Published online May 28, 2016. doi: 10.3748/wjg.v22.i20.4891
Peer-review started: January 30, 2016
First decision: March 7, 2016
Revised: March 24, 2016
Accepted: April 7, 2016
Article in press: April 7, 2016
Published online: May 28, 2016
Processing time: 111 Days and 19.8 Hours
AIM: To assess the diagnostic accuracy of multidetector-row computed tomography (MDCT) as compared with conventional magnetic resonance imaging (MRI), in identifying mesorectal fascia (MRF) invasion in rectal cancer patients.
METHODS: Ninety-one patients with biopsy proven rectal adenocarcinoma referred for thoracic and abdominal CT staging were enrolled in this study. The contrast-enhanced MDCT scans were performed on a 256 row scanner (ICT, Philips) with the following acquisition parameters: tube voltage 120 KV, tube current 150-300 mAs. Imaging data were reviewed as axial and as multiplanar reconstructions (MPRs) images along the rectal tumor axis. MRI study, performed on 1.5 T with dedicated phased array multicoil, included multiplanar T2 and axial T1 sequences and diffusion weighted images (DWI). Axial and MPR CT images independently were compared to MRI and MRF involvement was determined. Diagnostic accuracy of both modalities was compared and statistically analyzed.
RESULTS: According to MRI, the MRF was involved in 51 patients and not involved in 40 patients. DWI allowed to recognize the tumor as a focal mass with high signal intensity on high b-value images, compared with the signal of the normal adjacent rectal wall or with the lower tissue signal intensity background. The number of patients correctly staged by the native axial CT images was 71 out of 91 (41 with involved MRF; 30 with not involved MRF), while by using the MPR 80 patients were correctly staged (45 with involved MRF; 35 with not involved MRF). Local tumor staging suggested by MDCT agreed with those of MRI, obtaining for CT axial images sensitivity and specificity of 80.4% and 75%, positive predictive value (PPV) 80.4%, negative predictive value (NPV) 75% and accuracy 78%; while performing MPR the sensitivity and specificity increased to 88% and 87.5%, PPV was 90%, NPV 85.36% and accuracy 88%. MPR images showed higher diagnostic accuracy, in terms of MRF involvement, than native axial images, as compared to the reference magnetic resonance images. The difference in accuracy was statistically significant (P = 0.02).
CONCLUSION: New generation CT scanner, using high resolution MPR images, represents a reliable diagnostic tool in assessment of loco-regional and whole body staging of advanced rectal cancer, especially in patients with MRI contraindications.
Core tip: The introduction of new generation of multidetector-row computed tomography (MDCT) scanner allowed thin-collimation scanning and high spatial resolution, resulting in improved multiplanar reconstructions (MPRs) and could be potentially useful, in a single examination, for local staging and distant metastases evaluation in rectal cancer patients. On these basis in our study we assessed the accuracy of high row number MDCT for the prediction of tumor invasion of the mesorectal fascia, being MRI findings as reference standard, and whether the addition of high-resolution MPR images can provide greater accuracy.
- Citation: Ippolito D, Drago SG, Franzesi CT, Fior D, Sironi S. Rectal cancer staging: Multidetector-row computed tomography diagnostic accuracy in assessment of mesorectal fascia invasion. World J Gastroenterol 2016; 22(20): 4891-4900
- URL: https://www.wjgnet.com/1007-9327/full/v22/i20/4891.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i20.4891
Treatment options in rectal cancer patients, such as total mesorectal excision (TME) and preoperative neoadjuvant radiochemotherapy in advanced tumor stages[1,2], have greatly increased the importance of accurate preoperative staging to provide information about tumor location, size, configuration, and local infiltration[3]. One of the most important features of local rectal cancer staging is the assessment of the circumferential resection margin (CRM)[1] and relationship of the tumor to the mesorectal fascia (MRF), which actually defines the surgical CRM in TME surgery[4-6].
Magnetic resonance imaging (MRI) is today considered the “state-of-the-art” investigation for pre-operative evaluation of pelvic malignant disease due to the method’s multiplanar capabilities and its ability to visualize the rectum, the mesorectal fat and the MRF, urinary bladder and internal genitalia with high soft tissue contrast[7].
Although many studies have described the accuracy of computed tomography (CT) for predicting the depth of bowel wall and lymph node invasion[8-11], only few of them have addressed the problems of predicting tumor infiltration of the MRF with new generation of multidetector-row CT (MDCT). The current role of CT in the evaluation of patients with rectal cancer is controversial[3]. In a single examination, CT can assess the entire abdomen, pelvis and chest, allowing for local staging and distant metastases evaluation[12-15]. MRI is an integral part of the diagnostic work-up of patients with rectal cancer due to its proven efficacy to determine the tumor relationship to the MRF[4]. However, MRI does have the downside of limited availability, relatively long image acquisition time and high cost[4]. Moreover, not all patients can undergo MRI because of claustrophobia or the presence of metal in patients’ bodies. In addition, another important factor in the preoperative assessment of primary rectal cancer is the frequent presence of distant disease at the time of diagnosis. Modern CT techniques are better suited than MRI to search for the local tumor extent and distant metastases in the same imaging session[16,17]. These considerations on one hand, and improved spatial resolution of new MDCT scanner on the other hand, have revived the discussion whether to use CT or MRI for rectal cancer staging[4,17]. The introduction of MDCT allowed thin-collimation scanning and high spatial resolution[1], resulting in improved multiplanar reconstructions (MPR)[3,17]. MPR images can be potentially useful for local staging in rectal cancer as they can be aligned parallel or perpendicular to the axis of the tumor similar to MR imaging. The aim of the present study was to evaluate the accuracy of high row number MDCT for the prediction of tumor invasion of the MRF being MRI findings as reference standard, and whether the addition of high-resolution MPR images can provide greater accuracy.
One hundred and thirty-one patients with biopsy-proven adenocarcinoma of the rectum and distal margin of the tumor within 15 cm from the anal verge were enrolled in this retrospective study.
The standard workup for patients with a rectal cancer includes a pelvic MRI for the assessment of loco-regional staging and MDCT study to determine the whole body staging.
For this reason the inclusion criteria were: (1) a biopsy proven rectal cancer (0-15 cm from anal verge according to endoluminal biopsy); (2) availability of MRI study of lower abdomen; (3) availability of contrast enhanced MDCT of the chest and abdomen examinations; and (4) both MRI and CT images performed before application of any neo-adjuvant therapy or surgery.
Exclusion criteria were: (1) previous neo-adjuvant therapy for rectal cancer; (2) contraindications to MRI examination; (3) contraindications to contrast enhanced CT imaging (e.g., intolerance/allergy to iodine contrast medium); (4) insufficient MR imaging quality (e.g., movement artifact) and insufficient CT imaging quality (e.g., owing to metal implants); and (5) absence of one of the two diagnostic tools between MRI and CT .
Forty patients were excluded from this study: 2 patients had hip prostheses (important beam hardening artifacts reduced CT images quality); 6 patients were excluded due to movement-related artifacts in MR study; 19 patients had only MRI evaluation and 13 had a CT evaluation alone (patients in which the local MRI staging was performed in another Hospital).
A final cohort of 91 patients (65 male and 26 female, with a mean age of 69 years - range 30 to 89 years) satisfied the inclusion criteria and were enrolled in this study.
The mean interval time between the MRI and CT examination was 37 d (range 0-79 d).
The approval for this study was obtained by the ethical approval committee at our Institution.
All MDCT examinations were carried out without luminal rectal contrast media or air insufflation. All CT studies were performed on a 256-slice CT system (Brilliance iCT, Philips Medical Systems, Best, the Netherlands) with the following scan parameters: thickness 2 mm; increment 1 mm; collimation 128 × 0.625; pitch 0.915; rotation time 0.4 s; FOV 350; matrix 512 × 512. The scan images were acquired before and after the intravenous bolus injection of non-ionic iodinated contrast material (Xenetix 350; Guerbet, Aulnay, France), according to the body weight, at a rate of 3.5 mL/s, using a double-syringe injector (Medrad Stellant, Pittsburgh, PA, United States) and 18-gauge catheter positioned into the antecubital vein. Bolus tracking software was used to set individual acquisition times for the arterial, portal and equilibrium phases. Contrast material enhancement was automatically calculated by placing the region of interest cursor over the abdominal aorta, and the level of the trigger threshold was set to increase to 120 HU.
Thirteen seconds after the trigger threshold had been reached, arterial phase CT data acquisition began automatically. The portal venous and equilibrium phases were acquired after 60 and 140 s, respectively, after the trigger threshold had been reached.
Examinations were performed during one breath-hold from the thorax to the anus.
None of the patients received a contrast enema or bowel relaxation.
MRI imaging examination was performed for tumor staging before starting the treatment or surgery.
All MRI examinations were performed with a 1.5-T system (Achieva Plus; Philips, The Netherlands) in combination with a five-channel phased-array body coil.
After a planning scan, axial and sagittal T2 weighted turbo spin-echo (T2WI-TSE) images covering entire length of the rectum were acquired and used to plan high resolution scans.
Scan protocol consisted of axial TSE T1 weighted axial sequence turbo spin-echo (TSE) (slice thickness: 3 mm; slice: 20; gap: 3 mm; TR: 612 ms; TE: 14 ms; flip angle: 90°; FOV: 180; RFOV: 85; matrix: 272 × 320; NSA: 4; time: 4.43 min); sagittal TSE T2 sequence (slice thickness: 3 mm; slice: 32; gap: 0 mm; TR: 5501 ms; TE: 85 ms; flip angle: 90°; FOV: 220; RFOV: 105; matrix: 276 × 200; NSA: 4; time: 4.40 min); axial TSE T2 sequence (slice thickness: 3.5 mm; slice: 18; gap: 3.5 mm; TR: 4750 ms; TE: 120 ms; flip angle: 90°; FOV: 180; RFOV: 85; matrix: 256 × 256; NSA: 4; time: 3.05 min); coronal TSE T2 sequence (slice thickness: 3 mm; slice: 20; gap: 0.5 mm; TR: 5058 ms; TE: 125 ms; flip angle: 90°; FOV: 180; RFOV: 100; matrix: 256 × 256; NSA: 4; time: 3.47 min). The axial and coronal oblique images were performed orthogonal and parallel, respectively, to the long axis of the rectal cancer.
Afterwards diffusion weighted images with background body signal suppression (DWIBS) using a Multi-slice Spin Echo Eco-planar Single Shot (SE-EPI-SSh) sequence were obtained; DWIBS were combined with a short time inversion recovery (STIR) pre-pulse for fat saturation. The DWIBS sequences were acquired in a pure axial plane in order to avoid distortion artifacts, with b-value 0 and 1000 s/mm2 with following parameters: slice thickness: 6 mm; slice: 12; gap: 6 mm; TR: 3000 ms; TE: 74 ms; flip angle: 90◦; b-value: 0 and 700 s/mm2; FOV: 380; RFOV: 80; matrix: 240 × 256; NSA: 4; time: 1.30 min; SENSE factor: 1.5. According to recent literature no contrast enhanced dynamic or steady state T1 weighted or fat suppressed sequences were used[18,19].
All these sequences were obtained in free breathing. The total examination time was approximately 30 min. Patients did not undergo any preparation such as bowel cleaning or spasmolytic medication before the MR examinations. Luminal distention was achieved with rectal administration of a small amount (almost 100 mL) of sonography transmission gel to distend the rectal lumen.
In order to obtain an optimal contrast enhancement, the images of the pelvis were observed in the portal-venous contrast enhanced phase. Multiplanar CT reconstructions were performed from the same radiologist, (blinded to pathological evaluation, clinical and MRI patient data), that analyzed all the CT images and orientated MPR images axial plane along the tumor axis.
According to recent guidelines about clinical management of rectal cancer patients with MRI (recommendations from ESGAR, 2012)[18], sagittal reconstructions are used to determine the longitudinal tumor axis in order to angle the axial and coronal planes as perpendicular and parallel to the tumor axis as possible, respectively. MPR CT images were performed following the same recommendations to obtain axial and oblique coronal planes similar to MRI imaging.
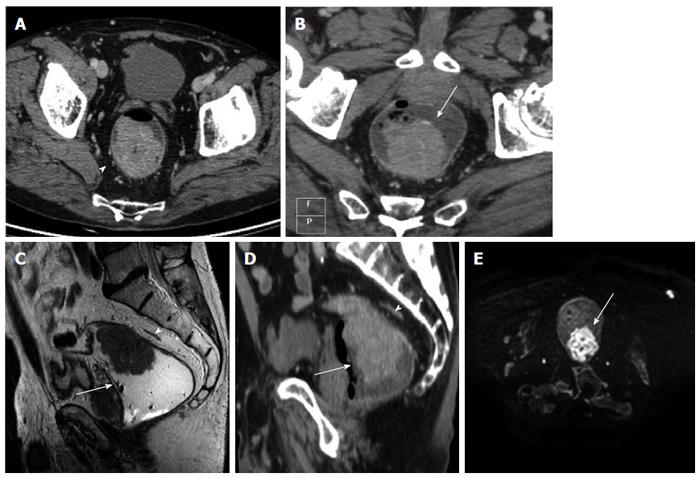
After iv contrast injection the tumor was seen as an intraluminal polypoid mass (Figure 1) or as asymmetric or circumferential mural thickening (> 6 mm)[20] with or without luminal narrowing (with abrupt transition from normal to abnormally thick-walled rectum) and smooth outer bowel margins. In some cases strands of the soft tissue extending from serosal surface into perirectal fat was observed (Figure 1).
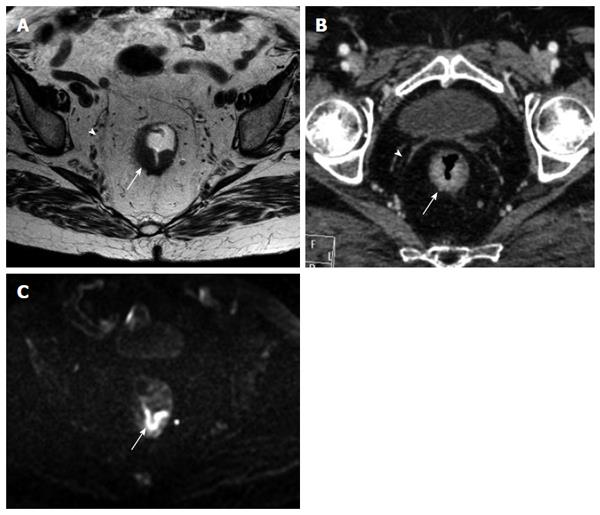
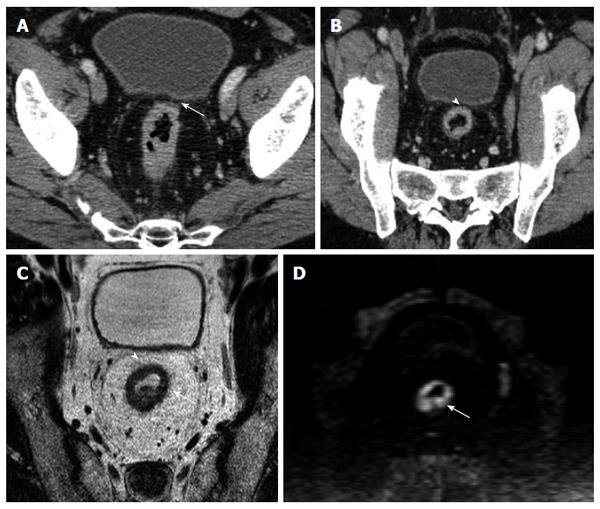
The MRF was seen as a thin, curvilinear structure surrounding the mesorectal fat with similar density to muscle adjacent to the rectum[1,21] (Figures 2 and 3). The main outcome parameter was the involvement of MRF defined as a visible fat line between the tumor and the MRF (Figure 3).
All CT axial images were observed first, in order to determine the tumor extension and direct involvement of the MRF; few days after (0-6 d), the same evaluation was done with MPR images.
For each patient a radiologist with 10 years of experience in abdominal imaging analyzed T2-weighted sequences and DWIBS images in order to detect and correctly localize the primary lesion. The presence of the tumor was diagnosed on T2-weighted sequences. Rectal cancer typically appeared hypointense as compared to the surrounding fat, and slightly hyperintense as compared to the muscles.
The mesorectal fascia was seen as a thin hypointense line surrounding the mesorectal fat[1].
DWIBS images were analyzed in order to obtain information about microscopic structures of biologic tissue through water proton mobility and to achieve a possible tool to monitor the response of tumor tissue after therapy[22].
These images were of diagnostic quality and adequate to identify the tumor region. When the anatomic details were unclear due to the low signal-to-noise (SNR) on DWIBS images, they were matched to T2WI images of same planes. The diagnostic criterion on DWI was defined as a focal mass with high signal intensity (SI) on b1000 DW, compared with the signal of the normal adjacent rectal wall or background of lower SI tissue[22].
During images analysis, the radiologist was blinded to clinical patient data and pathological evaluation.
Multiplanar T2 weighted sequences and axial T1 weighted sequences images were evaluated in order to assess the presence of the tumor, the involvement of the MRF and the adjacent structures.
All statistical analysis was performed using commercially available software (Med Calc, Med calc software 11.0, Mariakerke Belgium). The McNemar test was used to compare axial and MPR CT images with those of MRI imaging, which was considered as the reference standard, in order to determine the involvement of the MRF.
The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of axial and MPR images were assessed and the obtained data were then compared. Overall accuracy, sensitivity and specificity of the prediction of involvement of the MRF were calculated using cross-tabulation statistics.
In the native axial CT imaging analysis the MRF was involved by the tumor in 51 patients, while on MPR images the involvement of the MRF was observed in 50 patients. At MR image evaluation, the involvement of the MRF by the rectal cancer was observed in 51 patients.
DWIBS allowed to recognize the tumor as a focal mass with high signal intensity on high b-value images, compared with the signal of the normal adjacent rectal wall or with the lower tissue signal intensity background (Figures 1, 3 and 4).
The overall correlation of MPR and native axial findings with the MR images demonstrated (Table 1) that the number of patients correctly staged by evaluating the native axial images was 71 out of 91 patients (41 true positive, TP; 30 true negative, TN), while by using the MPR a total of 80 patients were correctly staged (45 TP and 35 TN) (Figure 4).
The number of false negative (FN) for axial and MPR was respectively 10 FN and 6 FN. The results obtained in our series of patients show an overall good diagnostic value of CT technique: considering the native axial CT images, the overall sensitivity and specificity were respectively 80.4% and 75%, PPV was 80.4%, NPV 75% and Accuracy was 78%. While analyzing the MPR images the sensitivity raised up to 88% and specificity up to 87.5%, PPV was 90%, NPV 85.36% and accuracy raise up to 88% (Table 2). The difference in performance between axial and MPR images was not statistically significant, in terms of sensitivity and specificity (McNemar test with P = 0.22 and P = 0.13 respectively) (Table 3), but considering the overall diagnostic accuracy, in terms of MRF involvement, the MPR images demonstrated to be superior (P = 0.02) in comparison with native axial images alone, as compared to the reference MR images (Table 4).
| Axial CT images | ||||
| Sensitivity | Specificity | PPV | NPV | Accuracy |
| 80.40% | 75% | 80.4% | 75% | 78% |
| MPR CT images | ||||
| Sensitivity | Specificity | PPV | NPV | Accuracy |
| 88% | 87.5% | 90% | 85.36% | 88% |
| Uncorrect subjects staged with AX/MPR CT images | Correct subjects staged with AX/MPR CT images | Total of correct subjects staged with MRI | |
| Accuracy1 (P = 0.02) | |||
| Uncorrect subjects staged with MPR/AX | 9 | 2 | 11 |
| Correct subjects staged with MPR/AX | 11 | 69 | 80 |
| Total of correct subjects staged with MRI | 20 | 71 | 91 |
To date, only few studies[1,4,16,17,21,23,24] analyzed the role of MDCT as possible and reliable imaging technique in assessment of MRF invasion by rectal cancer. According to recent literature[25], the appropriate angulation of the axial plane orthogonal to the tumor is essential in primary tumor staging, since incorrect plane obliquity leads to a pseudospiculated appearance that may lead to overstaging (Figures 2 and 4). Placement of the orthogonal plane is based on the definition of the tumor on sagittal T2-weighted images.
DWIBS are usually performed in pre-operative rectal cancer staging[22,25] in order to improve the detection and localization of rectal tumors, especially when the tumor is difficult to visualize with other sequences[25]. While these sequences have no role in the assessment of mesorectal fascia involvement, due to the intrinsic limitations of MRF visualization at high b-values. While the DWIBS are frequently employed in restaging of rectal cancer patients, due to the possibilities to offer information about structures of biologic tissue through water proton mobility, and suggested as a possible tool to monitor the response of tumor tissue after therapy[22,25].
Several problems frequently arise during this critical initial step, due to motion artifacts, small tumor size, low contrast between the tumor and the rectal wall on fast relaxation fast spin-echo (FSE) T2-weighted images, redundancy and tortuosity of the rectum. In addition, nodes along the pelvic sidewall and superior rectal vessels may fall outside the FOV of axial high-resolution images.
In this setting the clinical use of MDCT images combined with MPR along the different axis of rectal lumen, permits to overcoming some of these limitations, having also the possibility to include a large FOV and modify the different perpendicular axial plane in a less time consuming analysis in order to evaluate the tumor axis (also in case of tortuosity and redundancy of the rectum), the MRF involvement and distant lymph-nodes sites. Moreover the new generation multidetector row CT scanner permits to increase the spatial resolution, offering high detailed images combined with short acquisition time and avoiding or reducing possible motion artifacts. Unfortunately, for small size rectal tumor, there’s a lower contrast between the tumor and the rectal wall using CT images compared to MR images, especially if combined with use of DWIBS.
A recent survey of United Kingdom practice has revealed that less than 50% of patients were offered MR staging and up to 80% of patients who do not undergo MR staging have a CT examination[1]. The results obtained in this study may help to establish MDCT as an effective diagnostic technique in the evaluation of preoperative local staging of rectal cancer[3].
In our study we compared the diagnostic capability of MDCT images, with new generation of multi-row scanner, in the prediction of MRF involvement by rectal cancer, by evaluating native axial images and MPRs, as compared with MR images as reference standard[1,4,16,23]. In our series of patients a good diagnostic quality was achieved for both series of CT images, obtaining an accuracy of 78% for pure axial images and 88% for MPR (difference statistically significant, P = 0.02), while the sensitivity of pure axial images was 80.4% and these results arise to 88% with MPR. Previous studies reported high accuracy rates for CT[3,17,21], however, most patients in these early series had advanced disease[3,26]. In more recent reports, a less satisfactory results have been obtained, with accuracy rates ranging between 41% and 82%[3,4,16,24] in rectal cancer. Those results, probably, were related to the limited spatial collimation and insufficient reconstructions increments used in CT-protocol (i.e., thickening from 5 to 10 mm, no MPRs)[4,16,25] as well as the absence of standardized contrast agent injection protocol. Therefore, the spatial resolution of the scans was too low to make any reliable predictions on margin involvement, especially if compare with MR protocol (assumed with 3 mm thickness and with different orientation of the axial plane)[16,23,27]. In comparison with previous studies we obtained an higher PPV (90%); this result could be explained by the use of thinner slices (2 mm), increasing consequently the spatial resolution, close to MR images protocol (3 mm). The employed protocol, 2 mm thickness and 1 mm of increment, offers reliable results comparable with MR images, especially with the use of MPR.
Our findings are more similar to those of Shina[1] that found an accuracy rate in predicting the involvement of mesorectal fascia in comparison with histopathology of 96.5% and 91.2% on MPR and axial images, respectively. Multiplanar reconstructions images in addiction to axial images significantly improve the diagnostic accuracy in image interpretation (P = 0.02), even if the difference of sensitivity and specificity between axial images and MPR not reach the statistical significant (P > 0.05). In the study of Matsuoka[28] the accuracy of MDCT (4 slices, 5 mm thickness) and MRI was assessed using the histopatology as gold standard, with equal results between CT and MRI in the preoperative local staging of rectal carcinoma. In our series of patients the NPV of MDCT was 75% for axial images and 85.36% for MPRs, with specificity of 75% and 87.5% respectively.
One of the limitations of this study is represented by the use of MRI as reference standard, rather than histology, although this comparison is virtually impossible since patients with a MRF involvement are currently treated with long courses of chemoradiation therapy[4,29]. In addiction we did not perform any luminal distention on CT images and this could explaining some discrepancies of finally rectal cancer findings, between CT and MRI analysis. Another limitation of CT images is represented by the fact that in patients with small amounts of peri-rectal fat, the identification of the true extramural extension is more challenging due to smaller tissue interfaces, causing a higher rate of mistakes in assessment of involvement of the MRF. In our series, we did not considered the BMI of the patient as well as the amount of peri-rectal fat, in order to obtain a reliable data about sensitivity of CT images, in daily current clinical practice.
Moreover, as well known, CT-images do not allow accurate differentiation of different bowel layers, as compared with MRI, but the involvement of the MRF represents the main aim of rectal cancer imaging, since the MRF involvement determines the distinction between primary resectable and locally advanced tumors[1].
In conclusion, despite these limitations the CT imaging of rectal cancer patients with new generation MDCT scanner, demonstrated high sensitivity and high accuracy in assessment of MRF involvement, especially with the use of MPRs, and would become a potential one-step imaging tool. CT imaging could be useful as making decision therapy process during a whole-body staging workup, allowing accurate distant rectal staging and local involvement of the MRF in a single examination.
Treatment options in rectal cancer are total mesorectal excision (TME) or preoperative neoadjuvant radiochemotherapy in patients with locally advanced rectal cancer (LARC). One of the most important features of local rectal cancer staging is the assessment of the tumor relationship with the mesorectal fascia (MRF), which defines the circumferential resection margin (CRM) in TME surgery. To date MR imaging investigation is used for local staging and to identifying patients who may benefit from preoperative chemotherapy-radiation therapy (patients in which the MRF and the CRM could be involved by the tumor). However not all patients can undergo MRI because of claustrophobia or the presence of metal in patients’ bodies; moreover MRI has the downside of limited availability, relatively long image acquisition time and high cost. Another important factor in the preoperative assessment of primary rectal cancer is the frequent presence of distant disease at the time of diagnosis, which are assessed, routinely, with CT. For these reasons, the use of MDCT for local staging and distant metastases evaluation could offer high detailed images combined with low cost and short acquisition time.
New generation of high row number MDCT scans allow thin-collimation, high spatial resolution and better multiplanar reconstructions (MPRs). MPR images can be aligned parallel or perpendicular to the axis of the tumor similar to MR imaging and can be useful for predicting tumor infiltration of the MRF in local staging of rectal cancer. Therefore MDCT can assess in a single examination, the entire abdomen, pelvis and chest, allowing for local staging and distant metastases evaluation.
Considering the variability among the results in previous studies, the actual evidence suggests that old CT protocol, having a limited spatial collimation, an insufficient reconstructions increments and poor MPRs, could not be used for local staging in rectal cancer. New generation MDCT scanner used in modern clinical practice, with high sensitivity and high accuracy in assessment of MRF involvement, would become a potential one-step imaging tool for distant rectal cancer staging and local involvement of the MRF.
The importance of this work relies on the possibility to offer, in a single step examination, a new diagnostic approach (performed with new generation MDCT, ) that allows the non-invasive evaluation of MRF involvement in local rectal staging, as well as the assessment of distant metastases using high detailed images of the entire abdomen, pelvis and chest. Moreover in this manuscript the authors compared and commented our results with those of previous literature on this field by using the two different techniques modalities (i.e., CT and MRI).
TME is a surgical technique that entails en bloc resection of the primary tumor and the mesorectum by means of dissection along the mesorectal fascial plane or the CRM. MDCT are new generation of CT with high number of detector, which allow to obtain high spatial resolution images with thinner collimation. MPR is multiplanar reconstructions of the images are images obtained after a post-processing of native axial CT images. Thanks to high collimation of MDCT, all pure axial images can be orientated along different planes (i.e., coronal, sagittal, and oblique axis). MRF is mesorectal fascia, surrounds the mesorectal fat around the rectum. The mesorectal fascia runs along the anterior aspect of the sacrum, where it fuses with the presacral fascia, and then laterally on either side of the rectum. Anteriorly in males, it forms a dense band of connective tissue posterior to the seminal vesicle and prostate gland (the Denonvilliers fascia). The MRF is critical for surgical planning in TME. On T2-weighted images appears as a thin hypointense line surrounding the meserectal fat. On CT images is depicted as a thin line surrounding the mesorectal fat with similar density to the muscles.
Congratulations for the article. Often in daily clinic are situations where you can not perform an MRI either clinical or resource problems. Having information like that concludes this article endorse the decisions of physicians to such situations and allow proper staging of patients.
P- Reviewer: Palacios-Eito A, Razek AA S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Sinha R, Verma R, Rajesh A, Richards CJ. Diagnostic value of multidetector row CT in rectal cancer staging: comparison of multiplanar and axial images with histopathology. Clin Radiol. 2006;61:924-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 2. | Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;2:996-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1474] [Cited by in RCA: 1365] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 3. | Kulinna C, Eibel R, Matzek W, Bonel H, Aust D, Strauss T, Reiser M, Scheidler J. Staging of rectal cancer: diagnostic potential of multiplanar reconstructions with MDCT. AJR Am J Roentgenol. 2004;183:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Vliegen R, Dresen R, Beets G, Daniels-Gooszen A, Kessels A, van Engelshoven J, Beets-Tan R. The accuracy of Multi-detector row CT for the assessment of tumor invasion of the mesorectal fascia in primary rectal cancer. Abdom Imaging. 2008;33:604-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Quirke P, Dixon MF. The prediction of local recurrence in rectal adenocarcinoma by histopathological examination. Int J Colorectal Dis. 1988;3:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 245] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 552] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 7. | Blomqvist L, Holm T, Nyrén S, Svanström R, Ulvskog Y, Iselius L. MR imaging and computed tomography in patients with rectal tumours clinically judged as locally advanced. Clin Radiol. 2002;57:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Beets-Tan RG, Beets GL, Borstlap AC, Oei TK, Teune TM, von Meyenfeldt MF, van Engelshoven JM. Preoperative assessment of local tumor extent in advanced rectal cancer: CT or high-resolution MRI? Abdom Imaging. 2000;25:533-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Hadfield MB, Nicholson AA, MacDonald AW, Farouk R, Lee PW, Duthie GS, Monson JR. Preoperative staging of rectal carcinoma by magnetic resonance imaging with a pelvic phased-array coil. Br J Surg. 1997;84:529-531. [PubMed] |
| 10. | Heriot AG, Grundy A, Kumar D. Preoperative staging of rectal carcinoma. Br J Surg. 1999;86:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 89] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Goldman S, Arvidsson H, Norming U, Lagerstedt U, Magnusson I, Frisell J. Transrectal ultrasound and computed tomography in preoperative staging of lower rectal adenocarcinoma. Gastrointest Radiol. 1991;16:259-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Heo SH, Kim JW, Shin SS, Jeong YY, Kang HK. Multimodal imaging evaluation in staging of rectal cancer. World J Gastroenterol. 2014;20:4244-4255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (2)] |
| 13. | Dewhurst C, Rosen MP, Blake MA, Baker ME, Cash BD, Fidler JL, Greene FL, Hindman NM, Jones B, Katz DS. ACR Appropriateness Criteria pretreatment staging of colorectal cancer. J Am Coll Radiol. 2012;9:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Bipat S, Glas AS, Slors FJ, Zwinderman AH, Bossuyt PM, Stoker J. Rectal cancer: local staging and assessment of lymph node involvement with endoluminal US, CT, and MR imaging--a meta-analysis. Radiology. 2004;232:773-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 727] [Cited by in RCA: 721] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 15. | Samee A, Selvasekar CR. Current trends in staging rectal cancer. World J Gastroenterol. 2011;17:828-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Wolberink SV, Beets-Tan RG, de Haas-Kock DF, van de Jagt EJ, Span MM, Wiggers T. Multislice CT as a primary screening tool for the prediction of an involved mesorectal fascia and distant metastases in primary rectal cancer: a multicenter study. Dis Colon Rectum. 2009;52:928-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Ahmetoğlu A, Cansu A, Baki D, Kul S, Cobanoğlu U, Alhan E, Ozdemir F. MDCT with multiplanar reconstruction in the preoperative local staging of rectal tumor. Abdom Imaging. 2011;36:31-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2013;23:2522-2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 19. | van de Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, Beets-Tan RG, van den Broek CB, Brown G, Van Cutsem E. EURECCA colorectal: multidisciplinary management: European consensus conference colon & amp; rectum. Eur J Cancer. 2014;50:1.e1-1.e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 307] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 20. | Raman SP, Chen Y, Fishman EK. Evolution of imaging in rectal cancer: multimodality imaging with MDCT, MRI, and PET. J Gastrointest Oncol. 2015;6:172-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 21. | Dar RA, Chowdri NA, Parray FQ, Shaheen F, Wani SH, Mushtaque M. Pre-operative staging of rectal cancer using multi-detector row computed tomography with multiplanar reformations: single center experience. Indian J Cancer. 2014;51:170-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Monguzzi L, Ippolito D, Bernasconi DP, Trattenero C, Galimberti S, Sironi S. Locally advanced rectal cancer: value of ADC mapping in prediction of tumor response to radiochemotherapy. Eur J Radiol. 2013;82:234-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Wolberink SV, Beets-Tan RG, de Haas-Kock DF, Span MM, van de Jagt EJ, van de Velde CJ, Wiggers T. Conventional CT for the prediction of an involved circumferential resection margin in primary rectal cancer. Dig Dis. 2007;25:80-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Maizlin ZV, Brown JA, So G, Brown C, Phang TP, Walker ML, Kirby JM, Vora P, Tiwari P. Can CT replace MRI in preoperative assessment of the circumferential resection margin in rectal cancer? Dis Colon Rectum. 2010;53:308-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Kaur H, Choi H, You YN, Rauch GM, Jensen CT, Hou P, Chang GJ, Skibber JM, Ernst RD. MR imaging for preoperative evaluation of primary rectal cancer: practical considerations. Radiographics. 2014;32:389-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 26. | Maier A, Fuchsjäger M. Preoperative staging of rectal cancer. Eur J Radiol. 2003;47:89-97. [PubMed] |
| 27. | Taylor A, Slater A, Mapstone N, Taylor S, Halligan S. Staging rectal cancer: MRI compared to MDCT. Abdom Imaging. 2012;32:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Matsuoka H, Nakamura A, Masaki T, Sugiyama M, Takahara T, Hachiya J, Atomi Y. A prospective comparison between multidetector-row computed tomography and magnetic resonance imaging in the preoperative evaluation of rectal carcinoma. Am J Surg. 2003;185:556-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Aljebreen AM, Azzam NA, Alzubaidi AM, Alsharqawi MS, Altraiki TA, Alharbi OR, Almadi MA. The accuracy of multi-detector row computerized tomography in staging rectal cancer compared to endoscopic ultrasound. Saudi J Gastroenterol. 2013;19:108-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |