Published online Jun 14, 2016. doi: 10.3748/wjg.v22.i22.5154
Peer-review started: February 9, 2016
First decision: March 7, 2016
Revised: March 23, 2016
Accepted: April 7, 2016
Article in press: April 7, 2016
Published online: June 14, 2016
Processing time: 120 Days and 19.7 Hours
AIM: To develop a new rat model we wanted to gain a better understanding of stricture formation in Crohn’s disease (CD).
METHODS: Chronic colitis was induced locally by the administration of 2,4,6-trinitrobenzenesulfonic acid (TNBS). The relapsing inflammation characteristic to CD was mimicked by repeated TNBS treatments. Animals were randomly divided into control, once, twice and three times TNBS-treated groups. Control animals received an enema of saline. Tissue samples were taken from the strictured colonic segments and also adjacent proximally and distally to its 60, 90 or 120 d after the last TNBS or saline administrations. The frequency and macroscopic extent of the strictures were measured on digital photographs. The structural features of strictured gut wall were studied by light- and electron microscopy. Inflammation related alterations in TGF-beta 2 and 3, matrix metalloproteinases 9 (MMP9) and TIMP1 mRNA and protein expression were determined by quantitative real-time PCR and western blot analysis. The quantitative distribution of caspase 9 was determined by post-embedding immunohistochemistry.
RESULTS: Intestinal strictures first appeared 60 d after TNBS treatments and the frequency of them increased up to day 120. From day 90 an intact lamina epithelialis, reversible thickening of lamina muscularis mucosae and irreversible thickening of the muscularis externa were demonstrated in the strictured colonic segments. Nevertheless the morphological signs of apoptosis were frequently seen and excess extracellular matrix deposition was recorded between smooth muscle cells (SMCs). Enhanced caspase 9 expression on day 90 in the SMCs and on day 120 also in myenteric neurons indicated the induction of apoptosis. The mRNA expression profile of TGF-betas after repeated TNBS doses was characteristic to CD, TGF-beta 2, but not TGF-beta 3 was up-regulated. Overexpression of MMP9 and down-regulation of TIMP1 were demonstrated. The progressive increase in the amount of MMP9 protein in the strictures was also obvious between days 90 and 120 but TIMP1 protein was practically undetectable at this time.
CONCLUSION: These findings indicate that aligned structural and molecular changes in the gut wall rather than neuronal cell death play the primary role in stricture formation.
Core tip: Intestinal strictures in Crohn’s disease (CD) cause hardly treatable complications in patients. The aim of this study was to find the correlation between the intestinal stricture formation, the damaged innervation of smooth muscle cells (SMCs) and the changed expression of TGF-beta 2, 3 and MMP9/TIMP1 in rats with CD by using different light- and electron microscopic and molecular biological methods. Our findings indicate that disintegration of SMCs due to the up-regulation of TGF-beta 2 and off-balance in MMP9/TIMP1 expression rather than neuronal cell death play the primary role in the formation of intestinal strictures in CD.
- Citation: Talapka P, Berkó A, Nagy LI, Chandrakumar L, Bagyánszki M, Puskás LG, Fekete &, Bódi N. Structural and molecular features of intestinal strictures in rats with Crohn's-like disease. World J Gastroenterol 2016; 22(22): 5154-5164
- URL: https://www.wjgnet.com/1007-9327/full/v22/i22/5154.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i22.5154
Despite the fact that the formation of obstructive strictures is the leading cause of surgical intervention in patients with Crohn’s disease (CD), little is known about their etiopathogenesis, and no direct therapies are available for the effective prevention or reversal of this condition[1]. Lasting deep remission has emerged as a major therapeutic goal in CD[2,3]. This implies not only alleviation of the symptoms, but also the achievement of complete mucosal healing, with the accompanying decrease in the risk of irreversible pathological alterations of the gut wall[4]. However, total mucosal regeneration to prevent stricture formation is unattainable[5,6].
The spread of fibrosis deep into the gut wall leads to disorganization of the lamina muscularis mucosae (LMM) and thickening of all layers of the gut wall due to the accumulation of extracellular matrix (ECM) elements[5,6]. Previous studies have demonstrated that the cytokines transforming growth factor-beta (TGF-β) isoforms and the tissue-degrading matrix metalloproteinases (MMPs) are the key contributors to these processes[7,8]. Both TGF-beta and its receptors are overexpressed in the intestine of CD patients. However, the expression of the TGF-beta isoforms varies with the nature of the tissue. Fibrotic tissue exhibits a reduced expression of TGF-beta 3 and an enhanced expression of TGF-beta 2[9-11]. MMPs are secreted as inactive zymogens which must undergo proteolytic cleavage to become active, and their activity is regulated by specific tissue inhibitors of metalloproteinases (TIMPs)[12-14]. The MMPs do not simply degrade ECM as their name might suggest, but are also responsible for the homeostatic regulation of the ECM. Previous studies have shown that the gene transcription of MMP9 is inducible and that the promoter region is highly responsive to most growth factors and cytokines. They directly cleave and activate growth factors into active ligands, and therefore regulate their bioavailability and/or activity[15-17]. In consequence of these complex interactions of the regulatory processes, the development of the intestinal strictures characteristic of CD cannot be explained simply by the lower or higher expression of one or other of these factors. The key driver of stricture formation rather appears to be an off-balance between the TGF-betas, MMPs and TIMPs which develops in the chronic phase of inflammation. MMP9 is the most abundant MMP expressed in colonic tissue from CD patients, and may therefore be regarded as a biomarker in the evaluation of the clinical activity of inflammatory bowel diseases (IBDs)[18].
The intestinal symptoms common among CD patients are often related to enteric neuropathy. The evidence suggests that both the quantitative properties and function of the myenteric neurons are altered substantially by intestinal inflammation[19-22] and in fact complete loss of the myenteric neurons has been observed in the strictured regions[23]. However, the extent to which the deficient innervation of the smooth muscle cells (SMCs) and/or the imbalance in the regulation in the molecular events behind the tissue remodelling are responsible for the stricture formation remains unclear.
We recently reported on a rat model of chronic colitis where the mortality was negligible despite the severity of the intestinal symptoms. We demonstrated that experimentally provoked recurring periods of acute inflammation exerted a preconditioning effect against the mucosal damage and reduced the rapid, significant and widespread loss of myenteric neurons observed after the induction of the colitis[24]. In the present work, we used this model to investigate the long-term consequences of acute inflammation on the structural and molecular alterations in the strictured gut wall. The aim of the study was to investigate the possible coincidence between the expressions of TGF-betas, MMP9 and TIMP1 behind the structural remodelling of the strictured gut wall. The structural findings at the light- and electron microscopic levels and the molecular findings at the mRNA and protein levels will be discussed.
All procedures involving experimental animals were approved from the Local Ethics Committee for Animal Research Studies at the University of Szeged. Adult male Sprague-Dawley rats weighing 200-220 g were used throughout the experiments. The animal protocol was designed to minimize pain or discomfort to the animals. The rats were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for two weeks prior to experimentation. Colitis was induced locally under pentobarbital anaesthesia (45 mg/kg ip) by the administration of 2,4,6-trinitrobenzenesulfonic acid (TNBS; Sigma-Aldrich, St. Louis, MO, United States; 10 mg) dissolved in 0.25 mL of 25% ethanol, as described earlier[24]. Repetitive relapsing inflammation (RRI) was mimicked through repeated administration of the same TNBS doses. The rats were treated once (n = 8), twice (n = 7) or three times (n = 8) with TNBS, 2 weeks passed between the treatments. Control rats (n = 18) received an enema of 0.25 mL of 9 g/L saline at the same time as the TNBS was administered. The rats were weighed and monitored daily for activity, bloody diarrhoea and mortality and were sacrificed 60, 90 or 120 d after the last TNBS or saline administrations.
The animals were killed by cervical dislocation under pentobarbital anaesthesia. After this the last 8 cm region of the descending colon from the anus was dissected. Digital photographs were taken to evaluate the frequency and macroscopic extent of the strictures. Three colonic tissue samples were taken from each animal: the stricture itself and samples adjacent proximally and distally to it. Colonic samples of age-matched controls were also collected. Small pieces (2-3 mm) of the colonic segments for light- and electron microscopic morphometry and post-embedding immunohistochemistry were fixed in 20 g/L formaldehyde and 20 g/L glutaraldehyde solution and embedded in Epon (Electron Microscopy Sciences, Hatfield, PA, United States). Gut segments for molecular studies were cut along the mesentery and pinched flat. After longitudinal cutting, the mucosa and submucosa were removed. Half of the colon samples were immediately frozen in liquid N2 and later processed for western blot analysis. The other half were incubated overnight at 4 °C in RNA Later (Qiagen, Venlo, The Netherlands) and stored at -80 °C until processing for quantitative real-time PCR (qRT PCR).
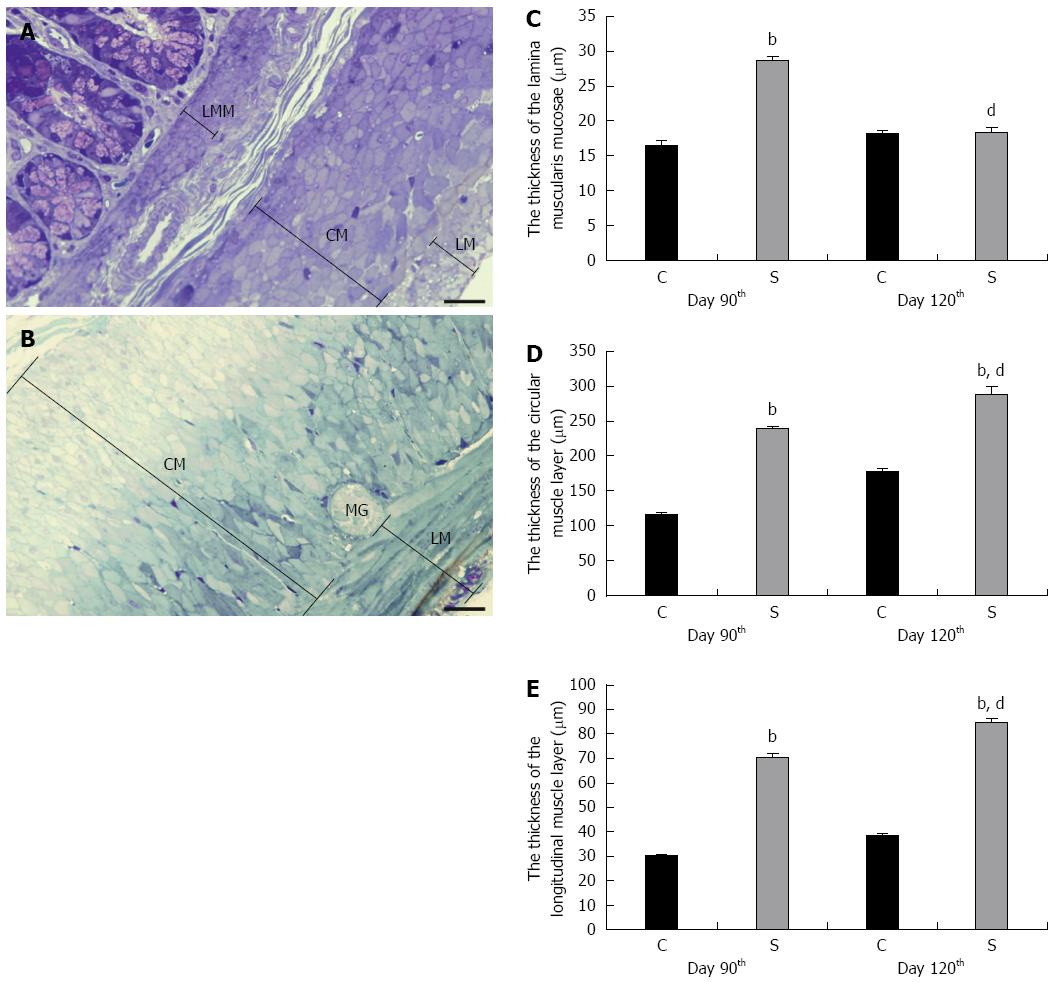
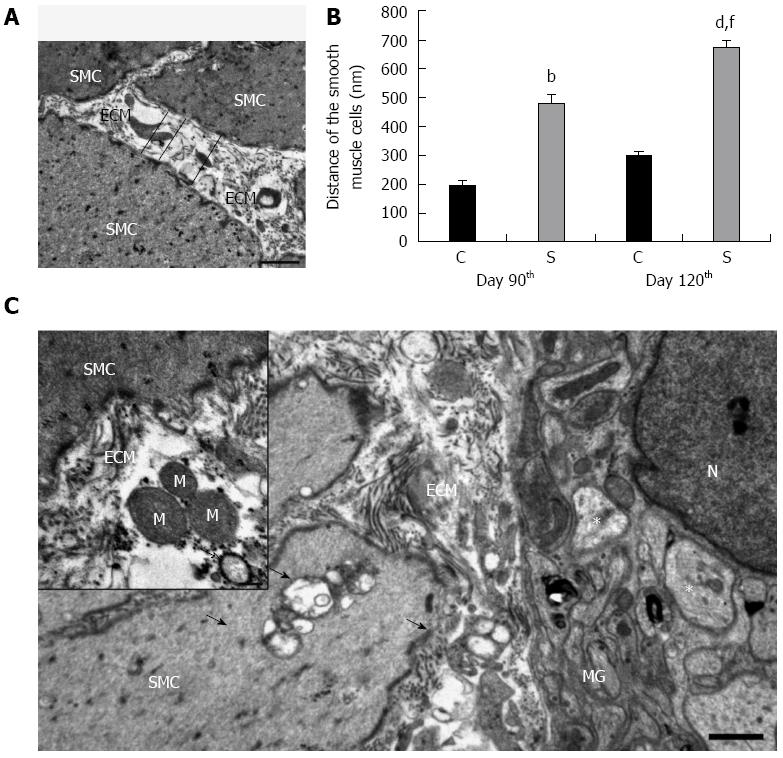
The Epon blocks were used to prepare semithin (0.7 μm) sections, which were stained with 10 g/L toluidine blue solution for the light-microscopic study. In the selected area of interest in the semithin cross-sections, all the layers of the gut wall were well oriented. The thicknesses of the LMM and the external circular (CM) and longitudinal (LM) smooth muscle layers were measured at random points with Image J 1.44 (National Institute of Health, Bethesda, MD, United States). The same Epon blocks were used to prepare ultrathin (70 nm) sections and the samples were mounted on nickel grids. Three grids per block were stained with uranyl acetate (Merck, Darmstadt, Germany) and lead citrate (Merck) and were examined and photographed with a Philips CM 10 electronmicroscope equipped with a MEGAVIEW II camera. The width of 15 tight junctions (TJs), i.e., the distance between adjacent enterocytes, was measured at a magnification of × 46000 in the control samples and in the strictures by using the AnalySIS 3.2 program (Soft Imaging System GmbH, Münster, Germany). The distance between SMCs was determined to evaluate the expansion of the ECM within the muscularis externa (ME). Ten montage photographs per intestinal segment were made at a magnification of × 10500 and the distance of SMCs was evaluated in limited-size (2000 nm × 2000 nm) grids for all images, at the intersection of the grid lines, perpendicularly to the cells and calculated by using the AnalySIS 3.2 program. The mean distance was calculated by using the AnalySIS 3.2 program.
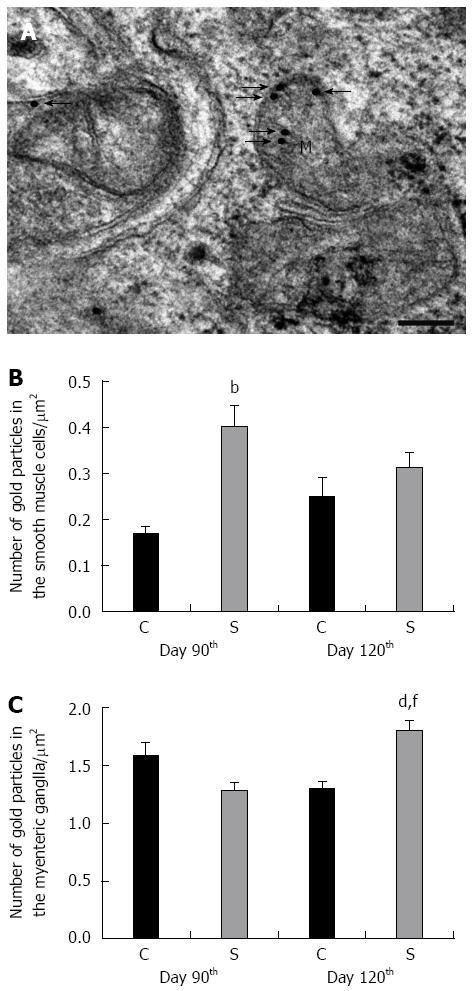
The Epon-embedded tissue blocks used previously for the morphometry also served for the post-embedding immunohistochemistry of caspase 9, as described earlier[25]. Briefly, ultrathin sections from each block were sequentially incubated with anti-caspase 9 (Sigma-Aldrich, St. Louis, MO, United States; final dilution 1:50) primary antibodies overnight, followed by protein A-gold-conjugated anti-rabbit (18 nm gold particles, Jackson ImmunoResearch, West Grove, PA, United States; final dilution 1:20) secondary antibodies for 3 h, with extensive washing between. Sections were counterstained with uranyl acetate and lead citrate, and then examined and photographed with a Philips CM10 electronmicroscope equipped with a MEGAVIEW II camera. The numbers of gold particles were counted on digital photographs at a magnification of × 25000 in 10 SMCs and at a magnification of × 34000 in 5 myenteric ganglia (MGs) per colonic segment in each experimental groups with the AnalySIS 3.2 program.
Statistical analysis of the histological results was performed by using one-way ANOVA and the Newman-Keuls test with GraphPad Prism 4.0 (GraphPad Software, La Jolla, CA, United States), and a probability P < 0.05 was set as the level of significance. The results were expressed as mean ± SE. The statistical methods of the study were reviewed by Mária Bagyánszki from University of Szeged.
Tissue samples were homogenized in AccuZol (Bioneer, Daejeon, South Korea) directly before qRT PCR. Total RNA was prepared from tissue homogenates as suggested by the manufacturer (Bioneer, Daejeon, Korea). The reverse transcription was achieved by using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, United States) as described earlier[24]. qRT PCR was performed in an Exicycler 96 (Bioneer, Daejeon, Korea) in a total volume of 20 μL containing 10 μL of FastStart SYBR Green PCR Master Mix, 1 μL of specific primer (0.5 pmol/μL) and 50 ng of cDNA template. The PCR program began with a 15-min initial step at 95 °C, followed by 45 cycles of 15 s at 95 °C for denaturation, 45 s at 60 °C for annealing and 25 s at 72 °C for extension. The sequences of primers were derived from NCBI RefSeq Database entry NM_031131.1 for TGF-beta 2 (forward: 5’ agtgggcagcttttgctc 3’ and reverse: 5’ gtagaaagtgggcgggatg 3’), NM_013174.2 for TGF-beta 3 (forward: 5’ gaagagggccctggacac 3’ and reverse: 5’ gcgcacacagcagttctc 3’), NM_031055.1 for MMP9 (forward: 5’cctctgcatgaagacgacataa 3’ and reverse: 5’ ggtcaggtttagagccacga 3’) and NM_053819.1 for TIMP1 (forward: 5’ cagcaaaaggccttcgtaa 3’ and reverse: 5’ tggctgaacagggaaacact 3’). Hypoxanthine guanine phosphoribosyltransferase (HPRT) (NBCI RefSeq Database entry: NM_012583.2; forward: 5’ gaccggttctgtcatgtcg 3’ and reverse 5’ acctggttcatcatcactaatcac 3’) was used as a housekeeping gene to normalize the expression data. The results were expressed as mean ± SD.
Tissue samples were homogenized in TRIS-mannitol buffer and the total cellular protein was then denaturated (mixing and boiling with v/v 20 mmol/L Tris 7-9, 3 mmol/L EDTA, 20 g/L sodium dodecyl sulphate (SDS), 100 g/L mercaptoethanol and 200 g/L glycerol) from each sample as described earlier[26]. Aliquots of 10 μg of total cellular protein were electrophoresed by 100 g/L SDS-polyacrilamide gel, and transferred to nitro-cellulose membrane (Amersham, Buckinghamshire, United Kingdom). Two hours after blocking (with PBS pH 7.4, 2.5 g/L Tween 20 (v/v) and 50 g/L non-fat dried milk), the membranes were probed with anti-MMP9 mouse monoclonal antibody (Abcam PLC, Cambridge, United Kingdom; final dilution 1:1000) or TIMP1 (H150) rabbit polyclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, United States; final dilution 1:1000) for 2 h, and then incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, United States; final dilution 1:2000) for 1 h at room temperature with extensive PBS-Tween 20 washing between. Immunoreaction was visualized with an Immobilon Western HRP Substrate enhanced chemiluminescence system (Millipore Corporation, Billerica, MA, United States) and scanned with a LI-COR C-DiGit™ Blot Scanner (Li-Cor Corporate, Lincoln, NE, United States).
The activity of MMP9 was determined by gelatine zymography, performed by diluting colonic homogenates in zymogram sample buffer (Bio-Rad, Hercules, CA, United States) and electrophoresing the samples in precast 100 g/L SDS-PAGE containing gelatine (20 mg/mL; Sigma-Aldrich, St. Louis, MO, United States) at 120 V until resolution was achieved. The gels were removed from their casings, gently rinsed in ddH2O, placed onto a shaker in 1X renaturation buffer (Bio-Rad, Hercules, CA, United States) for 40 min, and then placed in 1X development buffer (Bio-Rad Hercules, CA, United States). With change of the buffer once at 20 min, the gels were next incubated at 37 °C for 20 h and stained with Coomassie Blue (Bio-Rad, Hercules, CA, United States) for 40 min before being destained in water for 1 h and scanned with a LI-COR C-DiGit™ Blot Scanner.
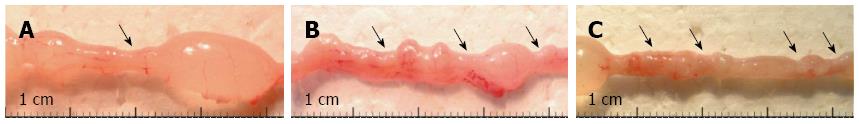
Despite the severity of the acute intestinal inflammation of the TNBS-treated rats, the mortality was negligible: only 2 rats died throughout the 120-d experimental period. By 1 d following the TNBS treatment, all the animals had developed symptoms such as weakness, weight loss and bloody diarrhoea. However, by 7 or 8 d after TNBS administrations, all the visible symptoms accompanied by acute inflammation had resolved. By day 60 following TNBS treatments, all the rats that had previously been exposed to acute colitis had regained their initial body weight and strictures had appeared in each TNBS-treated group. We, therefore, investigated the structural and molecular characteristics of the strictured gut wall from this timepoint on. Whereas the numbers and sizes of the strictures increased in time and with the number of TNBS treatments, they always developed within the previously inflamed colonic areas (Figure 1) and, once they had appeared, their structure and molecular characteristics did not differ. To avoid repetitions therefore, representative results will be presented here, obtained after the processing of tissue samples collected exclusively after the third TNBS administration.
Representative images of toluidine blue-stained semithin sections of colon where the thickness of the ME was measured are shown in Figure 2. Such colonic sections were collected for measurements on days 90 and 120 following TNBS administrations and also from age-matched controls. The strictured colonic regions displayed normal mucosal architecture and clearly defined, yet thickened muscle layers (not shown). Morphometric analyses revealed the approximately 2-fold thickening of the LMM and layers of the ME in the strictured region relative to the control samples on 90 d. While further significant thickening of the ME was measured beyond day 90 after TNBS administrations, the thickness of the LMM at later than 90 d was similar to that in the controls (Figure 2).
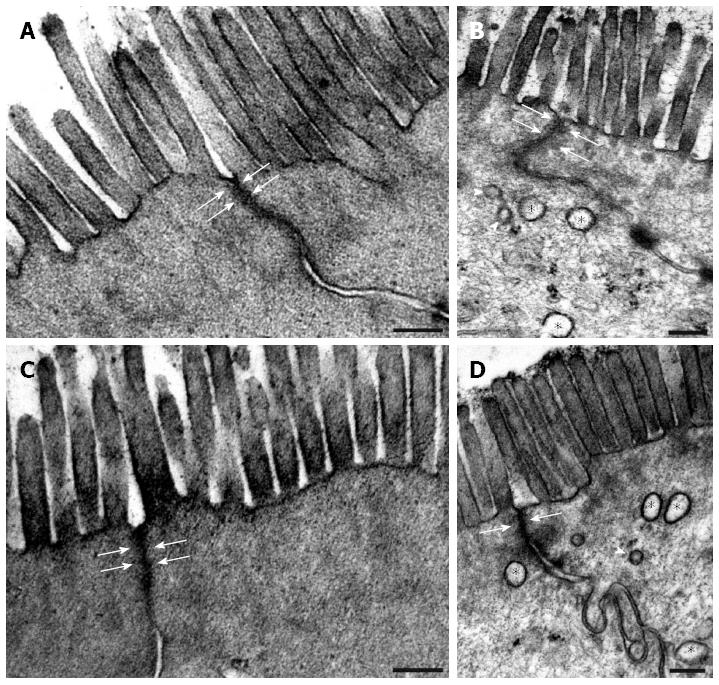
Transmission electronmicroscopic examination of the colonic epithelium in the strictured region on days 90 and 120 after TNBS administrations showed that the apical surface of the enterocytes with intact brush-border and closed TJs was similar to that in the controls (Figure 3). The width of the TJs between adjacent enterocytes was evaluated morphometrically and was always found to be less than 3 nm (data not shown). However, autophagosome-like double-membrane vesicles of different sizes were frequently seen within the enterocytes (Figure 3).
Because of the excess accumulation of ECM elements in the strictured colonic regions, the SMCs had moved away from each other significantly by day 90 after TNBS administrations, and by 120 d there was more than 2-fold increase in the distance between adjacent SMCs as compared with the controls (Figure 4). Because of the ECM deposition, the SMCs also moved away from the MGs (Figure 4). By day 120 post-TNBS treatments, swollen and empty confluent vacuoles and autophagosomes were frequently seen in the SMCs and also in their close environment, together with different cell organelles in the strictured colonic areas (Figure 4). The vast majority of the axons appeared normal, but necrotic axons were seen rarely in the MGs (Figure 4). Quantitative post-embedding immunohistochemistry in the strictured areas revealed a progressive increase in the number of gold particles indicating caspase 9 antigen in the SMCs and MGs relative to the control samples (Figure 5). The caspase 9-labelling gold particles in the MGs were mainly associated with the mitochondria (Figure 5), the ultrastructure of which was well preserved even 120 d after TNBS treatments.
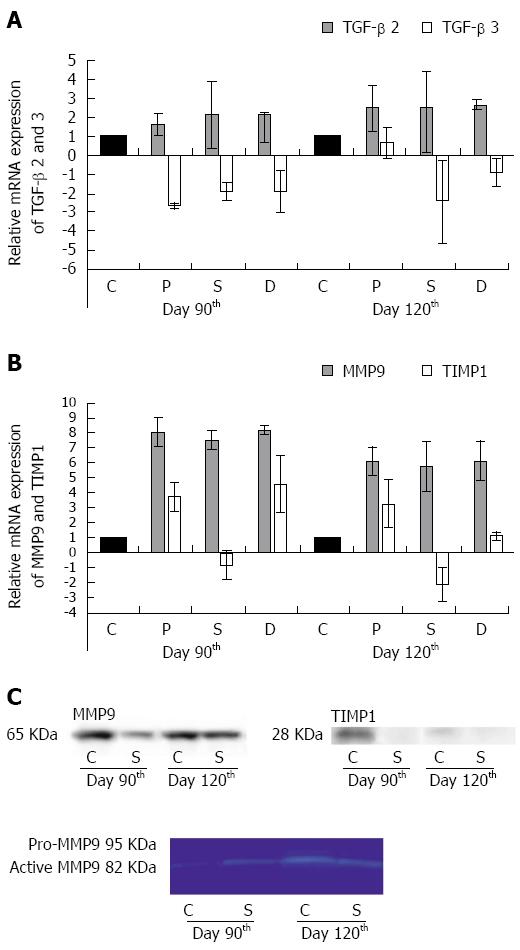
On day 90, the TGF-beta 2 mRNA was up-regulated, while the TGF-beta 3 mRNA was down-regulated in the strictured gut wall and also in the colonic segments adjacent proximally and distally to the strictures as compared with the controls. The TGF-beta 2 mRNA expression progressively increased, while the TGF-beta 3 mRNA expression further decreased by day 120 in all three segments (Figure 6A).
A marked overexpression of MMP9 mRNA was detected in all the colonic segments examined on days 90 and 120 after TNBS treatments (Figure 6B). At the same timepoints, the TIMP1 mRNA expression was up-regulated in the colonic segments adjacent proximally and distally to the strictures, but was down-regulated in the strictures themselves (Figure 6B). MMP9 and TIMP1 expression was also evaluated at the protein levels. Although a high amount of MMP9 protein was demonstrated in the tissue samples from the control rats, the progressive increase in the amount of MMP9 protein in the strictures was obvious between days 90 and 120 (Figure 6C). Gelatine zymography demonstrated that an active form of MMP9 protein rather than pro-MMP was expressed (Figure 6C). While the amount of TIMP1 protein also decreased acutely between days 90 and 120 in the control samples, it was practically undetectable in the strictures (Figure 6C).
We recently reported on a rat model in which alleviated inflammatory damage in association with the persistent up-regulation of HO-1 were salient features in the acute phase of intestinal inflammation induced by repeated TNBS administrations[24]. The same model was used in the present work to investigate the structural and molecular events leading to the formation of a strictured gut wall. Concerning the long-term consequences of the acute inflammation in this model, all the visible symptoms had resolved by day 60 after TNBS administration, the body weight of the treated rats was similar to that of the age-matched controls, and intestinal strictures developed in all of the rats that had previously displayed intestinal inflammation. These findings accord well with the clinical observations that mucosal healing and clinical remission alone cannot be treatment endpoints in CD, because this does not prevent later stricturing[27,28]. The increases in size and frequency of the strictures observed here after 60 d provide experimental evidence in favour of the view that strictures, once present, gradually progress and, once fibrosis develops, it cannot be reversed[29].
Aligned thickening of all the muscle layers in the strictured gut wall until up to day 90 after TNBS administrations was characteristic. Whereas the thickening of the ME progressed further and became a decisive element of the strictured gut wall, the thickness of the LMM did not change after day 90, and did not differ from that in the controls by the end of the experimental period. Since the LMM is most involved in maintaining the mucosal integrity[30], we suppose that the earlier cessation of excess ECM deposition in the LMM is a consequence of the differential regulation of inflammation-related events here through cytokines derived from the epithelium[31,32].
At 90 and 120 d following TNBS administrations, transmission electronmicroscopy showed that the structures of the epithelium necessary to maintain the barrier functions were intact. However, the frequent presence of double-membrane autophagosomes indicated high levels of intracellular stressors in the previously affected epithelium. It has been well documented that induction of autophagy is a determining factor for the maintenance of cellular homeostasis in chronic colitis[33,34]. The importance of autophagy in the pathogenesis of chronic intestinal inflammation has also been demonstrated by genome-wide association studies which indentified a link between the genes involved in autophagy regulation and IBDs[35,36].
While the rapid and widespread loss of myenteric neurons was a characteristic feature of the onset of acute inflammation[24], the precise timing of the cellular events in the chronic phase leading to the intestinal stricturing here showed that the SMCs in the ME were affected first in these processes. Since the excess deposition of ECM in the ME was sustained throughout the experimental period, the SMCs progressively moved away from each other and also from the MGs, leading eventually to deficient innervation and severe cellular damage. After day 60 following TNBS treatments, the appearance of autophagosomes, the leakage of cellular contents and the increasing number of gold particles labelling caspase 9 expression indicated that all three types of cell death mechanisms had already progressed in the SMCs by day 90 when necrotic axons were only rarely seen in the MGs. As the pathological environment became more extensive with time, by day 120 after TNBS administration locally severe neuronal injury also occurred in the strictured tissue as a significant sign of chronic inflammation, similarly as described in other models[23,37]. As regards the timing of the events, we presume suppose that the neuronal injury is a consequence and not the cause of the stricturing processes.
Evidence from both animal models[38,39] and human studies[18,40,41] has suggested that the up-regulation of TGF-beta 2 and of MMP9 may be considered to be biomarkers in the post-inflammatory tissue remodelling leading to stricturing in CD. The mRNA expression profile of the TGF-beta isoforms, the up-regulation of TGF-beta 2 and the down-regulation of TGF beta 3 in the colonic segments examined in our model accorded well with the distinctive expressional profile of the secreted TGF-beta isoforms in human CD primary intestinal myofibroblasts[41]. The spreading of this characteristic expression pattern both proximally and distally to the strictures indicated the bidirectional diffusion of the disease along the colon in our model. We also detected progressive up-regulation of MMP9 mRNA in all three colonic segments, suggesting again the proximally and distally directed diffusion of the pathological environment. However, the MMP9 up-regulation in the strictured gut wall was coupled with the down-regulation of TIMP1, and an increased amount of active MMP9, but no TIMP1 protein was detected here, indicating a stricture-specific off-balance in the production of proteases and their inhibitors. This expression pattern is very reminiscent of that which develops in the fistulae in approximately one-third of patients with CD[42]. The apparent differences in expression profiles between our study and the literature data in tissue samples prepared from the control guts could be explained by the different methodological approaches. The novelty of our studies was that we prepared tissue homogenates for molecular studies not from the mucosa overlying the strictures, but exclusively from the ME, where the background events of the chronic inflammation leading to stricture formation actually occurred.
In conclusion, The structural and molecular events leading to stricturing as a long-term consequence of acute intestinal inflammation that were demonstrated earlier in animal models and in human studies also characterized the stricture formation induced in our rat model by repeated TNBS administrations. Since the exact timing of the stricturing processes was possible in this model, we reached the conclusion that, in contrast with the general view, the ME, and not the epithelial barrier or the MGs, was the primary target of the events leading to stricture formation. Moreover, this TNBS-induced rat model has provided the first experimental demonstration of the molecular diffusion of the disease both proximally and distally along the gut wall. The off-balance in MMP9/TIMP1 expression profile found strictly within the border of the strictures may well allow use of this model to investigate the molecular mechanisms leading to fistulated CD.
Intestinal strictures are characteristic complications of Crohn’s disease (CD) affecting more than one third of all patients. Its can lead to partial or total intestinal obstruction with potentially life-threatening consequences. Although the treatment of the chronic complications of CD is a serious medical problem, the pathogenesis, factors, and cell types involved in stricture formation are largely unknown.
Despite of the huge amount of animal models and human studies, the structural and molecular events leading to stricturing as a long-term consequence of acute intestinal inflammation are still not clear until today. Besides, the ultrastructure of the intestinal strictures is still unknown.
This TNBS-induced rat model has provided the first experimental demonstration of that, in contrast with the general view, the muscularis externa, and not the epithelial barrier or the myenteric ganglia, was the primary target of the events leading to stricture formation.
The authors hypothesize form the results derived our rat model with chronic colitis and very low mortality that the experimentally provoked recurrent relapsing inflammations characteristic to CD can provoke the recrudescence of the strictures post-surgically despite of the complete mucosal healing and restoring myenteric neuronal injury.
The authors described earlier that experimentally provoked repetitive relapsing inflammations develop preconditioning effect by speeding up mucosal healing and restoring myenteric neuronal injury.
This is a well-written manuscript with carefully designed and described experiments. The observations are interesting and certainly add to our knowledge in the inflammation-induced fibrosis in the large intestine.
P- Reviewer: Bayraktar Y, Kuo SM, Nakajima H, Ohkohchi N S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Chang CW, Wong JM, Tung CC, Shih IL, Wang HY, Wei SC. Intestinal stricture in Crohn’s disease. Intest Res. 2015;13:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 661] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 3. | Tan M, Ong JP, Teo EK. Achieving deep remission in Crohn’s disease: treating beyond symptoms. Ann Acad Med Singapore. 2014;43:200-202. [PubMed] |
| 4. | Orlando A, Guglielmi FW, Cottone M, Orlando E, Romano C, Sinagra E. Clinical implications of mucosal healing in the management of patients with inflammatory bowel disease. Dig Liver Dis. 2013;45:986-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Burke JP, Mulsow JJ, O’Keane C, Docherty NG, Watson RW, O’Connell PR. Fibrogenesis in Crohn’s disease. Am J Gastroenterol. 2007;102:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 6. | Rieder F, Fiocchi C. Mechanisms of tissue remodeling in inflammatory bowel disease. Dig Dis. 2013;31:186-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Rieder F, Brenmoehl J, Leeb S, Schölmerich J, Rogler G. Wound healing and fibrosis in intestinal disease. Gut. 2007;56:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Hills CE, Squires PE. TGF-beta1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Am J Nephrol. 2010;31:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Shah M, Foreman DM, Ferguson MW. Neutralising antibody to TGF-beta 1,2 reduces cutaneous scarring in adult rodents. J Cell Sci. 1994;107:1137-1157. [PubMed] |
| 10. | Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108:985-1002. [PubMed] |
| 11. | Wang B, Koh P, Winbanks C, Coughlan MT, McClelland A, Watson A, Jandeleit-Dahm K, Burns WC, Thomas MC, Cooper ME. miR-200a Prevents renal fibrogenesis through repression of TGF-β2 expression. Diabetes. 2011;60:280-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 282] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 12. | Woessner JF. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145-2154. [PubMed] |
| 13. | Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197-250. [PubMed] |
| 14. | Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000;18:1135-1149. [PubMed] |
| 15. | Overall CM. Dilating the degradome: matrix metalloproteinase 2 (MMP-2) cuts to the heart of the matter. Biochem J. 2004;383:e5-e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1279] [Cited by in RCA: 1413] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 17. | Overall CM, Kleifeld O. Towards third generation matrix metalloproteinase inhibitors for cancer therapy. Br J Cancer. 2006;94:941-946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 267] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 18. | Kofla-Dlubacz A, Matusiewicz M, Krzystek-Korpacka M, Iwanczak B. Correlation of MMP-3 and MMP-9 with Crohn’s disease activity in children. Dig Dis Sci. 2012;57:706-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Lourenssen S, Wells RW, Blennerhassett MG. Differential responses of intrinsic and extrinsic innervation of smooth muscle cells in rat colitis. Exp Neurol. 2005;195:497-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Skobowiat C, Gonkowski S, Calka J. Phenotyping of sympathetic chain ganglia (SChG) neurons in porcine colitis. J Vet Med Sci. 2010;72:1269-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Skobowiat C, Calka J, Majewski M. Axotomy induced changes in neuronal plasticity of sympathetic chain ganglia (SChG) neurons supplying descending colon in the pig. Exp Mol Pathol. 2011;90:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Skobowiat C. Contribution of Neuropeptides and Neurotransmitters in colitis. J Veterinar Sci Technol. 2012;3: 1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Marlow SL, Blennerhassett MG. Deficient innervation characterizes intestinal strictures in a rat model of colitis. Exp Mol Pathol. 2006;80:54-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Talapka P, Nagy LI, Pál A, Poles MZ, Berkó A, Bagyánszki M, Puskás LG, Fekete É, Bódi N. Alleviated mucosal and neuronal damage in a rat model of Crohn’s disease. World J Gastroenterol. 2014;20:16690-16697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Bódi N, Talapka P, Poles MZ, Hermesz E, Jancsó Z, Katarova Z, Izbéki F, Wittmann T, Fekete É, Bagyánszki M. Gut region-specific diabetic damage to the capillary endothelium adjacent to the myenteric plexus. Microcirculation. 2012;19:316-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Horváth K, Varga C, Berkó A, Pósa A, László F, Whittle BJ. The involvement of heme oxygenase-1 activity in the therapeutic actions of 5-aminosalicylic acid in rat colitis. Eur J Pharmacol. 2008;581:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Osterman MT, Haynes K, Delzell E, Zhang J, Bewtra M, Brensinger C, Chen L, Xie F, Curtis JR, Lewis JD. Comparative effectiveness of infliximab and adalimumab for Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:811-817.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 255] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 29. | Rieder F, Zimmermann EM, Remzi FH, Sandborn WJ. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62:1072-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 385] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 30. | Kagnoff MF. The intestinal epithelium is an integral component of a communications network. J Clin Invest. 2014;124:2841-2843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 31. | Yang SK, Eckmann L, Panja A, Kagnoff MF. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology. 1997;113:1214-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 253] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 32. | Sansonetti PJ, Arondel J, Huerre M, Harada A, Matsushima K. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect Immun. 1999;67:1471-1480. [PubMed] |
| 33. | Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220-9231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1479] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 34. | Randall-Demllo S, Chieppa M, Eri R. Intestinal epithelium and autophagy: partners in gut homeostasis. Front Immunol. 2013;4:301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Hampe J, Franke A, Rosenstiel P, Till A, Teuber M, Huse K, Albrecht M, Mayr G, De La Vega FM, Briggs J. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet. 2007;39:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1519] [Cited by in RCA: 1454] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 36. | Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010;42:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2110] [Cited by in RCA: 2004] [Article Influence: 133.6] [Reference Citation Analysis (0)] |
| 37. | Lakhan SE, Kirchgessner A. Neuroinflammation in inflammatory bowel disease. J Neuroinflammation. 2010;7:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 162] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 38. | Medina C, Videla S, Radomski A, Radomski MW, Antolín M, Guarner F, Vilaseca J, Salas A, Malagelada JR. Increased activity and expression of matrix metalloproteinase-9 in a rat model of distal colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G116-G122. [PubMed] |
| 39. | Medina C, Santana A, Paz MC, Díaz-Gonzalez F, Farre E, Salas A, Radomski MW, Quintero E. Matrix metalloproteinase-9 modulates intestinal injury in rats with transmural colitis. J Leukoc Biol. 2006;79:954-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Baugh MD, Perry MJ, Hollander AP, Davies DR, Cross SS, Lobo AJ, Taylor CJ, Evans GS. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 255] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 41. | McKaig BC, Hughes K, Tighe PJ, Mahida YR. Differential expression of TGF-beta isoforms by normal and inflammatory bowel disease intestinal myofibroblasts. Am J Physiol Cell Physiol. 2002;282:C172-C182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 42. | Kirkegaard T, Hansen A, Bruun E, Brynskov J. Expression and localisation of matrix metalloproteinases and their natural inhibitors in fistulae of patients with Crohn’s disease. Gut. 2004;53:701-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 164] [Article Influence: 7.8] [Reference Citation Analysis (0)] |