Published online Jul 7, 2016. doi: 10.3748/wjg.v22.i25.5769
Peer-review started: March 4, 2016
First decision: April 1, 2016
Revised: April 12, 2016
Accepted: May 4, 2016
Article in press: May 4, 2016
Published online: July 7, 2016
Processing time: 123 Days and 21.5 Hours
AIM: To investigated the effects of urotensin II (UII) on hepatic insulin resistance in HepG2 cells and the potential mechanisms involved.
METHODS: Human hepatoma HepG2 cells were cultured with or without exogenous UII for 24 h, in the presence or absence of 100 nmol/L insulin for the last 30 min. Glucose levels were detected by the glucose-oxidase method and glycogen synthesis was analyzed by glycogen colorimetric/fluorometric assay. Reactive oxygen species (ROS) levels were detected with a multimode reader using a 2′,7′-dichlorofluorescein diacetate probe. The protein expression and phosphorylation levels of c-Jun N-terminal kinase (JNK), insulin signal essential molecules such as insulin receptor substrate -1 (IRS-1), protein kinase B (Akt), glycogen synthase kinase-3β (GSK-3β), and glucose transporter-2 (Glut 2), and NADPH oxidase subunits such as gp91phox, p67phox, p47phox, p40phox, and p22phox were evaluated by Western blot.
RESULTS: Exposure to 100 nmol/L UII reduced the insulin-induced glucose consumption (P < 0.05) and glycogen content (P < 0.01) in HepG2 cells compared with cells without UII. UII also abolished insulin-stimulated protein expression (P < 0.01) and phosphorylation of IRS-1 (P < 0.05), associated with down-regulation of Akt (P < 0.05) and GSK-3β (P < 0.05) phosphorylation levels, and the expression of Glut 2 (P < 0.001), indicating an insulin-resistance state in HepG2 cells. Furthermore, UII enhanced the phosphorylation of JNK (P < 0.05), while the activity of JNK, insulin signaling, such as total protein of IRS-1 (P < 0.001), phosphorylation of IRS-1 (P < 0.001) and GSK-3β (P < 0.05), and glycogen synthesis (P < 0.001) could be reversed by pretreatment with the JNK inhibitor SP600125. Besides, UII markedly improved ROS generation (P < 0.05) and NADPH oxidase subunit expression (P < 0.05). However, the antioxidant/NADPH oxidase inhibitor apocynin could decrease UII-induced ROS production (P < 0.05), JNK phosphorylation (P < 0.05), and insulin resistance (P < 0.05) in HepG2 cells.
CONCLUSION: UII induces insulin resistance, and this can be reversed by JNK inhibitor SP600125 and antioxidant/NADPH oxidase inhibitor apocynin targeting the insulin signaling pathway in HepG2 cells.
Core tip: We report our results on urotensin II (UII) in a newly developed insulin resistance model and the possible mechanisms involved. Exposure to UII may contribute to oxidative damage via the NADPH oxidase pathway and enhance the phosphorylation of c-Jun N-terminal kinase, which is associated with insulin signal transduction pathways. These may be the underlying mechanisms of UII-mediated insulin resistance in HepG2 cells. These findings confirm the important role of UII in hepatic insulin resistance, which shed light on new insight into hepatic insulin resistance.
- Citation: Li YY, Shi ZM, Yu XY, Feng P, Wang XJ. Urotensin II-induced insulin resistance is mediated by NADPH oxidase-derived reactive oxygen species in HepG2 cells. World J Gastroenterol 2016; 22(25): 5769-5779
- URL: https://www.wjgnet.com/1007-9327/full/v22/i25/5769.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i25.5769
Urotensin II (UII) is a vasoactive peptide first discovered in teleost fishes in 1985[1], and subsequently found in mammals and humans[2]. Ames identified a G protein-coupled UII receptor (GPR14) in 1999[3]. UII acts by binding to the UII receptor, and its expression has been detected in various tissues and organs, including cardiac and vascular tissues, the central nervous system, spinal cord, kidney, liver, and pancreas[4]. In addition to its significant role in the cardiovascular system[5], UII is also involved in metabolic syndrome (MetS) and plays an important role in type II diabetes mellitus (T2DM)[6]. Perfusion of rat pancreas with UII in vitro reduced glucose-induced insulin secretion[7]. The UII/UII receptor system was up-regulated, inhibiting insulin-stimulated 2-deoxyglucose uptake, in the skeletal muscle of diabetic mice[8], and impaired skeletal muscle glucose transport via the NADPH oxidase pathway[9]. In addition, UII expression was elevated in diethylnitrosamine-mediated precancerous rat liver lesions[10], and UII knockout mice had significantly decreased low-density lipoprotein cholesterol profiles and hepatic steatosis, consistent with dyslipidemia[11], with lower plasma insulin and glucose levels and increased tolerance[12].
Insulin resistance is a common pathophysiological condition in which higher concentrations of insulin are required to maintain normal insulin actions in target tissues such as liver, muscle, and adipose tissues[13]. Recent studies have shown that insulin resistance is induced by multiple factors, such as high glucose, free fatty acids, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and resistin[14-18]. Elevated plasma UII levels, which often accompany MetS and T2DM, may play a role in insulin resistance. However, the precise molecular mechanisms with regard to insulin resistance remain poorly understood.
Accumulating evidence suggests that oxidative stress associated with increased production of ROS, is an important trigger in the process of insulin resistance[19]. Much attention has been focused on NADPH oxidase as a primary source of ROS generation in insulin resistance[20]. Wei et al[21,22] found that NADPH oxidase activation played a crucial role in angiotensin II-induced insulin resistance in skeletal muscle, and our preliminary studies[9] showed a similar effect. UII expression was increased in precancerous liver lesions in rats and in human liver tumor tissue[10,23] and associated with a high risk of insulin resistance. However, the role of UII in hepatic insulin resistance has not been explored. The present study investigated the role of UII in mediating insulin resistance in HepG2 cells, a frequently used in vitro system for studying insulin resistance in hepatic cells, and determined its involvement in the insulin signaling pathway.
Human hepatoma HepG2 cells (China Infrastructure of Cell Line Resources) were cultured in Dulbeccos’ modified Eagle’s medium (DMEM)/high glucose (Hyclone, Beijing, China) containing 200 mL/L fetal bovine serum (Gibco, New York, United States) and 10 mL/L penicillin-streptomycin (Gibco). Cells were incubated at 37 °C in a humidified atmosphere of 50 mL/L CO2. Cells were incubated with recombinant human UII (Sigma-Aldrich, Missouri, United States), and in some experiments, cultured HepG2 cells were pretreated with apocynin (200 μmol/L, Sigma) or SP600125 (Selleck Chemicals, United States) to inhibit ROS and JNK, respectively.
HepG2 cells were cultured in serum- and phenol red-free DMEM (Gibco) with various concentrations (0, 1, 10, 100 nmol/L) of exogenous UII for 24 h, with the addition of 100 nmol/L insulin (Novolin30R, Novo Nordisk, North Carolina, United States) for the last 30 min. The supernatant was then removed and the glucose concentration was detected using the glucose-oxidase method (Applygen Technologies, Beijing, China). Glucose consumption was calculated as the glucose concentrations in the test wells subtracted from the concentration in the blank control wells, and normalized with cellular protein concentration[14,24].
HepG2 cells were treated with or without 100 nmol/L UII for 24 h, followed by serum-free DMEM (Gibco) for 4 h in the presence or absence of 100 nmol/L insulin for the last 30 min[18]. Glycogen levels were measured using a glycogen colorimetric/fluorometric assay kit (Biovision, California, United States).
The dye 2′,7′-dichlorofluorescein diacetate (DCF-DA) (Nanjing Jiancheng Biotechnology, Jiangsu, China) was used to measure changes in ROS levels. Briefly, HepG2 cells were incubated with 5 μmol/L DCF-DA for 30 min at 37 °C in 96-well clear-bottom, black assay plates (Corning Incorporated, NY, United States), and washed three times with phosphate-buffered saline. ROS levels were determined by analyzing the fluorescence using a multimode reader (Molecular Devices SpectraMax M2/M2e, Highcreation, China), and expressed as the ratio of fluorescence intensity to cell proliferation.
Cell lysates (40-60 μg protein) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes (Millipore, Massachusetts, United States), and then incubated with primary antibodies at 4 °C overnight, after blocking with 50 g/L nonfat dry milk for 1 h. The blots were subsequently incubated with horseradish peroxidase-conjugated secondary antibodies, followed by detection with enhanced chemiluminescence (Applygen). Antibodies against SAPK/JNK, phospho (p)-SAPK/JNK (Thr183/Tyr185), insulin receptor substrate-1 (IRS-1), pIRS-1 (Tyr895), Akt, pAkt (Thr308), glycogen synthase kinase 3β (GSK-3β), pGSK-3β (Ser9) and β-actin were all obtained from Cell Signaling. Antibodies against p22phox, p67phox, and p40phox were from Santa Cruz Biotechnology, and antibodies against glucose transporter-2 (Glut2), gp91phox, and p47phox were purchased from Bioworld Technology. Detailed information of all antibodies can be found in Supplementary Table 1.
Data from at least three independent experiments are presented as the mean ± SD. Statistical analyses were carried out by one-way analysis of variance followed by Bonferroni’s adjustment. The reference condition was set to 1. A P value < 0.05 was considered significant. Statistical analyses were performed using SPSS version 15.0 software (SPSS Inc., Chicago).
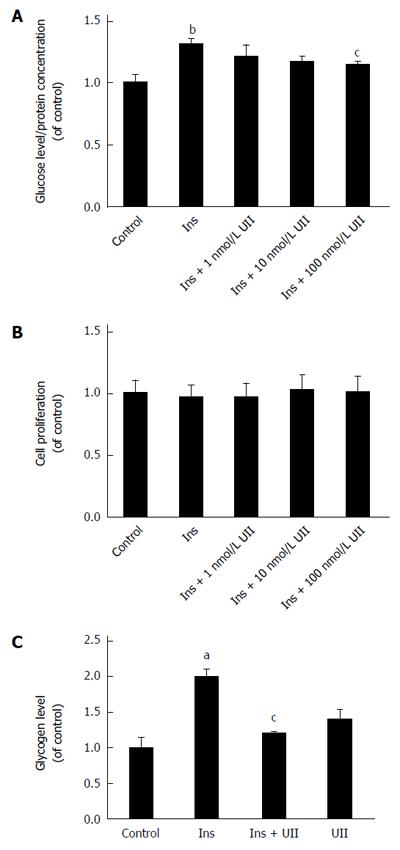
We examined the effect of UII on glucose consumption in human hepatoma HepG2 cells by incubating them with different concentrations of exogenous UII (0, 1, 10, or 100 nmol/L) for 24 h, with the addition of 100 nmol/L insulin for the last 30 min. UII decreased insulin-mediated glucose consumption in HepG2 cells in a dose-dependent manner (Figure 1A), in accord with the effect of UII on glucose transport in skeletal muscle[8]. Interestingly, glucose consumption was unaffected when HepG2 cells were treated with UII alone for 24 h (Supplementary Figure 1). To eliminate the possible influence of UII on apoptosis, we quantified cell viability by MTT assay and demonstrated no cytotoxic effect after exposure of HepG2 cells to UII for 24 h (Figure 1B). Previous studies demonstrated that 100 nmol/L UII inhibited glucose transport in C2C12 mouse myotube cells[9], and we therefore evaluated the effect of 100 nmol/L UII on glycogen synthesis, a metabolic endpoint of insulin action, in HepG2 cells. The insulin-induced glycogen content was significantly reduced in HepG2 cells after incubation with 100 nmol/L UII for 24 h, as analyzed using a glycogen-detection kit(Figure 1C).
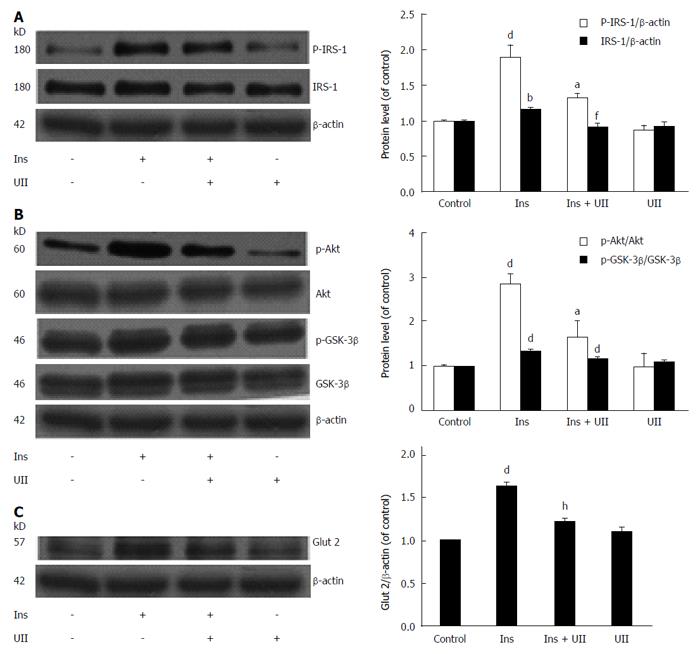
To determine if UII impairs the insulin signaling pathway in HepG2 cells, we subjected cell lysates to Western blot analysis. Expression levels of insulin-stimulated IRS-1 and tyrosine phosphorylation of IRS-1 were inhibited by UII treatment in HepG2 cells, while UII alone had no effect (Figure 2A). Downstream of IRS-1, insulin-mediated phosphorylation of Akt and GSK-3β was also impaired by 100 nmol/L UII, while total Akt and GSK-3β protein levels were unaffected (Figure 2B). Glut 2 is closely associated with glucose transport in liver cells. We observed that Glut 2 protein could be reduced by UII (Figure 2C), which potentially resulted in abnormal glucose metabolism. Overall, these results indicate that UII induces an insulin-resistant state in HepG2 cells.
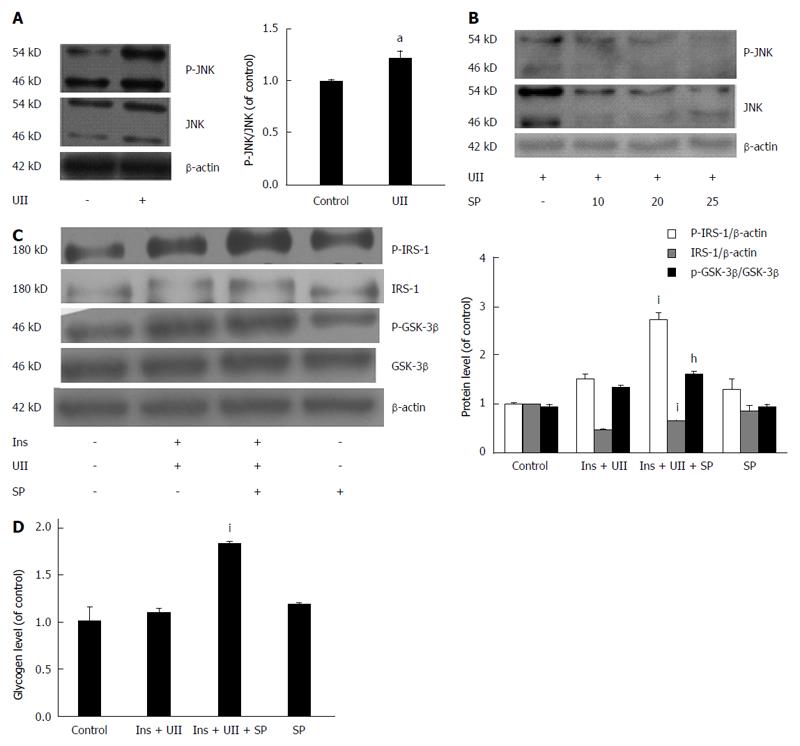
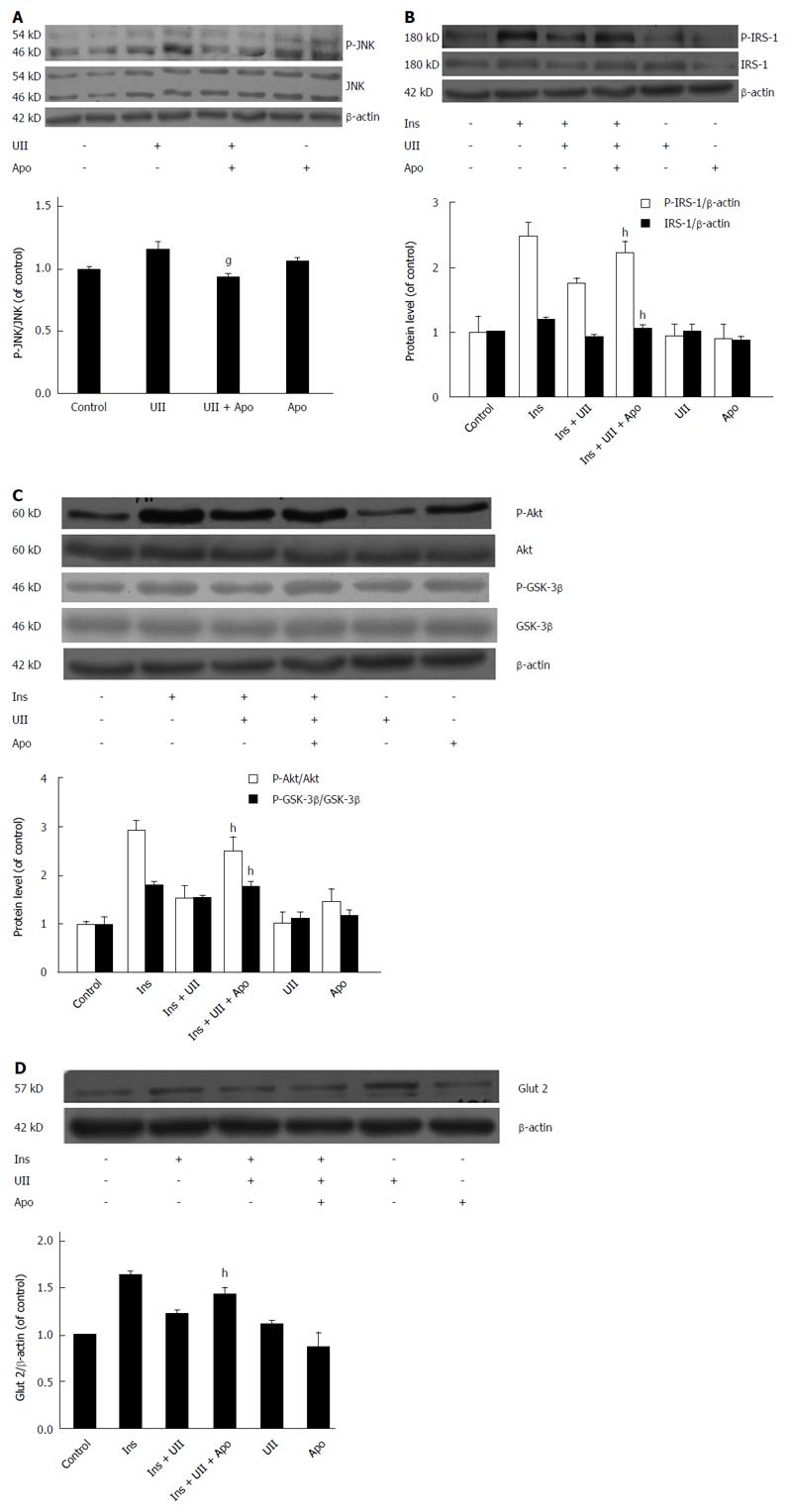
JNK has been reported to phosphorylate serine residues of IRS-1/-2[25,26], thus impairing IRS tyrosine phosphorylation and reducing insulin receptor-induced signaling. Furthermore, UII has been shown to increase JNK activity in human pulmonary artery smooth muscle cells (PASMCs)[27]. We therefore determined the role of UII in JNK activation and its correlation with insulin resistance in HepG2 cells. UII increased JNK phosphorylation (Figure 3A), and this effect was abrogated by the JNK inhibitor SP600125 in a dose-dependent manner, indicating that UII activated JNK (Figure 3B). To determine if UII-mediated JNK activation induced insulin resistance, cells were pretreated with SP600125. In parallel with decreased JNK phosphorylation, SP600125 (20 μmol/L) increased insulin-induced phosphorylation of IRS-1 and IRS proteins, followed by rescue of GSK-3β phosphorylation in HepG2 cells exposed to UII (Figure 3C). Moreover, SP600125 reversed the UII-induced decrease in cellular glycogen levels (Figure 3D). These results suggest that JNK activation by UII contributes to UII-induced insulin resistance in HepG2 cells.
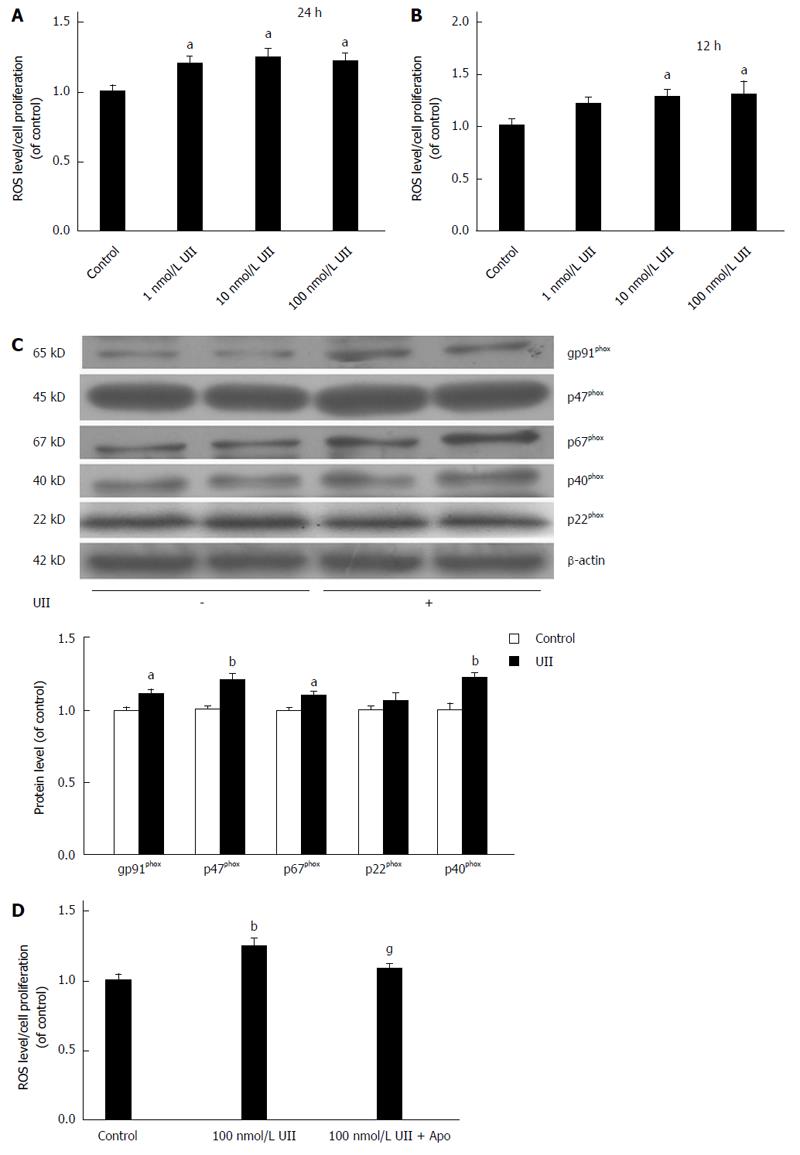
ROS generation is an important component of insulin resistance[28]. We thus further elucidated the molecular mechanisms responsible for UII-induced insulin resistance in HepG2 cells by assessing the effects of UII on ROS levels. HepG2 cells were exposed to different concentrations UII for 12 or 24 h, stained with DCF, and detected using a multimode reader. ROS levels were both dose- and time-dependently increased by UII in HepG2 cells (Figure 4A and B).
We also evaluated the relationship between ROS generation and NADPH oxidase by investigating NADPH oxidase subunit expression in response to UII and apocynin. NADPH oxidase subunits gp91phox, p67phox, p47phox, and p40phox protein levels were up-regulated in HepG2 cells treated with 100 nmol/L UII for 24 h (Figure 4C). Pretreatment with the NADPH synthase inhibitor apocynin for 30 min significantly inhibited the UII-induced generation of ROS (Figure 4D). Taken together, these results suggest that UII enhanced ROS production, at least in part, by increasing NADPH oxidase in HepG2 cells, consistent with its effects in PASMCs[27].
We investigated if JNK activation and ROS overproduction plays causal roles in UII-induced insulin resistance in HepG2 cells. ROS are known to facilitate the activation of JNK, and deletion or inhibition of JNK improved insulin sensitivity in mice[29]. In addition, the JNK pathway has been reported to mediate ROS generation in PASMCs induced by UII[27]. It is therefore possible that JNK may mediate ROS-induced insulin resistance. Our results showed that UII elevated the phosphorylation of JNK, and that this effect could be attenuated by apocynin (Figure 5A).
To determine if ROS and JNK are signaling intermediates in hepatic insulin resistance targeted by UII, HepG2 cells were pretreated with apocynin for 30 min. Inhibition of ROS by apocynin prevented UII-induced inhibition of insulin-stimulated total IRS-1 expression and IRS-1 tyrosine phosphorylation (Figure 5B). Similarly, pretreatment with apocynin abrogated UII-inhibited Akt and GSK-3β phosphorylation (Figure 5C) and Glut 2 expression (Figure 5D) in HepG2 cells. Overall, these results suggest that UII-induced insulin resistance is mediated by NADPH oxidase-derived ROS via JNK pathways in HepG2 cells, in accordance with previous studies of palmitate and TNF-α[24,30].
Insulin resistance is an essential factor in the pathogenesis of T2DM. Plasma UII levels were shown to be raised in diabetic mice and inhibit skeletal muscle glucose transport, suggesting a vital role for UII in the initiation and development of insulin resistance[8,9]. Insulin resistance involves decreased uptake and utilization of glucose promoted by insulin, and leads to impaired glucose consumption and glycogen synthesis in the liver, which is one of the main target organs of insulin resistance. Disruption of the liver microenvironment as a result of elevated UII levels may induce hepatic insulin resistance. However, animal models of insulin resistance are complex, and it is difficult to restrict the contribution of UII to the liver. We therefore assessed the effects of UII in HepG2 cells in vitro, and provided the first evidence for the induction of insulin resistance in HepG2 cells by UII.
However, the molecular link between UII and insulin resistance remains unclear. Intracellular JNK signaling pathways are known to be closely associated with insulin resistance[31]. We accordingly demonstrated that JNK played a vital role in the development of UII-induced insulin resistance in HepG2 cells, while the JNK inhibitor SP600125 rescued cells from UII-induced impairment of insulin signaling and glycogen synthesis. However, SP600125 failed to reverse Glut 2 protein expression (Supplementary Figure 2), suggesting that other pathways may also be involved in UII-induced insulin resistance in HepG2 cells.
Oxidative stress plays an important role in blunting insulin responsiveness. Our results also demonstrated that UII significantly increased ROS production, suggesting a link between UII and insulin resistance in HepG2 cells. Furthermore, NADPH oxidases are known to be a potential source of ROS, and we showed that UII up-regulated gp91phox, p67phox, p47phox, and p40phox protein levels in HepG2 cells, indicating that UII may increase ROS production through NADPH oxidases.
Apocynin rescued JNK by reducing ROS production, and also reversed the effects of UII on the insulin signaling pathway, suggesting that JNK is a downstream effector of ROS in UII-induced insulin resistance in HepG2 cells. In addition, the oxidative stress-induced decrease in insulin signaling contributes to inhibition of GSK-3β phosphorylation, the key enzyme in glycogen metabolism, and the facultative glucose transporter Glut2, which is located in/on the liver cell surface and transports molecules such as glucose and fructose in and out of the cells. These results are in accord with the findings in the liver of rats with high-fat diet-induced insulin resistance[32]. Our results demonstrated that UII alone did not alter GSK-3β phosphorylation and Glut 2 protein expression, but suppressed both of these in the presence of insulin, while pretreatment with either SP600125 or apocynin could reverse these effects. The changes in GSK-3β phosphorylation and/or Glut 2 expression suggest that the ability of insulin-stimulated HepG2 cells to take up glucose is partly blocked by exposure to UII, leading to decreased glucose consumption and hepatic glycogen synthesis. Collectively, these results demonstrate that UII impairs glucose consumption and glycogen synthesis in HepG2 cells, at least partly via a mechanism involving ROS production and the eventual occurrence of insulin resistance.
However, the effect of the ROS pathway may not be specific to UII, and chronic inflammation induced by adipokines and cytokines, associated with obesity or MetS, such as TNF-α and IL-6, is also known to induce insulin resistance in the liver[33,34]. Indeed, UII has a strong proinflammatory effect[6], and the role of inflammatory cytokines accompanied by ROS in the development of insulin resistance should be further explored. Furthermore, ROS have been reported to affect various signaling pathways involving not only JNK, but also Foxo, JAK/signal transducer and activator of transcription, mitogen-activated protein kinase, p53, and phosphoinositide 3-kinase, depending on various factors including the types of ROS and cells, and the duration of exposure[35]. In addition to impairing glucose consumption and glycogen synthesis, hepatic insulin resistance could also suppress protein synthesis, lipogenesis, and increase hepatic gluconeogenesis. However, further studies are needed to clarify the mechanisms responsible for UII-induced insulin resistance.
In summary, the results of the present study provide the first evidence demonstrating that UII enhances ROS levels by up-regulating NADPH oxidase subunits and promoting the phosphorylation of JNK, leading to reduced insulin sensitivity, glucose consumption, and glycogen synthesis in HepG2 cells. These results show that the effects of UII on insulin resistance are mediated by NADPH oxidase-derived ROS through the JNK pathway in HepG2 cells, and may be involved in the development of diabetes and MetS.
Insulin resistance is associated with many diseases, such as obesity, metabolic syndrome (MetS) and type II diabetes mellitus (T2DM). Urotensin II (UII) is also involved in MetS in addition to its significant role in the cardiovascular system, and plays an important role in the pathogenesis of insulin resistance and development of T2DM, but the exact mechanism remains unclear.
UII, a vasoactive peptide expressed in various tissues and organs, promotes endothelial cell proliferation, regulates blood pressure and is involved in glucose homeostasis. Recent studies show that UII gene polymorphism is positively correlated with insulin resistance. UII knockout mice had significantly decreased plasma insulin and glucose levels and increased tolerance. Moreover, elevated UII impairs insulin signaling in the muscle of diabetic model mice. The research hotspot is the mechanism of UII to induce insulin resistance.
In this article, the authors investigated the effects and mechanisms of UII in inducing insulin resistance in HepG2 cells. They provide the first evidence demonstrating that UII enhances ROS levels by up-regulating NADPH oxidase subunits and promotes the phosphorylation of JNK, which together lead to hepatic insulin resistance.
The study results suggest that UII induces insulin resistance, and may be a new target for preventing the development of MetS and diabetes in future.
UII, a vasoactive cyclic peptide, plays a variety of biological effects after binding to UII receptor. Studies have shown that UII participates in the pathological processes of many diseases, such as coronary heart disease, high blood pressure, diabetes and renal failure. In addition to its significant role in the cardiovascular system, the up-regulation of UII directly affects the pancreas, which results in impaired pancreatic beta cells, reduced glucose-induced insulin secretion, and damaged glucose tolerance.
In this study, Li and collaborators address, quite convincingly, that UII is an inducer of hepatic insulin resistance by studying the impact of a 24-h UII pre-treatment of hepatic cells HepG2 on subsequent insulin action. Authors show that pre-treatment of HepG2 cells with UII decreased hepatic glucose uptake, glycogen synthesis and diminished the activation of the key insulin-signaling molecules IRS1, PKB and GSK3 as assessed by the use of phospho-specific antibodies. Putative mechanisms are presented, including the upregulation of JNK activation and the NADPH oxidase compounds and consequent oxidative stress. This is a very well performed study, and the results are quite convincing.
Manuscript Source: Unsolicited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: China
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Pirola L, Zhang J S- Editor: Yu J L- Editor: Wang TQ E- Editor: Ma S
| 1. | Pearson D, Shively JE, Clark BR, Geschwind II, Barkley M, Nishioka RS, Bern HA. Urotensin II: a somatostatin-like peptide in the caudal neurosecretory system of fishes. Proc Natl Acad Sci USA. 1980;77:5021-5024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 294] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Coulouarn Y, Lihrmann I, Jegou S, Anouar Y, Tostivint H, Beauvillain JC, Conlon JM, Bern HA, Vaudry H. Cloning of the cDNA encoding the urotensin II precursor in frog and human reveals intense expression of the urotensin II gene in motoneurons of the spinal cord. Proc Natl Acad Sci USA. 1998;95:15803-15808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 316] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 3. | Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, Louden CS, Foley JJ, Sauermelch CF, Coatney RW. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 633] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 4. | Ross B, McKendy K, Giaid A. Role of urotensin II in health and disease. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1156-R1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 5. | Watanabe T, Arita S, Shiraishi Y, Suguro T, Sakai T, Hongo S, Miyazaki A. Human urotensin II promotes hypertension and atherosclerotic cardiovascular diseases. Curr Med Chem. 2009;16:550-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Barrette PO, Schwertani AG. A closer look at the role of urotensin II in the metabolic syndrome. Front Endocrinol (Lausanne). 2012;3:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Silvestre RA, Egido EM, Hernández R, Marco J. Characterization of the insulinostatic effect of urotensin II: a study in the perfused rat pancreas. Regul Pept. 2009;153:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Wang HX, Zeng XJ, Liu Y, Wang J, Lu LQ, Hao G, Zhang LK, Tang CS. Elevated expression of urotensin II and its receptor in skeletal muscle of diabetic mouse. Regul Pept. 2009;154:85-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Wang HX, Wu XR, Yang H, Yin CL, Shi LJ, Wang XJ. Urotensin II inhibits skeletal muscle glucose transport signaling pathways via the NADPH oxidase pathway. PLoS One. 2013;8:e76796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Wang H, Dong K, Xue X, Feng P, Wang X. Elevated expression of urotensin II and its receptor in diethylnitrosamine-mediated precancerous lesions in rat liver. Peptides. 2011;32:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Kiss RS, You Z, Genest J, Behm DJ, Giaid A. Urotensin II differentially regulates macrophage and hepatic cholesterol homeostasis. Peptides. 2011;32:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | You Z, Genest J, Barrette PO, Hafiane A, Behm DJ, D’Orleans-Juste P, Schwertani AG. Genetic and pharmacological manipulation of urotensin II ameliorate the metabolic and atherosclerosis sequalae in mice. Arterioscler Thromb Vasc Biol. 2012;32:1809-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42:1331-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 317] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 14. | Yin J, Hu R, Chen M, Tang J, Li F, Yang Y, Chen J. Effects of berberine on glucose metabolism in vitro. Metabolism. 2002;51:1439-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 195] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Pereira S, Park E, Mori Y, Haber CA, Han P, Uchida T, Stavar L, Oprescu AI, Koulajian K, Ivovic A. FFA-induced hepatic insulin resistance in vivo is mediated by PKCδ, NADPH oxidase, and oxidative stress. Am J Physiol Endocrinol Metab. 2014;307:E34-E46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Solomon SS, Buss N, Shull J, Monnier S, Majumdar G, Wu J, Gerling IC. Proteome of H-411E (liver) cells exposed to insulin and tumor necrosis factor-alpha: analysis of proteins involved in insulin resistance. J Lab Clin Med. 2005;145:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Senn JJ, Klover PJ, Nowak IA, Mooney RA. Interleukin-6 induces cellular insulin resistance in hepatocytes. Diabetes. 2002;51:3391-3399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 624] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 18. | Luo Z, Zhang Y, Li F, He J, Ding H, Yan L, Cheng H. Resistin induces insulin resistance by both AMPK-dependent and AMPK-independent mechanisms in HepG2 cells. Endocrine. 2009;36:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA. Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm. 2010;2010:453892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 20. | Delbosc S, Paizanis E, Magous R, Araiz C, Dimo T, Cristol JP, Cros G, Azay J. Involvement of oxidative stress and NADPH oxidase activation in the development of cardiovascular complications in a model of insulin resistance, the fructose-fed rat. Atherosclerosis. 2005;179:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 176] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, Stump CS. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am J Physiol Endocrinol Metab. 2008;294:E345-E351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM, Clark SE, Morris EM, Szary N, Manrique C, Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;281:35137-35146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 236] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 23. | Yu XT, Wang PY, Shi ZM, Dong K, Feng P, Wang HX, Wang XJ. Up-regulation of urotensin II and its receptor contributes to human hepatocellular carcinoma growth via activation of the PKC, ERK1/2, and p38 MAPK signaling pathways. Molecules. 2014;19:20768-20779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Gao D, Nong S, Huang X, Lu Y, Zhao H, Lin Y, Man Y, Wang S, Yang J, Li J. The effects of palmitate on hepatic insulin resistance are mediated by NADPH Oxidase 3-derived reactive oxygen species through JNK and p38MAPK pathways. J Biol Chem. 2010;285:29965-29973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 25. | Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2445] [Cited by in RCA: 2457] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 26. | Nakatani Y, Kaneto H, Kawamori D, Hatazaki M, Miyatsuka T, Matsuoka TA, Kajimoto Y, Matsuhisa M, Yamasaki Y, Hori M. Modulation of the JNK pathway in liver affects insulin resistance status. J Biol Chem. 2004;279:45803-45809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 27. | Djordjevic T, BelAiba RS, Bonello S, Pfeilschifter J, Hess J, Görlach A. Human urotensin II is a novel activator of NADPH oxidase in human pulmonary artery smooth muscle cells. Arterioscler Thromb Vasc Biol. 2005;25:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 128] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51:993-999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 450] [Cited by in RCA: 431] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 29. | Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka TA, Matsuhisa M, Kajimoto Y, Ichijo H, Yamasaki Y, Hori M. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10:1128-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 259] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 30. | Li L, He Q, Huang X, Man Y, Zhou Y, Wang S, Wang J, Li J. NOX3-derived reactive oxygen species promote TNF-alpha-induced reductions in hepatocyte glycogen levels via a JNK pathway. FEBS Lett. 2010;584:995-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Pal M, Febbraio MA, Lancaster GI. The roles of c-Jun NH2-terminal kinases (JNKs) in obesity and insulin resistance. J Physiol. 2016;594:267-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 32. | Gan KX, Wang C, Chen JH, Zhu CJ, Song GY. Mitofusin-2 ameliorates high-fat diet-induced insulin resistance in liver of rats. World J Gastroenterol. 2013;19:1572-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Khodabandehloo H, Gorgani-Firuzjaee S, Panahi G, Meshkani R. Molecular and cellular mechanisms linking inflammation to insulin resistance and β-cell dysfunction. Transl Res. 2016;167:228-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 34. | Xu L, Kitade H, Ni Y, Ota T. Roles of Chemokines and Chemokine Receptors in Obesity-Associated Insulin Resistance and Nonalcoholic Fatty Liver Disease. Biomolecules. 2015;5:1563-1579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 149] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 35. | Zephy D, Ahmad J. Type 2 diabetes mellitus: Role of melatonin and oxidative stress. Diabetes Metab Syndr. 2015;9:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |