Published online Jul 7, 2016. doi: 10.3748/wjg.v22.i25.5728
Peer-review started: March 21, 2016
First decision: May 12, 2016
Revised: May 26, 2016
Accepted: June 15, 2016
Article in press: June 15, 2016
Published online: July 7, 2016
Processing time: 108 Days and 8.5 Hours
Hepatopulmonary syndrome (HPS) is characterized by abnormalities in blood oxygenation caused by the presence of intrapulmonary vascular dilations (IPVD) in the context of liver disease, generally at a cirrhotic stage. Knowledge about the subject is still only partial. The majority of the information about the etiopathogenesis of HPS has been obtained through experiments on animals. Reported prevalence in patients who are candidates for a liver transplantation (LT) varies between 4% and 32%, with a predominance of mild or moderate cases. Although it is generally asymptomatic it does have an impact on their quality of life and survival. The diagnosis requires taking an arterial blood gas sample of a seated patient with alveolar-arterial oxygen gradient (AaO2) ≥ 15 mm Hg, or ≥ 20 mm Hg in those over 64 years of age. The IPVD are identified through a transthoracic contrast echocardiography or a macroaggregated albumin lung perfusion scan (99mTc-MAA). There is currently no effective medical treatment. LT has been shown to reverse the syndrome and improve survival rates, even in severe cases. Therefore the policy of prioritizing LT would appear to increase survival rates. This paper takes a critical and clinical look at the current understanding of HPS, as well as the controversies surrounding it and possible future research.
Core tip: Hepatopulmonary syndrome is a frequent complication which influences the quality of life and ultimately the survival of patients with cirrhosis. Knowledge about the condition is still limited and this complicates clinical decision making. The most widely used methods for establishing a diagnosis are an arterial blood gas analysis and a contrast echocardiography. There is currently no effective medical treatment and other means of supporting patients have barely been evaluated. Liver transplantation has been demonstrated to reverse it and improve survival levels, although there are controversies in the policies of prioritization in terms of the waiting lists for transplantation. This review examines current knowledge about the syndrome from a practical and analytical approach.
- Citation: Grilo-Bensusan I, Pascasio-Acevedo JM. Hepatopulmonary syndrome: What we know and what we would like to know. World J Gastroenterol 2016; 22(25): 5728-5741
- URL: https://www.wjgnet.com/1007-9327/full/v22/i25/5728.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i25.5728
Hepatopulmonary syndrome (HPS) is defined as a defect in arterial oxygenation caused by the presence of intrapulmonary vascular dilatations (IPVD) in the context of liver disease[1,2].
It was in 1884 that Flückiger first described the case of a woman with liver cirrhosis, cyanosis and acropachy, which could correspond to a patient with HPS. The term HPS was coined by Kennedy and Knudson[3] in 1977. New definitions for the syndrome were suggested by Krowka, Cortese and Rodríguez Roisín at the beginning of the 90s. They described HPS as a syndrome characterized by a clinical triad comprising the presence of advanced chronic liver disease, gas exchange abnormalities, ultimately leading to hypoxemia, and the presence of IPVD, without the presence of intrinsic pulmonary diseases[4]. Krowka et al[5] added precision to the definition, observing that the syndrome can coexist with cardiopulmonary diseases and can also appear in cases of hepatitis, portal hypertension not associated with liver cirrhosis, alpha 1 antitrypsin deficiency and Wilson’s disease.
Until 1988 HPS was considered to be a contraindication for liver transplantation (LT). Later however, it was observed that transplantations led to a reversal in hypoxemia and that post LT survival stood at around 70%. This finding, combined with the lack of an effective medical treatment for the syndrome, the progressive nature of hypoxemia and the higher mortality levels in these patients, meant that HPS became an indication for LT[6].
The majority of the information about the etiopathogenesis of HPS has been obtained through experiments on animals. The most widely used is performing a common bile duct ligation in rats to provoke a secondary biliary cirrhosis. This results in an alteration in the blood oxygenation and IPVD which can be measured in vivo and is similar to the changes in HPS in humans, although it is more common in animals[7]. Nevertheless, some of these findings have also been confirmed in humans.
The main mediators involved in the onset of IPVD, which are fundamental to the pathogenesis of HPS, are nitric oxide (NO) and carbon monoxide (CO). In the case of animals, the related molecules are: endothelin 1 (ET-1) and its receptors A and B; the heme oxygenase-1; TNF-alpha and its effect on endothelial NO synthase (eNOS) and the inducible NO synthase (iNOS)[8-20]. In humans, it has been observed that the levels of exhaled NO are higher in patients with HPS and that the administration of L-NAME and methylene blue as well as two nitric oxide synthase inhibitors (NOS) and their mediators, improve some HPS parameters[21-26]. The exhaled nitric oxide reflects an excess in NO produced in the alveoli which does not come from the liver[21,27,28]. Angiogenesis is also considered to be an important phenomenon in the development of HPS. Endothelial growth factor and other related molecules may be associated with this phenomenon[17].
Studies in animals keep leading to the discovery of new molecules which offer innovative perspectives in terms of the understanding of the etiopathogenesis of HPS and which could provide future targets for medical treatment of the syndrome[29-41]. There are a number of recent reviews which examine these developments in greater detail but this is not the aim of our review[42-44].
The principal abnormality which defines HPS is the dilatation of pre and post-capillary pulmonary vessels in the alveolar regions. The diameter of these vessels in normal conditions ranges between 8 and 15 μm, whereas when HPS is present, this rises to between 15 and 500 μm[1,19,45].
With HPS there is an increase in the alveolar-arterial gradient of O2 (AaO2) and hypoxemia which is caused by three mechanisms. The main one is a mismatch between the ventilation and perfusion of alveolar units which is evident in even the mildest cases of HPS. The vasodilatation of alveolar capillaries results in an excessive amount of blood flowing into the normally ventilated alveoli, which causes a decline in the ventilation perfusion quotient, resulting in increased AaO2 and/or arterial hypoxemia[2]. The other two mechanisms are firstly the shunt effect, unventilated units which are still perfused and can be explained by the presence of arteriovenous communication, and secondly the alteration in oxygen diffusion, which is correlated with a reduction in the lung’s diffusing capacity for CO (DLCO), and could be explained by the distance between the alveoli and the central flow in blood capillaries. This distance is too great to permit correct gas exchange and could also be related to the depositing of collagen in the capillaries and alveolar venules[1,46-48]. These mechanisms can be seen in both moderate and severe cases of the disease.
The prevalence of the syndrome has not been fully established since figures depend on the method employed for the diagnosis and the profile of the patients studied.
In terms of diagnostic methodology, there are differences in the criteria of alteration of arterial oxygenation which define the syndrome[49]. There are also differences according to the method used for the diagnosis of IPVD.
Different results are obtained depending on whether a contrast-enhanced echocardiography or macroaggregated albumin lung perfusion scan (99mTc-MAA) is used and if the echocardiography is transthoracic or transoesophageal. They also depend on which agents are used to generate contrast bubbles in the echocardiography[50,51]. It is important to unify criteria for the diagnosis of HPS so as to obtain comparable results from the various different studies.
In terms of the profiles of the patients studied, in cirrhotic patients the average prevalence of HPS was 15%, in those with chronic viral hepatitis, with or without cirrhosis, it was approximately 10% and in Budd-Chiari syndrome, 28%[52,53]. In patients listed for LT the different studies have shown figures of between 4% and 32%[49-51,54-64].
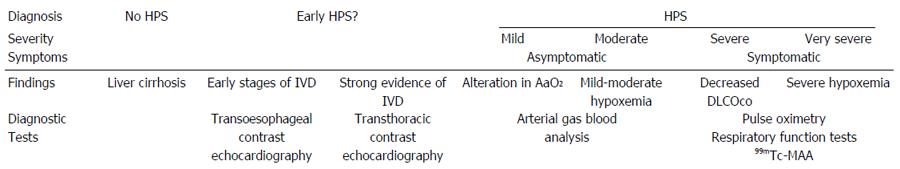
Four degrees of severity can be distinguished according to levels of hypoxemia: mild [partial oxygen pressure (pO2) is ≥ 80 mmHg], moderate (pO2 < 80 mmHg and ≥ 60 mmHg), severe (pO2 < 60 mmHg and ≥ 50 mmHg) and very severe (pO2 < 50 mmHg,) which is often associated with pO2 < 300 mmHg when the patient is administered oxygen at 100%[1]. Systematic HPS screening in cirrhosis patients listed for LT shows that the majority of HPS patients are mild or moderate (77%-88%). Severe cases (12%-17%) and very severe cases (4%-6.3%) are less common[56,65]. Previously it appeared that there were a higher number of more severe cases of HPS but this was due to the fact that in many earlier studies systematic screening was not used. Diagnosis was based on clinical criteria and this led to the more serious cases being selected rather than those which were less acute[57,59,66-70].
The symptoms which have classically been associated with HPS are dyspnea and platypnea[71]. In the largest study covering patients listed for LT, dyspnea was present in 48% of HPS patients, and was more frequent than in patients without HPS, with significant differences[55]. In another study it was found that dyspnea was more frequent in HPS patients with pO2 lower than 70 mmHg, (57%), compared with those who were diagnosed due to an increase in AaO2[49]. Platypnea, which means a worsening in dyspnea when a patient is standing rather than lying down, is considered a pathognomonic characteristic of HPS. This phenomenon is associated with orthodeoxia or a decreased pO2 when the patient changes from the lying down to the standing position. It has been suggested that increased perfusion at the base of the lungs when the HPS patients are standing up increases the shunt effect, resulting in orthodeoxia and platypnea[19]. In a study with 20 HPS patients the orthodeoxia phenomenon showed a prevalence of 25% and a decrease ≥ 5% or ≥ 4 mmHg in arterial pO2 was established as the cut off value for its diagnosis[72]. However, a prospective study found a 30% prevalence of orthodeoxia in cirrhotic patients evaluated for LT, with no differences found between patients with or without HPS[56]. Furthermore, in another study it was found that the opposite phenomenon to platypnea, orthopnea, or the worsening in dyspnea when lying down, is more frequent in patients with HPS (25%) than in those without it, with significant differences[55].
Among the exploration findings, acropachy, cyanosis and asterixis were rare (lower than 20%) although there was a significant association in HPS patients[55]. The studies focusing on the presence of vascular spiders and their connection with HPS are contradictory[49,55,73,74].
Extrapulmonary complications as a result of the presence of left-to-right shunt, such as cerebral abscesses, intracranial hemorrhages and polycythemia have also been described[13,75,76].
HPS patients have a lower quality of life, rank higher in the New York Heart Association functional classification and suffer significant oxygen desaturation whilst sleeping[55,77].
The available data on the symptoms and exploration findings in HPS patients is mainly based on those with cirrhosis and therefore should not be extrapolated to all HPS patients. Nevertheless, the data reveals that most patients are asymptomatic, the most frequent symptom is dyspnea, and platypnea and orthodeoxia do not seem to be characteristic HPS phenomena. Therefore further studies are required to clarify these findings.
The diagnostic criteria proposed for HPS are the presence of liver disease, an AaO2≥ 15 mmHg or ≥ 20 mmHg in patients over 64 years old detected by arterial blood gas analysis in a seating position, and the demonstration of IPVD by means of a positive contrast-enhanced echocardiography[1].
Although these criteria were established in 2004, there were other previously existing parameters: the presence of an AaO2 > 20 mmHg, regardless of age, and/or a pO2 < 70 mmHg obtained in any position, lying down, sitting or standing, were the most widely accepted gasometric criteria[66-68,70,78,79]. Other studies focused on the existence of an AaO2 greater than that theoretically established according to age. There are also differences in the formula used to calculate this value[58,59].
Arterial blood samples are obtained by radial artery puncture with the patient in a stable condition and breathing room air. There are also differences between studies in terms of the position of the patient when the sample was obtained: lying down, standing and/or sitting.
The establishing of standardized criteria allows us to unify the diagnostic methods and further our understanding of the disease. However these are based on a consensus of experts. There are hardly any studies on the application of different criteria for the diagnosis of HPS and how these might affect the prevalence or prognosis of the syndrome. A prospective study with 98 patients observed that prevalence of HPS is 32% when the cut off value for AaO2 is ≥ 15 mmHg, 31% when AaO2 > 20 mmHg and 28% when AaO2 was calculated according to age. This study also found that the prevalence is lower when pO2 rather than AaO2 was used in the diagnosis of HPS[49]. It is also worth noting an interesting study which proposed performing two arterial blood gas analyses to obtain a precise diagnosis of HPS and detect those cases in which the disease was more advanced. This study also analyzes different arterial gas criteria for the diagnosis of HPS[80]. In general, there are very few studies which have validated the arterial gas criteria which are currently used for the diagnosis of HPS as opposed to others. This is an issue which is extremely important to furthering our understanding of the disease, enabling us to compare studies.
A contrast echocardiography is a sensitive, qualitative and non-invasive method which allows the screening of IPVD, which are the main characteristic of HPS. It is considered to be the gold standard technique for the diagnosis of HPS[1,2]. It consists of injecting a liquid medium with bubbles into a peripheral vein so as to observe the liquid entering the right auricle and ascertain whether or not it subsequently passes into the left cavities. Under physiological conditions, once the bubbles are visualized in the right auricle they become trapped in the pulmonary vascular bed and therefore cannot be visualized in the left heart. However, in HPS, bubbles bypass the pulmonary circulation and can be seen in the left cavities. The presence of an intracardiac shunt is another condition in which bubbles can be seen in the left cavities. However, while in intracardiac shunts the transfer of bubbles occurs earlier, between the first and third heart beats, with HPS it takes place between the fourth and sixth beat[1].
The various agents used as a medium to produce bubbles offer different levels sensitivity for the diagnosis of IPVD. These include: saline solution, mannitol, polygeline, indocyanine green and other gelatinous solutions[50,81-84]. Of these, a saline solution at 0.9% is currently recommended as the medium of choice[1].
The transthoracic contrast echocardiography is preferred to the transoesophageal option due to the potential risk of damage that the latter can cause to oesophageal varices, although the studies carried out to date have not found this complication[50,51,81]. There is greater technique sensitivity for the diagnosis of HPS against transthoracic ultrasound scan, although some interpretation discrepancies exist regarding very early stages of IPVD without HPS presence. The current recommendation is to use it in the event of poor echo window and high probability of HPS[50,51,81].
A recent study compared the performance of transcranial doppler ultrasonography for the diagnosis of IPVD with transthoracic echocardiography. It found that transcranial doppler ultrasonography was effective diagnostically with AUC = 0.813% (95%CI: 0.666-0.959; P = 0.001), sensitivity: 76.2% (95%CI: 54.9-89.4) and specificity: 90% (95%CI: 63.9-96.5)[85]. Although the study was conducted with a small group of patients, it offers a new avenue of study for the diagnosis of HPS.
The 99mTc-MAA is another technique which is capable of detecting the presence of IPVD. The basis of this approach is similar to that of a contrast echocardiography. In this case, Tc99 tagged albumin particles are able to reach extrapulmonary sites due to the presence of IPVD. Cerebral uptake is considered to be pathological when greater than or equal to 6%[68]. However, some studies establish a cut off value at 5%, and others at 7% or even higher[86-88]. Nevertheless, for better interpretation, the results of the lung perfusion scan should be reported in terms of uptake values rather than positive or negative.
Its main advantage is its capacity to quantify IPVD and determine its role in hypoxemia in patients with organic respiratory comorbidity. It has also been given a prognostic role, indicating that cerebral uptake ≥ 20% and/or hypoxemia ≤ 55 mmHg were associated with greater post LT mortality[67,68]. However, subsequent studies have not confirmed a correlation between 99mTc-MAA-based cerebral uptake values and postransplantation survival[86]. Its main disadvantages on the other hand are its incapacity to differentiate IPVD from intracardiac shunting and its lower sensitivity in the diagnosis of IPVD. In this sense, the studies which have assessed this technique offer a variable sensitivity of between 20% and 96%, which seems to correlate with the severity of HPS, showing high sensitivity in severe and very severe cases and low in those which are mild and moderate[53,59,67,68,89,90]. These findings are consistent with the results of a sub-analysis (unpublished) of our work[56]. Further studies are required to determine the influence of the severity of HPS and the sensitivity of this technique as well as its standardization and prognostic role.
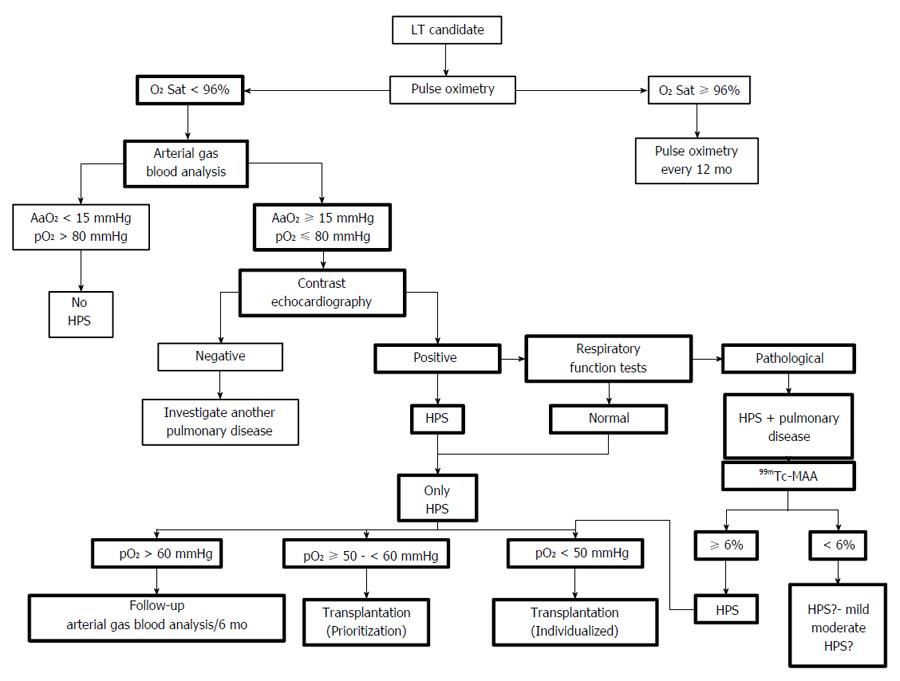
Pulse oximetry: Pulse oximetry is a cheap, rapid and painless method of estimating arterial pO2. This is the reason why it is considered a useful tool for the screening and monitoring of cirrhotic patients listed for LT, especially children[91-93]. The selection of the cut off value for pulse oximetry is based on a study with a group of 120 patients listed for LT, which found that levels of saturation lower than 96% had 100% sensitivity and that specificity in detecting levels of hypoxemia lower than 60 mmHg was 88%[94]. This cut off value was considered to be optimum since it was especially relevant in terms of its influence on transplantation list priority and patient prognosis. However, in view of the latest survival studies, the clinical importance of using this or other cut off values as references need to be reevaluated[86,95]. Furthermore, the use of pulse oximetry, a cut off value lower than 97% to perform arterial blood gas analysis, and a contrast echocardiography for LT candidates seem to be cost-effective measures for the screening of HPS, as opposed to the lack of screening, or the use of fatigue and dyspnea rates. There was no direct comparison with the use of arterial blood gas analysis[96].
However, some authors believe that this would not be sufficiently precise to replace arterial blood gas analysis, since pulse oximetry overestimates arterial oxygenation, which is not dependant on liver disease[1,94].
In any case, pulse oximetry alone is insufficient for the diagnosis of HPS. Therefore a contrast echocardiography followed by an arterial blood gas analysis is proposed[91].
Thoracic X-ray: Thoracic X-rays can be used to effectively rule out other concomitant pulmonary diseases. In the case of HPS, they are mostly normal although interstitial markings are more frequently found[1,55].
Thorax computed tomography scan: Thoracic computed tomography (CT) scans are proposed as a complementary technique to rule out another underlying pulmonary pathology[1,91] although there is little information regarding their specific role in the diagnosis of HPS. It is suggested that measurement of the caliber of the peripheral arteries and the bronchial/arterial relationship can be useful in the diagnosis of the syndrome[97,98]. Furthermore, CT scans offer the advantage of defining the vascular pattern of HPS in a similar manner to arteriography. A technique which combines the study of the pulmonary perfusion by means of a SPECT scan and fusion with CT (SPECT-CT) imaging has been used in two HPS patients, thereby offering a possible alternative to the use of pulmonary arteriography for the location of IPVD[99].
Pulmonary arteriography: Pulmonary arteriography permits us to distinguish two different types of vascular patterns in HPS patients. Firstly type I or diffuse, which is in turn divided into two subtypes. Subtype I minimal is characterized by the presence of normal or minimally dilated vessels in the shape of diffuse vascular spiders. Subtype I advanced presents more evident dilatations with a spongy, diffused and speckled appearance. Type II or focal, is characterized by the presence of arteriovenous shunts similar to those present in hereditary telangiectasia[100]. The application of this technique is suggested for cases in which type II HPS is suspected due to the presence of severe hypoxemia and lack of response to 100% oxygen supplement, and due to the possibility of embolotherapy[101]. However, the number of published cases of examples which were responsive to embolotherapy for both type I and type II HPS are insufficient to establish a strong recommendation in this respect[100,102-106].
Respiratory function tests: In HPS patients both spirometry and static volumes have characteristically been found to be normal in the absence of concomitant pulmonary diseases. However, although this has been mentioned in various different HPS reviews, it is essentially based on theoretical assumptions about the disease rather than observational studies[83]. In fact, more recent studies suggest that a reduction in forced vital capacity and maximum forced expiratory volume during the first second (FEV1) is more frequent in HPS patients. However, there is no hypothesis to explain this finding and therefore further studies for its corroboration and analysis are required[55,56]. Frequently, there is a moderate to severe reduction in pulmonary capacity for the diffusion of CO (DLCO) and in that corrected for hemoglobin (DLCOco)[55,56,64,107]. This has been related to the increase in the distance between the alveolus and the capillary, due to vascular dilatation, and possible accumulation of collagen between the capillaries and pulmonary venules and the alveoli[19,46-48]. However, its role in the diagnosis of HPS is limited, since it is more frequent in severe and very severe cases than in mild and moderate ones[56,64].
Laboratory tests: A study has found that the levels of the von Willebrand factor antigen in the blood of HPS patients are increased and that this is correlated with gasometric abnormalities[108]. This test could be useful for screening HPS although further studies are needed to confirm these findings (Table 1).
| Diagnosis methods | Findings in HPS patients | Limitations | Aims |
| Arterial blood gas analysis | AaO2≥ 15 mmHg or ≥ 20 mmHg in patients over 64 years old | Consensus criteria | Diagnosis of HPS |
| Invasive | |||
| Pulse oximetry | O2 Saturation < 96% or 97% | Low sensitivity | Screening HPS and follow up |
| Contrast echocardiography | Bubbles in the left cavities between the fourth and sixth beat | Various agents used | Diagnosis of IPVD |
| Transthoracic vs transoesophageal | |||
| 99mTc-MAA | Cerebral uptake ≥ 6% | Low sensitivity | Diagnosis and quantify of IPVD |
| Thoracic X-ray | Interstitial markings | Unspecific | Rule out other concomitant pulmonary diseases |
| Thorax CT scan | Measurement of the caliber of the peripheral arteries and the bronchial/arterial relationship | More studies needed | Rule out other concomitant pulmonary diseases |
| Vascular patterns | Location of IPVD | ||
| Pulmonary arteriography | Vascular patterns | Low sensitivity | Type II HPS suspected |
| Invasive | Embolotherapy? | ||
| Respiratory function tests | Normal or reduction FVC or FEV1 | Unspecific | Rule out other concomitant pulmonary diseases |
| Reduction in DLCOco | Low sensitivity | ||
| Laboratory tests | von Willebrand factor antigen elevated | More studies needed | Screening HPS |
The differential diagnosis of HPS in cirrhotic patients is essentially mandatory in the following situations: the presence of dyspnea, hypoxemia or abnormal AaO2. In these cases it is necessary to rule out the coexistence of other cardiopulmonary diseases or complications due to the cirrhosis itself such as ascitis, hepatic hydrothorax or portopulmonary hypertension. The differential diagnosis is particularly complex in the context of a pulmonary disease coexisting with IPVD. In this case the patient could be wrongly diagnosed as suffering from HPS, since the presence of IPVD is frequent in cirrhotic patients without HPS and gasometric abnormalities could be due to a coexisting pulmonary disease (false positive). Early definitions of HPS included the need to rule out the existence of cardiopulmonary diseases as a requirement to reach a diagnosis of the syndrome[4]. Later this requirement was ruled out, since from a physiopathological viewpoint, HPS can coexist with other processes[5]. Currently, the main recommendation in these cases is to complement studies with a 99mTc-MAA so as to quantify the extent of the shunt[91,101]. However, no studies have demonstrated the validity of this strategy. As we said earlier, 99mTc-MAA is a less sensitive technique and a positive result seems to be related to the severity of HPS, which means that mild cases of HPS may not be diagnosed as such (false negative). In view of this, it would be necessary to conduct further studies to validate the efficacy of 99mTc-MAA or other techniques for the differential diagnosis. In view of this, it seems reasonable to recommend that patients with concomitant cardiopulmonary disease are excluded from HPS research studies so as to prevent false positives and negatives.
The natural history of HPS is unknown. Existing studies have been conducted in cirrhotic patients for whom the presence of HPS worsens their survival rate, independently of their age, sex, race, Child-Pugh score, blood urea levels and MELD score[55,57,68]. The results regarding the influence on survival in terms of the severity of the HPS and the levels of hypoxemia or AaO2 are contradictory[55,57].
Based on data from cirrhotic patients, especially those listed for LT, we could establish the following hypothesis for the natural history of the disease. Firstly, IPVD develop, but this wouldn’t initially cause gasometric abnormalities. They have been found to be frequent in cirrhotic patients without HPS criteria[81]. Their significance has not been fully evaluated, but they may be associated with HPS in its initial or early stages and their presence can be detected by contrast-enhanced echocardiography. The progression of the IPVD and hemodynamic changes in cirrhotic patients result in gasometric changes, initially including AaO2 abnormalities and mild levels of hypoxemia[109]. This corresponds to the asymptomatic period of HPS, at mild and moderate levels, in which survival may not be especially compromised[55,56]. However, pulse oximetry and 99mTc-MAA imaging may not be able to diagnose this patient group[53,67,68,89,90,94]. If the disease progresses, hypoxemia becomes more severe and other abnormalities are detected in respiratory function tests, such as a decreased DLCOco or a positive 99mTc-MAA[53,56,64,67,68,89-90]. This is the symptomatic period of HPS, with severe to very severe cases in which survival would probably be compromised, resulting in death if the patient does not receive an LT[55,57,68] (Figure 1).
Different substances have been used to treat HPS. Nevertheless the majority of studies have been carried out on animals and the studies carried out on humans lack the necessary design and sufficient sample numbers to allow them to be applied to clinical practice. Substances which have been tested without producing any clearly favourable results include somatostatin analogues[100,110], norfloxacin[12,13,111], inhaled nitric oxide[112], cyclooxygenase inhibitors such as aspirin[113] and indomethacin[114], immunosuppressants such as mycophenolate mofetil[115], cyclophosphamide[116] and sorafenib[117,118], quercetin[20,119], beta blockers[120], paroxetine[121], rosuvastatin[122], caspase-3 inhibitors[37], methylene blue[26,123,124] and inhaled iloprost[125]. Amongst the substances used in human tests are pentoxifylline and garlic. Pentoxifylline, which has been seen to yield positive results in animal tests[11,17], has also been tried on adults and children. However, samples in each study have been fewer than 10 patients, with contradictory results in terms of improvements in oxygenation and frequent gastrointestinal side effects[126-128]. The use of garlic as a treatment for HPS has not been tested on animals and its active mechanism is not understood. Nevertheless, in a comparative study using garlic oil capsules and a salt capsule placebo, with a total sample of 41 HPS patients, and in another study without a placebo involving 15 patients, favorable results were observed, with improvements in oxygenation and other symptoms[129,130]. For pharmaceutical studies it would be necessary to carry out multicentre controlled trials using these and other substances against placebos. A study is currently taking place to evaluate the safety of sorafenib vs placebo, and its effects in blood oxygenation (ClinicalTrials.gov Identifier: NCT02021929).
It is recommended that HPS patients with severe hypoxemia at rest receive oxygen therapy[1,131]. Nevertheless, there is no available data concerning effectiveness, tolerance, cost-effectiveness, compliance and effects on survival rates of this therapy[1]. Only 2 case studies have been published involving the long term treatment of HPS with mild and severe hypoxemia using oxygen therapy in a home environment. In both cases an improvement in liver function and oxygenation was observed[132].
There are very few published cases of HPS patients being treated with a transjugular intrahepatic portosystemic shunt (TIPS), and those which do exist have shown varied short-term results regarding pulmonary oxygen exchange. 12 cases have been studied, and the largest series is 3 patients[133-139]. As such, there is not sufficient data to propose the compassionate use of TIPS in cases of HPS[1,138].
Budd-Chiari syndrome shows a high prevalence of HPS and there are descriptions of improvements in cases where the obstruction is resolved through cavoplasty or other methods[87,139].
As has been described in a few isolated cases, embolization has also been used for the treatment of type 1 and type 2 HPS with positive results[100,102-106].
In both instances the information has been obtained from reports about isolated cases and therefore it is not possible to make recommendations about their use.
The most widely studied treatment is LT. At the moment it is the only effective treatment for HPS and is proven to improve survival rates[68,86].
When evaluating a patient listed for LT, it is recommended that they are tested for the presence of HPS. This can be done through arterial blood gas analysis, contrast-enhanced echocardiography or pulse oximetry, the latter being proposed more frequently as the option of choice[1,42,91,101]. In patients with oxygen saturation lower than 96%, the most effective way of confirming the existence of HPS is through an arterial blood gas analysis, followed by a contrast echocardiography, or vice versa[42,91]. These screening models are based on the detection through pulse oximetry of HPS patients with significant hypoxemia, due to existing implications in prioritization and post-transplantation survival of these patients. As we have already mentioned, the findings of new studies could question this form of screening[86,95].
In terms of mortality rates for those on the active transplantation list, in a prospective study, no differences were observed between patients who had HPS and those who did not. In the study, the majority of cases were mild or moderate and MELD scores were not taken into account. This means that the survival of HPS patients was evaluated without the “distorting” effect associated with the inclusion of these scores[56]. Other retrospective studies, which include large samples, have found that mortality rates for HPS patients on the list are lower than those for patients without HPS, a situation which is explained by the addition of MELD points to severe or very severe cases of HPS, thereby favoring transplantations in these patients as opposed to those who do not have HPS[86,95].
Hypoxemia can worsen in HPS patients who are on the active transplantation list, with a median decrease in pO2 of 5.2 mmHg per year[68]. It has been suggested that an arterial blood gas analysis should be carried out every 6 mo, although there are no studies which have evaluated the method for carrying out this follow-up process (arterial blood gas analysis vs pulse oximetry) nor how frequently it should be carried out[42,91] (Figure 2).
Anesthetics and post LT intensive care: There are very few studies concerning the influence of HPS in anesthetic procedures or in the intensive care unit immediately following a LT and those that do exist are based on very small samples. It would appear that inhaled general anesthetics have a worse immediate effect on hypoxemia than intravenous anesthetics, but after an hour there is no apparent difference[140]. Inhaled nitric oxide, methylene blue, extracorporeal membrane oxygenation and non-invasive ventilation have all been suggested as ways of improving oxygenation in immediate post-surgery[141-143]. More studies are required in order to increase our understanding of HPS in terms of both anesthetics and immediate post-surgery so as to improve patient care.
Post LT survival: Ten years survival after LT in HPS patients stands at 64%[86]. The majority of published studies analyze gross mortality rate of transplanted HPS patients retrospectively. Post LT mortality rates obtained in these studies range between 7.7% and 33%. Retrospective and later prospective studies show that hypoxemia ≤ 50 mmHg and cerebral uptake on 99mTc-MAA ≥ 20% are predictors of post operative mortality, but patient sample figures have been small[66,67,79,83]. Since 2007, in the United States, as a result of these findings, and due to the progression of hypoxemia on the transplantation list, it has been recommended to assign a 22 MELD score to HPS patients with pO2 < 60 mmHg, with increases every 3 mo[144]. Nevertheless, later studies have failed to confirm these findings[86,145].
Recently, a number of comparative studies have been carried out comparing survival rates for patients who have HPS and those who do not. In the majority of retrospective studies there were no statistically significant differences[65,69,146] and this was also true in a prospective study[56]. Finally, two prospective studies analyzing transplantation data of the UNOS, and therefore a larger sample of HPS patients, showed a better rate of survival in HPS patients, probably due to lower waiting list mortality and a similar level of post-transplantation mortality, influenced by the MELD score, as a previous study suggested[86,95,147]. Another finding from one of the studies was greater mortality when pO2 is ≤ 44 mmHg, but this was not the case in lower levels of hypoxemia to which MELD scores are currently being applied in an exceptional manner[95]. These recent findings have opened a debate concerning the suitability of the MELD scoring system as applied to HPS, which prioritizes patients for LT, and there could well be changes to this policy in the near future[134].
Reversibility after LT: The improvement in the parameters which define HPS after LT has mainly been evaluated in retrospective studies which have shown total reversibility figures ranging from 52% to 100% over a 6 to 12 mo period. The definition of reversibility has used a range of different criteria[58,67-70,145,148,149].
There is a prospective study which analyzes HPS reversibility at 6, 9 and 12 mo after LT based on the different criteria used to define the syndrome. It shows that full reversibility of HPS can be seen after 12 mo and that the process is rapid, since even after 6 mo, in mainly mild and moderate cases there is a 95.8% reversal. In terms of the evolution of the characteristic parameters of HPS, pO2 and AaO2 levels improve more quickly than in intrapulmonary shunt demonstrated by means of a contrast echocardiography.
It has also been observed that there is a post transplantation improvement in DLCO, although not in all patients[56]. Previously, the reversibility of this parameter was not described[148]. It should be noted that the sample in both studies was small.
Current knowledge about HPS is limited and is essentially based on studies of cirrhotic patients listed for LT or animal experiments. These studies have enabled us to establish the fact that HPS is a frequent complication for these patients, and although it is generally asymptomatic it does have an impact on their quality of life and survival. It has also been established that LT is an efficient method of treating the syndrome and has positive post-transplantation survival results, even in severe cases. Nevertheless, there is still much to do, particularly in terms of increasing the number of multi-centre studies which confirm the etiopathogenic findings of animal models in humans and aid the development of pharmacological treatments. It is also important to focus on improving the clinical use of diagnostic or screening techniques as well as clarifying the prioritization and selection of patients for LT.
Manuscript Source: Invited manuscript
Specialty Type: Gastroenterology and Hepatology
Country of Origin: Spain
Peer-Review Report Classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ramsay MA, Tumgor G S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Rodríguez-Roisin R, Krowka MJ, Hervé P, Fallon MB. Pulmonary-Hepatic vascular Disorders (PHD). Eur Respir J. 2004;24:861-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 507] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 2. | Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome--a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Kennedy TC, Knudson RJ. Exercise-aggravated hypoxemia and orthodeoxia in cirrhosis. Chest. 1977;72:305-309. [PubMed] |
| 4. | Rodriguez-Roisin R, Roca J. Hepatopulmonary syndrome: the paradigm of liver-induced hypoxaemia. Baillieres Clin Gastroenterol. 1997;11:387-406. [PubMed] |
| 6. | Krowka MJ. Hepatopulmonary syndrome: recent literature (1997 to 1999) and implications for liver transplantation. Liver Transpl. 2000;6:S31-S35. [PubMed] |
| 7. | Fallon MB, Abrams GA, McGrath JW, Hou Z, Luo B. Common bile duct ligation in the rat: a model of intrapulmonary vasodilatation and hepatopulmonary syndrome. Am J Physiol. 1997;272:G779-G784. [PubMed] |
| 8. | Zhang J, Ling Y, Tang L, Luo B, Pollock DM, Fallon MB. Attenuation of experimental hepatopulmonary syndrome in endothelin B receptor-deficient rats. Am J Physiol Gastrointest Liver Physiol. 2009;296:G704-G708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Ling Y, Zhang J, Luo B, Song D, Liu L, Tang L, Stockard CR, Grizzle WE, Ku DD, Fallon MB. The role of endothelin-1 and the endothelin B receptor in the pathogenesis of hepatopulmonary syndrome in the rat. Hepatology. 2004;39:1593-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Zhang M, Luo B, Chen SJ, Abrams GA, Fallon MB. Endothelin-1 stimulation of endothelial nitric oxide synthase in the pathogenesis of hepatopulmonary syndrome. Am J Physiol. 1999;277:G944-G952. [PubMed] |
| 11. | Sztrymf B, Libert JM, Mougeot C, Lebrec D, Mazmanian M, Humbert M, Herve P. Cirrhotic rats with bacterial translocation have higher incidence and severity of hepatopulmonary syndrome. J Gastroenterol Hepatol. 2005;20:1538-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Rabiller A, Nunes H, Lebrec D, Tazi KA, Wartski M, Dulmet E, Libert JM, Mougeot C, Moreau R, Mazmanian M. Prevention of gram-negative translocation reduces the severity of hepatopulmonary syndrome. Am J Respir Crit Care Med. 2002;166:514-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Añel RM, Sheagren JN. Novel presentation and approach to management of hepatopulmonary syndrome with use of antimicrobial agents. Clin Infect Dis. 2001;32:E131-E136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Sztrymf B, Rabiller A, Nunes H, Savale L, Lebrec D, Le Pape A, de Montpreville V, Mazmanian M, Humbert M, Hervé P. Prevention of hepatopulmonary syndrome and hyperdynamic state by pentoxifylline in cirrhotic rats. Eur Respir J. 2004;23:752-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Luo B, Liu L, Tang L, Zhang J, Ling Y, Fallon MB. ET-1 and TNF-alpha in HPS: analysis in prehepatic portal hypertension and biliary and nonbiliary cirrhosis in rats. Am J Physiol Gastrointest Liver Physiol. 2004;286:G294-G303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Arguedas MR, Drake BB, Kapoor A, Fallon MB. Carboxyhemoglobin levels in cirrhotic patients with and without hepatopulmonary syndrome. Gastroenterology. 2005;128:328-333. [PubMed] |
| 17. | Zhang J, Luo B, Tang L, Wang Y, Stockard CR, Kadish I, Van Groen T, Grizzle WE, Ponnazhagan S, Fallon MB. Pulmonary angiogenesis in a rat model of hepatopulmonary syndrome. Gastroenterology. 2009;136:1070-1080. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Tumgor G, Berdeli A, Arikan C, Levent E, Aydogdu S. Mcp-1, eNOS, tPA and PAI-1 gene polymorphism and correlation of genotypes and phenotypes in hepatopulmonary syndrome. Dig Dis Sci. 2008;53:1345-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Zhang XJ, Katsuta Y, Akimoto T, Ohsuga M, Aramaki T, Takano T. Intrapulmonary vascular dilatation and nitric oxide in hypoxemic rats with chronic bile duct ligation. J Hepatol. 2003;39:724-730. [PubMed] |
| 20. | Tieppo J, Cuevas MJ, Vercelino R, Tuñón MJ, Marroni NP, González-Gallego J. Quercetin administration ameliorates pulmonary complications of cirrhosis in rats. J Nutr. 2009;139:1339-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Rolla G. Is nitric oxide the ultimate mediator in hepatopulmonary syndrome? J Hepatol. 2003;38:668-670. [PubMed] |
| 22. | Matsumoto A, Ogura K, Hirata Y, Kakoki M, Watanabe F, Takenaka K, Shiratori Y, Momomura S, Omata M. Increased nitric oxide in the exhaled air of patients with decompensated liver cirrhosis. Ann Intern Med. 1995;123:110-113. [PubMed] |
| 23. | Cremona G, Higenbottam TW, Mayoral V, Alexander G, Demoncheaux E, Borland C, Roe P, Jones GJ. Elevated exhaled nitric oxide in patients with hepatopulmonary syndrome. Eur Respir J. 1995;8:1883-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 129] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 24. | Dinh-Xuan AT, Texereau J. Measuring exhaled nitric oxide: Not only a matter of how - But also why - Should we do it? Eur Respir J. 1998;12:1005-1007. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Brussino L, Bucca C, Morello M, Scappaticci E, Mauro M, Rolla G. Effect on dyspnoea and hypoxaemia of inhaled N(G)-nitro-L-arginine methyl ester in hepatopulmonary syndrome. Lancet. 2003;362:43-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Schenk P, Madl C, Rezaie-Majd S, Lehr S, Müller C. Methylene blue improves the hepatopulmonary syndrome. Ann Intern Med. 2000;133:701-706. [PubMed] |
| 27. | Gómez FP, Barberà JA, Roca J, Burgos F, Gistau C, Rodríguez-Roisin R. Effects of nebulized N(G)-nitro-L-arginine methyl ester in patients with hepatopulmonary syndrome. Hepatology. 2006;43:1084-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Delclaux C, Mahut B, Zerah-Lancner F, Delacourt C, Laoud S, Cherqui D, Duvoux C, Mallat A, Harf A. Increased nitric oxide output from alveolar origin during liver cirrhosis versus bronchial source during asthma. Am J Respir Crit Care Med. 2002;165:332-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Zeng J, Chen L, Chen B, Lu K, Belguise K, Wang X, Yi B. MicroRNA-199a-5p Regulates the Proliferation of Pulmonary Microvascular Endothelial Cells in Hepatopulmonary Syndrome. Cell Physiol Biochem. 2015;37:1289-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Wang L, Zhuang L, Rong H, Guo Y, Ling X, Wang R, Yu X, Zhang W. MicroRNA-101 inhibits proliferation of pulmonary microvascular endothelial cells in a rat model of hepatopulmonary syndrome by targeting the JAK2/STAT3 signaling pathway. Mol Med Rep. 2015;12:8261-8267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Gao J, Chen L, Zeng J, Cui J, Ning JL, Wang GS, Belguise K, Wang X, Qian GS, Lu KZ. The involvement of aquaporin 1 in the hepatopulmonary syndrome rat serum-induced migration of pulmonary arterial smooth muscle cells via the p38-MAPK pathway. Mol Biosyst. 2015;11:3040-3047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Liu C, Chen L, Zeng J, Cui J, Ning JN, Wang GS, Belguise K, Wang X, Qian GS, Lu KZ. Bone morphogenic protein-2 regulates the myogenic differentiation of PMVECs in CBDL rat serum-induced pulmonary microvascular remodeling. Exp Cell Res. 2015;336:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 33. | Yeh DY, Yang YC, Wang JJ. Hepatic Warm Ischemia-Reperfusion-Induced Increase in Pulmonary Capillary Filtration Is Ameliorated by Administration of a Multidrug Resistance-Associated Protein 1 Inhibitor and Leukotriene D4 Antagonist (MK-571) Through Reducing Neutrophil Infiltration and Pulmonary Inflammation and Oxidative Stress in Rats. Transplant Proc. 2015;47:1087-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Oswald-Mammosser M, Rashid S, Boehm N, Agin A, Geny B, Schini-Kerth V, Charloux A. Effect of the oestrogen receptor antagonist fulvestrant on the cirrhotic rat lung. Fundam Clin Pharmacol. 2015;29:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 35. | Chen L, Li YS, Cui J, Ning JN, Wang GS, Qian GS, Lu KZ, Yi B. MiR-206 controls the phenotypic modulation of pulmonary arterial smooth muscle cells induced by serum from rats with hepatopulmonary syndrome by regulating the target gene, annexin A2. Cell Physiol Biochem. 2014;34:1768-1779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | Yang W, Hu B, Wu W, Batra S, Blackburn MR, Alcorn JL, Fallon MB, Zhang J. Alveolar type II epithelial cell dysfunction in rat experimental hepatopulmonary syndrome (HPS). PLoS One. 2014;9:e113451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Chen B, Ning JL, Gu JT, Cui J, Yang Y, Wang Z, Zeng J, Yi B, Lu KZ. Caspase-3 inhibition prevents the development of hepatopulmonary syndrome in common bile duct ligation rats by alleviating pulmonary injury. Liver Int. 2015;35:1373-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Zhang H, Lv M, Zhao Z, Jia J, Zhang L, Xiao P, Wang L, Li C, Ji J, Tian X. Glucose-regulated protein 78 may play a crucial role in promoting the pulmonary microvascular remodeling in a rat model of hepatopulmonary syndrome. Gene. 2014;545:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Nacif LS, Andraus W, Kubrusly MS, Molan N, Chaib E, D’Albuquerque LA. Myeloperoxidase activity is increased in hepatopulmonary syndrome in rats. Arq Bras Cir Dig. 2013;26:293-295. [PubMed] |
| 40. | Chen Y, Yi B, Wang Z, Gu J, Li Y, Cui J, Lu K. Paxillin suppresses the proliferation of HPS rat serum treated PASMCs by up-regulating the expression of cytoskeletal proteins. Mol Biosyst. 2014;10:759-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Zhang H, Lv M, Jia J, Zhao Z, Zhang L, Lai L, Wu Y, Li B, Li C, Ji J. Expression of the 78 kD glucose-regulated protein is induced by endoplasmic reticulum stress in the development of hepatopulmonary syndrome. Gene. 2014;537:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 42. | Raevens S, Geerts A, Van Steenkiste C, Verhelst X, Van Vlierberghe H, Colle I. Hepatopulmonary syndrome and portopulmonary hypertension: recent knowledge in pathogenesis and overview of clinical assessment. Liver Int. 2015;35:1646-1660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Lv Y, Fan D. Hepatopulmonary Syndrome. Dig Dis Sci. 2015;60:1914-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Tumgor G. Cirrhosis and hepatopulmonary syndrome. World J Gastroenterol. 2014;20:2586-2594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 45. | Schraufnagel DE, Kay JM. Structural and pathologic changes in the lung vasculature in chronic liver disease. Clin Chest Med. 1996;17:1-15. [PubMed] |
| 46. | Berthelot P, Walker JG, Sherlock S, Reid L. Arterial changes in the lungs in cirrhosis of the liver--lung spider nevi. N Engl J Med. 1966;274:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 232] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Krowka MJ, Cortese DA. Hepatopulmonary syndrome. Current concepts in diagnostic and therapeutic considerations. Chest. 1994;105:1528-1537. [PubMed] |
| 48. | Stanley NN, Williams AJ, Dewar CA, Blendis LM, Reid L. Hypoxia and hydrothoraces in a case of liver cirrhosis: correlation of physiological, radiographic, scintigraphic, and pathological findings. Thorax. 1977;32:457-471. [PubMed] |
| 49. | Schenk P, Fuhrmann V, Madl C, Funk G, Lehr S, Kandel O, Müller C. Hepatopulmonary syndrome: prevalence and predictive value of various cut offs for arterial oxygenation and their clinical consequences. Gut. 2002;51:853-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 209] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 50. | Aller R, Moya JL, Moreira V, García-Lledo A, Sanromán AL, Paino C, Boixeda D. Diagnosis and grading of intrapulmonary vascular dilatation in cirrhotic patients with contrast transesophageal echocardiography. J Hepatol. 1999;31:1044-1052. [PubMed] |
| 51. | Aller R, Moya JL, Moreira V, Boixeda D, Cano A, Picher J, García-Rull S, de Luis DA. Diagnosis of hepatopulmonary syndrome with contrast transesophageal echocardiography: advantages over contrast transthoracic echocardiography. Dig Dis Sci. 1999;44:1243-1248. [PubMed] |
| 52. | Rodríguez-Roisin R, Agustí AG, Roca J. The hepatopulmonary syndrome: new name, old complexities. Thorax. 1992;47:897-902. [PubMed] |
| 53. | Fallon MB, Abrams GA. Pulmonary dysfunction in chronic liver disease. Hepatology. 2000;32:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 54. | Lima BL, França AV, Pazin-Filho A, Araújo WM, Martinez JA, Maciel BC, Simões MV, Terra-Filho J, Martinelli AL. Frequency, clinical characteristics, and respiratory parameters of hepatopulmonary syndrome. Mayo Clin Proc. 2004;79:42-48. [PubMed] |
| 55. | Fallon MB, Krowka MJ, Brown RS, Trotter JF, Zacks S, Roberts KE, Shah VH, Kaplowitz N, Forman L, Wille K. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology. 2008;135:1168-1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 56. | Pascasio JM, Grilo I, López-Pardo FJ, Ortega-Ruiz F, Tirado JL, Sousa JM, Rodriguez-Puras MJ, Ferrer MT, Sayago M, Gómez-Bravo MA. Prevalence and severity of hepatopulmonary syndrome and its influence on survival in cirrhotic patients evaluated for liver transplantation. Am J Transplant. 2014;14:1391-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 57. | Schenk P, Schöniger-Hekele M, Fuhrmann V, Madl C, Silberhumer G, Müller C. Prognostic Significance of the Hepatopulmonary Syndrome in. Gastroenterology. 2003;5085:1042-1052. [DOI] [Full Text] |
| 58. | Stoller JK, Lange PA, Westveer MK, Carey WD, Vogt D, Henderson JM. Prevalence and reversibility of the hepatopulmonary syndrome after liver transplantation. The Cleveland Clinic experience. West J Med. 1995;163:133-138. [PubMed] |
| 59. | Abrams GA, Nanda NC, Dubovsky EV, Krowka MJ, Fallon MB. Use of macroaggregated albumin lung perfusion scan to diagnose hepatopulmonary syndrome: a new approach. Gastroenterology. 1998;114:305-310. [PubMed] |
| 60. | Abrams GA, Sanders MK, Fallon MB. Utility of pulse oximetry in the detection of arterial hypoxemia in liver transplant candidates. Liver Transpl. 2002;8:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 61. | Yang YY, Lin HC, Lee WC, Hou MC, Lee FY, Chang FY, Lee SD. Portopulmonary hypertension: distinctive hemodynamic and clinical manifestations. J Gastroenterol. 2001;36:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | De BK, Sen S, Biswas PK, Biswas J, Maity AK. Clinical and haemodynamic aspects of hepatopulmonary syndrome in Indian patients with cirrhosis. J Gastroenterol Hepatol. 2000;15:412-416. [PubMed] |
| 63. | Przybyłowski T, Krenke R, Fangrat A, Nasilowski J, Grabczak EM, Styczynski G, Pruszczyk P, Krawczyk M, Chazan R. Gas exchange abnormalities in patients listed for liver transplantation. J Physiol Pharmacol. 2006;57 Suppl 4:313-323. [PubMed] |
| 64. | Martínez GP, Barberà JA, Visa J, Rimola A, Paré JC, Roca J, Navasa M, Rodés J, Rodriguez-Roisin R. Hepatopulmonary syndrome in candidates for liver transplantation. J Hepatol. 2001;34:651-657. [PubMed] |
| 65. | Deberaldini M, Arcanjo AB, Melo E, da Silva RF, Felício HC, Arroyo PC, Duca WJ, Cordeiro JA, da Silva RC. Hepatopulmonary syndrome: morbidity and survival after liver transplantation. Transplant Proc. 2008;40:3512-3516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Krowka MJ, Wiseman GA, Burnett OL, Spivey JR, Therneau T, Porayko MK, Wiesner RH. Hepatopulmonary syndrome: a prospective study of relationships between severity of liver disease, PaO(2) response to 100% oxygen, and brain uptake after (99m)Tc MAA lung scanning. Chest. 2000;118:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 165] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 67. | Arguedas MR, Abrams GA, Krowka MJ, Fallon MB. Prospective evaluation of outcomes and predictors of mortality in patients with hepatopulmonary syndrome undergoing liver transplantation. Hepatology. 2003;37:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 238] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 68. | Swanson KL, Wiesner RH, Krowka MJ. Natural history of hepatopulmonary syndrome: Impact of liver transplantation. Hepatology. 2005;41:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 291] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 69. | Schiffer E, Majno P, Mentha G, Giostra E, Burri H, Klopfenstein CE, Beaussier M, Morel P, Hadengue A, Pastor CM. Hepatopulmonary syndrome increases the postoperative mortality rate following liver transplantation: a prospective study in 90 patients. Am J Transplant. 2006;6:1430-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 70. | Taillé C, Cadranel J, Bellocq A, Thabut G, Soubrane O, Durand F, Ichaï P, Duvoux C, Belghiti J, Calmus Y. Liver transplantation for hepatopulmonary syndrome: a ten-year experience in Paris, France. Transplantation. 2003;75:1482-1489; discussion 1446-1447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 71. | Lange PA, Stoller JK. The hepatopulmonary syndrome. Ann Intern Med. 1995;122:521-529. [PubMed] |
| 72. | Gómez FP, Martínez-Pallí G, Barberà JA, Roca J, Navasa M, Rodríguez-Roisin R. Gas exchange mechanism of orthodeoxia in hepatopulmonary syndrome. Hepatology. 2004;40:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 73. | Andrivet P, Cadranel J, Housset B, Herigault R, Harf A, Adnot S. Mechanisms of impaired arterial oxygenation in patients with liver cirrhosis and severe respiratory insufficiency. Effects of indomethacin. Chest. 1993;103:500-507. [PubMed] |
| 74. | Rodriguez-Roisin R, Roca J, Agusti AG, Mastai R, Wagner PD, Bosch J. Gas exchange and pulmonary vascular reactivity in patients with liver cirrhosis. Am Rev Respir Dis. 1987;135:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 75. | Molleston JP, Kaufman BA, Cohen A, Shackelford PG, Keating JP, Lowell JA, Howard TK. Brain abscess in hepatopulmonary syndrome. J Pediatr Gastroenterol Nutr. 1999;29:225-226. [PubMed] |
| 76. | Shijo H, Sasaki H, Nishimaru K, Okumura M. Recurrent intracranial hemorrhagic episodes in hepatopulmonary syndrome. Intern Med. 1992;31:786-790. [PubMed] |
| 77. | Palma DT, Philips GM, Arguedas MR, Harding SM, Fallon MB. Oxygen desaturation during sleep in hepatopulmonary syndrome. Hepatology. 2008;47:1257-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 78. | Collisson EA, Nourmand H, Fraiman MH, Cooper CB, Bellamy PE, Farmer DG, Vierling JM, Ghobrial RM, Busuttil RW. Retrospective analysis of the results of liver transplantation for adults with severe hepatopulmonary syndrome. Liver Transpl. 2002;8:925-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 79. | Krowka MJ, Mandell MS, Ramsay MA, Kawut SM, Fallon MB, Manzarbeitia C, Pardo M, Marotta P, Uemoto S, Stoffel MP. Hepatopulmonary syndrome and portopulmonary hypertension: a report of the multicenter liver transplant database. Liver Transpl. 2004;10:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 281] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 80. | Gupta S, Nayyar D, Pomier-Layrargues G. Variability of oxygenation in possible hepatopulmonary syndrome: effects of requiring two abnormal arterial blood gas results for diagnosis. Dig Dis Sci. 2015;60:1848-1855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 81. | Vedrinne JM, Duperret S, Bizollon T, Magnin C, Motin J, Trepo C, Ducerf C. Comparison of transesophageal and transthoracic contrast echocardiography for detection of an intrapulmonary shunt in liver disease. Chest. 1997;111:1236-1240. [PubMed] |
| 82. | Kim BJ, Lee SC, Park SW, Choi MS, Koh KC, Paik SW, Lee SH, Hong KP, Park JE, Seo JD. Characteristics and prevalence of intrapulmonary shunt detected by contrast echocardiography with harmonic imaging in liver transplant candidates. Am J Cardiol. 2004;94:525-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 83. | Krowka MJ, Porayko MK, Plevak DJ, Pappas SC, Steers JL, Krom RA, Wiesner RH. Hepatopulmonary syndrome with progressive hypoxemia as an indication for liver transplantation: case reports and literature review. Mayo Clin Proc. 1997;72:44-53. [PubMed] |
| 84. | Krowka MJ, Tajik AJ, Dickson ER, Wiesner RH, Cortese DA. Intrapulmonary vascular dilatations (IPVD) in liver transplant candidates. Screening by two-dimensional contrast-enhanced echocardiography. Chest. 1990;97:1165-1170. [PubMed] |
| 85. | Ramírez Moreno JM, Millán Núñez MV, Rodríguez Carrasco M, Ceberino D, Romaskevych-Kryvulya O, Constantino Silva AB, Muñoz-Vega P, García-Corrales C, Guiberteau-Sánchez A, Roa Montero A. [Detection of an intrapulmonary shunt in patients with liver cirrhosis through contrast-enhanced transcranial Doppler. A study of prevalence, pattern characterization, and diagnostic validity]. Gastroenterol Hepatol. 2015;38:475-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 86. | Iyer VN, Swanson KL, Cartin-Ceba R, Dierkhising RA, Rosen CB, Heimbach JK, Wiesner RH, Krowka MJ. Hepatopulmonary syndrome: favorable outcomes in the MELD exception era. Hepatology. 2013;57:2427-2435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 87. | Kaymakoglu S, Kahraman T, Kudat H, Demir K, Cakaloglu Y, Adalet I, Dincer D, Besisik F, Boztas G, Sözen AB. Hepatopulmonary syndrome in noncirrhotic portal hypertensive patients. Dig Dis Sci. 2003;48:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 88. | de Queirós AS, Brandão SC, Macedo LG, Ourem MS, Mota VG, Leite LA, Lopes EP, Domingues AL. Evaluation of normality and reproducibility parameters of scintigraphy with (99m)Tc-MAA in the diagnosis of intrapulmonary vascular dilatations. Ann Nucl Med. 2015;29:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 89. | Zhao H, Tsauo J, Ma HY, Li X. The role of macroaggregated albumin lung perfusion scan in hepatopulmonary syndrome: are we ready to draw conclusions? Liver Int. 2015;35:1918-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 90. | Abrams GA, Jaffe CC, Hoffer PB, Binder HJ, Fallon MB. Diagnostic utility of contrast echocardiography and lung perfusion scan in patients with hepatopulmonary syndrome. Gastroenterology. 1995;109:1283-1288. [PubMed] |
| 91. | Machicao VI, Balakrishnan M, Fallon MB. Pulmonary complications in chronic liver disease. Hepatology. 2014;59:1627-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 92. | Kochar R, Tanikella R, Fallon MB. Serial pulse oximetry in hepatopulmonary syndrome. Dig Dis Sci. 2011;56:1862-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Noli K, Solomon M, Golding F, Charron M, Ling SC. Prevalence of hepatopulmonary syndrome in children. Pediatrics. 2008;121:e522-e527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 94. | Arguedas MR, Singh H, Faulk DK, Fallon MB. Utility of pulse oximetry screening for hepatopulmonary syndrome. Clin Gastroenterol Hepatol. 2007;5:749-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 95. | Goldberg DS, Krok K, Batra S, Trotter JF, Kawut SM, Fallon MB. Impact of the hepatopulmonary syndrome MELD exception policy on outcomes of patients after liver transplantation: an analysis of the UNOS database. Gastroenterology. 2014;146:1256-1265.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 96. | Roberts DN, Arguedas MR, Fallon MB. Cost-effectiveness of screening for hepatopulmonary syndrome in liver transplant candidates. Liver Transpl. 2007;13:206-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 97. | Köksal D, Kaçar S, Köksal AS, Tüfekçioğlu O, Küçükay F, Okten S, Saşmaz N, Arda K, Sahin B. Evaluation of intrapulmonary vascular dilatations with high-resolution computed thorax tomography in patients with hepatopulmonary syndrome. J Clin Gastroenterol. 2006;40:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 98. | Lee KN, Lee HJ, Shin WW, Webb WR. Hypoxemia and liver cirrhosis (hepatopulmonary syndrome) in eight patients: comparison of the central and peripheral pulmonary vasculature. Radiology. 1999;211:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 99. | Suga K, Kawakami Y, Iwanaga H, Tokuda O, Matsunaga N. Findings of hepatopulmonary syndrome on breath-hold perfusion SPECT-CT fusion images. Ann Nucl Med. 2009;23:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 100. | Krowka MJ, Dickson ER, Cortese DA. Hepatopulmonary syndrome. Clinical observations and lack of therapeutic response to somatostatin analogue. Chest. 1993;104:515-521. [PubMed] |
| 101. | Porres-Aguilar M, Altamirano JT, Torre-Delgadillo A, Charlton MR, Duarte-Rojo A. Portopulmonary hypertension and hepatopulmonary syndrome: a clinician-oriented overview. Eur Respir Rev. 2012;21:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 102. | Grady K, Gowda S, Kingah P, Soubani AO. Coil embolization of pulmonary arteries as a palliative treatment of diffuse type I hepatopulmonary syndrome. Respir Care. 2015;60:e20-e25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 103. | Ryu JK, Oh JH. Hepatopulmonary syndrome: angiography and therapeutic embolization. Clin Imaging. 2003;27:97-100. [PubMed] |
| 104. | Saad NE, Lee DE, Waldman DL, Saad WE. Pulmonary arterial coil embolization for the management of persistent type I hepatopulmonary syndrome after liver transplantation. J Vasc Interv Radiol. 2007;18:1576-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 105. | Lee HW, Suh KS, Kim J, Shin WY, Yi NJ, Jae HJ, Chung JW, Oh SW, Kang KW, Lee KU. Pulmonary artery embolotherapy in a patient with type I hepatopulmonary syndrome after liver transplantation. Korean J Radiol. 2010;11:485-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 106. | Poterucha JJ, Krowka MJ, Dickson ER, Cortese DA, Stanson AW, Krom RA. Failure of hepatopulmonary syndrome to resolve after liver transplantation and successful treatment with embolotherapy. Hepatology. 1995;21:96-100. [PubMed] |
| 107. | Márquez Martín E, Jara Palomares L, Ortega Ruiz F, Grilo Bensusán I, López-Campos JL, Cejudo Ramos P, Pascasio JM, Rodríguez Becerra E. [Hepatopulmonary syndrome in patients with advanced hepatic disease: study of a series of 24 cases]. Med Clin (Barc). 2008;130:98-102. [PubMed] |
| 108. | Horvatits T, Drolz A, Roedl K, Herkner H, Ferlitsch A, Perkmann T, Müller C, Trauner M, Schenk P, Fuhrmann V. Von Willebrand factor antigen for detection of hepatopulmonary syndrome in patients with cirrhosis. J Hepatol. 2014;61:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 109. | Alonso Martínez JL, Zozaya Urmeneta JM, García Sanchotena JL, Olaz-Preciado F, Estébanez-Estébanez C, Berjón-Reyero J. [Hepatopulmonary syndrome: relationship with liver dysfunction and systemic hemodynamic disorder]. Med Clin (Barc). 2004;123:721-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 110. | Söderman C, Juhlin-Dannfelt A, Lagerstrand L, Eriksson LS. Ventilation-perfusion relationships and central haemodynamics in patients with cirrhosis. Effects of a somatostatin analogue. J Hepatol. 1994;21:52-57. [PubMed] |
| 111. | Gupta S, Faughnan ME, Lilly L, Hutchison S, Fowler R, Bayoumi AM. Norfloxacin therapy for hepatopulmonary syndrome: a pilot randomized controlled trial. Clin Gastroenterol Hepatol. 2010;8:1095-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 112. | Jounieaux V, Leleu O, Mayeux I. Cardiopulmonary effects of nitric oxide inhalation and methylene blue injection in hepatopulmonary syndrome. Intensive Care Med. 2001;27:1103-1104. [PubMed] |
| 113. | Song JY, Choi JY, Ko JT, Bae EJ, Kim HS, Noh CI, Yoon YS. Long-term aspirin therapy for hepatopulmonary syndrome. Pediatrics. 1996;97:917-920. [PubMed] |
| 114. | Shijo H, Sasaki H, Yuh K, Sakaguchi S, Okumura M. Effects of indomethacin on hepatogenic pulmonary angiodysplasia. Chest. 1991;99:1027-1029. [PubMed] |
| 115. | Moreira Silva H, Reis G, Guedes M, Cleto E, Vizcaíno JR, Kelly D, Gennery AR, Santos Silva E. A case of hepatopulmonary syndrome solved by mycophenolate mofetil (an inhibitor of angiogenesis and nitric oxide production). J Hepatol. 2013;58:630-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 116. | Cadranel JL, Milleron BJ, Cadranel JF, Fermand JP, Andrivet P, Brouet JC, Adnot S, Akoun GM. Severe hypoxemia-associated intrapulmonary shunt in a patient with chronic liver disease: improvement after medical treatment. Am Rev Respir Dis. 1992;146:526-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 117. | Yang W, Zhang J, Hu B, Wu W, Venter J, Alpini G, Fallon MB. The role of receptor tyrosine kinase activation in cholangiocytes and pulmonary vascular endothelium in experimental hepatopulmonary syndrome. Am J Physiol Gastrointest Liver Physiol. 2014;306:G72-G80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 118. | Chang CC, Chuang CL, Lee FY, Wang SS, Lin HC, Huang HC, Teng TH, Hsu SJ, Hsieh HG, Lee SD. Sorafenib treatment improves hepatopulmonary syndrome in rats with biliary cirrhosis. Clin Sci (Lond). 2013;124:457-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 119. | Vercelino R, Tieppo J, Forgiarini Junior LA, Dias AS, Marroni CA, Marroni NP. Experimental models for assessment of pulmonary alterations in hepatopulmonary syndrome. J Bras Pneumol. 2008;34:453-460. [PubMed] |
| 120. | Lambrecht GL, Malbrain ML, Coremans P, Verbist L, Verhaegen H. Orthodeoxia and platypnea in liver cirrhosis: effects of propranolol. Acta Clin Belg. 1994;49:26-30. [PubMed] |
| 121. | Yilmaz S, Dursum M, Canoruç F, Bayan K, Karabulut A, Akay H. A severe (type II) hepatopulmonary syndrome in a patient with idiopathic portal hypertension and treatment with paroxetine. Neth J Med. 2005;63:448-452. [PubMed] |
| 122. | Chang CC, Wang SS, Hsieh HG, Lee WS, Chuang CL, Lin HC, Lee FY, Lee SD, Huang HC. Rosuvastatin improves hepatopulmonary syndrome through inhibition of inflammatory angiogenesis of lung. Clin Sci (Lond). 2015;129:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 123. | Rolla G, Bucca C, Brussino L. Methylene blue in the hepatopulmonary syndrome. N Engl J Med. 1994;331:1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 124. | Miyamoto A, Katsuta Y, Zhang XJ, Li HL, Ohsuga M, Komeichi H, Shimizu S, Akimoto T, Mizuno K. Effect of chronic methylene blue administration on hypoxemia in rats with common bile duct ligation. Hepatol Res. 2010;40:622-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 125. | Krug S, Seyfarth HJ, Hagendorff A, Wirtz H. Inhaled iloprost for hepatopulmonary syndrome: improvement of hypoxemia. Eur J Gastroenterol Hepatol. 2007;19:1140-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 126. | Tanikella R, Philips GM, Faulk DK, Kawut SM, Fallon MB. Pilot study of pentoxifylline in hepatopulmonary syndrome. Liver Transpl. 2008;14:1199-1203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 127. | Gupta LB, Kumar A, Jaiswal AK, Yusuf J, Mehta V, Tyagi S, Tempe DK, Sharma BC, Sarin SK. Pentoxifylline therapy for hepatopulmonary syndrome: a pilot study. Arch Intern Med. 2008;168:1820-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 128. | Kianifar HR, Khalesi M, Mahmoodi E, Afzal Aghaei M. Pentoxifylline in hepatopulmonary syndrome. World J Gastroenterol. 2012;18:4912-4916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 129. | De BK, Dutta D, Pal SK, Gangopadhyay S, Das Baksi S, Pani A. The role of garlic in hepatopulmonary syndrome: a randomized controlled trial. Can J Gastroenterol. 2010;24:183-188. [PubMed] |
| 130. | Abrams GA, Fallon MB. Treatment of hepatopulmonary syndrome with Allium sativum L. (garlic): a pilot trial. J Clin Gastroenterol. 1998;27:232-235. [PubMed] |
| 131. | Porres-Aguilar M, Gallegos-Orozco JF. Hepatopulmonary syndrome: Is it time to redefine the MELD exception score for better organ allocation and outcomes? Ann Hepatol. 2014;13:468-470. [PubMed] |
| 132. | Fukushima KY, Yatsuhashi H, Kinoshita A, Ueki T, Matsumoto T, Osumi M, Matsuoka Y. Two cases of hepatopulmonary syndrome with improved liver function following long-term oxygen therapy. J Gastroenterol. 2007;42:176-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 133. | Corley DA, Scharschmidt B, Bass N, Somberg K, Gold W. Lack of efficacy of TIPS for hepatopulmonary syndrome. Gastroenterology. 1997;113:728-730. [PubMed] |
| 134. | Allgaier HP, Haag K, Ochs A, Hauenstein KH, Jeserich M, Krause T, Heilmann C, Gerok W, Rössle M. Hepato-pulmonary syndrome: successful treatment by transjugular intrahepatic portosystemic stent-shunt (TIPS). J Hepatol. 1995;23:102. [PubMed] |
| 135. | Lasch HM, Fried MW, Zacks SL, Odell P, Johnson MW, Gerber DA, Sandhu FS, Fair JH, Shrestha R. Use of transjugular intrahepatic portosystemic shunt as a bridge to liver transplantation in a patient with severe hepatopulmonary syndrome. Liver Transpl. 2001;7:147-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 136. | Riegler JL, Lang KA, Johnson SP, Westerman JH. Transjugular intrahepatic portosystemic shunt improves oxygenation in hepatopulmonary syndrome. Gastroenterology. 1995;109:978-983. [PubMed] |
| 137. | Selim KM, Akriviadis EA, Zuckerman E, Chen D, Reynolds TB. Transjugular intrahepatic portosystemic shunt: a successful treatment for hepatopulmonary syndrome. Am J Gastroenterol. 1998;93:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 138. | Siramolpiwat S. Transjugular intrahepatic portosystemic shunts and portal hypertension-related complications. World J Gastroenterol. 2014;20:16996-17010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 139. | De BK, Sen S, Biswas PK, Sanyal R, Majumdar D, Biswas J. Hepatopulmonary syndrome in inferior vena cava obstruction responding to cavoplasty. Gastroenterology. 2000;118:192-196. [PubMed] |
| 140. | Kim JA, Lee JJ, Kim CS, Chung IS, Gwak MS, Kim GS. Does general anesthesia with inhalation anesthetics worsen hypoxemia in patients with end-stage liver disease and an intrapulmonary shunt? Transplant Proc. 2011;43:1665-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 141. | Monsel A, Mal H, Brisson H, Luo R, Eyraud D, Vézinet C, Do CH, Lu Q, Vaillant JC, Hannoun L. Extracorporeal membrane oxygenation as a bridge to liver transplantation for acute respiratory distress syndrome-induced life-threatening hypoxaemia aggravated by hepatopulmonary syndrome. Crit Care. 2011;15:R234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 142. | Chihara Y, Egawa H, Tsuboi T, Oga T, Handa T, Yamamoto K, Mishima M, Tanaka K, Uemoto S, Chin K. Immediate noninvasive ventilation may improve mortality in patients with hepatopulmonary syndrome after liver transplantation. Liver Transpl. 2011;17:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 143. | Fleming GM, Cornell TT, Welling TH, Magee JC, Annich GM. Hepatopulmonary syndrome: use of extracorporeal life support for life-threatening hypoxia following liver transplantation. Liver Transpl. 2008;14:966-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 144. | Fallon MB, Mulligan DC, Gish RG, Krowka MJ. Model for end-stage liver disease (MELD) exception for hepatopulmonary syndrome. Liver Transpl. 2006;12:S105-S107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 145. | Gupta S, Castel H, Rao RV, Picard M, Lilly L, Faughnan ME, Pomier-Layrargues G. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am J Transplant. 2010;10:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 146. | Kim HY, Choi MS, Lee SC, Park SW, Lee JH, Koh KC, Paik SW, Yoo BC, Rhee JC. Outcomes in patients with hepatopulmonary syndrome undergoing liver transplantation. Transplant Proc. 2004;36:2762-2763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 147. | Sulieman BM, Hunsicker LG, Katz DA, Voigt MD. OPTN policy regarding prioritization of patients with hepatopulmonary syndrome: does it provide equitable organ allocation? Am J Transplant. 2008;8:954-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 148. | Martínez-Palli G, Gómez FP, Barberà JA, Navasa M, Roca J, Rodríguez-Roisin R, Burgos F, Gistau C. Sustained low diffusing capacity in hepatopulmonary syndrome after liver transplantation. World J Gastroenterol. 2006;12:5878-5883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 149. | Egawa H, Kasahara M, Inomata Y, Uemoto S, Asonuma K, Fujita S, Kiuchi T, Hayashi M, Yonemura T, Yoshibayashi M. Long-term outcome of living related liver transplantation for patients with intrapulmonary shunting and strategy for complications. Transplantation. 1999;67:712-717. [PubMed] |