Published online Jul 7, 2016. doi: 10.3748/wjg.v22.i25.5831
Peer-review started: March 13, 2016
First decision: March 31, 2016
Revised: April 21, 2016
Accepted: May 4, 2016
Article in press: May 4, 2016
Published online: July 7, 2016
Processing time: 113 Days and 2.6 Hours
AIM: To evaluate the hemostatic effect of topical hemocoagulase spray in digestive endoscopy.
METHODS: Eighty-nine patients who developed oozing bleeding during endoscopic treatment from September 2014 to October 2014 at Center for Digestive Endoscopy, Tianjin Medical University General Hospital were randomly divided into either a study group (n = 39) or a control group (n = 50). The study group was given topical hemocoagulase spray intraoperatively, while the control group was given traditional 8% norepinephrine spray. Hemostatic efficacy was compared between the two groups. Bleeding site, wound cleanliness and perforation were recorded, and the rates of perforation and late bleeding were compared.
RESULTS: Successful hemostasis was achieved in 39 (100%) patients of the study group and in 47 (94.0%) patients of the control group, and there was no significant difference in the rate of successful hemostasis between the two groups. Compared with the control group, after topical hemocoagulase spray in the study group, the surgical field was clearer, the bleeding site was more easily identified, and the wound was cleaner. There was no significant difference in the rate of perforation between the study and control groups (16.7% vs 35.0%, P = 0.477), but the rates of late bleeding (0% vs 15.8%, P = 0.048) and overall complications (P = 0.032) were significantly lower in the study group.
CONCLUSION: Topical hemocoagulase spray has a definite hemostatic effect for oozing bleeding in digestive endoscopy, and this method is convenient, safe, and reliable. It is expected to become a new method for endoscopic hemostasis.
Core tip: In this study, we evaluated the hemostatic effect of topical hemocoagulase spray in digestive endoscopy. There was no significant difference in the rate of perforation between the study and control groups. There was no significant difference in the rate of successful hemostasis between the two groups, but the rates of late bleeding and overall complications of the hemocoagulase group were significantly lower than the 8% norepinephrine group. The surgical field was clearer, the bleeding site was more easily identified, and the wound was cleaner in the study group.
- Citation: Wang T, Wang DN, Liu WT, Zheng ZQ, Chen X, Fang WL, Li S, Liang L, Wang BM. Hemostatic effect of topical hemocoagulase spray in digestive endoscopy. World J Gastroenterol 2016; 22(25): 5831-5836
- URL: https://www.wjgnet.com/1007-9327/full/v22/i25/5831.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i25.5831
With the development of endoscopic techniques, more and more gastrointestinal diseases can be treated endoscopically, such as endoscopic resection of gastrointestinal polyps and endoscopic submucosal dissection (ESD) of tumors. Compared with traditional open surgery, endoscopic surgery can greatly reduce the trauma to patients. However, bleeding and perforation are the main complications of endoscopic treatment[1-3]. Effectively reducing the development of intraoperative bleeding and maintaining adequate visualization of the surgical field are the keys to reducing the incidence of complications with endoscopic treatment and ensuring successful endoscopic treatment.
Hemocoagulase for injection (Baquting), a hemostatic agent extracted and purified from the venom of Bothrops jararacussu, has thrombin- and thrombokinase-like effects[4,5]. It can cause platelet aggregation at the bleeding site, accelerate the hydrolysis of fibrin to form fibrin I monomer and polymer, and promote the formation of white thrombi, thereby achieving hemostatic effects[6]. Currently, hemocoagulase has been widely used to manage bleeding in various clinical settings, such as obstetrics, orthopaedics[7,8], and general surgery[9]. Hemocoagulase combined with proton pump inhibitors has been used to manage peptic ulcer bleeding. These studies indicated that hemocoagulase has a definite hemostatic effect. However, hemocoagulase was delivered via an intravenous route in most of previous studies. A study[10] showed that topical application of hemocoagulase at the bleeding site had dose-related effects in promoting fibrinogen polymerization, which was not inhibited by any plasma thrombin inhibitor or anticoagulant. Thus, hemocoagulase can be used as a topical hemostatic. A recent study[11] showed that local injection of hemocoagulase can achieve rates of successful hemostasis of 100% and 88.9%, respectively, for portal veins with inner diameters of < 1 mm and 1-2 mm, and the maximum time to achieve hemostasis was 24.0 ± 7.2 s, suggesting that hemocoagulase has a more obvious hemostatic effect in tiny blood vessels (diameter < 1 mm).
The purpose of the present study was to evaluate the hemostatic effect of hemocoagulase spraying on oozing bleeding in digestive endoscopy.
Eighty-nine patients who developed oozing bleeding (non-small artery or vein bleeding) during endoscopic treatment from September 2014 to October 2014 at Center for Digestive Endoscopy, Tianjin Medical University General Hospital were included in this study, including 32 patients who underwent ESD for gastric muscularis propria tumors, 22 patients who underwent esophageal tunneling techniques (including STER and POEM), and 35 patients who underwent ESD for gastric mucosal or submucosal tumors. Patients with coagulation disorders or drug allergies were excluded. The patients were randomly divided into a study group (n = 39) and a control group (n = 50). The study group was given topical hemocoagulase spray intraoperatively, while the control group was given traditional 8% norepinephrine (in normal saline) spray. There were no significant differences in demographic data between the two groups (Table 1).
| Study group | Control group | |
| Drug spray | Hemocoagulase | 8% norepinephrine |
| Patients | 39 | 50 |
| Gender | ||
| Male | 18 | 20 |
| Female | 21 | 30 |
| Age | 52.90 ± 1.73 | 53.10 ± 1.76 |
| Category of endoscopic treatments | ||
| ESD | 28 | 39 |
| Submucosal tunneling | 11 | 11 |
| (POEM and STER) | ||
| Hemostatic effect | ||
| Successful | 39 | 47 |
| Failed | 0 | 3 |
| Complication | 2 | 10 |
| Perforation | 2 | 7 |
| Late bleeding | 0 | 3 |
All patients were preoperatively given an intravenous drip of esomeprazole (40 mg + 0.9% normal saline 100 mL) once every 24 h. Patients who developed oozing bleeding (non-small artery or vein bleeding) during endoscopic treatment were randomly divided into a study group and a control group. The study group was treated with hemocoagulase for injection (Baquting).The hemocoagulase solution was prepared by dissolving 4U hemocoagulase in 60 mL normal saline. The solution (20 mL) was sprayed to the wound site at 30 s intervals until the bleeding stopped. The control group was treated with 8% norepinephrine spray at 30 s intervals. If active bleeding persisted 5 min after spraying, failed hemostasis was considered. For patients who had failed hemostasis and were found to have small artery or vein bleeding, electrocoagulation or clipping was performed to achieve hemostasis. Hemostatic efficacy was compared between the two groups. Bleeding site, wound cleanliness and perforation were observed. After wound processing was performed, the endoscope was withdrawn. Postoperatively, gastrointestinal decompression was carried out, and the patients were fasted and given parenteral nutrition support and an intravenous drip of esomeprazole (40 mg + 0.9% normal saline 100 mL) once every 12 h. Patients with perforation were treated with antibiotics to prevent wound infection. Late bleeding was observed and recorded. Statistical methods were then used to compare the rates of successful hemostasis, perforation and late bleeding between the two groups.
There were no significant differences in demographic data such as gender or age between the two groups.
Successful hemostasis was achieved in 39 (100%) patients of the study group and in 47 (94.0%) patients of the control group, and there was no significant difference in the rate of successful hemostasis between the two groups (χ2 = 3.541, P = 0.060) (Table 2). Three patients in the control group had failed hemostasis, and all of them underwent ESD for gastric mucosal or submucosa tumors. After endoscopic electrocoagulation or clipping was performed, successful hemostasis was achieved in all the three cases.
| Group | Study | Control | Total |
| Successful hemostasis | 39 (100) | 47 (94) | 86 (97) |
| Failed hemostasis | 0 (0) | 3 (6) | 3 (3) |
| Total | 39 | 50 | 89 |
There were a total of nine cases of perforation, all of which occurred in the gastric muscularis propria, including 2 (16.7%) cases in the study group and 7 (35.0%) cases in the control group. Patients with perforation after clipping hemostasis did not show any sign of peritonitis such as obvious abdominal pain. There was no significant difference in the rate of perforation between the two groups (χ2 = 0.505, P = 0.477) (Table 3). No late bleeding occurred in the study group, but there were 3 (15.8%) cases in the control group, all of which occurred in patients who underwent ESD for gastric mucosal or submucosal tumors. The rate of late bleeding was significantly lower in the study group than in the control group (χ2 = 3.901, P = 0.048) (Table 3). The rate of overall complications was also significantly lower in the study group than in the control group (χ2 = 4.576, P = 0.032) (Table 3).
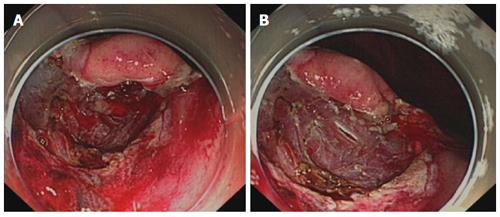
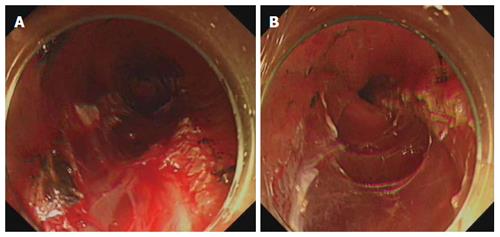
Compared with the control group, after hemocoagulase spray in the study group, bleeding stopped more rapidly, the surgical field was clearer, the bleeding site was more easily identified, and the world was cleaner (Figures 1 and 2).
With the wide application of digestive endoscopy in clinical practice, minimally invasive endoscopic techniques have gradually been advocated by more and more patients. Bleeding and perforation are common complications of endoscopic treatment[1-3]. Therefore, effective, fast endoscopic hemostasisis is very important for endoscopic treatment. Currently commonly used endoscopic hemostatic methods include mechanical hemostasis methods (such as electrocoagulation and clipping), topical spray of drugs, and local injection of drugs[12-14].
Clipping hemostasis is achieved by using titanium clips to mechanically clamp the bleeding vessels and surrounding tissues[15], with a definite and reliable hemostatic effect. However, clipping hemostasis is mainly suitable for exposed large or deep blood vessels[16], and its application in endoscopic tunneling techniques (such as STER and POEM) is limited. Electrocoagulation hemostasis is achieved by using local high-frequency heat energy to make local tissue necrotic or coagulated[17], and this method is particularly effective for small blood vessel hemorrhage[18]. However, the above hemostatic methods have a high requirement for operation skill, are expensive and more suitable for local hemostasis, and are often associated with unclean wound, which makes it difficult to identify the bleeding site. Thus, their application value in diffuse oozing bleeding is limited.
Many studies[11,14,19-21] showed that endoscopic injection or spray of hemostatic drugs can be used for topical hemostasis, with a definite hemostatic effect. Common topical hemostatic drugs include hypertonic saline, Yunnan Baiyao, thrombin, adrenaline and so on. Hypertonic saline can induce tissue edema at the injection site, local compression, fibrosis of the vessel wall, and vascular lumen thrombosis, thus achieving hemostasis. However, the application of hypertonic saline affects the observation of the wound and bleeding site, and for this reason, hypertonic saline was less applied clinically. Adrenaline does not damage the tissue, and after injection it can induce local vasoconstriction, swelling of the surrounding tissue to compress blood vessels, and platelet aggregation, thus achieving temporary hemostasis, with a rate of successful hemostasis of about 80%[20]. However, studies have shown that with the increase of the dose of adrenaline, it can lead to high blood pressure and increased heart rate. Therefore, its use in patients with cardiovascular diseases is restricted[22]. In addition, the hemostatic effect of adrenaline is short-acting, and it is associated with high rates of pseudo hemostasis and late bleeding.
Hemocoagulase is a hemostatic agent extracted and purified from the venom of Bothrops jararacussu, and it has advantages of high efficiency, rapid action, definite topical hemostatic effect, reduced local tissue inflammation and accelerated wound healing. It can not only promote the fibrin formation and result in rapid blood solidification to form blood clots, but also promote irreversible platelet aggregation and platelet release reaction, accelerate blood clotting, promote vascular epithelial cell growth, and accelerate wound healing, thus achieving a good hemostatic effect[11,23]. In this study, we used local hemocoagulase spray to manage oozing bleeding in digestive endoscopic treatment. The results showed that hemocoagulase had a comparable hemostatic effect to norepinephrine and was associated with a lower incidence of complications. In addition, no systemic adverse reactions such as elevated blood pressure and heart rate were observed. Compared with norepinephrine, hemocoagulase had a longer hemostatic effect. After endoscopic hemocoagulase spray, the wound was cleaner and the broken ends of small blood vessels were clearly shown, which makes the identification of bleeding sites easier. Therefore, topical hemocoagulase spray can not only achieve hemostasis, but also help provide good wound conditions for other hemostatic methods such as electrocoagulation and clipping to improve the efficiency of hemostasis, increase the success rate of hemostasis, reduce the damage of electrocoagulation to the wound, and decrease the rates of perforation and late bleeding associated with electrocoagulation.
In conclusion, topical hemocoagulase spray has a definite hemostatic effect for oozing bleeding in digestive endoscopy, and this method is simple and has low cost. Since the wound after topical hemocoagulase spray is clean, the bleeding site is easily identified, which is conductive to postoperative recovery. Topical hemocoagulase spray is associated with a low rate of late bleeding, and it is expected to be widely used in endoscopic therapy. A study has shown that topical hemocoagulase spray can be used for the treatment of upper gastrointestinal mucosal bleeding in hepatitis B patients[24], but its curative effect needs further validation. However, topical hemocoagulase spray is more suitable for oozing non-small artery or vein bleeding, and its hemostatic effect in large vessel bleeding is poorer than clipping, which is the main limitation of topical hemocoagulase spray. In addition, the sample size of this study is small, and multicenter studies with larger sample sizes are warranted to confirm the hemostatic efficacy of topical hemocoagulase spray.
Bleeding and perforation are the main complications of endoscopic treatment, how to reduce the incidence of complications remains to be studied. Many studies indicated that hemocoagulase has a definite hemostatic effect and can be used as a topical hemostatic. The purpose of the present study was to evaluate the hemostatic effect of hemocoagulase spraying on oozing bleeding in digestive endoscopy.
Hemocoagulase has thrombin- and thrombokinase-like effects, and it has been widely used to manage bleeding in various clinical settings. A study showed that topical application of hemocoagulase at the bleeding site had dose-related effects in promoting fibrinogen polymerization. However, no one has used it for endoscopic hemostasis, or explored its effectiveness.
The authors compared the rate of successful hemostasis between hemocoagulase spray and 8% norepinephrine spray, and showed that there was no significant difference in the rate of successful hemostasis, but the rates of late bleeding and overall complications of the hemocoagulase group were significantly lower than the 8% norepinephrine group. The surgical field was clearer, the bleeding site was more easily identified, and the wound was cleaner in the study group.
Topical hemocoagulase spray has a definite hemostatic effect for oozing bleeding in digestive endoscopy, and this method is convenient, safe, and reliable. It is expected to become a new method for endoscopic hemostasis.
Endoscopic hemocoagulase spray is a method of spraying the hemocoagulase solution to the wound site at 30 s intervals until the bleeding stops.
This study is an interesting study about the hemostatic effect of topical hemocoagulase spray in digestive endoscopy. Compared with the control group, after topical hemocoagulase spray in the study group, the surgical field was clearer, the bleeding site was more easily identified, and the wound was cleaner. There was no significant difference in the rate of perforation between the study and control groups, but the rates of late bleeding and overall complications were significantly lower in the study group. Over all, the study is well designed and the manuscript can be accepted. Large-sample studies should be performed in the future.
Manuscript Source: Unsolicited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: China
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sogabe I, Toth E S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Takeda S, Mitoro A, Namisaki T, Yoshida M, Sawai M, Yamao J, Yoshiji H, Uejima M, Moriya K, Douhara A. Gastric adenocarcinoma of fundic gland type (chief cell predominant type) with unique endoscopic appearance curatively treated by endoscopic submucosal resection. Acta Gastroenterol Belg. 2015;78:340-343. [PubMed] |
| 2. | Zhang Y, Chen Y, Qu CY, Zhou M, Ni QW, Xu LM. Effects of medical adhesives in prevention of complications after endoscopic submucosal dissection. World J Gastroenterol. 2013;19:2704-2708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Salah W, Faigel DO. When to puncture, when not to puncture: Submucosal tumors. Endosc Ultrasound. 2014;3:98-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Marsh N, Williams V. Practical applications of snake venom toxins in haemostasis. Toxicon. 2005;45:1171-1181. [PubMed] |
| 5. | Braud S, Bon C, Wisner A. Snake venom proteins acting on hemostasis. Biochimie. 2000;82:851-859. [PubMed] |
| 6. | Stocker KF. Snake venom proteins affecting hemostasis and fibrinolysis. Medical Use of Snake Venom Proteins. Boca Raton: CRC Press Inc 1990; 97-160. |
| 7. | Hu HM, Chen L, Frary CE, Chang CC, Hui H, Zhang HP, Huang DG, Liu ZK, Zhao YT, He SM. The beneficial effect of Batroxobin on blood loss reduction in spinal fusion surgery: a prospective, randomized, double-blind, placebo-controlled study. Arch Orthop Trauma Surg. 2015;135:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Xu C, Wu A, Yue Y. Which is more effective in adolescent idiopathic scoliosis surgery: batroxobin, tranexamic acid or a combination? Arch Orthop Trauma Surg. 2012;132:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Zeng Z, Xiao P, Chen J, Wei Y. Are batroxobin agents effective for perioperative hemorrhage in thoracic surgery? A systematic review of randomized controlled trials. Blood Coagul Fibrinolysis. 2009;20:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Meier J, Stocker K. Effects of snake venoms on hemostasis. Crit Rev Toxicol. 1991;21:171-182. [PubMed] |
| 11. | Luo Y, Liu Q, Jiao Z, Wu R, Tang J, Lv F. A comparison study of local injection and radiofrequency ablation therapy for traumatic portal vein injure guided by contrast-enhanced ultrasonography. Ann Hepatol. 2012;11:249-256. [PubMed] |
| 12. | Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, Sinclair P. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152:101-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 754] [Cited by in RCA: 710] [Article Influence: 47.3] [Reference Citation Analysis (1)] |
| 13. | Sung JJ, Chan FK, Chen M, Ching JY, Ho KY, Kachintorn U, Kim N, Lau JY, Menon J, Rani AA. Asia-Pacific Working Group consensus on non-variceal upper gastrointestinal bleeding. Gut. 2011;60:1170-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 14. | Holster IL, Kuipers EJ. Update on the endoscopic management of peptic ulcer bleeding. Curr Gastroenterol Rep. 2011;13:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Raju GS, Gajula L. Endoclips for GI endoscopy. Gastrointest Endosc. 2004;59:267-279. [PubMed] |
| 16. | Ljubicic N. Efficacy of endoscopic clipping and long-term follow-up of bleeding Dieulafoy’s lesions in the upper gastrointestinal tract. Hepatogastroenterology. 2006;53:224-227. [PubMed] |
| 17. | Lei KF, Chen KH, Tsui PH, Tsang NM. Real-time electrical impedimetric monitoring of blood coagulation process under temperature and hematocrit variations conducted in a microfluidic chip. PLoS One. 2013;8:e76243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Hui AJ, Sung JJ. Endoscopic Treatment of Upper Gastrointestinal Bleeding. Curr Treat Options Gastroenterol. 2005;8:153-162. [PubMed] |
| 19. | Changela K, Papafragkakis H, Ofori E, Ona MA, Krishnaiah M, Duddempudi S, Anand S. Hemostatic powder spray: a new method for managing gastrointestinal bleeding. Therap Adv Gastroenterol. 2015;8:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 20. | Calvet X, Vergara M, Brullet E, Gisbert JP, Campo R. Addition of a second endoscopic treatment following epinephrine injection improves outcome in high-risk bleeding ulcers. Gastroenterology. 2004;126:441-450. [PubMed] |
| 21. | Chung SC, Leong HT, Chan AC, Lau JY, Yung MY, Leung JW, Li AK. Epinephrine or epinephrine plus alcohol for injection of bleeding ulcers: a prospective randomized trial. Gastrointest Endosc. 1996;43:591-595. [PubMed] |
| 22. | Liou TC, Lin SC, Wang HY, Chang WH. Optimal injection volume of epinephrine for endoscopic treatment of peptic ulcer bleeding. World J Gastroenterol. 2006;12:3108-3113. [PubMed] |
| 23. | Jin Y. Hemostasis mechanism of Hemocoagulase atrox for injection. Zhongguo Yiyuan Yongyao Pingjia Yu Fenxi. 2012;12:488-490. |
| 24. | Wang X, Wang Z, Jin B, Xiang L, Li H, Han J. Efficacy of endoscopic spray blood coagulation in the treatment of patients with hepatitis B. Zhongguo Yiyao. 2010;11:1092. [DOI] [Full Text] |