Published online Jan 14, 2016. doi: 10.3748/wjg.v22.i2.887
Peer-review started: June 1, 2015
First decision: September 29, 2015
Revised: October 20, 2015
Accepted: November 30, 2015
Article in press: December 1, 2015
Published online: January 14, 2016
Processing time: 222 Days and 19.5 Hours
Skin toxicity is a common symptom of anti-epidermal growth factor receptor (EGFR) antibody treatment and is also a predictive marker of its efficacy in colorectal cancer patients. However, severe skin disorders induced by such antibodies negatively impact on the quality of life of patients and decreases drug compliance during treatment. If we can predict the high-risk group susceptible to severe skin toxicity before treatment, we can undertake the early management of any arising skin disorders and formulate a more accurate prognosis for anti-EGFR antibody treatment. Previous studies have identified molecular markers of skin toxicity induced by anti-EGFR antibody, such as EGFR polymorphisms, the expression of inflammatory chemokines and serum levels of EGFR ligands. A clinical trial was undertaken involving the escalation of cetuximab doses, guided by the grade of skin toxicity observed, such as no or low-grade, in metastatic colorectal cancer (the EVEREST study). The dose escalation of cetuximab was confirmed by a safety profile and had the tendency to achieve a higher response rate in KRAS wild-type patients. A large, prospective randomized trial is now ongoing (EVEREST 2) and the results of this trial may contribute to personalized medicine in KRAS wild-type colorectal cancer patients.
Core tip: Skin toxicity is a well-known biomarker used in the prognosis of anti-epidermal growth factor receptor (EGFR) antibody treatment of colorectal cancer patients. Previous retrospective studies indicated a change of the polymorphism of EGFR intron-1, chemokines and ligands were predictive markers of skin toxicity induced by anti-EGFR antibody. Such biomarkers used in predicting skin toxicity will enable the earlier management of skin toxicity as well as improve patients’ quality of life; however, further validations of prospective studies are needed. For patients with no/mild skin toxicity, a clinical trial of a dose escalation strategy is under evaluation and ongoing in the form of the EVEREST 2 study.
- Citation: Kubo A, Hashimoto H, Takahashi N, Yamada Y. Biomarkers of skin toxicity induced by anti-epidermal growth factor receptor antibody treatment in colorectal cancer. World J Gastroenterol 2016; 22(2): 887-894
- URL: https://www.wjgnet.com/1007-9327/full/v22/i2/887.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i2.887
Colorectal cancer is one of the most common causes of death from cancer, in both men and women, around the world[1]. Owing to the development of diagnostic skills and chemotherapeutic drugs, prognoses concerning colorectal cancer patients have improved in the last decade. Although patients with early-stage colorectal cancer can undergo curative resection by endoscopy or surgery to achieve long survival after treatment, the 5-year survival rate of advanced colorectal cancer patients continues to be low because of a high rate of recurrence after surgical treatment. For the treatment of patients with metastatic or recurrent colorectal cancer, a variety of agents, including anti-vascular endothelial growth factor (VEGF) antibody, anti-epithelial growth factor receptor (EGFR) antibody, regorafenib and TAS-102 have recently been approved in Japan[2-7]. Unfortunately, most patients eventually acquire resistance to these drugs, leading to poor survival times.
Cetuximab (Erbitax®, Merck Serono) and panitummab (Vectibix®, Amgen) are anti-EGFR antibodies, which were initially approved for KRAS exon 2 wild-type patients with metastatic or recurrent colorectal cancer. Recently, genomic analyses of the EGFR downstream signal pathway, such as minor KRAS (exon 3 and 4), NRAS (exon 3, 4 and 5), BRAF V600E and PIK3CA (exon 9, 20) were performed and it was found that these genomic alterations were associated with a poor prognosis in KRAS exon2 wild-type patients treated with anti-EGFR antibodies[8-10]. Retrospective analyses of several prospective trials indicated that the RAS mutation, which consists of KRAS (exon 2, 3, 4) and NRAS (exon 2, 3, 4) mutations, is a newly predictive biomarker. The BRAF V600E mutation is also considered a prognostic factor in anti-EGFR antibody treatment of patients with metastatic colorectal cancer[11-13].
Besides the genomic mutations of the EGFR downstream pathway, several studies have indicated that the grade of skin toxicity is a biomarker for predicting the efficacy of anti-EGFR antibody treatment for several cancers[14-16]. Skin toxicity is a typical side effect of anti-EGFR antibodies and causes various types of cutaneous changes, such as acneiform eruptions, dry skin and paronychia, during treatment. Although severe skin toxicity is associated with a better response to anti-EGFR antibodies, it negatively affects the quality of life (QOL) of patients and decreases drug compliance. Prophylaxis for skin toxicity, such as moisturizers, sunscreen, topical steroids, and oral doxycycline, is known to decrease the frequency of cutaneous disorders due to anti-EGFR antibodies and to improve the QOL of patients[17]. Molecular biomarkers for predicting the subgroup that will have severe skin toxicity due to anti-EGFR antibodies before treatment have been investigated, but there are no established markers for use in clinical practice.
In this review, we describe previous findings concerning the mechanism of skin toxicity in EGFR inhibition, biomarkers of skin toxicity for anti-EGFR antibodies, and treatment approaches guided by the severity of skin toxicity of anti-EGFR antibodies in colorectal cancer.
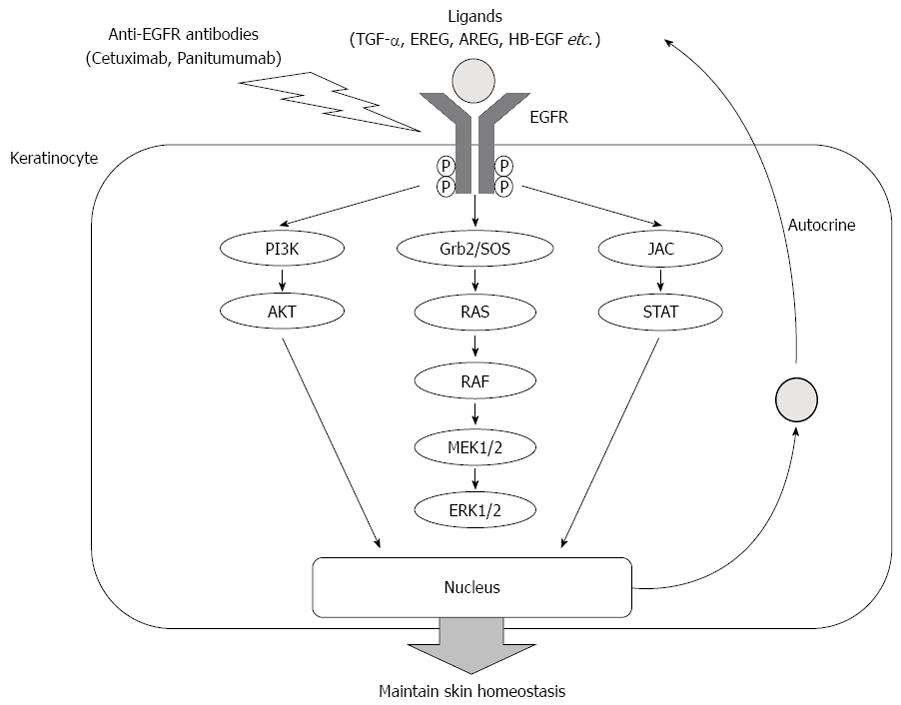
EGFR inhibition induces various symptoms of skin disorders and an acneiform rash is commonly observed on the scalp and face, particularly the cheeks, nose, nasolabial folds, chin, perioral regions, and the forehead, within the first 2-4 wk of treatment[18,19]. The EGFR is normally expressed in proliferating keratinocytes in the basal and supra-basal layers of the epidermis, outer layers of the hair follicle, sebaceous and eccrine sweat glands. It is believed that the EGFR plays a significant role in several processes of skin homeostasis, such as the regulation of cell survival, keratinocyte proliferation, differentiation and migration, wound healing and carcinogenesis[20]. Inhibition of the EGFR leads to the impairment of epidermal thickness and barrier function, and to the abnormal proliferation and differentiation of cells that express the EGFR in the skin and follicular epithelium[21,22]. Intracellular signaling pathway and mechanism of EGFR inhibition are shown in Figure 1.
According to previous reports, several ligands of EGFR as well as chemokines play significant roles in the skin inflammatory reaction caused by EGFR inhibition. Keratinocytes in cutaneous tissues are a rich source of EGFR ligands, such as transforming growth factor-alpha, epiregulin (EREG), amphiregulin (AREG) and heparin-binding EGF[23]. These ligands are known to stimulate the EGFR and maintain the cutaneous cell cycle of wound healing and EGFR-driven inflammatory reactions in keratinocytes[24]. EREG is known as an autocrine growth factor in normal human keratinocytes, and organizes the epidermal structure by regulating keratinocyte proliferation and differentiation[25]. The expression of AREG is developmentally regulated in the epithelium and mesenchyme of human skin during morphogenesis[26]. In addition, the EGFR is also activated by hepatocyte growth factor (HGF) stimulation in human epidermal keratinocytes. Transactivation of the EGFR by HGF-induced stimulation is associated with wound healing and mortality in epithelial cells[27,28]. EGFR inhibition causes an imbalance of cutaneous homeostasis, which is controlled by several ligands, and a skin inflammatory reaction induced by several chemokines.
An EGFR inhibitor decreases the activity of EGFR downstream signals and induces the expression of several chemokines, which enhance the skin’s inflammatory and immune response[29,30]. Previous reports indicated that the inhibition of EGFR induces tumor necrosis factor-alpha and interleukin-1 (IL-1) during the development of skin rash in mice[31]. These chemokines induce IL-8 secretion by fibroblasts and keratinocytes, which activate neutrophil migration in cutaneous tissues[32-34]. Bangsgaard et al[35] revealed that the neutralization of IL-8 by HuMab-10F8 prevented the skin toxicity induced by an EGFR inhibitor. In addition, EGFR blockage is known to increase CC-chemokine ligand 2 (CCL2), CCL5, and C-X-C motif chemokine 10 (CXCL10), and reduce CXCL8 expression in keratinocytes[36]. Recently, Paul et al[37] reported that increased CCL2, CCL5 and decreased IL-8 or CXCL8 expression was observed in keratinocytes treated by EGFR inhibitor. In patients treated with EGFR inhibitor, a low level of serum CXCL8, corresponding to stronger EGFR inhibition, was associated with a higher grade of skin toxicity.
Most patients treated with anti-EGFR antibodies have an increased risk of skin toxicity. If we could predict the development of skin toxicities before the initiation of anti-EGFR antibody treatment, we could manage skin toxicity early and improve the patient’s QOL. Previous reports which evaluated the predictive factors of skin toxicity of anti-EGFR antibody treatment in patients with colorectal cancer are summarized in Table 1.
| Ref. | Methods | Predictive markers | Risk factors of severe skin toxicity |
| Jatoi et al[39] (2009) | Patient's backgrounds | Age | Younger |
| Gender | Men | ||
| Graziano et al[40] (2008) | EGFR polymorphism | CA repeat in EGFR intron-1 | EGFR intron-1 S/S variant |
| Vallböhmer et al[42] (2005) | mRNA expression | Cox-2 | Low expression |
| Takahashi et al[43,44] (2014, 2015) | Serum levels of ligands | AREG, EREG, HGF | Low levels of ligands at pre-treatment |
A North Central Cancer Treatment Group N0147 trial, which was a randomized phase III trial of oxaliplatin plus 5-fluorouracil/leucovorin, with or without cetuximab, after curative resection of stage III colon cancer, investigated the risk factors for severe rash (≥ grade 3) in 933 patients[38,39]. More men (OR = 2.12, P = 0.017) and younger patients (< 70-year-old; OR = 0.21, P = 0.032) developed a severe rash compared with women and older patients.
Pharmacogenomic analyses of EGFR polymorphisms and several genomic mutations have been undertaken to determine their predictive value in the development of skin toxicity after anti-EGFR antibody treatment. A previous study indicated that a polymorphism of the EGFR intron-1 (CA single sequence repeat; short [S]/long [L] variant) was associated with the severity of skin toxicity (grade 0-1 vs 2-3) in colorectal cancer patients treated with irinotecan plus cetuximab as second-line chemotherapy[40]. EGFR intron-1 S/S carriers showed significantly more frequent grade 2-3 skin toxicity (P = 0.001) and a treatment response (P = 0.008) than EGFR intron-1 L/L carriers. The EGFR intron-1 S/S genotype was also associated with better survival in patients treated with cetuximab. Other polymorphisms of EGF and EGFR, such as EGF 61A>G, EGFR 216G>T and EGFR 497G>A were not associated with the severity of skin toxicity after cetuximab treatment in this study. On the other hand, prognostic analyses of a randomized phase II trial of first-line chemotherapy with cetuximab in the AIO CRC Study Group indicated that the LL variant of the CA repeat in the EGFR intron-1 had a significant tendency to predict the severity of skin toxicity compared with the SS variant (P = 0.07)[41]. A heterogeneity in results was apparent in terms of polymorphisms of the CA repeat in the EGFR intron-1 because these studies were relatively small-sized and the optimized cut-off values for the CA repeat were not determined in colorectal cancer. Further validation by other translational analyses in a large prospective study is required to solve the heterogeneity of biomarkers.
As well as the above, a small scale analysis was conducted that measured the mRNA expression of cyclo-oxygenase 2 (Cox-2), cyclin D1, IL-8 and VEGF, and evaluated their predictive role in skin toxicity by cetuximab in patients of a phase II open-label multicenter study (IMCL-0144). Of these genes, the low expression of Cox-2 was associated with a high grade of skin toxicity[42]. There was no association between the expression level of other genes and the severity of skin toxicity.
EGFR ligands have been considered significant modulators of cutaneous homeostasis and the inflammatory reaction in cutaneous tissues according to previous reports. As alterative markers of skin toxicity, we focused on the serum levels of ligands, which were associated with the EGFR signaling pathway. We evaluated the relationship between serum levels of EGFR ligands and the severity of skin toxicity, and the prognostic roles of these serum ligands in metastatic colorectal cancer patients who received anti-EGFR antibodies[43,44]. Our study indicated that low pre-treatment levels of serum AREG, EREG and HGF were associated with severe skin toxicity induced by anti-EGFR antibodies and a better prognosis in KRAS wild-type patients with metastatic colorectal cancer.
Molecular markers predicting skin toxicity, such as the CA repeat variant of the EGFR intron-1 and serum levels of particular ligands, are associated with the efficacy of the anti-EGFR antibody. Unfortunately, these potent findings were evaluated in small-scale studies and have not been validated as yet by other research studies; therefore the role of these biomarkers for clinical use is, at the moment, inconclusive.
Several prospective studies of colorectal cancer have described the severity and frequency of acneiform skin toxicity as a significant signature of the activity of anti-EGFR antibody, and are summarized in Table 2[4,41,45-48]. These reports on the treatment of colorectal cancer with anti-EGFR antibody indicated that severe skin toxicity was associated with a higher response to antibody and a better prognosis, compared with no or mild skin toxicity. For patients with genetic alterations in the EGFR downstream pathway such as a RAS mutation, dose escalation of cetuximab for those without a high-grade skin reaction may be inefficient because of the permanent activation of the EGFR downstream pathway by such genetic alterations. On the other hand, in patients without genetic alterations of the EGFR downstream pathway, a dose escalation of anti-EGFR antibody may be effective and improve patient survival, especially for patients with tumor tissues not saturated with anti-EGFR antibodies. Skin toxicity may be a significant signature that affects the saturation of the anti-EGFR antibody in tumor tissues.
| Ref. | Number | Treatment | Clinical trial | Objective response rate | Overall survival | |||
| Grade of skin toxicity | P value | Grade of skin toxicity (mo) | P value | HR (95%CI) | ||||
| Jonker et al[45] (2007) | 283 | Cetuximab | Phase III | - | - | 2.6 vs 4.8 vs 8.4 (grade 0 vs grade 1 vs≥ grade 2) | < 0.001 | - |
| Van Cutsem et al[46] (2007) | 200 | Panitumumab + BSC | Phase III | 14% vs 86% (grade 1 vs grade 2-3) | - | ND | - | 0.59 (0.42-0.85) |
| Cunningham et al[4] (2004) | 218 | IRI + cetuximab | Phase III | 6.3% vs 25.8% (grade 0 vs any grade) | 0.005 | 3.0 vs 9.1 (grade 0 vs any grade) | - | - |
| 111 | Cetuximab | 0% vs 13.0% (grade 0 vs any grade) | 2.5 vs 8.1 (grade 0 vs any grade) | - | - | |||
| Sobrero et al[47] (2008) | 648 | IRI + cetuximab (2nd-line) | Phase III | - | - | 5.8 vs 11.7 vs 15.6 (grade 0 vs grade 1-2 vs≥ grade 3) | - | - |
| Bokemeyer et al[48] (2009) | 169 | FOLFOX + cetuximab (1st-line) | Phase II | 13.0 vs 43.2% vs 53.2% vs 66.7% (grade 0 vs 1 vs 2 vs 3) | - | - | - | - |
| Stintzing et al[41] (2013) | 149 | CAPIRI/CapOX + cetuximab (1st-line) | Phase II | 41% vs 62% (grade 0-1 vs grade 2-3) | 0.021 | 18.0 vs 30.3 (grade 0-1 vs grade 2-3) | 0.161 | 0.75 (0.50-1.12) |
Two prospective studies have evaluated the dose escalation of anti-EGFR antibodies in colorectal cancer[49,50]. Fora et al[49] reported on a phase II trial of high-dose cetuximab (500 mg/m2) plus irinotecan in KRAS wild-type patients after progression of standard-dose cetuximab plus irinotecan. This study was small-scale, but nine out of twenty patients who received high-dose cetuximab plus irinotecan achieved disease control lasting more than 12 wk; the toxicity profile was also tolerable, except for grade 3/4 hypomagnesemia (25%). The efficacy of dose escalation of cetuximab after progression of a standard dose of cetuximab was limited and further selection of patients, such as those with low-grade skin toxicity or a response to a previous standard dose of cetuximab, may have been necessary in this study.
The EVEREST study was a prospective randomized study to evaluate the efficacy of dose escalation of cetuximab, compared with the 250 mg/m2 per week standard regimen, in patients who had developed no or a mild skin reaction in the first three weeks[50]. This study revealed that a dose escalation of cetuximab up to 500 mg/m2 achieved a safety profile comparable to a standard dose of cetuximab (250 mg/m2). Among KRAS mutant-type patients, there was no efficacy such as an increased objective response rate (ORR) and overall survival (OS) by the dose escalation of cetuximab. Among KRAS wild-type patients, the results of an ORR, for this subgroup, of a dose escalation of cetuximab were better than that for the subgroup with a standard dose of cetuximab (ORR: 43% vs 30%). However, the OS was similar between the two subgroups. The reasons for these results were considered, such as the small sample size of each group and the effects of negative genomic biomarkers such as NRAS, BRAF and other mutations. Data was not presented in this study on the difference in the change of severity of skin toxicity after dose escalation of cetuximab in KRAS wild-type and mutant-type patients.
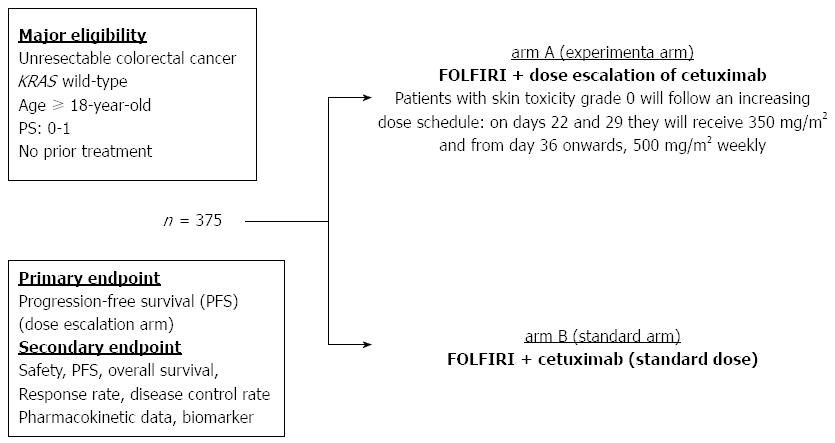
Another EVEREST study has not drawn a conclusion on the efficacy of a dose escalation strategy for anti-EGFR antibody in a large-scale phase II trial (EVEREST 2), which was designed to compare the efficacy of dose escalation of a cetuximab plus 5-fluorouracil/leucovorin/irinotecan (FOLFIRI) regimen, as first-line chemotherapy, with a standard dose of FOLFIRI plus cetuximab in KRAS wild-type patients with metastatic colorectal cancer; this study is ongoing (NCT01251536) and the schema of this trial is shown in Figure 2. If such a dose escalation of anti-EGFR antibody achieves favorable results, a therapeutic decision on anti-EGFR antibody treatment for RAS wild-type patients with a low-grade skin toxicity may change dramatically and contribute to personalized treatment for this target population in future.
Although several studies have investigated the predictive markers of skin toxicity induced by anti-EGFR antibody in colorectal cancer patients, potent markers such as polymorphisms of EREG intron1, the expression of Cox-2 and serum levels of several ligands, such as HGF, EREG and AREG, have already been identified. Further validation of these biomarkers by other, larger studies, and translational research of novel markers to predict the skin toxicity of anti-EGFR antibody, are presently required.
P- Reviewer: Ju JF S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25546] [Article Influence: 1824.7] [Reference Citation Analysis (7)] |
| 2. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7735] [Article Influence: 368.3] [Reference Citation Analysis (1)] |
| 3. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2302] [Cited by in RCA: 2272] [Article Influence: 133.6] [Reference Citation Analysis (0)] |
| 4. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3767] [Cited by in RCA: 3708] [Article Influence: 176.6] [Reference Citation Analysis (1)] |
| 5. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1394] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 6. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2194] [Cited by in RCA: 2120] [Article Influence: 176.7] [Reference Citation Analysis (0)] |
| 7. | Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero J, Komatsu Y. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 1011] [Article Influence: 101.1] [Reference Citation Analysis (0)] |
| 8. | De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, Kalogeras KT, Kotoula V, Papamichael D, Laurent-Puig P. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1491] [Cited by in RCA: 1656] [Article Influence: 110.4] [Reference Citation Analysis (1)] |
| 9. | Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705-5712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1242] [Article Influence: 73.1] [Reference Citation Analysis (0)] |
| 10. | Loupakis F, Ruzzo A, Cremolini C, Vincenzi B, Salvatore L, Santini D, Masi G, Stasi I, Canestrari E, Rulli E. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101:715-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 448] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 11. | Van Cutsem E, Lenz HJ, Köhne CH, Heinemann V, Tejpar S, Melezínek I, Beier F, Stroh C, Rougier P, van Krieken JH. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. 2015;33:692-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 632] [Article Influence: 63.2] [Reference Citation Analysis (0)] |
| 12. | Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1610] [Cited by in RCA: 1733] [Article Influence: 144.4] [Reference Citation Analysis (0)] |
| 13. | Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011-2019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1314] [Cited by in RCA: 1452] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 14. | Bonner JA, Harari PM, Giralt J, Cohen RB, Jones CU, Sur RK, Raben D, Baselga J, Spencer SA, Zhu J. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010;11:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1453] [Cited by in RCA: 1380] [Article Influence: 92.0] [Reference Citation Analysis (1)] |
| 15. | Gatzemeier U, von Pawel J, Vynnychenko I, Zatloukal P, de Marinis F, Eberhardt WE, Paz-Ares L, Schumacher KM, Goddemeier T, O’Byrne KJ. First-cycle rash and survival in patients with advanced non-small-cell lung cancer receiving cetuximab in combination with first-line chemotherapy: a subgroup analysis of data from the FLEX phase 3 study. Lancet Oncol. 2011;12:30-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Xiong HQ, Rosenberg A, LoBuglio A, Schmidt W, Wolff RA, Deutsch J, Needle M, Abbruzzese JL. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II Trial. J Clin Oncol. 2004;22:2610-2616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 337] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 17. | Lacouture ME, Mitchell EP, Piperdi B, Pillai MV, Shearer H, Iannotti N, Xu F, Yassine M. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-Emptive Skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:1351-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 340] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 18. | Pérez-Soler R, Delord JP, Halpern A, Kelly K, Krueger J, Sureda BM, von Pawel J, Temel J, Siena S, Soulières D. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. Oncologist. 2005;10:345-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 189] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Nanney LB, Stoscheck CM, King LE, Underwood RA, Holbrook KA. Immunolocalization of epidermal growth factor receptors in normal developing human skin. J Invest Dermatol. 1990;94:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 136] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Jost M, Kari C, Rodeck U. The EGF receptor - an essential regulator of multiple epidermal functions. Eur J Dermatol. 2000;10:505-510. [PubMed] |
| 21. | Galimont-Collen AF, Vos LE, Lavrijsen AP, Ouwerkerk J, Gelderblom H. Classification and management of skin, hair, nail and mucosal side-effects of epidermal growth factor receptor (EGFR) inhibitors. Eur J Cancer. 2007;43:845-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Lacouture ME. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat Rev Cancer. 2006;6:803-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 551] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 23. | Rittié L, Varani J, Kang S, Voorhees JJ, Fisher GJ. Retinoid-induced epidermal hyperplasia is mediated by epidermal growth factor receptor activation via specific induction of its ligands heparin-binding EGF and amphiregulin in human skin in vivo. J Invest Dermatol. 2006;126:732-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 24. | Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. J Invest Dermatol. 2008;128:1365-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 347] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 25. | Morita S, Shirakata Y, Shiraishi A, Kadota Y, Hashimoto K, Higashiyama S, Ohashi Y. Human corneal epithelial cell proliferation by epiregulin and its cross-induction by other EGF family members. Mol Vis. 2007;13:2119-2128. [PubMed] |
| 26. | Piepkorn M, Underwood RA, Henneman C, Smith LT. Expression of amphiregulin is regulated in cultured human keratinocytes and in developing fetal skin. J Invest Dermatol. 1995;105:802-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Spix JK, Chay EY, Block ER, Klarlund JK. Hepatocyte growth factor induces epithelial cell motility through transactivation of the epidermal growth factor receptor. Exp Cell Res. 2007;313:3319-3325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Xu KP, Yu FS. Cross talk between c-Met and epidermal growth factor receptor during retinal pigment epithelial wound healing. Invest Ophthalmol Vis Sci. 2007;48:2242-2248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Woodworth CD, Michael E, Marker D, Allen S, Smith L, Nees M. Inhibition of the epidermal growth factor receptor increases expression of genes that stimulate inflammation, apoptosis, and cell attachment. Mol Cancer Ther. 2005;4:650-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Pastore S, Mascia F, Mariotti F, Dattilo C, Mariani V, Girolomoni G. ERK1/2 regulates epidermal chemokine expression and skin inflammation. J Immunol. 2005;174:5047-5056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | Surguladze D, Deevi D, Claros N, Corcoran E, Wang S, Plym MJ, Wu Y, Doody J, Mauro DJ, Witte L. Tumor necrosis factor-alpha and interleukin-1 antagonists alleviate inflammatory skin changes associated with epidermal growth factor receptor antibody therapy in mice. Cancer Res. 2009;69:5643-5647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Han SS, Lee M, Park GH, Bang SH, Kang YK, Kim TW, Lee JL, Chang HM, Ryu MH. Investigation of papulopustular eruptions caused by cetuximab treatment shows altered differentiation markers and increases in inflammatory cytokines. Br J Dermatol. 2010;162:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 33. | Larsen CG, Anderson AO, Oppenheim JJ, Matsushima K. Production of interleukin-8 by human dermal fibroblasts and keratinocytes in response to interleukin-1 or tumour necrosis factor. Immunology. 1989;68:31-36. [PubMed] |
| 34. | Hoffmann TK, Schirlau K, Sonkoly E, Brandau S, Lang S, Pivarcsi A, Balz V, Müller A, Homey B, Boelke E. A novel mechanism for anti-EGFR antibody action involves chemokine-mediated leukocyte infiltration. Int J Cancer. 2009;124:2589-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Bangsgaard N, Houtkamp M, Schuurhuis DH, Parren PW, Baadsgaard O, Niessen HW, Skov L. Neutralization of IL-8 prevents the induction of dermatologic adverse events associated with the inhibition of epidermal growth factor receptor. PLoS One. 2012;7:e39706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 36. | Mascia F, Mariani V, Girolomoni G, Pastore S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 213] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 37. | Paul T, Schumann C, Rüdiger S, Boeck S, Heinemann V, Kächele V, Steffens M, Scholl C, Hichert V, Seufferlein T. Cytokine regulation by epidermal growth factor receptor inhibitors and epidermal growth factor receptor inhibitor associated skin toxicity in cancer patients. Eur J Cancer. 2014;50:1855-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 38. | Alberts SR, Sargent DJ, Nair S, Mahoney MR, Mooney M, Thibodeau SN, Smyrk TC, Sinicrope FA, Chan E, Gill S. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 359] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 39. | Jatoi A, Green EM, Rowland KM, Sargent DJ, Alberts SR. Clinical predictors of severe cetuximab-induced rash: observations from 933 patients enrolled in north central cancer treatment group study N0147. Oncology. 2009;77:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 40. | Graziano F, Ruzzo A, Loupakis F, Canestrari E, Santini D, Catalano V, Bisonni R, Torresi U, Floriani I, Schiavon G. Pharmacogenetic profiling for cetuximab plus irinotecan therapy in patients with refractory advanced colorectal cancer. J Clin Oncol. 2008;26:1427-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 41. | Stintzing S, Kapaun C, Laubender RP, Jung A, Neumann J, Modest DP, Giessen C, Moosmann N, Wollenberg A, Kirchner T. Prognostic value of cetuximab-related skin toxicity in metastatic colorectal cancer patients and its correlation with parameters of the epidermal growth factor receptor signal transduction pathway: results from a randomized trial of the GERMAN AIO CRC Study Group. Int J Cancer. 2013;132:236-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Vallböhmer D, Zhang W, Gordon M, Yang DY, Yun J, Press OA, Rhodes KE, Sherrod AE, Iqbal S, Danenberg KD. Molecular determinants of cetuximab efficacy. J Clin Oncol. 2005;23:3536-3544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 43. | Takahashi N, Yamada Y, Furuta K, Nagashima K, Kubo A, Sasaki Y, Shoji H, Honma Y, Iwasa S, Okita N. Association between serum ligands and the skin toxicity of anti-epidermal growth factor receptor antibody in metastatic colorectal cancer. Cancer Sci. 2015;106:604-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Takahashi N, Yamada Y, Furuta K, Honma Y, Iwasa S, Takashima A, Kato K, Hamaguchi T, Shimada Y. Serum levels of hepatocyte growth factor and epiregulin are associated with the prognosis on anti-EGFR antibody treatment in KRAS wild-type metastatic colorectal cancer. Br J Cancer. 2014;110:2716-2727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Jonker DJ, O’Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040-2048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1489] [Article Influence: 82.7] [Reference Citation Analysis (1)] |
| 46. | Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1473] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 47. | Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J. EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol. 2008;26:2311-2319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 711] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 48. | Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1241] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 49. | Fora AA, McMahon JA, Wilding G, Groman A, Ma WW, Romano KS, Fakih MG. A phase II study of high-dose cetuximab plus irinotecan in colorectal cancer patients with KRAS wild-type tumors who progressed after standard dose of cetuximab plus irinotecan. Oncology. 2013;84:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 50. | Van Cutsem E, Tejpar S, Vanbeckevoort D, Peeters M, Humblet Y, Gelderblom H, Vermorken JB, Viret F, Glimelius B, Gallerani E. Intrapatient cetuximab dose escalation in metastatic colorectal cancer according to the grade of early skin reactions: the randomized EVEREST study. J Clin Oncol. 2012;30:2861-2868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 7.7] [Reference Citation Analysis (0)] |