Published online Jun 14, 2016. doi: 10.3748/wjg.v22.i22.5165
Peer-review started: January 19, 2016
First decision: February 18, 2016
Revised: March 1, 2016
Accepted: March 14, 2016
Article in press: March 14, 2016
Published online: June 14, 2016
Processing time: 135 Days and 14.4 Hours
AIM: To determine whether high-protein, high-fat, and low-carbohydrate diets can cause lesions in rat livers.
METHODS: We randomly divided 20 female Wistar rats into a control diet group and an experimental diet group. Animals in the control group received an AIN-93M diet, and animals in the experimental group received an Atkins-based diet (59.46% protein, 31.77% fat, and 8.77% carbohydrate). After 8 wk, the rats were anesthetized and exsanguinated for transaminases analysis, and their livers were removed for flow cytometry, immunohistochemistry, and light microscopy studies. We expressed the data as mean ± standard deviation (SD) assuming unpaired and parametric data; we analyzed differences using the Student’s t-test. Statistical significance was set at P < 0.05.
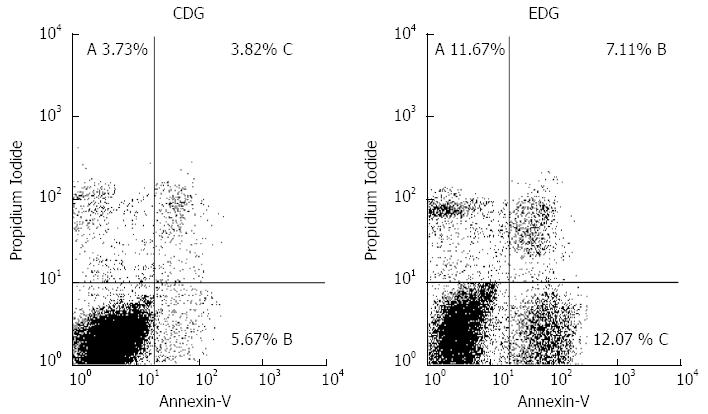
RESULTS: We found that plasma alanine aminotransferase and aspartate aminotransferase levels were significantly higher in the experimental group than in the control group. According to flow cytometry, the percentages of nonviable cells were 11.67% ± 1.12% for early apoptosis, 12.07% ± 1.11% for late apoptosis, and 7.11% ± 0.44% for non-apoptotic death in the experimental diet group and 3.73% ± 0.50% for early apoptosis, 5.67% ± 0.72% for late apoptosis, and 3.82% ± 0.28% for non-apoptotic death in the control diet group. The mean percentage of early apoptosis was higher in the experimental diet group than in the control diet group. Immunohistochemistry for autophagy was negative in both groups. Sinusoidal dilation around the central vein and small hepatocytes was only observed in the experimental diet group, and fibrosis was not identified by hematoxylin-eosin or Trichrome Masson staining in either group.
CONCLUSION: Eight weeks of an experimental diet resulted in cellular and histopathological lesions in rat livers. Apoptosis was our principal finding; elevated plasma transaminases demonstrate hepatic lesions.
Core tip: Obesity is a serious and growing health problem. A high-protein, high-fat, and low-carbohydrate diet known as the Atkins diet has been adopted since the 1970s. Many people adhere to this diet in an attempt to lose weight, and it has recently been introduced for children with difficult-to-control seizures and elderly suffering from Alzheimer’s and Parkinson’s diseases. The benefits and effects of the Atkins diet remain unclear, especially in hepatic metabolism. Since the primary metabolic reactions involving macronutrients occur in the liver, it is essential to understand the potential hepatic lesions that can result from dietary modifications.
- Citation: Monteiro MEL, Xavier AR, Oliveira FL, Filho PJ, Azeredo VB. Apoptosis induced by a low-carbohydrate and high-protein diet in rat livers. World J Gastroenterol 2016; 22(22): 5165-5172
- URL: https://www.wjgnet.com/1007-9327/full/v22/i22/5165.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i22.5165
The prevalence of obesity is increasing worldwide among adults, youth, and children; this serious health problem requires widespread public mobilization in the search for solutions. Extremely high numbers of people adhere to special diets in an attempt to lose weight[1]. A high-protein, high-fat, and low-carbohydrate diet was announced by the American cardiologist Robert C. Atkins in the mid-1970s as the best and healthiest way to become slim[2]. This so-called “Atkins diet” or “protein diet” has continued to be viewed as “a matter of love or hate”[3]. This diet was recently introduced for children with difficult-to-control seizures and elderly with Alzheimer’s and Parkinson’s diseases[4]. Despite numerous medical publications related to the Atkins diet, the results from a majority of such studies are inconclusive and have failed to demonstrate benefits and effects, especially in hepatic metabolism[5].
Several studies have reported associations between a high-protein diet and alterations in the liver[6], intestinal mucosa[7], kidneys[8,9], pancreas[10], adipose tissue[11], and bones[9,12]. Since the primary metabolic reactions involving macronutrients occur in the liver, it is essential to understand the potential hepatic lesions that may result from dietary modifications. It has been shown that high-fat or low-carbohydrate diets can cause hepatic steatosis related to excessive demand for fatty acids from diet and from adipose tissues as a consequence of gluconeogenesis[5]. In a recently published study, a high-protein diet (independent of the amount and type of fat or carbohydrate) was found not to lead to steatosis and may actually reverse it[13].
Hepatic cells are important targets for lesions in the presence of excessive dietary components since they are absorbed through the intestinal mucosa and quickly reach the liver through the portal vein[6]. Among macronutrients, carbohydrates and fat are largely responsible for the observed alterations since they promote changes in gene transcription and glycolytic and lipogenic enzymes [sterol responsive binding protein 1/2 (SREBP) and the mammalian target of rapamycin - mTOR), insulin, and adipokines][14].
Hepatocytes respond to injuries via various mechanisms, of which the most important are autophagy, apoptosis, and non-apoptotic death[15]. Autophagy may be considered to be an adaptive process associated with different types of liver injury, such as nutrient deprivation, insufficient growth factor, hypoxia, and the accumulation of fat in hepatocytes. Autophagy can be reversed if conditions improve. The cell digests its own components for use as an energy substrate; and when these components are insufficient to maintain cell homeostasis, either apoptosis or non-apoptotic death occurs[16,17]. Apoptosis, a cellular suicide program, is a natural process that is necessary to remove damaged, senescent, or mutagenic cells that have completed their mission. However, this process may lead to the development of various liver diseases. It is responsible for the development of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH) when the capacity of hepatocytes to store free fatty acids from the diet is exceeded[18]. Apoptosis is an active process that requires energy; when energy is insufficient, the process decelerates and non-apoptotic death begins. Non-apoptotic death differs from apoptosis in many ways since it sparks an inflammatory response that can aggravate the initial lesion[15,18].
Additional histopathological studies of livers of animals fed a low-carbohydrate and high-protein diet are required since the results to date have been conflicting[19,20].
Here, we hypothesized that a high-protein (59.46%), high-fat (31.77%), and very low-carbohydrate (8.77%) diet can cause hepatocyte lesions, as identified by flow cytometry and immunohistochemistry (IHC). We aimed to correlate the cytometry findings with light microscopy alterations and plasma transaminase levels.
The experimental study was performed from March-May 2015 in the Experimental Nutrition Laboratory of the Nutrition College, Fluminense Federal University, Niterói, RJ, Brazil. The animal protocol minimized pain and discomfort to the rats. The experiment used 20 female Wistar rats (Rattus norvegicus) ranging in age from 11-13 wk. The animals weighed 211-249 g and were reared at the Laboratory Animal Facility of the Oswaldo Cruz Foundation, Ministry of Health, Rio de Janeiro, Brazil. The animals were kept in group cages with four animals each, for adaptation, over the course of 5 d, receiving water and laboratory diet ad libitum. After this period, the rats were separated randomly into two groups of 10 animals each [the control diet group (CDG) and the experimental diet group (EDG)] and individually housed in polypropylene cages with controlled temperature (24 ± 2 °C) and humidity (60% ± 10%) and an alternating light-dark cycle consisting of 12 h of lightness and darkness.
The CDG diet consisting of the AIN 93M diet[21] was formulated for the maintenance of adult rats by the American Institute of Nutrition in 1993; we based the elaborated EDG diet on the Atkins diet. Both groups received water and an ad libitum diet for 8 wk. The diets were prepared by Pragsoluções Biociências Comércio e Serviços, LTD, Jaú, São Paulo, Brazil. The control diet had the following composition: carbohydrate (76.98%), protein (13.56%), and fat (9.46%). The experimental diet was composed of carbohydrate (8.77%), protein (59.46%), and fat (31.77%). The amount of vitamins, minerals, L-cysteine, choline, and fiber were the same in the two groups, and tert-butylhydroquinone was calculated as 0.002 mg per gram of fat, all based on AIN 93M determinations (Tables 1 and 2).
| Ingredients | g/100 g | CH (g) | PTN (g) | LIP (g) | FI (g) |
| Cornstarch | 46.5 | 39.52 | |||
| L-cysteine | 0.18 | 0.18 | |||
| Choline bitartrate | 0.25 | 0.25 | |||
| Mineral mix | 3.50 | 0.77 | |||
| Vitamin mix | 1 | 0.97 | |||
| Tert-butylhydroquinone | 0.008 | ||||
| Fiber | 5 | 5 | |||
| Soybean oil | 4 | 4 | |||
| Casein (> 85% Protein) | 14 | 11.06 | |||
| Sucrose | 10 | 10.00 | |||
| Dextrinized cornstarch | 15.5 | 13.95 | |||
| Kcal (%) | 338.8 | 260.84 | 45.96 | 32 | |
| Macronutrients (%) | 100 | 76.98 | 13.56 | 9.46 |
| Ingredient | (g/100 g) | CH (g) | PTN (g) | LIP (g) | FI(g) |
| Agar | 2 | 2 | |||
| L-cysteine | 0.18 | 0.18 | |||
| Choline bitartrate | 0.25 | 0.25 | |||
| Mineral mix | 3.5 | 0.77 | |||
| Vitamin mix | 1 | 0.97 | |||
| Tert-butylhydroquinone | 0.028 | ||||
| Fiber | 5 | 5 | |||
| Sucrose | 6 | 6 | |||
| Casein(> 85% protein) | 20 | 16 | |||
| Powdered chicken breast | 60 | 36 | 12 | ||
| Soybean oil | 2 | 2 | |||
| Kcal (%) | 352.68 | 30.96 | 209.72 | 112 | |
| Macronutrients (%) | 100 | 8.77 | 59.46 | 31.77 |
On the morning of the day of sacrifice, all of the animals underwent vaginal smears to determine their estrous cycle phase. Animals in estrus were separated and given no more access to food. After 8 h of fasting, the animals were anesthetized via an intraperitoneal injection of a solution containing 11.50 mg/100 g body mass of ketamine and 0.10 mg/100 g body mass of xylazine and were exsanguinated by cardiac puncture[22]. They were then sacrificed one at a time, alternating between the experimental and control group. The blood was placed in a heparinized tube and centrifuged for 20 min at 314 rad/s, and the plasma was separated and stored at -80 °C until analysis. The liver was removed after withdrawing the blood, and six liver fragments measuring 1 cm3 each were washed gently with NaCl 0.9%, submerged in a recipient with the same solution and stored in the freezer at -4 °C for 2 h prior to performing flow cytometry.
We measured plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels using automatic analysis (Vitalab Selectra E, Vital Scientific, Spankaren, Netherlands) with commercial kits from BioSystems Reagents and Instruments (Barcelona, Spain) located in the Multidisciplinary Research Support Laboratory (LAMAP), School of Medicine, UFF, Niterói, RJ, Brazil. The flow cytometry used the following fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit I components: 10X Annexin V Binding Buffer; FITC Annexin V; propidium iodide solution from BD Pharmingen (San Diego, CA, United States). The flow cytometer was the FACF-Calibur BD model. The liver fragments were fixed in Bouin’s solution, processed in graded alcohols and xylene, embedded in paraffin blocks, stained for optical microscopy [hematoxylin-eosin (HE) and Trichrome Masson (TM) stains], and prepared for IHC. Alexa fluor 647 rat anti-mouse blimp-1 from BD Pharmingen was used for identifying autophagy by IHC. We performed flow cytometry and IHC in the Biomedical Science Institute of the Federal University of Rio de Janeiro, Rio de Janeiro, RJ, Brazil. We relied on a Zeiss (Oberkochen, Germany) Axioscop 20 microscope and Canon (Tokyo, Japan) G10 camera JPG with 14.7 megapixels at the Pathology Department of the School of Medicine, UFF, Niterói, RJ, Brazil for the optical microscopy.
The results of this study are presented using descriptive statistics, such as arithmetic mean and standard deviation. The two-tailed unpaired Student’t test was used to compare means between the two groups. It was considered that 95% confidence interval contains the true difference between the means (P < 0.05). An F test was performed to prove that the data came from two groups that have identical standard deviations (and thus identical variances). The computer program used was Graphpad-Prism version 6.0e (La Jolla, CA, United States) for Mac OS X, 2015, and WinMDI 2.9 was used for flow cytometry. The statistical analyses were reviewed by a biomedical statistician. The IHC and optical microscopy were based on observational evaluation by an expert.
Plasma ALT and AST levels were significantly higher in the EDG than in the CDG. The mean ALT in the EDG was 61.70 ± 4.16 U/L compared with 28.10 ± 4.06 U/L in the CDG (P < 0.0001). AST in the EDG was 238.30 ± 15.85 U/L and 179.20 ± 13.86 U/L in the CDG (P = 0.0117).
Flow cytometry revealed a significantly higher percentage of nonviable cells in the EDG compared with the CDG (30.85% ± 2.20% and 13.22% ± 1.43%, respectively; P < 0.0001).
In the EDG, the percentages of nonviable cells were 11.67% ± 1.12% for early apoptosis, 12.07% ± 1.11% for late apoptosis, and 7.11% ± 0.44% for non-apoptotic death. In the CDG, the comparable values were 3.73% ± 0.50% for early apoptosis, 5.67% ± 0.72% for late apoptosis, and 3.82% ± 0.28% for non-apoptotic death (Figure 1).
When comparing the nonviable cells in the two groups, only the mean percentage of early apoptosis was statistically significant (Table 3).
Considering only non-apoptotic death and total apoptosis (early + late), the EDG demonstrated 23.99% ± 2.12% non-apoptotic death and 76.01% ± 2.12% total apoptosis (P < 0.0001); the CDG exhibited 29.20% ± 1.29% non-apoptotic death and 70.80% ± 1.29% total apoptosis (P < 0.0001).
IHC was negative for autophagy in both groups.
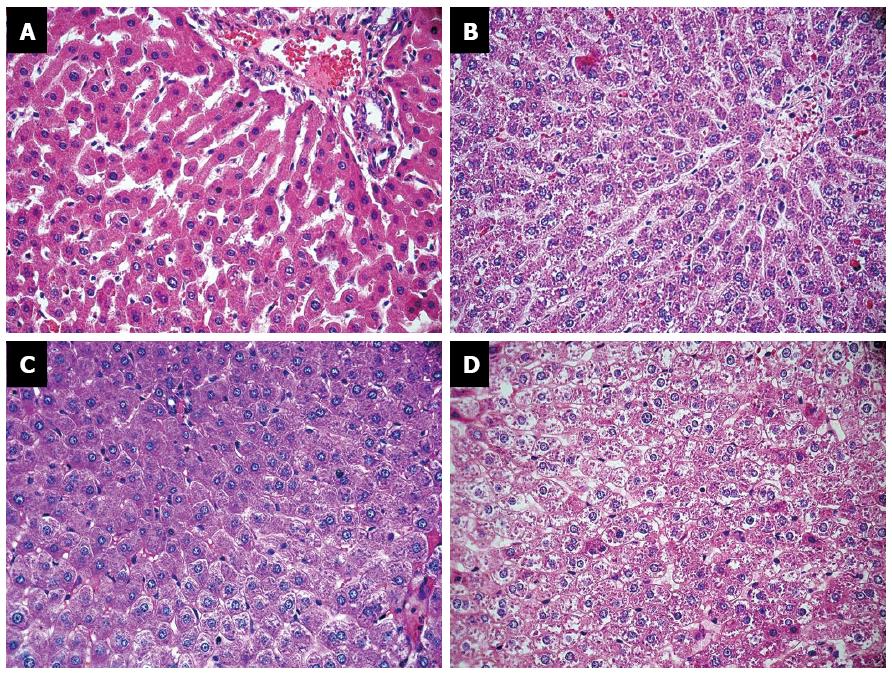
Upon examining the livers of the rats in the EDG, the pathologist identified marked sinusoidal dilation around the central vein, with smaller perisinusoidal hepatocytes compared with rats in the CDG, which showed no alteration. The central vein was normal in both groups. Five animals in the CDG had isolated periportal cytoplasmic microvesicles compared with three animals in the EDG. Small and heterogeneously distributed structures were found in the liver of all animals in both groups; these structures likely corresponded to deposition of glycogen. In the EDG, these structures decreased and even absent in some zones. The pathologist observed acute and chronic inflammatory periportal cells in both groups, which are considered to be normal in rats. Fibrosis was not identified by HE or TM in either group (Figure 2).
As expected, we found that a high-protein, high-fat, and low-carbohydrate diet caused cellular and histopathological lesions in the livers of experimental rodents. ALT and AST were increased in the EDG compared with the CDG. Since these tests are considered to be precise liver function tests, our results confirmed the presence of liver damage involving hepatocyte destruction with plasmatic membrane disruption and late-phase apoptosis and non-apoptotic death in the EDG[23,24].
In a study by Jean et al[25], a group of animals that received a diet consisting of 50% protein exhibited high ALT and normal AST compared with controls that received a modified AIN-93M diet. The results were interpreted as hepatic lesions since ATL is a specific liver enzyme located in the hepatocyte cytoplasm; AST can also be expressed by muscles and kidneys[23,25]. Oarada et al[6] demonstrated that when rats were fed increasing amounts of protein (35%, 40%, 45%, and 50%), ALT and AST increased to the same degree. These authors accordingly concluded that protein-independent of other macronutrients and energy consumption-was a risk factor for liver injury. In a recent study, Kostogrys et al[26] found no changes in plasma transaminase levels with a diet of 50.0% protein, 37.7% fat, and 12.3% carbohydrate, although the liver was enlarged compared to animals receiving the AIN93-M diet. Comparing our results with those noted in the literature may be difficult since the percentages of macronutrients fed to the rats vary from one study to another.
Flow cytometry confirmed the hepatic damage, as demonstrated by increased plasma transaminase levels; 30.85% of the hepatocytes were nonviable in the EDG compared with 13.22% in control animals. Nonviable cells in the CDG included 3.73% early apoptosis, 5.67% late apoptosis, and 3.82% non-apoptotic death. These findings in the control group can be considered to be physiological since apoptosis and non-apoptotic death represent a continuous process that is responsible for maintaining the balance between proliferation and cellular death. Non-apoptotic death is part of the same process since it is the ultimate fate of cells that undergo apoptosis[24].
Apoptosis was markedly increased in the EDG, with 11.67% early apoptosis, 12.07% late apoptosis, and 7.11% non-apoptotic cells exhibiting a non-physiological state. Any dysregulation of apoptosis is deleterious and results in tissue damage[15]. Similar results were found by Chiang et al[27] who demonstrated that mice receiving an 8-wk diet with 60% protein exhibited changes in bodyweight, liver histology, and expression of apoptosis and fibrosis.
Another important finding of the present study was that the percentage of early apoptosis, degeneration of mRNA, was significantly higher in the EDG (37.34%) compared with that in the CDG (28.43%) (P < 0.0001), indicating that apoptosis was progressing in the liver[18]. This finding might be evidence that the rats did not adapt to the experimental diet over the 8 wk of the study.
IHC was negative for autophagy in both groups. Autophagy was likely not found in this study because the percentage of nonviable cells increased (i.e., the cytoprotective mechanism was probably suppressed), and apoptosis continued to be active since early apoptosis was higher in the experimental group than in the control group. The two pathways are controlled by common mechanisms: when autophagy is inhibited, apoptosis is induced[16-18]. For comparison, we note that Garbow et al[5] fed animals a similar diet and found autophagy among others alterations.
A histological examination of the rats’ livers in the EDG revealed sinusoidal dilation around the central vein with smaller perisinusoidal cells compared to the livers of control animals. Similarly Bollo et al[28] found small hepatocytes around the central vein in alpine chamois during winter and considered this change an adaptation to under-nutrition. Bollo et al[28] asserted that the central vein region was a metabolic zone from which nutrients were distributed to the rest of the organ. The hepatocytes atrophied, and the size of the core was reduced. This probable adaptation to inadequate nutrition could be considered a strategy to minimize energy expenditures.
Cytoplasmic microvesicles were observed in five rats fed the control diet and three rats fed the experimental diet. The stain method that we used was unable to differentiate fat from water. Other authors have recovered similar results. Lacroix et al[19], in an experimental study with rats fed a high-protein diet (50%) versus a normal-protein diet (14%) over the course of 6 mo, found no serious histological lesions in either group. Rats in the normal-protein diet group exhibited more microvesicular hepatic steatosis than rats in the high-protein group. Caraballo et al[13] showed that rats fed a high-protein and high-fat diet did not develop steatosis compared with rats fed a high-fat and low-protein diet; they concluded that the high-protein diet had an anti-steatotic effect on rat livers regardless of the amounts of others macronutrients that the rats ingested. On the other hand, Garbow et al[5] found hepatocellular damage, inflammatory response, severe hepatic steatosis, apoptosis, and autophagy in mice that were fed a ketogenic diet (low-carbohydrate, low-protein, high-fat) for 12 wk. Only Caraballo et al[13] used a stain specific to fat. For energy, high-protein diet associated gluconeogenesis improves glucose disposal from amino acids and glycogen and reduces fat deposition; neo-lipogenesis does not occur[20]. York et al[29] showed that a low-carbohydrate diet might be an option to treat patients with NAFLD and NASH since such a diet improves liver histology and reduces fat deposits, insulin resistance, and metabolic syndrome. Although the prevalence of NAFLD in rich countries is between 20% and 30% and this condition is considered to be part of the Metabolic Syndrome, which is responsible for high morbidity and mortality, many aspects of its pathology and treatment remain only partially understood. Apoptosis is considered an important point of NAFLD lesion and a common mechanism of hepatic injury[15,30].
We found that the amount of glycogen was likely lower in the experimental group and that both groups exhibited a heterogeneous glycogen distribution. Since the animals of each group were sacrificed in an alternating fashion, changed in glycogen content cannot be due to the duration of fasting. It was not possible to be certain that it was glycogen since no specific stain (periodic acid-Schiff reagent PAS) was conducted. Caraballo et al[13] reported similar results in animals fed a high-protein and high-fat diet. However, with a low-fat and low-protein diet, glycogen was not decreased and was instead concentrated in the periportal area. With a high-fat and low-protein diet, the glycogen was concentrated in the pericentral area[28]. In contrast, when Azzout-Marnich et al[20] compared two diets with 14% and 50% protein, respectively, glycogen levels were not different between the groups, but hey considered glyconeogenesis to be the primary pathway of the high-protein diet metabolism. Caraballo et al and Azzout-Marniche et al[20] also did not use a specific stain for glycogen.
We found no evidence for inflammation in the liver, as expected, since apoptosis (76.01%) was the primary mechanism of hepatocyte damage in the experimental group, not non-apoptotic death (23.99%). Furthermore, literature results have shown that apoptosis does not lead to a local inflammatory response[18].
Although neither group exhibited evidence of fibrosis, apoptosis may be considered not only an important mechanism of liver injury but also a contributor to liver fibrosis[27,31,32]. It is probable that if the rats had received the experimental diet for a longer period of time, fibrosis may have eventually appeared.
Additional studies are necessary to determine the exact mechanism by which changes in the percentage of macronutrients induce apoptosis. The lack of suitable nutrients may be considered to be a possible hypothesis. Gluconeogenesis, the primary metabolic pathway associated with a low-carbohydrate diet, leads to an increased production of keto acids and fatty acids, but they may not be the better energy substrate for hepatic cells[33]. When gluconeogenesis occurs, mitochondria exert important functions in energy metabolism (i.e., the oxidation of fatty acids and oxidative phosphorylation for the production of adenosine triphosphate). Dysregulation of this pathway results in energy deficiencies and/or the production of reactive oxygen species that may be responsible for cell damage[34-36].
In conclusion, this study furthers our understanding of the hepatic cellular and histological changes caused by a high-protein, high-fat, and low-carbohydrate diet. The findings revealed that rats fed this diet had elevated levels of plasma transaminases and a higher percentage of nonviable cells in flow cytometry, which is evidence of hepatic lesions. Apoptosis was the principal pathway of hepatic injury. The primary findings from the optical microscopy-small hepatocytes and a decreased amount of glycogen-correlated well with changes in flow cytometry and can be attributed to modification of essential macronutrients to the liver. No inflammation or fibrosis was found in the livers of either the experimental or control animals. A better understanding of the mechanism of hepatic lesions associated with a high-protein, high-fat, and low-carbohydrate diet requires investigating the metabolic effects of diet in the liver.
The authors wish to acknowledge the collaboration of the Experimental Nutrition Laboratory, School of Nutrition, UFF, Niterói, RJ, Brazil; the Laboratory Animal Facility of the Oswaldo Cruz Foundation, Rio de Janeiro, Ministry of Health, Brazil; the Biomedical Science Institute, UFRJ, Rio de Janeiro, RJ, Brazil, the Multidisciplinary Support Laboratory for Research, School of Medicine, UFF, Niterói, RJ, Brazil, and the School of Pharmacy of UFF, Niterói, RJ, Brazil. They also thank Dr. Prof. Antônio Orestes de Salvo Castro for his valuable collaboration with the statistical analysis.
The authors did an experimental study comparing two groups of rats, one fed an experimental diet based on Atkins' (59.46% protein, 31.77% fat, and 8.77% carbohydrate) and the other an AIN-93M diet, so that they could study the effect of the experimental diet on hepatic metabolism. The modification of dietary components might result in hepatic cell damage since they are important targets for lesions when excessive macronutrients are absorbed and go through the intestinal mucosa to reach quickly the liver through the portal vein.
The prevalence of obesity is increasing and may be considered a serious health problem. Worldwide, many people adopt various diets to lose weight without a particular orientation. The Atkins diet is a low-carbohydrate and high protein diet formulated by Dr. Robert Atkins in 1972. More recently, it was introduced for children with difficult-to-control seizures and elderly with Alzheimer’s and Parkinson’s diseases. Despite these indications, the effect of the diet on hepatic metabolism remains unclear.
This study demonstrated a strong association between low-carbohydrate, high-protein, and high fat diet and hepatic apoptosis, as identified by flow cytometry. These changes were correlated with alterations found by optical microscopy and may be due to metabolic changes.
A better understanding of the mechanism underlying hepatic lesions associated with this diet in rats and its effects on human metabolism is essential. The article emphasizes that the use of this diet should be used with caution for children, adults, and elders.
Alanine aminotransferase) and aspartate aminotransferase are two enzymes found mainly in the liver that are considered hepatic markers of liver damage. Flow cytometry is a biophysical laser technology that was employed in this cell study; it detects cells in early apoptosis, late apoptosis, and nonapoptotic death.
Rats fed a low-carbohydrate, high-protein, and high-fat diet for 8 wk have hepatic cellular damage, as demonstrated by flow cytometry.
P- Reviewer: Tarantino G S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Zhang DN
| 1. | Huang TT, Cawley JH, Ashe M, Costa SA, Frerichs LM, Zwicker L, Rivera JA, Levy D, Hammond RA, Lambert EV. Mobilisation of public support for policy actions to prevent obesity. Lancet. 2015;385:2422-2431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 2. | Atkins RC. Atkins new diet revolution. New York: Harper Collins 1999; . |
| 3. | Frigolet ME, Ramos Barragán VE, Tamez González M. Low-carbohydrate diets: a matter of love or hate. Ann Nutr Metab. 2011;58:320-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Bielohuby M, Menhofer D, Kirchner H, Stoehr BJ, Müller TD, Stock P, Hempel M, Stemmer K, Pfluger PT, Kienzle E. Induction of ketosis in rats fed low-carbohydrate, high-fat diets depends on the relative abundance of dietary fat and protein. Am J Physiol Endocrinol Metab. 2011;300:E65-E76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Garbow JR, Doherty JM, Schugar RC, Travers S, Weber ML, Wentz AE, Ezenwajiaku N, Cotter DG, Brunt EM, Crawford PA. Hepatic steatosis, inflammation, and ER stress in mice maintained long term on a very low-carbohydrate ketogenic diet. Am J Physiol Gastrointest Liver Physiol. 2011;300:G956-G967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Oarada M, Tsuzuki T, Nikawa T, Kohno S, Hirasaka K, Gonoi T. Refeeding with a high-protein diet after a 48 h fast causes acute hepatocellular injury in mice. Br J Nutr. 2012;107:1435-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Lan A, Andriamihaja M, Blouin JM, Liu X, Descatoire V, Desclée de Maredsous C, Davila AM, Walker F, Tomé D, Blachier F. High-protein diet differently modifies intestinal goblet cell characteristics and mucosal cytokine expression in ileum and colon. J Nutr Biochem. 2015;26:91-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Hammond KA, Janes DN. The effects of increased protein intake on kidney size and function. J Exp Biol. 1998;201:2081-2090. [PubMed] |
| 9. | Cuenca-Sánchez M, Navas-Carrillo D, Orenes-Piñero E. Controversies surrounding high-protein diet intake: satiating effect and kidney and bone health. Adv Nutr. 2015;6:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Hara H, Narakino H, Kiriyama S, Kasai T. Induction of pancreatic growth and proteases by feeding a high amino acid diet does not depend on cholecystokinin in rats. J Nutr. 1995;125:1143-1149. [PubMed] |
| 11. | Brito MN, Brito NA, Migliorini RH. Thermogenic capacity of brown adipose tissue is reduced in rats fed a high protein, carbohydrate-free diet. J Nutr. 1992;122:2081-2086. [PubMed] |
| 12. | Heaney RP. Excess dietary protein may not adversely affect bone. J Nutr. 1998;128:1054-1057. [PubMed] |
| 13. | Garcia-Caraballo SC, Comhair TM, Verheyen F, Gaemers I, Schaap FG, Houten SM, Hakvoort TB, Dejong CH, Lamers WH, Koehler SE. Prevention and reversal of hepatic steatosis with a high-protein diet in mice. Biochim Biophys Acta. 2013;1832:685-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Tarantino G, Capone D. Inhibition of the mTOR pathway: a possible protective role in coronary artery disease. Ann Med. 2013;45:348-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 15. | Rust C, Gores GJ. Apoptosis and liver disease. Am J Med. 2000;108:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Czaja MJ, Ding WX, Donohue TM, Friedman SL, Kim JS, Komatsu M, Lemasters JJ, Lemoine A, Lin JD, Ou JH. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9:1131-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 368] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 17. | Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5421] [Cited by in RCA: 5289] [Article Influence: 311.1] [Reference Citation Analysis (0)] |
| 18. | Afford S, Randhawa S. Apoptosis. Mol Pathol. 2000;53:55-63. [PubMed] |
| 19. | Lacroix M, Gaudichon C, Martin A, Morens C, Mathé V, Tomé D, Huneau JF. A long-term high-protein diet markedly reduces adipose tissue without major side effects in Wistar male rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R934-R942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Azzout-Marniche D, Gaudichon C, Blouet C, Bos C, Mathé V, Huneau JF, Tomé D. Liver glyconeogenesis: a pathway to cope with postprandial amino acid excess in high-protein fed rats? Am J Physiol Regul Integr Comp Physiol. 2007;292:R1400-R1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939-1951. [PubMed] |
| 22. | He S, Atkinson C, Qiao F, Chen X, Tomlinson S. Ketamine-xylazine-acepromazine compared with isoflurane for anesthesia during liver transplantation in rodents. J Am Assoc Lab Anim Sci. 2010;49:45-51. [PubMed] |
| 23. | Heeringa M, Hastings A, Yamazaki S, de Koning P. Serum biomarkers in nonalcoholic steatohepatitis: value for assessing drug effects? Biomark Med. 2012;6:743-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Krysko DV, Vanden Berghe T, D’Herde K, Vandenabeele P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 2008;44:205-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 481] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 25. | Jean C, Rome S, Mathé V, Huneau JF, Aattouri N, Fromentin G, Achagiotis CL, Tomé D. Metabolic evidence for adaptation to a high protein diet in rats. J Nutr. 2001;131:91-98. [PubMed] |
| 26. | Kostogrys RB, Franczyk-Żarów M, Maślak E, Topolska K. Effect of low carbohydrate high protein (LCHP) diet on lipid metabolism, liver and kidney function in rats. Environ Toxicol Pharmacol. 2015;39:713-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Chiang WD, Shibu MA, Lee KI, Wu JP, Tsai FJ, Pan LF, Huang CY, Lin WT. Lipolysis-stimulating peptide VHVV ameliorates high fat diet induced hepatocyte apoptosis and fibrosis. J Funct Foods. 2014;11:482-492. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Bollo E, Bassano B, Peracino V, Biolatti B. Effect of emanciation on liver histology of alpine chamois during winter. J Wildl Dis. 1999;35:770-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | York LW, Puthalapattu S, Wu GY. Nonalcoholic fatty liver disease and low-carbohydrate diets. Annu Rev Nutr. 2009;29:365-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Tarantino G. Should nonalcoholic fatty liver disease be regarded as a hepatic illness only? World J Gastroenterol. 2007;13:4669-4672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002;123:1323-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 226] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 32. | Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 799] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 33. | Garcia Caraballo SC, Comhair TM, Dejong CH, Lamers WH, Köhler SE. A high-protein diet is anti-steatotic and has no pro-inflammatory side effects in dyslipidaemic APOE2 knock-in mice. Br J Nutr. 2014;112:1251-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Yuzefovych LV, Musiyenko SI, Wilson GL, Rachek LI. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One. 2013;8:e54059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 207] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 35. | Koo SH. Nonalcoholic fatty liver disease: molecular mechanisms for the hepatic steatosis. Clin Mol Hepatol. 2013;19:210-215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 314] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 36. | Galloway CA, Yoon Y. Mitochondrial morphology in metabolic diseases. Antioxid Redox Signal. 2013;19:415-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |