Published online Jun 14, 2016. doi: 10.3748/wjg.v22.i22.5201
Peer-review started: January 25, 2016
First decision: February 18, 2016
Revised: February 28, 2016
Accepted: March 18, 2016
Article in press: March 18, 2016
Published online: June 14, 2016
Processing time: 130 Days and 17.4 Hours
AIM: To explore the preventive and therapeutic effects of Faecalibacterium prausnitzii (F. prausnitzii) supernatant on dextran sulfate sodium (DSS) induced colitis in mice.
METHODS: Forty C57BL/6J male mice were randomly divided into four groups: control group, model group, treatment group, and prevention group. Mice were weighed daily. On day 10, the colon length was measured, the colorectal histopathologic damage score (HDS) was assessed, and plasma interleukin (IL)-17A, IL-6, and IL-4 levels were detected by enzyme-linked immunosorbent assay. The expression of transcription factor retinoic acid-related orphan receptor-γt (RORγt) and IL-17A in colon inflammatory mucosa tissue were determined by immunohistochemical assay, and the expression levels of RORγt mRNA, IL-17A mRNA, and IL-6 mRNA were detected by real-time quantitative polymerase chain reaction (PCR). The proportion of Th17 in mononuclear cells in spleen was assayed by fluorescence activated cell sorter.
RESULTS: When compared with the model group, the colon length (P < 0.05) and body weight (P < 0.01) in the treatment and prevention groups were significantly increased, and the colon HDS was decreased (P < 0.05 and P < 0.01). There was no statistical difference between the treatment group and prevention group. After treatment with F. prausnitzii supernatant, the plasma levels of IL-17A and IL-6 (P < 0.05), the protein and mRNA expression of IL-17A and RORγt, and the Th17 cell ratio of spleen cells (P < 0.01) were significantly decreased compared to the model group. Plasma IL-4 level in the prevention group was significantly higher than that in the model group (P < 0.05), but there was no significant difference between these two groups in the expression of IL-6 in both the plasma and colon mucosa tissues.
CONCLUSION: F. prausnitzii supernatant exerts protective and therapeutic effects on DSS-induced colitis in mice, probably via inhibition of Th17 differentiation and IL-17A secretion in the plasma and colon mucosa tissues. It can also improve colitis in mice by downregulating IL-6 and prevent colitis by upregulating IL-4.
Core tip:Faecalibacterium prausnitzii (F. prausnitzii) supernatant has anti-inflammatory and immune regulatory activity. This study showed that the preventive and therapeutic use of F. prausnitzii supernatant could ameliorate dextran sulfate sodium (DSS)-induced colitis in mice by inhibiting Th17 cell differentiation and inflammatory cytokines release.
- Citation: Huang XL, Zhang X, Fei XY, Chen ZG, Hao YP, Zhang S, Zhang MM, Yu YQ, Yu CG. Faecalibacterium prausnitzii supernatant ameliorates dextran sulfate sodium induced colitis by regulating Th17 cell differentiation. World J Gastroenterol 2016; 22(22): 5201-5210
- URL: https://www.wjgnet.com/1007-9327/full/v22/i22/5201.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i22.5201
Inflammatory bowel diseases (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are multifactorial ailments characterized by intestinal inflammation. Although the precise etiology and pathogenesis of IBD are not fully elucidated, multiple factors contribute to IBD, including genetic background, environment, intestinal flora imbalance, and immune disorder[1-4]. It has been hypothesized that an undesired intestinal mucosal immune response to intestinal flora imbalance contributes to the onset of IBD in genetically susceptible individuals.
A T helper (Th)17 cell is defined as a cell producing the cytokine interleukin (IL)-17A, but it also can secrete many other cytokines, such as IL-17F, IL-6, and IL-23, during an inflammatory response[5]. Th17 cells are characterized by the expression of the transcription factor retinoic acid-related orphan receptor (RORγt), and there is growing evidence that Th17 cells are paramount in the development of human autoimmune diseases, including IBD[6-8]. In the intestine of IBD patients, elevated numbers of Th17 cells and increased RORγt and IL-17 levels are found[9]. The differentiation of Th17 cells from naïve CD4+ T cells is known to be affected by multiple cytokines, such as transforming growth factor (TGF)-β, IL-6, IL-4, and IL-23[10,11]. IL-6 plays a key role in cooperating with TGF-β to initiate Th17 differentiation, while IL-4 inhibits Th17 differentiation.
Faecalibacterium prausnitzii (F. prausnitzii) is the major bacterium of the Clostridium leptum group, and is one of the most abundant anaerobic bacteria in the human gut[12]. F. prausnitzii plays an important role in maintaining the intestinal health and providing energy to the colonocytes[13]. A recent study indicated that F. prausnitzii levels were decreased in IBD patients compared with healthy controls[14]. Previously, we confirmed in animals that both the bacteria and its supernatant relieved trinitro-benzene-sulfonic acid induced colitis in rats[15]. Nevertheless, the specific mechanism is largely unclear.
Dextran sulfate sodium (DSS) induced colitis is a well-established animal model for IBD pathogenesis, and it has been used in preclinical studies for over two decades[16,17]. Furthermore, it has been shown that the clinical features and pathological changes of DSS-induced colitis in mice were similar to human UC[18]. Here, we determined whether the F. prausnitzii supernatant could relieve DSS-induced colitis in mice by reducing Th17 cells and inflammatory cytokines.
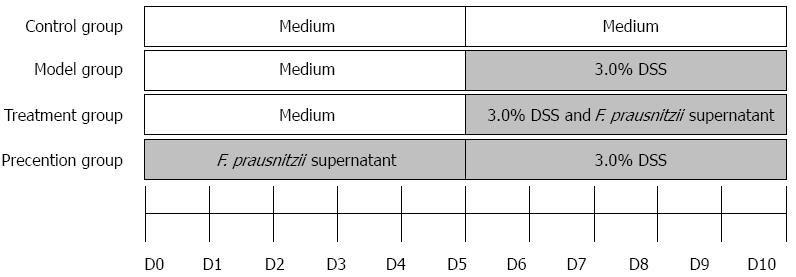
All experiments were approved by the Experimental Animal Ethical Committee of Nanjing Drum Tower Hospital, the Affiliated Hospital of Nanjing University Medical School. Forty male C57BL/6J mice aged 8-10 wk and weighing 18-22 g were obtained from the Animal Center, Nanjing Drum Tower Hospital (Nanjing, China). The mice were allocated equally and randomly to four groups: control group, model group, treatment group, and prevention group. The group divisible design is shown in Figure 1. The period of observation was 10 d. In the first 5 d, the mice in the prevention group were given supernatant of F. prausnitzii (five times concentrated, 0.1 mL/10 g) through gavage once a day, while the other groups received the same dosage of medium. For the next 5 d, all groups, except for the control group, were treated with 3.0% DSS in their drinking water ad libitum, the treatment group was fed F. prausnitzii supernatant by gavage once a day.
Mice were weighed daily and sacrificed by cervical dislocation at day 10. Colons were dissected, and the distance from cecum to anus was measured. The colon tissues were fixed in 4% formalin for later pathological examination and immunohistochemical study. The peripheral blood and spleen were isolated for testing Th17 cells and cytokines.
F. prausnitzii (ATCC 27766, Manassas, VA, United States) was cultured anaerobically at 37 °C in LYHBHI medium [main component of brain-heart infusion medium (37 g/L, BD, Franklin Lakes, NJ, United States), yeast extract (5 g/L, Oxoid, Basingstoke, United Kingdom), cellobiose ( 1 g/L, Sigma, St. Louis, MO, United States), maltose (1 g/L, Amresco, Solon, OH, United States), hemin (5 mg/L, Sigma), and cysteine (0.5 g/L, Sigma)]. The number of live bacteria (colony-forming units, CFU) was calculated according to optical density (OD) at 600 nm. The supernatant was collected from cultures with 109-1010 CFU/mL (OD = 1.9). Sterile culture medium acted as placebo. Bacterial supernatant and sterile culture medium were lyophilized and stored at -80 °C. They were thawed and diluted to five times concentrated solution with phosphate buffered saline (PBS) before administration.
The histopathologic grading of colon damage was scored by two blinded pathologists under microscope based on Neurath Scoring criteria as previously described[19]. In short, 4: transmural leukocyte infiltrations, high vascular density, loss of goblet cells, and thickening of the colon wall; 3: high level of leukocyte infiltration, thickening of the colon wall, high vascular density; 2: low level of leukocyte infiltration; 1: very low level of leukocyte infiltration; and 0: no inflammation.
Splenic mononuclear cells were isolated from spleens through Ficoll–Isopaue density gradient centrifugation[20]. Fresh spleens were placed in Roswell Park Memorial Institute (RPMI)-1640 (Gibco, Carlsbad, NY, United States) and mechanically disrupted by a 2 mL syringe plunger into cell suspensions. Cell suspensions were repeatedly aspirated with a sterile Pasteur pipette and gently filtered through a 200 μm strainer. Splenic single-cell suspensions were layered over an equal volume of Ficoll-Hypaque Solution (Haoyang BioScience Corporation, Tianjin, China) per spleen and centrifuged at 1500 rpm for 20 min. The band of leukocyte enriched fraction at the interface was collected after centrifugation at 1800 rpm for 10 min without brake. The resulting splenic mononuclear cell density was counted in a hemocytometer, and viability was assessed by Trypan blue staining.
Flow cytometry followed routine procedures by using 2 × 106 cells per sample. The splenic mononuclear cells were stimulated by phorbol-12-myristate-13-acetate (PMA), ionomycin, and brefeldin A for 5 h at 37 °C in a 5% CO2 incubator, then labeled with fluorescein isothiocyanate (FITC) anti-mouse CD4 (eBioscience, San Diego, CA, United States) and APC anti-mouse CD3 (eBioscience). After permeabilization and fixed treatment, cells were labeled with PE anti-mouse IL-17 (eBioscience). The stained cells were tested by flow cytometry (BD, San Jose, CA, United States) and analyzed by the Cell Quest software.
Cytokines (IL-17A, IL-6, IL-4) were measured using a commercially available enzyme-linked immunosorbent assay kit (Yunhan Biological Technology Corporation, Shanghai, China) according to the manufacturers’ instructions.
Total RNAs were extracted from mid-colon samples taken from mice in each group using the Trizol reagent (Invitrogen, Carlsbad, CA, United States) with the following procedure. The concentration was determined by NanoDrop TM 1100 (NanoDrop Technologies, Wilmington, DE, United States). Total RNA was reversely transcribed into cDNA using reverse transcription kit. The polymerase chain reaction (PCR) reactions were performed in a 96-well Optical Reaction Plate (Applied Biosystems, Foster City, CA, United States) with the following procedure: degeneration 95 °C for 30 s, annealing 95 °C for 5 s, 40 cycles of 60 °C for 34 s. All primers and probes used in this study are listed in Table 1.
| Target gene | Primer sequence | Product length (bp) |
| ROR-γt | forward: GACGGCCAACTTACTCTTGG | 109 |
| reverse: AGAAACTGGGAATGCAGTGG | ||
| IL-17A | forward: TCCCTCTGTGATCTGGGAAG | 154 |
| reverse: CTCGACCCTGAAAGTGAAGG | ||
| IL-6 | forward:CGGAGAGGAGACTTCACAGAG | 105 |
| reverse: CATTTCCACGATTTCCCAGA | ||
| GAPDH | forward: CATGGCCTTCCGTGTTCCTA | 83 |
| reverse:TGTCATCATACTTGGCAGGTTTCT |
Paraffin slides of colon were re-hydrated in different concentrations of ethanol and washed in PBS. Sections were microwaved in sodium citrate buffer. After blocking with 10% goat serum for 30 min, sections were incubated with rabbit anti-rat IL-17 antibodies (Abcam, Cambridge, United Kingdom) overnight at 4 °C. Slides were then incubated with the corresponding secondary antibody (Zsbio, Beijing, China), labelled with horseradish peroxidase, developed using a diaminobenzidine (DAB) reaction, and counterstained with hematoxylin. Cells stained with the antibodies were calculated by random selection of five fields under a microscope at 200 × magnification.
The GraphPad Prism version 5.0 (La Jolla, CA, United States) was used for data analysis. Data are presented as mean ± SD and were analyzed using one-way analysis of variance. P < 0.05 was considered to be statistically significant.
Mice became symptomatic (e.g., bloody diarrhea, weight loss, shakes andsloth) by day 3 of drinking 3.0% DSS ad libitum. The symptoms worsened with prolonged 3.0% DSS drinking time.
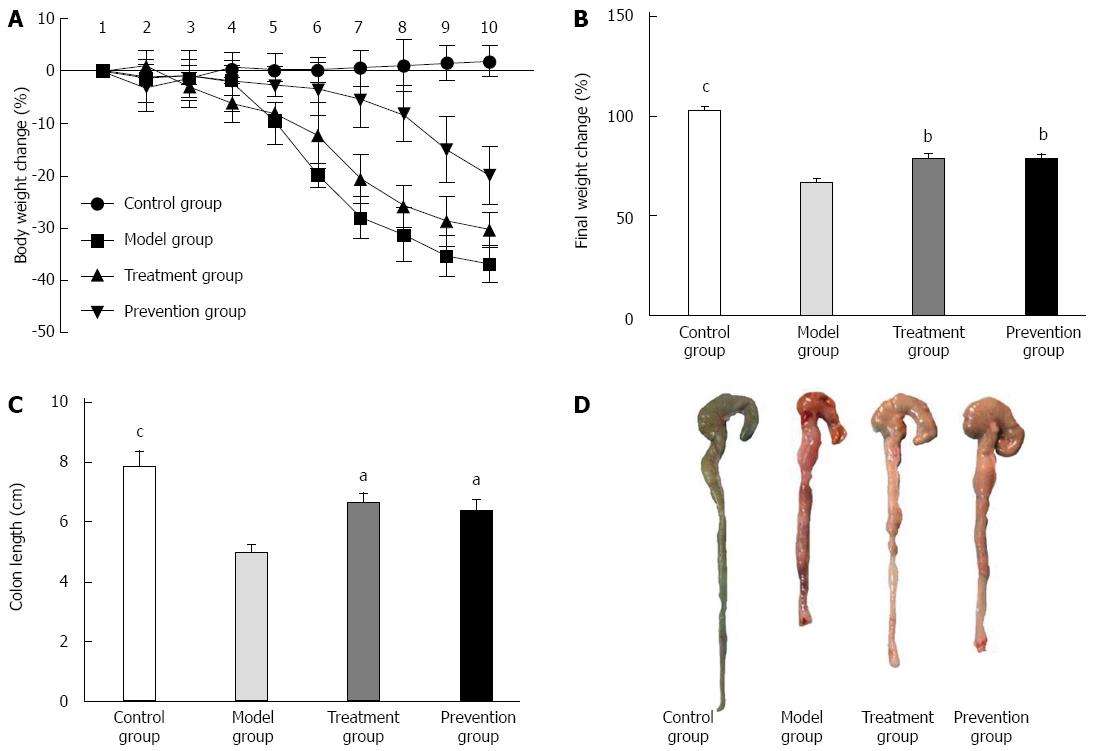
The mice in the model group had obvious weight loss compared to the control group (P < 0.001), and the mice from the model group weighed significantly less than those from the treatment and prevention groups. There was no significant difference in weight loss between the treatment group and prevention group (Figure 2).
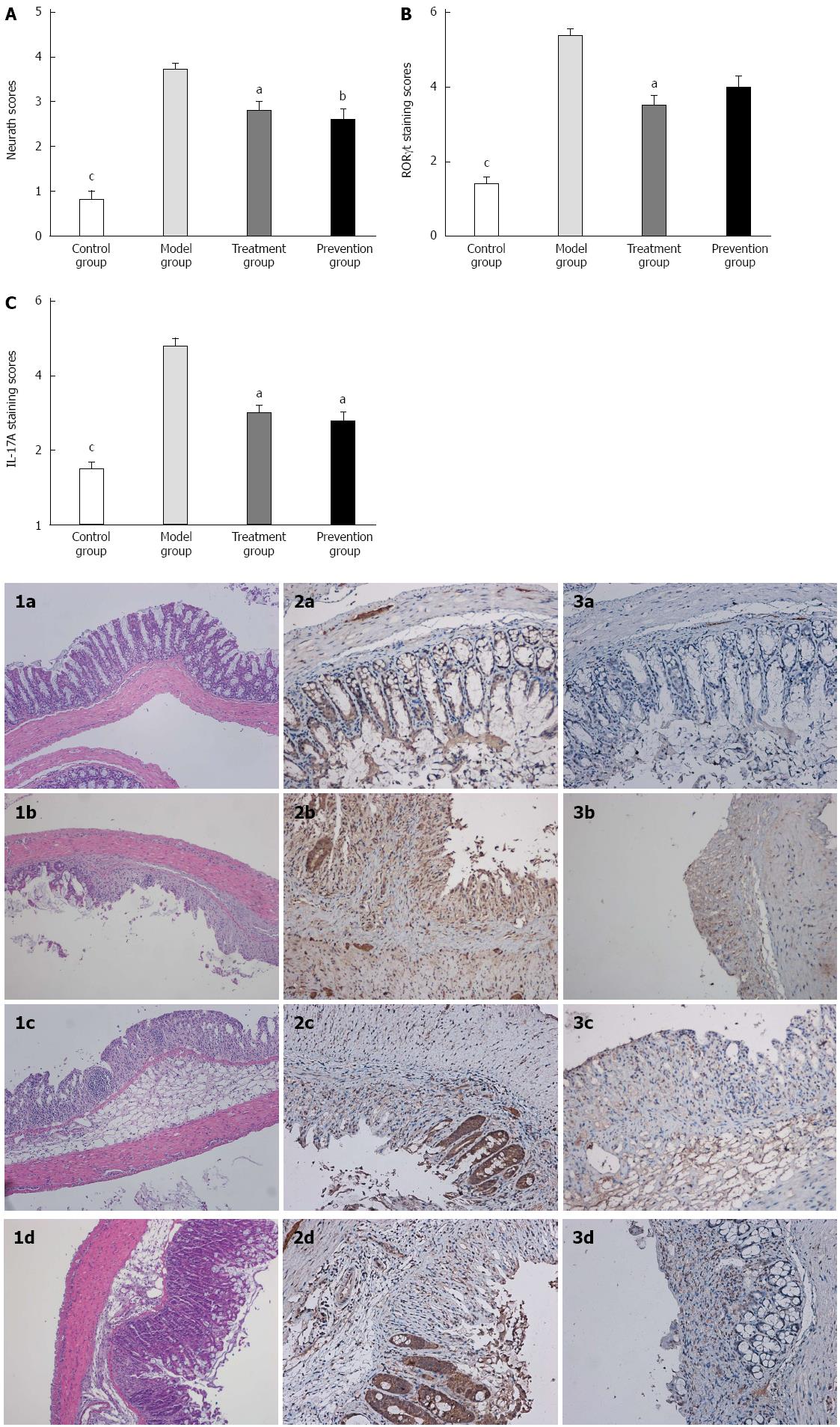
Compared with the control group, the mice in the model group had markedly shorter colon length (7.89 ± 1.536 vs 4.92 ± 0.925, P < 0.001), more serious colon damage, and higher histopathologic damage scores (0.8 ± 0.632 vs 3.7 ± 0.483, P < 0.01). Histological examination of model group mice showed that the normal colon mucous membrane structure disappeared, extensive ulceration developed, and a large number of inflammation cells infiltrated. However, culturing supernatant of F. prausnitzii in treatment and prevention group mice significantly ameliorated the colon damage by increasing colon length (P < 0.01 and P < 0.05) and reducing high histopathologic damage scores (P < 0.05) as compared with model group (Figure 2).
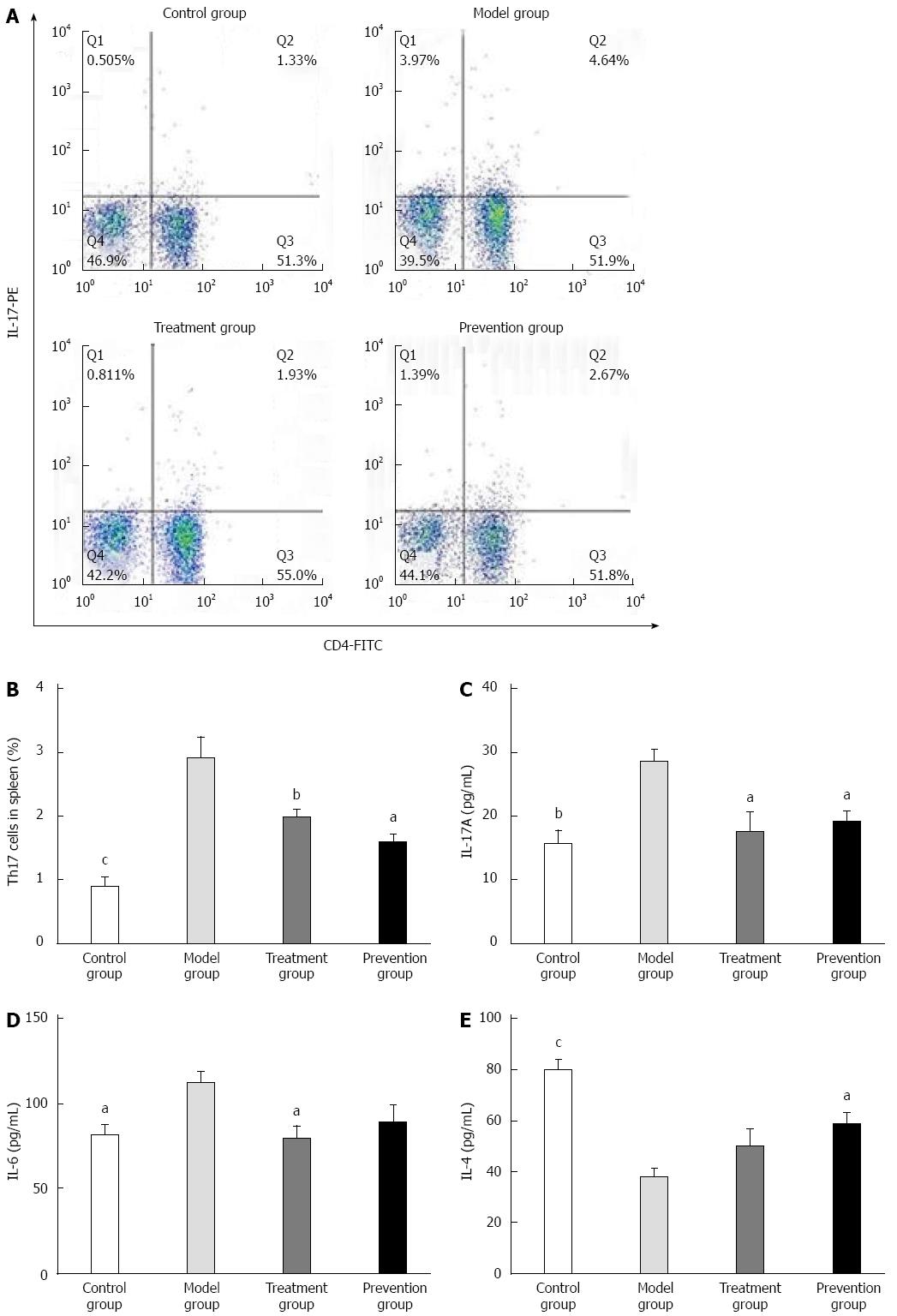
The ratio of Th17 cells in splenic mononuclear cells of the model group was significantly higher than that of the control group (4.02 ± 1.111 vs 1.34 ± 0.417, P < 0.001). It was obviously decreased after preventive and therapeutic application of F. prausnitzii supernatant (4.02 ± 1.111 vs 2.60 ± 0.839, P < 0.01 and 4.02 ± 1.111 vs 2.21 ± 1.030, P < 0.05), and there was no significant difference between the treatment and prevention groups (Figure 3).
Plasma IL-17A, IL-6, and IL-4 levels of the control group were significantly different from the model group [15.73 ± 4.382 (pg/mL) vs 28.44 ± 4.116 (pg/mL) P < 0.01, 81.19 ± 13.609 (pg/mL) vs 111.82 ± 14.369 (pg/mL) P < 0.05, 79.91 ± 12.245 (pg/mL) vs 38.16 ± 9.507 (pg/mL) P < 0.001]. The plasma levels of IL-17A in the treatment and prevention groups were significantly lower than that in the model group (P < 0.05). Plasma IL-6 level in the treatment group was also significantly less than that in the model group (P < 0.05), but the difference was not statistically significant between the prevention group and model group. On the contrary, level of plasma IL-4 in the prevention group was obviously higher than that in the model group (P < 0.05), while no difference was found between the treatment group and the model group (Figure 3).
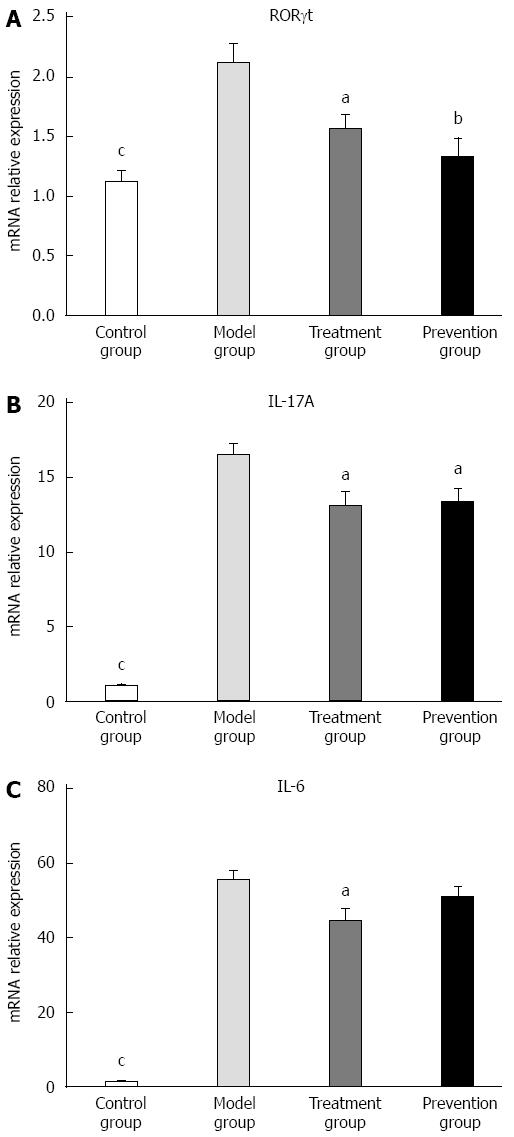
The expression of IL-17A, IL-6, and RORγt mRNA in colon tissue of mice in the model group was significantly higher than that in the control (P < 0.001) and treatment groups (P < 0.05). When compared with the model group, the expression of IL-17A and RORγt mRNA in colon inflammatory tissue of the treatment and prevention groups was significantly decreased (P < 0.01 or P < 0.05). There was no difference, however, in IL-6 between the model group and prevention group. As shown in Figure 4, the expression of cytokines and RORγt mRNA in colon mucosal tissue did not significantly differ between the treatment and prevention groups.
To investigate the effects of IL-17A and RORγt on colon tissue, we conducted immunohistochemical staining of proinflammatory cytokines in tissue sections. Consistent with the results of quantitative real time PCR, the expression of IL-17A and RORγt in the colon tissue of model group mice was significantly increased compared to that in the control group (P < 0.001) and treatment group (P < 0.05). Although the expression of RORγt in colon tissue was declined after protective use of F. prausnitzii, there was no difference between the model and prevention groups (Figure 5).
In this study, we found that F. prausnitzii supernatant ameliorated colitis in mice by regulating Th17 cell differentiation and inhibiting the excretion of relevant inflammatory cytokines. We also found that F. prausnitzii supernatant was effective in the treatment and prevention of DSS-induced mice colitis by inhibiting differentiation of Th17 cell.
Both living F. prausnitzii and F. prausnitzii supernatant, which contains a mixture of secreted products, have been shown to have an anti-inflammatory effect[21]. Compared to F. prausnitzii, its supernatant could be more effective therapeutically, as it may have a longer shelf-life, which would facilitate delivery, handling, and administration[22]. However, the exact composition and the anti-inflammatory mechanism of F. prausnitzii supernatant are currently largely unknown. Therefore, we explored the effects and immune mechanisms of F. prausnitzii supernatant on DSS-deduced colitis. Our study showed that the plasma levels of IL-17A and IL-6, the protein and mRNA expression of IL-17A and RORγt in intestinal mucosa, and the Th17 cell ratio of spleen cells (P < 0.01) in supernatant treatment group were significantly decreased compared to those in the model group. This finding indicated that the therapeutic use of F. prausnitzii supernatant could ameliorate DSS-induced colitis through inhibiting Th17 cells. Carlsson et al[23] previously demonstrated that the supernatant of F. prausnitzii affected the function of the intestinal barrier.
Th17-related gene polymorphisms are associated with IBD susceptibility[24]. Th17-derived cytokines, such as IL-17A, IL-6, and IL-22, have been shown to be upregulated in the inflamed intestine of IBD patients[25,26]. IL-17A is a strong inflammatory cytokine, which can enhance cell permeability and promote the generation of other pro-inflammatory cytokines and chemokines[27]. Animal experiments, however, have found that neither IL-17A knockout nor neutralization of IL-17 could protect DSS-administrated mice from colitis, suggesting that the role of IL-17 in intestinal inflammation may not be entirely pathogenic[14,28]. Adequate expression of IL-17A plays an important role in maintaining intestinal immune function. Consistent with previous studies, we found that IL-17A levels in the plasma, spleen, and colon tissue were significantly increased in mice with colitis and that these levels were remarkably downregulated in mice treated with F. prausnitzii culture supernatant. Therefore, F. prausnitzii supernatant could attenuate DSS-induced mice colitis, possibly by inhibiting the expression of IL-17A[15,29].
We also found that levels of IL-6 in plasma and colon tissues of colitis mice were significantly reduced after F. prausnitzii supernatant treatment. F. prausnitzii supernatant could alleviate mice colitis by downregulating IL-6 levels and inhibiting Th17 cell differentiation, thus leading to reduced secretion of inflammatory cytokines (such as IL-17A and IL-6) and attenuation of the local inflammatory response. However, the regulation of IL-6 expression in the treatment and prevention groups was inconsistent, suggesting that there might be other ways of inhibiting Th17 differentiation. Fu et al[29] demonstrated that boosting of Th2 associated cytokines (IL-4, IL-13, and IL-10) can reverse Th17-mediated intestinal inflammation. We also found that plasma IL-4 levels in mice of the prevention group were significantly greater than those in the model group.
In conclusion, F. prausnitzii supernatant can prevent DSS-deduced colitis in mice by inhibiting the generation of Th17 cells in the spleen and intestinal mucosa, leading to a reduction of IL-17A and IL-6 levels and attenuation of intestinal inflammation. This study provides the theoretical basis for the application of F. prausnitzii supernatant in UC treatment and prevention. However, what specific substances in the supernatant of F. prausnitzii possess biological activity needs to be elucidated in future studies. The safety and efficacy of F. prausnitzii supernatant also warrant further investigation by more large scale clinical trials.
The study has an internal teamwork from Institute of Digestive Disease, Nanjing Drum Tower Hospital. We thank the people who generously gave their precious time to take part in this study. We are also grateful to professor Jun Yang (pathology, Nanjing Tower Hospital) for his help on pathological diagnosis.
Inflammatory bowel disease (IBD) is a multifactorial ailment characterized by intestinal inflammation, and its etiology is complicated and ambiguous. Factors that contribute to IBD include genetic background, environment, intestinal flora imbalance, and immune disorder as well as the interactions between them.
Faecalibacterium prausnitzii (F. prausnitzii) is a common anaerobic bacteria that colonizes the human gut, and it plays a critical role in IBD. F. prausnitzii supernatant has anti-inflammatory and immune regulatory activity. Previously, the authors showed in animals that both the bacteria and its supernatant relieved trinitro-benzene-sulfonic acid-induced colitis in rats. However, the specific mechanism is largely unclear.
This study is the first to show that the preventive and therapeutic use of F. prausnitzii supernatant could ameliorate dextran sulfate sodium (DSS) induced mice colitis through inhibiting Th17 cells. The molecular mechanism of proliferation and differentiation of Th17 cells was different. F. prausnitzii supernatant may treat colitis in mice by downregulating IL-6 and preventing the upregulation of IL-4.
This study investigated the molecular mechanism of the preventive and therapeutic use of F. prausnitzii supernatant for IBD and provided evidence for the prevention and treatment of the disease.
F. prausnitzii is the major bacterium of the Clostridium leptum group and is one of the most abundant anaerobic bacteria in human gut.
The study investigates the preventive and therapeutic role of F. prausnitzii supernatant in a mouse model of DSS-induced ulcerative colitis. The topic is interesting, and the design and methods have clear scientific values. The data are clear and well presented.
P- Reviewer: Decorti G, Howarth GS, Wittmann T S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Ma S
| 1. | Ghosh S, Dai C, Brown K, Rajendiran E, Makarenko S, Baker J, Ma C, Halder S, Montero M, Ionescu VA. Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status, and ion transporter gene expression. Am J Physiol Gastrointest Liver Physiol. 2011;301:G39-G49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, Ferguson DJ, Campbell BJ, Jewell D, Simmons A. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 818] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 3. | Scharl M, Rogler G. Inflammatory bowel disease pathogenesis: what is new? Curr Opin Gastroenterol. 2012;28:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Steck N, Mueller K, Schemann M, Haller D. Republished: bacterial proteases in IBD and IBS. Postgrad Med J. 2013;89:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Annunziato F, Romagnani S. Heterogeneity of human effector CD4+ T cells. Arthritis Res Ther. 2009;11:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012;181:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 468] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 7. | Lubberts E. Th17 cytokines and arthritis. Semin Immunopathol. 2010;32:43-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Brucklacher-Waldert V, Stuerner K, Kolster M, Wolthausen J, Tolosa E. Phenotypical and functional characterization of T helper 17 cells in multiple sclerosis. Brain. 2009;132:3329-3341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 337] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 9. | Hundorfean G, Neurath MF, Mudter J. Functional relevance of T helper 17 (Th17) cells and the IL-17 cytokine family in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 10. | Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 979] [Cited by in RCA: 958] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 11. | Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2372] [Cited by in RCA: 2487] [Article Influence: 130.9] [Reference Citation Analysis (0)] |
| 12. | Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM. Enterotypes of the human gut microbiome. Nature. 2011;473:174-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5822] [Cited by in RCA: 5009] [Article Influence: 357.8] [Reference Citation Analysis (2)] |
| 13. | Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1462] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 14. | Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, Wang YH, Schluns KS, Broaddus RR, Zhu Z. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205:1063-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 575] [Cited by in RCA: 616] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 15. | Zhang M, Qiu X, Zhang H, Yang X, Hong N, Yang Y, Chen H, Yu C. Faecalibacterium prausnitzii inhibits interleukin-17 to ameliorate colorectal colitis in rats. PLoS One. 2014;9:e109146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1253] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 17. | Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 595] [Cited by in RCA: 645] [Article Influence: 49.6] [Reference Citation Analysis (1)] |
| 18. | Melgar S, Karlsson L, Rehnström E, Karlsson A, Utkovic H, Jansson L, Michaëlsson E. Validation of murine dextran sulfate sodium-induced colitis using four therapeutic agents for human inflammatory bowel disease. Int Immunopharmacol. 2008;8:836-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Neurath MF, Fuss I, Kelsall BL, Stüber E, Strober W. Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med. 1995;182:1281-1290. [PubMed] |
| 20. | Sippel TR, Shimizu T, Strnad F, Traystman RJ, Herson PS, Waziri A. Arginase I release from activated neutrophils induces peripheral immunosuppression in a murine model of stroke. J Cereb Blood Flow Metab. 2015;35:1657-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermúdez-Humarán LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci USA. 2008;105:16731-16736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2747] [Cited by in RCA: 3188] [Article Influence: 187.5] [Reference Citation Analysis (0)] |
| 22. | Prisciandaro L, Geier M, Butler R, Cummins A, Howarth G. Probiotics and their derivatives as treatments for inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1906-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Carlsson AH, Yakymenko O, Olivier I, Håkansson F, Postma E, Keita AV, Söderholm JD. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand J Gastroenterol. 2013;48:1136-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 24. | Yu P, Shen F, Zhang X, Cao R, Zhao X, Liu P, Tu H, Yang X, Shi R, Zhang H. Association of single nucleotide polymorphisms of IL23R and IL17 with ulcerative colitis risk in a Chinese Han population. PLoS One. 2012;7:e44380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Brand S. Crohn’s disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn’s disease. Gut. 2009;58:1152-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 518] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 26. | Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474:307-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2019] [Cited by in RCA: 1878] [Article Influence: 134.1] [Reference Citation Analysis (2)] |
| 27. | Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1048] [Cited by in RCA: 1048] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 28. | Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 404] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 29. | Fu SH, Lin MH, Yeh LT, Wang YL, Chien MW, Lin SH, Chang DM, Sytwu HK. Targeting tumour necrosis factor receptor 1 assembly reverses Th17-mediated colitis through boosting a Th2 response. Gut. 2015;64:765-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |