Published online May 28, 2016. doi: 10.3748/wjg.v22.i20.4926
Peer-review started: February 10, 2016
First decision: March 8, 2016
Revised: March 26, 2016
Accepted: April 7, 2016
Article in press: April 7, 2016
Published online: May 28, 2016
Processing time: 99 Days and 14.1 Hours
AIM: To evaluate the performance of elastography by ultrasound with acoustic radiation force impulse (ARFI) in determining fibrosis stage in patients with alcoholic liver disease (ALD) undergoing alcoholic detoxification in relation to biopsy.
METHODS: Eighty-three patients with ALD undergoing detoxification were prospectively enrolled. Each patient underwent ARFI imaging and a liver biopsy on the same day. Fibrosis was staged according to the METAVIR scoring system. The median of 10 valid ARFI measurements was calculated for each patient.
RESULTS: Sixty-nine males and thirteen females (one patient excluded due to insufficient biopsy size) were assessed with a mean alcohol consumption of 132.4 ± 128.8 standard drinks per week and mean cumulative year duration of 17.6 ± 9.5 years. Sensitivity and specificity were respectively 82.4% (0.70-0.95) and 83.3% (0.73-0.94) (AUROC = 0.87) for F ≥ 2 with a cut-off value of 1.63m/s; 82.4% (0.64-1.00) and 78.5% (0.69-0.89) (AUROC = 0.86) for F ≥ 3 with a cut-off value of 1.84m/s; and 92.3% (0.78-1.00] and 81.6% (0.72-0.90) (AUROC = 0.89) for F = 4 with a cut-off value of 1.94 m/s.
CONCLUSION: ARFI is an accurate, non-invasive and easy method for assessing liver fibrosis in patients with ALD undergoing alcoholic detoxification.
Core tip: The aim of this study was to evaluate the performance of elastography by ultrasound with acoustic radiation force impulse (ARFI) in determining fibrosis stage in patients with alcoholic liver disease (ALD) undergoing alcoholic detoxification. Compared to biopsy, ARFI is an accurate, non-invasive and easy method for assessing liver fibrosis in patients with ALD undergoing alcoholic detoxification, with a good sensitivity and specificity.
- Citation: Kiani A, Brun V, Lainé F, Turlin B, Morcet J, Michalak S, Le Gruyer A, Legros L, Bardou-Jacquet E, Gandon Y, Moirand R. Acoustic radiation force impulse imaging for assessing liver fibrosis in alcoholic liver disease. World J Gastroenterol 2016; 22(20): 4926-4935
- URL: https://www.wjgnet.com/1007-9327/full/v22/i20/4926.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i20.4926
Chronic alcohol abuse is a major public health problem worldwide. Alcoholic liver disease (ALD) is one of the most common complications and a leading cause of alcohol-related death, due to liver cirrhosis and its complications. In 2004, 3.8% of all global deaths and 4.6% of global disability-adjusted life-years were attributable to alcohol consumption. The treatment of alcoholic liver disease generates substantial costs for the healthcare system[1].
Three histological lesions characterise ALD: steatosis, steatohepatitis and fibrosis. There are different stages of fibrosis, the last of which is cirrhosis. In ALD, it is important to know the fibrosis stage in order to guide management decisions and estimate prognosis. The appropriate intervention strategies can prevent serious long-term outcomes[2]. Patients with cirrhosis run a greater risk of complications (portal hypertension, hepatocellular carcinoma, ascites, etc.) and need closer follow-up. Informing patients of their cirrhosis could also provide an incentive to stop alcohol consumption. However, patients with severe fibrosis or cirrhosis are completely clinically asymptomatic for a long period of time and can be difficult to diagnose.
Liver biopsy is the gold standard for assessment of liver fibrosis, evaluating fibrosis, steatosis and the necroinflammatory stage at the same time. However, biopsy is an invasive procedure associated with morbidity and minor complications (local discomfort at the biopsy site, pain and transient hypotension due to a vasovagal reaction) reported in 5%-20% of cases and major complications (bleeding and peritonitis) in 0.3%-0.6% of cases. The mortality rate is 0.01%-0.3%[3]. Moreover, there are other considerations such as contraindications (ascites, intrahepatic biliary duct dilation, coagulation disorders), insufficient sampling and inter-observer variability. A 1-d hospital stay is also necessary, leading to significant costs.
This led to the development of alternative non-invasive methods for assessing hepatic fibrosis in alcoholic liver disease. Serum markers alone or in combination with specific algorithms have been used for the non-invasive assessment of liver fibrosis. Examples include Fibrotest®, Forns index and APRI[4-6]. The limitations of these tests are the influence of comorbid conditions and a lack of liver specificity. Another method involves measuring the elasticity of liver tissue (liver stiffness) which is markedly influenced by the stage of fibrosis. The most popular method for measuring liver stiffness is transient elastography (TE) by Fibroscan®, which has been validated for hepatitis C liver disease patients. Some authors have even combined TE and serum markers[7].
A new ultrasound technique has recently emerged: acoustic radiation force impulse (ARFI) elastography. ARFI could be of great utility in the measurement of liver fibrosis in alcoholic liver disease. This non-invasive method has the particular advantage of combining conventional ultrasound and liver stiffness measurement. Ultrasound is the primary imaging technique used worldwide to evaluate diffuse hepatic diseases. Acoustic radiation force is a phenomenon associated with the propagation of acoustic waves in attenuating media. The device generates a short-duration (262 ms) acoustic pulse by ultrasound. This pulse creates mechanical excitation and displacement of tissue. The deformation induced by the acoustic pulse is followed by a relaxation process after which the tissue returns to its original configuration, generating a shear wave. The speed of this wave is calculated, providing a quantitative measurement. The shear wave speed of the tissue can be reconstructed as soft tissues are elastic and deformed more easily than rigid tissue.
In the past few years, ARFI has started to be assessed in comparison to biopsy, TE and biological markers. These studies mainly involved hepatitis B, hepatitis C and non-alcoholic steatohepatitis (NASH).
The aim of our prospective study was to evaluate the performance of ARFI in determining fibrosis stage in patients with alcoholic liver disease in relation to biopsy.
The local ethics committee approved this study. All patients gave written informed consent prior to enrolment. This study is an ancillary single-centre study of a larger, ongoing, multi-centre trial on validation of non-invasive fibrosis tests in ALD. Clinical Trials Identifier: NCT01789008.
From February 2013 to June 2015, the study was offered to all patients referred to the University Hospital of Rennes, France, who were admitted to the addiction treatment unit in the liver disease department for detoxification with an indication of liver biopsy for alcoholic liver disease. The inclusion criteria were: patients over 18 years old, hospitalisation for alcoholic detoxification, high-risk alcohol consumption (more than 210 g of alcohol per week for men and 140 g of alcohol per week for women) for a cumulative period of more than 5 years, a rise in serum aspartate transferase (AST) greater than 1.5 the upper limit of the normal range, associated with a rise in gamma-glutamyl transferase (GGT) and not explained by another cause of liver disease. When patients had features of metabolic syndrome or were obese, liver disease was felt to be principally due to alcohol consumption when the AST/alanine amino transferase (ALT) ratio was greater than one, and GGT was markedly high, and when the abnormalities decreased with alcohol withdrawal. Non-inclusion criteria were: cirrhosis already known or obvious due to clinical and biological signs (ascites, increased prothrombin time or oesophageal varices), other causes of hepatic disease (viral, autoimmune or cholestatic disease) or contraindication to biopsy. The interval between alcohol cessation and the procedure was not more than 10 d. Eighty-three patients were prospectively enrolled.
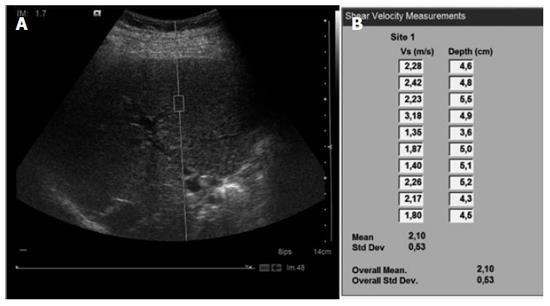
ARFI imaging was performed with a Siemens Acuson S2000TM ultrasound system (Siemens AG, Erlangen, Germany), with software version VB10D and a 4C1 curved ultrasound probe. The region of interest (ROI) of 10 mm length and 5 mm width was placed while performing B-mode imaging in the right lobe of the liver at a maximum depth of 8 cm, avoiding large vessels, biliary ducts and potential lesions (Figure 1). The operator applied the minimum pressure required to take the image.
Patients were in fasted state. None had cardiac disease. They were in the supine position with the right arm in maximum abduction and were asked to stop normal breathing for a moment and not inhale or exhale deeply. The aim was to minimise breathing motion and avoid inhaling/exhaling, which are known influencing factors[8]. The probe was placed between and parallel to the seventh to tenth intercostal space.
Ten valid acquisitions were obtained for each patient, in the same intercostal space but with different locations and at different depths. Each acquisition period was about 10-15 s long. The median of all 10 acquisitions was calculated and considered as indicative of fibrosis severity. The results were expressed in m/s. For each measurement, the depth of the box was given. Measurements were obtained at a depth of 1 cm from the liver capsule down to a maximum depth of 8 cm below the transducer. If the measurement was technically evaluated as non-reliable by the device, X.XX was displayed on the screen. Reliable, successful liver stiffness measurements were defined as the median of 10 valid measurements with a success rate ≥ 60% (based on TE).
The operator was blinded for all patient characteristics including clinical, biological and histological data.
Liver and abdominal ultrasound imaging was performed at the same time for all patients using the same device and probe as for the ARFI examination. We recorded the right liver lobe size (right liver arrow), the distance between the skin and the superficial liver capsule, the liver structure and any focal liver lesion.
Liver biopsy was performed under percutaneous ultrasound guidance after ARFI acquisition on the same day. The liver samples were fixed and for each patient three slides were stained with hematoxylin-eosin and Sirius red. To avoid sampling errors, specimens under 15 mm long were excluded. A senior pathologist, blinded to clinical, histological, biological and ARFI data, assessed the liver biopsies according to the METAVIR scoring system[9]. Fibrosis was staged from 0 to 4 determined according to the METAVIR score: F = fibrosis. F0: no fibrosis; F1: portal fibrosis without septa (minimal fibrosis); F2: portal fibrosis with rare septa (moderate fibrosis); F3: numerous septa without cirrhosis (severe fibrosis); and F4: cirrhosis. Perisinusoidal fibrosis was evaluated according to Brunt’s score[10].
Biological parameters were measured prior to liver biopsy and ARFI. These included: prothrombin, alkaline phosphatase, albumin, γ-globulin, platelets, AST, ALT, γ-glutamyl transferase, iron and ferritin. Other parameters were age, sex, body mass index (BMI) and hypertension.
Groups of patients were formed according to fibrosis stage and data were expressed as mean ± SD if normally distributed and median (range) if not normally distributed. Comparisons between groups were made using t-tests for normally distributed variables, Mann Whitney U test for non-normally distributed variables and the χ2 test or Fisher’s exact test for categorical variables. Spearman’s analysis was used to determine any correlations. Optimal cut-off values for fibrosis stages F ≥ 2, F ≥ 3 and F = 4 were determined by optimisation of Youden’s index from the AUROC curve analysis. Sensitivity, specificity, negative predictive value (NPV) and positive predictive value (PPV) were also calculated. The variables tested in the univariate analysis were ARFI, effects of prothrombin, alkaline phosphatase, albumin, γ-globulin, platelets, AST, ALT, γ-glutamyl transferase, iron, ferritin, age, sex, BMI and hypertension (P < 0.2). Multivariate ordinal logistic regression analysis using fibrosis stage (in three classes: F0-F1/F2/F3-F4) as the outcome variable was used to assess the strength of the relationship with ARFI even after adjustments for other factors associated with fibrosis progression or confusion factors. A P value of < 0.05 was considered statistically significant. An algorithm was developed using the clustering method. Statistical analyses were performed using SAS V9.4 software (SAS Institute, United States).
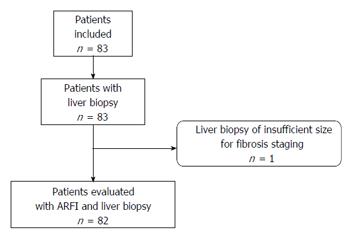
Eighty-two patients (69 males and 13 females) were evaluated in the analysis. One enrolled patient was excluded due to insufficient biopsy size (Figure 2). The mean age of the patients was 43.8 ± 10 years and the mean BMI was 22.9 ± 4.3 kg/m2. Mean alcohol consumption was 132.4 ± 128.8 standard drinks (defined as 10 g of pure alcohol per standard drink in France) per week with a mean cumulative year duration of 17.6 ± 9.5 years. Mean biopsy size was 30.7 ± 10.5 mm. Patient characteristics and fibrosis stages are summarised in Tables 1 and 2 respectively. Successful liver stiffness measurements (10 valid measurements) were obtained in 100% of patients measured with ARFI imaging.
| Characteristic | Normal values | Patients included(n = 82) |
| Sex (male/female) | NA | 69/13 |
| Age (yr) | NA | 43.8 ± 10 |
| Body mass index (kg/m2) | NA | 22.9 ± 4.3 |
| AST (IU/L) | 0-35 | 62.0 (44-98) |
| ALT (IU/L) | 0-35 | 67.0 (40-105) |
| γ-glutamyl transpeptidase (IU/L) | 5-36 | 316.0 (141-654) |
| γ-globulin (g/L) | 7-15 | 8.7 (7.4-10.4) |
| Alkaline phosphatase (IU/L) | 30-120 | 90.5 (66-121) |
| Prothrombin (%) | 70-130 | 101.2 ± 12.4 |
| Platelets (× 109/L) | 180-390 | 191.5 ± 71.7 |
| Iron (μmol/L) | 18-22 | 16.2 ± 8.1 |
| Ferritin (μg/L) | Male: 30-300 | 478.0 (310.5-787.5) |
| Female: 20-150 | 404.0 (216.0-783.0) | |
| Albumin (g/L) | 40-60 | 39.0 ± 4.9 |
| Fibrosis stage | Number of patients | ARFI (m/s) |
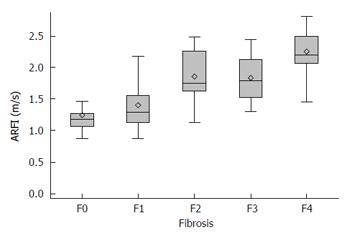
| F0 | 13 | 1.25 ± 0.31 |
| F1 | 35 | 1.40 ± 0.36 |
| F2 | 17 | 1.86 ± 0.42 |
| F3 | 4 | 1.83 ± 0.47 |
| F4 | 13 | 2.25 ± 0.36 |
| P value | < 0.0001 |
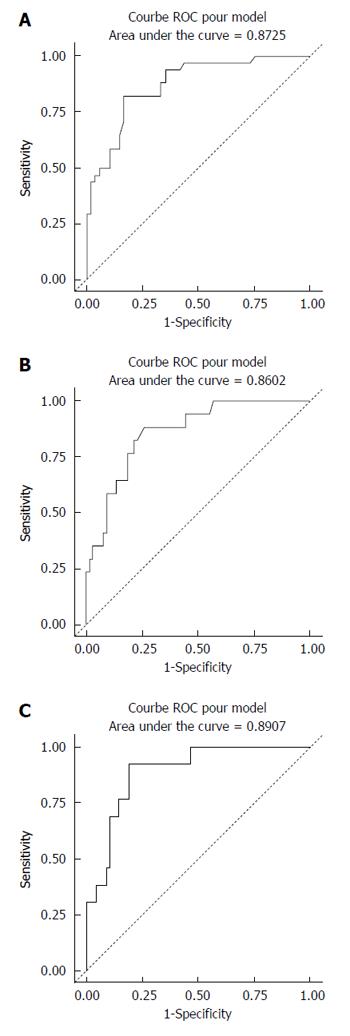
The median values for ARFI imaging according to fibrosis stage are described in Table 2 (mean of medians ± SD). The results showed a significant and strong correlation between ARFI measurements and the histological fibrosis stage (P < 0.0001) (Figure 3). Analyses to determine the optimal ARFI cut-off values were performed according to stages of clinical interest, for F ≥ 2, ≥ F3 and F = 4. AUROC values were respectively 0.87, 0.86 and 0.89 (Figure 4). Sensitivity, specificity, PPV and NPV are shown in Table 3 according to the cut-off values.
| Diagnostic parameters | F ≥ 2 | F ≥ 3 | F = 4 |
| ARFI cut-off (m/s) | 1.63 | 1.84 | 1.94 |
| Sensitivity (%) | 82.4 (0.70-0.95) | 82.4 (0.64-1.00) | 92.3 (0.78-1.00) |
| Specificity (%) | 83.3 (0.73-0.94) | 78.5 (0.69-0.89) | 81.6 (0.72-0.90) |
| Area under curve (AUROC) | 0.87 | 0.86 | 0.89 |
| PPV (%) | 77.8 (0.64-0.91) | 50.0 (0.31-0.69) | 48.0 (0.28-0.68) |
| NPV (%) | 87.0 (0.77-0.97) | 94.4 (0.88-1.00) | 98.2 (0.95-1.00) |
Significant variables in univariate analyses (P < 0.2) as described previously were entered into the multivariate model (Table 4). The proportional odds assumption was verified (P = 0.76) and the coefficient of determination (R²) was 61%. Age ≥ 50 [OR = 4.73 (1.43-15.66)] and γ-globulin ≥ 10 g/L [OR = 9.67 (2.19-42.63)] were independently associated with fibrosis stage. Moreover, the relationship between fibrosis and ARFI was still significant [40.71 (9.94-166.7)] after adjusting for those additional parameters.
| Characteristic | Univariate analysis | Multivariate analysis |
| OR (95%) | OR (95%) | |
| P value | R2 = 0.61; | |
| STPOA1: 0.76 | ||
| ARFI | 29.06 [8.59-98.33] | 40.71 [9.94-166.7] |
| P < 0.0001 | P < 0.0001 | |
| Sex | ||
| Female | 1 | |
| Male | 2.43 [0.79-7.42] | |
| P = 0.12 | ||
| Age | ||
| < 50 yr | 1 | 1 |
| ≥ 50 yr | 4.01 [1.55-10.39] | 4.73 [1.43-15.66] |
| P = 0.004 | P = 0.01 | |
| Body mass index | 1.07 [0.97-1.18] | |
| P = 0.19 | ||
| AST | 1.00 [0.992-1.01] | |
| P = 0.98 | ||
| ALT | 0.99 [0.98-0.999] | |
| P = 0.04 | ||
| γ-glutamyl transpeptidase | 1.00 [1.000-1.002] | |
| P = 0.05 | ||
| γ-globulin | ||
| < 10 | 1 | 1 |
| ≥ 10 | 7.42 [2.34-23.56] | 9.67 [2.19-42.63] |
| P = 0.003 | P = 0.004 | |
| Alkaline phosphatase | 1.01 [1.001-1.012] | |
| P = 0.02 | ||
| Prothrombin | 0.002 [0.001-0.06] | |
| P = 0.001 | ||
| Platelets | 1.00 [0.99-1.01] | |
| P = 0.93 | ||
| Iron | 1.03 [0.97-1.08] | |
| P = 0.33 | ||
| Ferritin | 1.001 [1.000-1.002] | |
| P = 0.06 | ||
| Albumin | 0.90 [0.82-1.00] | |
| P = 0.05 |
The correlation between the mean and median of the 10 values was excellent with a Spearman correlation coefficient of 0.98 (P < 0.001).
Medians were calculated using a different number of values (2-9) to determine whether or not there was a need for 10 values. The values were taken in order. Spearman’s correlation coefficient is 0.98 between the median of 10 and 6 values (P < 0.0001).
This study demonstrates that ARFI imaging could be used for the assessment of liver fibrosis in ALD. We suggest that a median of 1.63 m/s could be used as an ARFI diagnostic threshold for diagnosing significant liver fibrosis (F ≥ 2) with sensitivity and specificity of respectively 82.4% and 83.3% (AUROC = 0.87). Moreover, the threshold of 1.94 m/s provided a diagnosis of cirrhosis with a sensitivity of 92.3% and specificity of 81.6%.
In alcoholic liver disease, the identification of cirrhosis (F4) is important for optimal patient care. The follow-up schedule includes endoscopy every 3-4 years, ultrasonography every 6 mo, hepatitis vaccination and contraindication to certain drugs. The identification of stage ≥ F2 is less important clinically than in viral hepatitis but can lead to closer medical surveillance of fibrosis with ARFI, and even serve as an incentive for detoxification. For both stages, the AUROC curve value was close to 1, indicating good diagnostic accuracy[11].
To date, the predominant and most reliable non-invasive method for the diagnosis of liver fibrosis in alcoholic liver disease is transient elastography (TE)[12]. Compared to TE, ARFI has several advantages. First, B-mode evaluation of the liver (and other organs such as the spleen) is possible with the same device and can therefore be incorporated into routine ultrasound protocols, thereby reducing costs. The use of B-mode can also determine optimal ROI placement, preserving structures such as lesions, large blood vessels, biliary ducts or even heterogeneous areas. Second and probably most importantly is the liver stiffness measurement success rate of 100%, which was reported both in the literature and in our study, whereas in some studies TE has a success rate of under 70%[13,14]. This is a major strength of the ARFI method compared to TE. Third, ARFI imaging can be performed in some cases where TE is not possible[15]. Bota et al[16] demonstrated that the presence of ascites did not influence the ARFI measurement reliability rate, whereas TE cannot be performed in the case of ascites. TE is unreliable for overweight and obese patients whereas ARFI can be performed to a maximum depth of 8 cm. Published data also suggest that ARFI may not be influenced by steatosis grade, unlike TE[14,17,18]. This is a clear advantage in our population, as steatosis is often associated with ALD[19]. ARFI is also a good alternative for patients with contraindications to biopsy or TE. The ARFI measurement area size of 1 cm (compared to 4 cm for TE) can easily be offset by the possibility of several measurements in different parts of the liver.
Recently, studies have also started to evaluate ARFI on hepatitis B, hepatitis C and NASH[20-23]. ARFI has good intra-operator and inter-operator reproducibility, as described in the Bota et al[24] study, with an intraclass correlation coefficient (ICC) of 0.90 and 0.81 for intra- and inter-operator reproducibility respectively. The cut-off values reported in the literature are different, however. In hepatitis B, hepatitis C and NASH, cut-off values in m/s for F = 4 were respectively 1.84 for Dong et al[18] 1.55 for Sporea et al[21] and 1.9 for Yoneda et al[25]. These differences suggest that ARFI values differ depending on the disease, as shown in the meta-analysis by Nierhoff et al[23]. There is therefore a need to define cut-off values for each diffuse liver disease. To our knowledge, there is only one other study, by Zhang et al[26] evaluating the performance of ARFI imaging for the assessment of liver fibrosis in patients with ALD in comparison to biopsy, with an AUROC value of 0.89 for F = 4. However, the study populations are very different. In their international multi-centre study, Sporea et al[21] showed that the cut-off values predictive of fibrosis stages differ between European and Asian populations. This could explain why the cut-off values are respectively 1.27 and 1.65 for F ≥ 2 and F = 4 in the Chinese study by Zhang and 1.63 and 1.94 in our study, using the same ultrasound device.
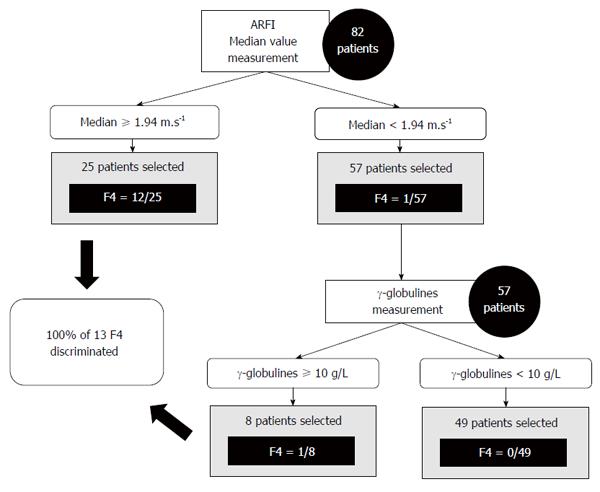
Our study showed good sensitivity and specificity, as described previously. But the excellent negative predictive value of ARFI (98.2% for F = 4) can open the possibility of using ultrasound elastography as a screening test rather than a diagnostic test. A decision tree of clinical value is proposed in Figure 5. Other larger studies are clearly needed to confirm these results.
Our study included patients undergoing alcoholic detoxification. Bardou-Jacquet et al[27] recently suggested that the alcohol consumption greatly influences TE and by extension liver stiffness, but could be a useful tool in the follow-up of patient as an indicator of alcohol consumption beyond the sole fibrosis evaluation. Fibrosis evaluation made on patients undergoing detoxification is the most common clinical situation. So, ARFI values may also be influenced by alcohol consumption and alcohol cessation. This may explain some mismatches between ARFI and biopsies.
In our study, ARFI was performed according to guidelines. In certain debatable conditions, the neutral condition was chosen. For example, according to the literature and the device provider’s instructions, 10 measurements were taken and the median value was calculated, as for TE, with the patient gently holding their breath. Ten measurements were taken for each patient in our study, and the median was calculated for each one. Karlas et al[28] reported that deep inhalation on measurements could increase values by an average of 13%, while Horster et al[29] and Goertz et al[8] reported no difference. In our study, the patients were therefore asked to stop normal breathing for a moment. As previous studies reported that ARFI results could be influenced by food intake, we decided to perform ARFI in a fasted state[30]. An interlobar difference was found in the literature[31]. Our measurements were therefore taken in the right liver lobe in the intercostal space. This location was chosen for several reasons. First, operator pressure on the liver may produce false positives due to direct probe compression, which occurs when measurements are taken in the left liver lobe. The measurements were taken in the intercostal spaces so that the ribs limit this compressive effect. Moreover, the operator exerted minimal pressure. Heartbeat artefacts could falsify the measurements when performed in the left lobe. Second, the aim was to use the same location as the biopsy. ARFI imaging was performed prior to liver biopsy to prevent the interaction of artefacts (such as from haematoma).
However, some findings are discordant with the results of the biopsy, which is considered the gold standard. One reason may be that ARFI produces mean values for a large area in the right liver lobe whereas liver biopsy involves taking a sample. The specimen obtained represents only 1/50000 of the total liver volume and it is well known that fibrosis has an uneven distribution within the liver[32]. In order to be comparable and reliable, multiple biopsies from different locations in the right liver lobe should be taken to gain an accurate comparison with the ARFI values obtained in different locations in the right liver lobe in the same intercostal space[33,34]. This requirement is ethically disputable. Another solution would be to compare ARFI values and hepatic explant findings.
In the literature, many factors have been reported to influence ARFI values, including sex, BMI, age, ethnicity, fasted state, depth of ROI, inflammation grade, obstructive cholestasis and certain other biological markers (alanine transaminase, platelets, prothrombin time, albumin, hyaluronic acid, cholesterol, γ-globulin)[18,21,28,30,35-41]. In our study, in the multivariate analysis, statistically significant correlations were only found for γ-globulin and age. This suggests that liver stiffness and hence fibrosis stage should be interpreted in view of the biological and clinical findings.
Millonig et al[42] suggested that liver stiffness is a direct function of central venous pressure and Goertz et al[8] reported that heart dysfunction may impair ARFI accuracy. None of the patients analysed in our study had heart failure.
In the literature, the median of the values is reported as being more accurate than the mean, and is used by convention. In our study we also calculated the mean of the 10 values for each patient. The correlation between the mean and median was almost perfect with a correlation factor of 0.98. This suggests that the mean of ten values could have been used instead of the median on our cohort of patients. Larger studies are needed to confirm this observation.
Another disputable point is the number of 10 values chosen for the median calculation. In many articles, the recommended number is 10. However, even if ARFI is a fast technique, obtaining ten values takes time. Therefore, in our study we analysed medians calculated from 2 to 9 values (the first values) and compared them to the median of 10 values. It would appear that a number of 6 values is sufficient to determine an accurate median with an excellent correlation coefficient of 0.98.
In addition to the benefits of ARFI as a non-invasive technique, our study has numerous strengths. This was a prospective study of a homogeneous population of alcoholic liver disease patients, with a delay no more than 10 d between the procedures and the beginning of alcohol withdrawal. The clinician, operator and pathologist were blinded to the results. Fibrosis was assessed by biopsy. The mean biopsy size was 30.7 ± 10.5 mm with the majority larger than 25 mm. Factors reported to influence ARFI results in the literature were taken into consideration and generally included in the multivariate analysis. Guidelines on the ARFI technique were summarised and applied to the measurements for each patient. To our knowledge, only one other study evaluating the performance of ARFI in predicting liver fibrosis in ALD has been published, but concerned a different ethnic population.
There were also limitations to our study. One is sample size. Larger studies or meta-analyses are needed to confirm the ARFI threshold in ALD. The comparison with TE was not done and would also be useful. The literature suggests that liver stiffness is influenced by inflammation[43,44]. Inflammation was only assessed and confirmed by transaminase levels in our study and not by histology. Correlation with steatosis grade was not assessed, but published data suggest that moderate/severe steatosis is not a significant error factor for ARFI elastography[14,18].
ARFI is an accurate, non-invasive and easy method for assessing liver fibrosis in patients with ALD. This imaging technique can be easily incorporated into routine patient care. Cut-off values are suggested and require further confirmation in larger studies. A comparison with TE and supersonic shear-wave elastography (Aixplorer Supersonic®) would be interesting for a complete live liver assessment.
We received support from the national clinical research program for public hospitals of France. Thanks to Tracey Westcott for the language help.
Acoustic radiation force impulse (ARFI) elastography has the particular advantage of combining conventional ultrasound and liver stiffness measurement. ARFI imaging is an accurate, non-invasive and easy method for assessing liver fibrosis in patients with hepatitis B and C. The aim of this study was to evaluate the performance of ARFI in determining fibrosis stage in patients with alcoholic liver disease (ALD).
Liver biopsy is the gold standard for assessment of liver fibrosis. However, biopsy is an invasive procedure. This study suggests that ARFI imaging, a non-invasive method, could be used for the assessment of liver fibrosis in ALD.
The study showed the algorithm between ARFI and biochemical parameters for the prediction of presence of cirrhosis. Sensibility and specificity of ARFI were good. The investigation was carried out within European population of patients with ALD undergoing alcoholic detoxification.
This study is helpful for further research in Acoustic Radiation Force Impulse imaging among patients undergoing alcoholic detoxification.
The study showed the interesting algorithm between ARFI and biochemical parameters for the prediction of presence of cirrhosis. Interestingly, the investigation was carried out within European population of patients with ALD undergoing alcoholic detoxification. The paper makes original contribution and it is clinically exhaustive. The manuscript is well written, seems accurate and well organized. The authors clearly presented any doubts concerning the investigation conducted by them.
P- Reviewer: Domagalski K, Kayadibi H S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. The Lancet. 2009;373:2223-2233. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2298] [Cited by in RCA: 2314] [Article Influence: 144.6] [Reference Citation Analysis (0)] |
| 2. | Mueller S, Seitz HK, Rausch V. Non-invasive diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:14626-14641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (2)] |
| 3. | Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 203] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 4. | Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1037] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 5. | Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 721] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 6. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3230] [Article Influence: 146.8] [Reference Citation Analysis (0)] |
| 7. | Lee HJ, Seo YS, Kim DJ, Kang HS, An H, Kim JH, Cheong JY, Yim HJ, Yeon JE, Lee HS. Application of the HALF index obviates the need for liver biopsy in half of all patients with chronic hepatitis B. J Gastroenterol Hepatol. 2011;26:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Goertz RS, Egger C, Neurath MF, Strobel D. Impact of food intake, ultrasound transducer, breathing maneuvers and body position on acoustic radiation force impulse (ARFI) elastometry of the liver. Ultraschall Med. 2012;33:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2860] [Cited by in RCA: 3072] [Article Influence: 105.9] [Reference Citation Analysis (0)] |
| 10. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2702] [Cited by in RCA: 2878] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 11. | Swets JA. Measuring the accuracy of diagnostic systems. Science. 1988;240:1285-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6200] [Cited by in RCA: 4924] [Article Influence: 133.1] [Reference Citation Analysis (0)] |
| 12. | Fernandez M, Trépo E, Degré D, Gustot T, Verset L, Demetter P, Devière J, Adler M, Moreno C. Transient elastography using Fibroscan is the most reliable noninvasive method for the diagnosis of advanced fibrosis and cirrhosis in alcoholic liver disease. Eur J Gastroenterol Hepatol. 2015;27:1074-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Castéra L, Foucher J, Bernard PH, Carvalho F, Allaix D, Merrouche W, Couzigou P, de Lédinghen V. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 405] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 14. | Bota S, Herkner H, Sporea I, Salzl P, Sirli R, Neghina AM, Peck-Radosavljevic M. Meta-analysis: ARFI elastography versus transient elastography for the evaluation of liver fibrosis. Liver Int. 2013;33:1138-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 328] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 15. | Foucher J, Castéra L, Bernard PH, Adhoute X, Laharie D, Bertet J, Couzigou P, de Lédinghen V. Prevalence and factors associated with failure of liver stiffness measurement using FibroScan in a prospective study of 2114 examinations. Eur J Gastroenterol Hepatol. 2006;18:411-412. [PubMed] |
| 16. | Bota S, Sporea I, Sirli R, Popescu A, Danila M, Jurchis A, Gradinaru-Tascau O. Factors associated with the impossibility to obtain reliable liver stiffness measurements by means of Acoustic Radiation Force Impulse (ARFI) elastography--analysis of a cohort of 1,031 subjects. Eur J Radiol. 2014;83:268-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Mueller S, Millonig G, Sarovska L, Friedrich S, Reimann FM, Pritsch M, Eisele S, Stickel F, Longerich T, Schirmacher P. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J Gastroenterol. 2010;16:966-972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 160] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Dong DR, Hao MN, Li C, Peng Z, Liu X, Wang GP, Ma AL. Acoustic radiation force impulse elastography, FibroScan®, Forns’ index and their combination in the assessment of liver fibrosis in patients with chronic hepatitis B, and the impact of inflammatory activity and steatosis on these diagnostic methods. Mol Med Rep. 2015;11:4174-4182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Brunt PW, Kew MC, Scheuer PJ, Sherlock S. Studies in alcoholic liver disease in Britain. I. Clinical and pathological patterns related to natural history. Gut. 1974;15:52-58. [PubMed] |
| 20. | Sporea I, Sirli R, Bota S, Popescu A, Sendroiu M, Jurchis A. Comparative study concerning the value of acoustic radiation force impulse elastography (ARFI) in comparison with transient elastography (TE) for the assessment of liver fibrosis in patients with chronic hepatitis B and C. Ultrasound Med Biol. 2012;38:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Sporea I, Bota S, Peck-Radosavljevic M, Sirli R, Tanaka H, Iijima H, Badea R, Lupsor M, Fierbinteanu-Braticevici C, Petrisor A. Acoustic Radiation Force Impulse elastography for fibrosis evaluation in patients with chronic hepatitis C: an international multicenter study. Eur J Radiol. 2012;81:4112-4118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 22. | Fierbinteanu Braticevici C, Sporea I, Panaitescu E, Tribus L. Value of acoustic radiation force impulse imaging elastography for non-invasive evaluation of patients with nonalcoholic fatty liver disease. Ultrasound Med Biol. 2013;39:1942-1950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Nierhoff J, Chávez Ortiz AA, Herrmann E, Zeuzem S, Friedrich-Rust M. The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: a meta-analysis. Eur Radiol. 2013;23:3040-3053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 24. | Bota S, Sporea I, Sirli R, Popescu A, Danila M, Costachescu D. Intra- and interoperator reproducibility of acoustic radiation force impulse (ARFI) elastography--preliminary results. Ultrasound Med Biol. 2012;38:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Yoneda M, Suzuki K, Kato S, Fujita K, Nozaki Y, Hosono K, Saito S, Nakajima A. Nonalcoholic fatty liver disease: US-based acoustic radiation force impulse elastography. Radiology. 2010;256:640-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 26. | Zhang D, Li P, Chen M, Liu L, Liu Y, Zhao Y, Wang R. Non-invasive assessment of liver fibrosis in patients with alcoholic liver disease using acoustic radiation force impulse elastography. Abdom Imaging. 2015;40:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Bardou-Jacquet E, Legros L, Soro D, Latournerie M, Guillygomarc’h A, Le Lan C, Brissot P, Guyader D, Moirand R. Effect of alcohol consumption on liver stiffness measured by transient elastography. World J Gastroenterol. 2013;19:516-522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Karlas T, Pfrepper C, Wiegand J, Wittekind C, Neuschulz M, Mössner J, Berg T, Tröltzsch M, Keim V. Acoustic radiation force impulse imaging (ARFI) for non-invasive detection of liver fibrosis: examination standards and evaluation of interlobe differences in healthy subjects and chronic liver disease. Scand J Gastroenterol. 2011;46:1458-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Horster S, Mandel P, Zachoval R, Clevert DA. Comparing acoustic radiation force impulse imaging to transient elastography to assess liver stiffness in healthy volunteers with and without valsalva manoeuvre. Clin Hemorheol Microcirc. 2010;46:159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 30. | Popescu A, Bota S, Sporea I, Sirli R, Danila M, Racean S, Suseanu D, Gradinaru O, Ivascu Siegfried C. The influence of food intake on liver stiffness values assessed by acoustic radiation force impulse elastography-preliminary results. Ultrasound Med Biol. 2013;39:579-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | D’Onofrio M, Gallotti A, Mucelli RP. Tissue quantification with acoustic radiation force impulse imaging: Measurement repeatability and normal values in the healthy liver. AJR Am J Roentgenol. 2010;195:132-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Cholongitas E, Senzolo M, Standish R, Marelli L, Quaglia A, Patch D, Dhillon AP, Burroughs AK. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol. 2006;125:710-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 33. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1397] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 34. | Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523-525. [PubMed] |
| 35. | Liao LY, Kuo KL, Chiang HS, Lin CZ, Lin YP, Lin CL. Acoustic radiation force impulse elastography of the liver in healthy patients: test location, reference range and influence of gender and body mass index. Ultrasound Med Biol. 2015;41:698-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Rifai K, Cornberg J, Mederacke I, Bahr MJ, Wedemeyer H, Malinski P, Bantel H, Boozari B, Potthoff A, Manns MP. Clinical feasibility of liver elastography by acoustic radiation force impulse imaging (ARFI). Dig Liver Dis. 2011;43:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 37. | Zhang D, Chen M, Wang R, Liu Y, Zhang D, Liu L, Zhou G. Comparison of acoustic radiation force impulse imaging and transient elastography for non-invasive assessment of liver fibrosis in patients with chronic hepatitis B. Ultrasound Med Biol. 2015;41:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Attia D, Pischke S, Negm AA, Rifai K, Manns MP, Gebel MJ, Lankisch TO, Potthoff A. Changes in liver stiffness using acoustic radiation force impulse imaging in patients with obstructive cholestasis and cholangitis. Dig Liver Dis. 2014;46:625-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Pfeifer L, Strobel D, Neurath MF, Wildner D. Liver stiffness assessed by acoustic radiation force impulse (ARFI) technology is considerably increased in patients with cholestasis. Ultraschall Med. 2014;35:364-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Nishikawa T, Hashimoto S, Kawabe N, Harata M, Nitta Y, Murao M, Nakano T, Mizuno Y, Shimazaki H, Kan T. Factors correlating with acoustic radiation force impulse elastography in chronic hepatitis C. World J Gastroenterol. 2014;20:1289-1297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Raghuwanshi B, Jain N, Jain M. Normal values in healthy liver in central India by acoustic radiation force impulse imaging. J Clin Diagn Res. 2013;7:2498-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Millonig G, Friedrich S, Adolf S, Fonouni H, Golriz M, Mehrabi A, Stiefel P, Pöschl G, Büchler MW, Seitz HK. Liver stiffness is directly influenced by central venous pressure. J Hepatol. 2010;52:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 402] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 43. | Arena U, Vizzutti F, Corti G, Ambu S, Stasi C, Bresci S, Moscarella S, Boddi V, Petrarca A, Laffi G. Acute viral hepatitis increases liver stiffness values measured by transient elastography. Hepatology. 2008;47:380-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 594] [Cited by in RCA: 572] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 44. | Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, Diehl AM, Nightingale KR. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol. 2011;55:666-672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 248] [Article Influence: 17.7] [Reference Citation Analysis (0)] |