, Oda I, Tamakawa K, Ueda H, Kobayashi T, Kakizoe T. Prospective clinical trial of magnetic-anchor-guided endoscopic submucosal dissection for large early gastric cancer (with videos). Gastrointest Endosc. 2009;69:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 50. | Ho KY, Phee SJ, Shabbir A, Low SC, Huynh VA, Kencana AP, Yang K, Lomanto D, So BY, Wong YY. Endoscopic submucosal dissection of gastric lesions by using a Master and Slave Transluminal Endoscopic Robot (MASTER). Gastrointest Endosc. 2010;72:593-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Chiu PW, Phee SJ, Bhandari P, Sumiyama K, Ohya T, Wong J, Poon CC, Tajiri H, Nakajima K, Ho KY. Enhancing proficiency in performing endoscopic submucosal dissection (ESD) by using a prototype robotic endoscope. Endosc Int Open. 2015;3:E439-E442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Bartel MJ, Brahmbhatt BS, Wallace MB. Management of colorectal T1 carcinoma treated by endoscopic resection from the Western perspective. Dig Endosc. 2016;28:330-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 53. | Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100-107. [PubMed] |
| 54. | Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 2013;27:3262-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 55. | Uraoka T, Ishikawa S, Kato J, Higashi R, Suzuki H, Kaji E, Kuriyama M, Saito S, Akita M, Hori K. Advantages of using thin endoscope-assisted endoscopic submucosal dissection technique for large colorectal tumors. Dig Endosc. 2010;22:186-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 56. | Imaeda H, Hosoe N, Ida Y, Nakamizo H, Kashiwagi K, Kanai T, Iwao Y, Hibi T, Ogata H. Novel technique of endoscopic submucosal dissection by using external forceps for early rectal cancer (with videos). Gastrointest Endosc. 2012;75:1253-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Takeda T, Murakami T, Sakamoto N, Goto SP, Ritsuno H, Ueyama H, Mori H, Matsumoto K, Shibuya T, Osada T. Traction device to remove an adenoma in the appendiceal orifice by endoscopic submucosal dissection. Endoscopy. 2013;45 Suppl 2 UCTN:E239-E240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Sakamoto N, Osada T, Shibuya T, Beppu K, Matsumoto K, Shimada Y, Konno A, Kurosawa A, Nagahara A, Ohkusa T. The facilitation of a new traction device (S-O clip) assisting endoscopic submucosal dissection for superficial colorectal neoplasms. Endoscopy. 2008;40 Suppl 2:E94-E95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 59. | Sakamoto N, Osada T, Shibuya T, Beppu K, Matsumoto K, Mori H, Kawabe M, Nagahara A, Otaka M, Ogihara T. Endoscopic submucosal dissection of large colorectal tumors by using a novel spring-action S-O clip for traction (with video). Gastrointest Endosc. 2009;69:1370-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 60. | Ritsuno H, Sakamoto N, Osada T, Goto SP, Murakami T, Ueyama H, Mori H, Matsumoto K, Beppu K, Shibuya T. Prospective clinical trial of traction device-assisted endoscopic submucosal dissection of large superficial colorectal tumors using the S-O clip. Surg Endosc. 2014;28:3143-3149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 61. | Osada T, Sakamoto N, Shibuya T, Beppu K, Matsumoto K, Shimada Y, Mori H, Konno A, Kurosawa A, Nagahara A. “Loops-attached rubber band” facilitation of endoscopic submucosal dissection of superficial colorectal neoplasm. Endoscopy. 2008;40 Suppl 2:E101-E102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Tomiki Y, Ishiyama S, Sugimoto K, Takahashi M, Kojima Y, Tanaka M, Sakamoto K. Colorectal endoscopic submucosal dissection by using latex-band traction. Endoscopy. 2011;43 Suppl 2 UCTN:E250-E251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Saito Y, Emura F, Matsuda T, Uraoka T, Nakajima T, Ikematsu H, Gotoda T, Saito D, Fujii T. A new sinker-assisted endoscopic submucosal dissection for colorectal cancer. Gastrointest Endosc. 2005;62:297-301. [PubMed] |
| 64. | Yamasaki Y, Takeuchi Y, Hanaoka N, Higashino K, Uedo N, Ishihara R, Iishi H. A novel traction method using an endoclip attached to a nylon string during colonic endoscopic submucosal dissection. Endoscopy. 2015;47 Suppl 1 UCTN:E238-E239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Yamasaki Y, Takeuchi Y, Uedo N, Kato M, Hamada K, Aoi K, Tonai Y, Matsuura N, Kanesaka T, Yamashina T. Traction-assisted colonic endoscopic submucosal dissection using clip and line: a feasibility study. Endosc Int Open. 2016;4:E51-E55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Yamada S, Doyama H, Ota R, Takeda Y, Tsuji K, Tsuji S, Yoshida N. Impact of the clip and snare method using the prelooping technique for colorectal endoscopic submucosal dissection. Endoscopy. 2016;48:281-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |