Published online May 28, 2016. doi: 10.3748/wjg.v22.i20.4901
Peer-review started: January 20, 2016
First decision: February 18, 2016
Revised: February 29, 2016
Accepted: March 14, 2016
Article in press: March 14, 2016
Published online: May 28, 2016
Processing time: 121 Days and 1.5 Hours
AIM: To investigate clinical profiles and mutations of ABCB11 in Koreans with progressive familial intrahepatic cholestasis 2 and review the differences between Koreans and others.
METHODS: Of 47 patients with neonatal cholestasis, five infants had chronic intrahepatic cholestasis with normal γ-glutamyl transpeptidase. Direct sequencing analyses of ABCB11, including exons and introns, were performed from peripheral blood.
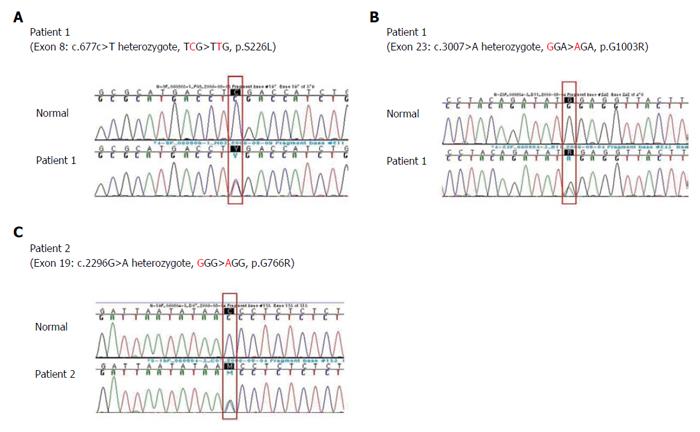
RESULTS: Living donor-liver transplantation was performed in four patients because of rapidly progressive hepatic failure and hepatocellular carcinoma. Three missense mutations were found in two patients: compound heterozygous 677C>T (S226L)/3007G>A (G1003R) and heterozygous 2296G>A (G766R). The mutations were located near and in the transmembranous space.
CONCLUSION: Alterations in the transmembrane of the bile salt export pump in the Korean infants were different from those previously reported in Chinese, Japanease, Taiwanese, and European patients.
Core tip: Reports of progressive familial intrahepatic cholestasis (PFIC) mutations in Asian countries have been less than those in Western countries because of time consuming and expensive diagnostic tools. Recently, reports on mutations of ABCB11 in Asian patients with PFIC have been increasing. In this study, the authors report mutations of ABCB11 in Korean infants with PFIC2 and compare Korean mutations with previously reported mutations.
- Citation: Park JS, Ko JS, Seo JK, Moon JS, Park SS. Clinical and ABCB11 profiles in Korean infants with progressive familial intrahepatic cholestasis. World J Gastroenterol 2016; 22(20): 4901-4907
- URL: https://www.wjgnet.com/1007-9327/full/v22/i20/4901.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i20.4901
Progressive familial intrahepatic cholestasis (PFIC) is an autosomal recessive disorder that manifests as cholestasis during the neonatal period due to defective bile secretion. PFIC is divided into types 1, 2, and 3 according to their different clinical manifestations and genetics. In PFIC1 and PFIC2, cholestasis develops during the neonatal period, and γ-glutamyl transpeptidase (GGT) is within normal limits. PFIC3 develops later than PFIC1 and PFIC2, and features a positive prenatal history of maternal cholestasis. Generally, cholestasis with elevated GGT is associated with PFIC3 rather than PFIC1 and PFIC2. Persistent or repetitive cholestasis develops within 1 year of age and rapidly progresses to liver cirrhosis and hepatic failure in patients with PFIC. Mutations of biliary transporters associated with PFIC have been discovered, which can aid in understanding the diagnosis and pathogenesis. The genes are ATP8B1, ABCB11, and ABCB4, which encode familial intrahepatic cholestasis 1 protein (FIC1), bile salt export pump (BSEP), and multidrug resistance protein 3 (MDR3) in PFIC1, 2, and 3, respectively.
ABCB11 is located on chromosome 2q24. It encodes BSEP, which plays a role in the secretion of conjugated bile acids, including taurocholates. BSEP defect can cause cholestasis with a normal range of GGT because of a bile secretion defect[1]. Over 82 different ABCB11 mutations have been reported[2,3]. Of them, E297G and D482G account for 30% of BSEP mutations in European patients with PFIC2. In Asia, mutations of BSEP in Chinese, Japanese, and Taiwanese patients with PFIC2 were reported[4-7].
To the best of our knowledge, there have been fewer reports of ABCB11 (BSEP) mutations in Asians with PFIC2 than in Europeans[2,4-7]. Because PFIC2 features rapid progression to liver cirrhosis and hepatic failure within the first decade, rapid diagnosis, management, and prediction of prognosis are important. In the present study, the authors investigated clinical profiles of Korean infants with PFIC and performed a mutation analysis on the ABCB11 gene. The authors obtained clearance from the ethical board of the hospital (GNUH 2015-09-004-001).
Between 2005 and 2006, 47 patients visited the Department of Pediatrics in Seoul National University Children’s Hospital for neonatal cholestasis. Examinations included abdominal ultrasonography, duodenal intubation, a hepatobiliary scan, and liver biopsy. Inborn error of metabolism, total parenteral nutrition, drug related cholestasis, congenital infection, and cholestasis secondary to sepsis were excluded. PFIC was suspected based on intrahepatic cholestasis with normal ranged GGT or on the results of the genetic analyses. None had a family history of PFIC.
Genetic analyses were performed for diagnosis with parental consent. Direct sequencing analysis of ABCB11 was done using peripheral blood. Exons and flanking intron sequences of the ABCB11 gene (NC_000002.10) were amplified by polymerase chain reaction (PCR) from total genomic DNA. PCR products were purified by ExoSAP-IT (USB, Cleveland, OH, United States) and subjected to DNA sequencing using the BigDye v3.1 Terminator Chemistry (PE Applied Biosystems, Foster City, CA, United States), followed by separation on an ABI 3100 DNA sequencer (PE Applied Biosystems). Sequence data were analyzed manually and were assembled with the Seqscape v2.5 (PE Applied Biosystems). As reference control, the ABCB11 genomic sequence was obtained from http://pharmacogenetics.ucsf.edu/set1/BSEPrefseq.html.
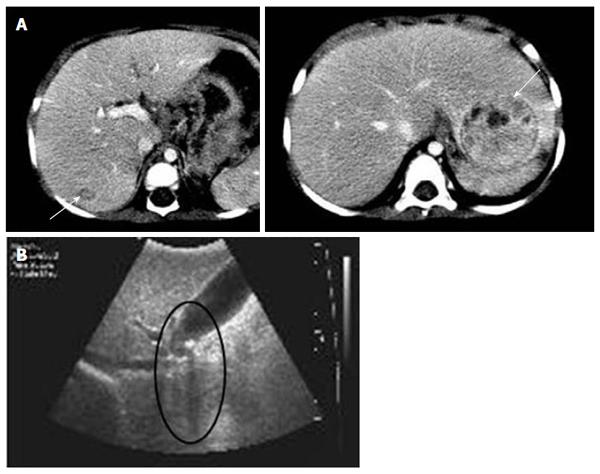
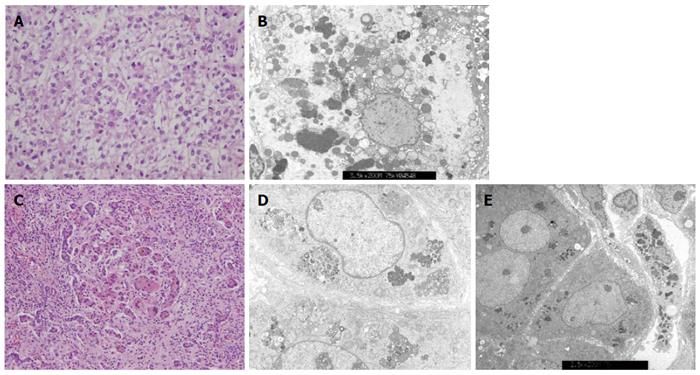
Among the 47 patients with cholestatic jaundice presented during the 2-year period, extrahepatic biliary atresia was diagnosed in eleven, congenital infection with TORCH in four, neonatal intrahepatic cholestasis caused by citrin deficiency in three, arthrogryposis, renal dysfunction, cholestasis (ARC) syndrome in two, neonatal Dubin-Johnson syndrome in two, Alagille syndrome in one, and non-syndromic bile duct paucity in one[8]. PFIC was suspected in five patients with intrahepatic cholestasis and normal GGT. Table 1 summarizes the clinical and laboratory findings of the patients. The chief complaint was cholestatic jaundice in all patients, and onset of the symptom ranged from 20 d to 9 mo after birth. Gallstone was developed in patient 1 and 2, and hepatocellular carcinoma (HCC) was developed in patient 1 (Figure 1). Hepatic pathologic examinations were performed in all patients. Various degrees of periportal fibrosis and inflammatory cell infiltration, canalicular and cytoplasmic bile pigments, cholestasis, and bile ductular dilatation and proliferation were noted in all hepatic specimens of the patients. HCC was confirmed from an excised liver in patient 1 (Figure 2). Living donor liver transplantations were performed in four patients due to hepatic failure between 2.5 mo and 10 years after their initial visits. Three of the 10 alleles examined showed mutations: compound heterozygous 677C>T (S226L)/3007G>T (G1003R) in patient 1 and heterozygous 2296G>A (G776R) in patient 2 (Figure 3). Three of the five patients showed no mutation of ABCB11. The PolyPhen program (http://genetics.bwh.harvard.edu/pph2) predicted that S226L is probably benign with a score of 0.175, but the SIFT program (http://sift.jcvi.org) predicted that S226L deleteriously affects protein function. G1003R and G776R were predicted to be probably damaging with scores of 1.000 by the PolyPhen-2 program and to affect deleteriously protein functions. 3007G/T (G1003R) was a novel mutation of ABCB11.
| Patient | Sex | Age of symptom onset | Age at LTx | Associated sign | AST/ALT (IU/L)(0-37/0-41) | T/D. bil (mg/dL)(0-1.2/0-0.5) | GGT (IU/L)(6-71) | AFP (ng/mL)(0-7.0) | Mutations of ABCB11 |
| 1 | F | 20 d | 24 mo | Gallstone, HCC | 602/242 | 11.3/7.9 | 29 | 3070 | S226L/ |
| G1003R | |||||||||
| 2 | F | 9 mo | 10 yr | Gallstone | 178/242 | 8.2/5.2 | 25 | < 5 | G776R |
| 3 | M | 5 d | 6 mo | - | 416/93 | 35.1/14 | 25 | 21500 | No |
| 4 | M | 1 mo | 3.5 mo | - | 1467/250 | 44.5/25.1 | 50 | - | No |
| 5 | M | 2 mo | - | - | 951/677 | 14.3/8.0 | 52 | 770000 | No |
Three mutations were found in two patients with the PFIC phenotype: 677C>T in exon 8 (S226L) and 3007G>A in exon 23 (G1003R) in patient 1 and 2296G>A in exon 19 (G766R) in patient 2 (Figure 3). Patients with mutations presented with chronic intrahepatic cholestasis with normal GGT from the infantile period, rapidly declining liver functions, gallstone without intravascular hemolysis, and HCC (Table 1, Figures 1-3).
PFICs are developed by mutations of bile transporters, and the incidence of the mutation has not yet been established[9]. In addition, its incidence might be underestimated in Korea because diagnosis in a patient with suspected PFIC is often time consuming and expensive because of clinical methods such as clinical courses, laboratory findings, pathologic examination, and genetic analysis. Differentiation between PFIC1 and PFIC2 is difficult based on clinical manifestations and pathologic findings because of their marked clinical overlap[1]. PFIC1 patients usually present with more diverse extrahepatic symptoms, including diarrhea, than patients with PFIC2[10]. The patients did not show diarrhea or pruritus, but patient 1 did develop gallstones and HCC.
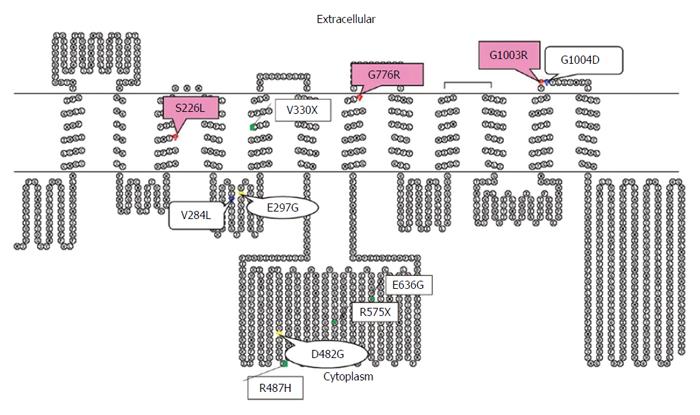
To the best our knowledge, Asian reports on PFIC2 are relatively lacking in comparison to Western reports[2,4-7]. However, reports on mutations of BSEP in Asian patients with PFIC2 are increasing by denaturing high performance liquid chromatography, high-resolution melting analysis, and direct sequencing. The reported Asian BSEP mutations were as follows: R575X, E636G, R487H, and V330X in Japanese patients; 1 bp deletion (position 1145), V284L, and G1004D in Taiwanese patients; and 20 mutations, including A167T, in Chinese patients[4,7,11,12]. Of the hundreds of BSEP mutations, E297G and D482G were the most common mutations in European patients[13]. Most of the previously reported mutations in Chinese and Europeans are located in the canalicular cytoplasm[2,4,12]. However, three different missense mutations (S226L, G1003R, and G775R) in the present study were located at and near the transmembranous (TM) part of BSEP (Figure 4). The TM alterations of BSEP in Korean infants might be due to ethnic differences. Further study, however, is needed because the number of mutations in the Korean patients with PFIC2 was low.
There was no mutation of ABCB11 in patient 3, 4, and 5. TPJ2 mutations can also cause a PFIC2 like phenotype and further genetic analyses of TPJ2 are necessary[14]. Patient 5 showed elevated GGT levels and decreased serum total bilirubin in a relatively short clinical course. Therefore, the possibilities of PFIC1 and PFIC2 are less, but careful follow-ups are essential with the possibility of benign recurrent intrahepatic cholestasis.
Compound heterozygotes of 677C>T (S226L) and 3007G>A (G1003R) in patient 1, and one missense mutation of 2296G>A (G766R) in patient 2 were noted (Figure 3). The mutations were predicted to be damaging or deleterious to BSEP, based on the clinical course of the patients and the results of PolyPhen-2 and SIFT program. Unfortunately, the authors could not perform genetic analyses of ABCB11 of the parents.
HCC developed in patient 1 (Figures 2 and 3A). Knisely et al[15] reported BSEP dysfunctions in 10 patients who presented with HCC under the age of 5 years. Chronic intrahepatic bile acids or suppressed DNA ligase by protein dysfunctions were suggested previously[16,17], but specific factors contributing to the development of HCC have not been evident. Cholangiocarcinoma and hepatoblastoma in patients with PFIC2 has also been reported[18,19]. The level of serum alpha fetoprotein (AFP) are high in > 60% of patients with HCC and hepatoblastoma, and AFP increased markedly to 204000 ng/mL at 21 mo of age in patient 1. In patient 5, a significantly high level of AFP was noted on the first laboratory examination. There was no evidence of hepatic mass on liver ultrasonography (USG) and inborn errors of metabolism on laboratory examination. The level of AFP was decreased to 530000 ng/mL after 1 mo. The decline of AFP and no occurrence of hepatic mass on liver USG could rule out the development of hepatic tumor in patient 5. Therefore, the increment of serum AFP and hepatic imaging can be useful modalities for early detection of hepatic tumors in a patient with PFIC2.
Various degrees of periportal fibrosis, inflammatory cell infiltrates, intracytoplasmic and intracanalicular cholestasis, giant cell transformation, and bile ductular proliferation on the pathologic examinations were noted in the present study (Figure 2). Bile duct paucity or bile ductular proliferation was not a typical finding in patients with PFIC, but pathologic findings might depend on the clinical moment when the biopsy was performed. Pathologic examinations in the present study did not show typical findings for PFIC because our hepatic specimens were obtained at the time of liver transplantation, except in patient 5. Pathologic differentiation from PFIC1 to PFIC2 depends on the severity of the aforementioned findings and characters of canalicular bile salts. Coarse granular bile salts can suggest PFIC1, while amorphous and filiform bile salts under electron microscopic examination can suggest PFIC2[1]. However, pathologic differentiation seems to depend significantly on clinical moments for biopsy and experience or skill of the pathologist.
In conclusion, the present study is the first report on Korean infants with PFIC, including early onset HCC, living donor liver transplantations, and novel mutation and ethnic differences of ABCB11. We tentatively suggest the suspicion of PFIC1 or PFIC2 when children are suffering from chronic intrahepatic cholestasis with normal GGT and without other associated anomalies from their infantile periods, regardless of family history. Early genetic analysis for PFIC1 or PFIC2 might be helpful to diagnose, predict prognosis, and make an early treatment plan.
Because progressive familial intrahepatic cholestasis (PFIC)2 features rapid progression to liver cirrhosis and hepatic failure within the first decade, rapid diagnosis, management, and prediction of prognosis are important. The authors investigated the clinical profiles of Korean infants with PFIC, performed mutation analysis on the ABCB11 gene, and reviewed the differences between Korean and other previous mutations.
In the present study, a novel mutation of ABCB11 in Korean infants with PFIC2 and the sites of amino acid alterations were different from those previously identified in Europeans.
The authors report a rare PFIC2 in Korean patients and its genetic novel mutations. These mutations affected amino acid substitutions of BSEP, and the sites were different from previous reports. The number of mutations, however, was low.
The described novel mutations of ABCB11 in Korean infants with PFIC2 might be helpful to understand racial differences in the future. However, further study is warranted, including a larger single nucleotide polymorphism database of Koreans.
There were concerns about the number of mutations in Korean infants with PFIC2. However, the focus of the manuscript is potentially interesting, and the present report is significant because of the rarity in character of PFIC2, especially in Asian groups.
P- Reviewer: Kamimura K, Vij M, Wang JS S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Wang CH
| 1. | Harris MJ, Le Couteur DG, Arias IM. Progressive familial intrahepatic cholestasis: genetic disorders of biliary transporters. J Gastroenterol Hepatol. 2005;20:807-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Strautnieks SS, Byrne JA, Pawlikowska L, Cebecauerová D, Rayner A, Dutton L, Meier Y, Antoniou A, Stieger B, Arnell H. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology. 2008;134:1203-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 263] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 3. | Francalanci P, Giovannoni I, Candusso M, Bellacchio E, Callea F. Bile salt export pump deficiency: A de novo mutation in a child compound heterozygous for ABCB11. Laboratory investigation to study pathogenic role and transmission of two novel ABCB11 mutations. Hepatol Res. 2013;43:315-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 4. | Hu G, He P, Liu Z, Chen Q, Zheng B, Zhang Q. Diagnosis of ABCB11 gene mutations in children with intrahepatic cholestasis using high resolution melting analysis and direct sequencing. Mol Med Rep. 2014;10:1264-1274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Chen HL, Liu YJ, Su YN, Wang NY, Wu SH, Ni YH, Hsu HY, Wu TC, Chang MH. Diagnosis of BSEP/ABCB11 mutations in Asian patients with cholestasis using denaturing high performance liquid chromatography. J Pediatr. 2008;153:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Chen ST, Chen HL, Su YN, Liu YJ, Ni YH, Hsu HY, Chu CS, Wang NY, Chang MH. Prenatal diagnosis of progressive familial intrahepatic cholestasis type 2. J Gastroenterol Hepatol. 2008;23:1390-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Goto K, Sugiyama K, Sugiura T, Ando T, Mizutani F, Terabe K, Ban K, Togari H. Bile salt export pump gene mutations in two Japanese patients with progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 2003;36:647-650. [PubMed] |
| 8. | Ko JS, Song JH, Park SS, Seo JK. Neonatal intrahepatic cholestasis caused by citrin deficiency in Korean infants. J Korean Med Sci. 2007;22:952-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Jansen PL, Strautnieks SS, Jacquemin E, Hadchouel M, Sokal EM, Hooiveld GJ, Koning JH, De Jager-Krikken A, Kuipers F, Stellaard F. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology. 1999;117:1370-1379. [PubMed] |
| 10. | Alissa FT, Jaffe R, Shneider BL. Update on progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 2008;46:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Chen HL, Chang PS, Hsu HC, Ni YH, Hsu HY, Lee JH, Jeng YM, Shau WY, Chang MH. FIC1 and BSEP defects in Taiwanese patients with chronic intrahepatic cholestasis with low gamma-glutamyltranspeptidase levels. J Pediatr. 2002;140:119-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Liu LY, Wang ZL, Wang XH, Zhu QR, Wang JS. ABCB11 gene mutations in Chinese children with progressive intrahepatic cholestasis and low gamma glutamyltransferase. Liver Int. 2010;30:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Hayashi H, Takada T, Suzuki H, Akita H, Sugiyama Y. Two common PFIC2 mutations are associated with the impaired membrane trafficking of BSEP/ABCB11. Hepatology. 2005;41:916-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Carlton VE, Pawlikowska L, Bull LN. Molecular basis of intrahepatic cholestasis. Ann Med. 2004;36:606-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 29] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, Bull LN, Pawlikowska L, Bilezikçi B, Ozçay F. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 16. | Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589:47-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 458] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 17. | Prieto-Alamo MJ, Laval F. Deficient DNA-ligase activity in the metabolic disease tyrosinemia type I. Proc Natl Acad Sci USA. 1998;95:12614-12618. [PubMed] |
| 18. | Scheimann AO, Strautnieks SS, Knisely AS, Byrne JA, Thompson RJ, Finegold MJ. Mutations in bile salt export pump (ABCB11) in two children with progressive familial intrahepatic cholestasis and cholangiocarcinoma. J Pediatr. 2007;150:556-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Richter A, Grabhorn E, Schulz A, Schaefer HJ, Burdelski M, Ganschow R. Hepatoblastoma in a child with progressive familial intrahepatic cholestasis. Pediatr Transplant. 2005;9:805-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |