Published online Jan 14, 2016. doi: 10.3748/wjg.v22.i2.641
Peer-review started: April 15, 2015
First decision: May 18, 2015
Revised: August 4, 2015
Accepted: October 12, 2015
Article in press: October 13, 2015
Published online: January 14, 2016
Processing time: 268 Days and 15.3 Hours
Over the past several years, the severity of Helicobacter pylori (H. pylori) infections has not significantly diminished. After successful eradication, the annual H. pylori recurrence rate is approximately 13% due to oral H. pylori infection. Established clinical diagnostic techniques do not identify an oral etiologic basis of H. pylori prior to gastric infection. There has been disagreement as to whether oral infection of H. pylori exists or not, with no definite conclusion. In medical practice, negative results with the urea breath test suggest that the stomach infection of H. pylori is cured in these patients. In fact, patients can present negative urea breath test results and yet exhibit H. pylori infection due to oral infection. The present paper provides evidence that H. pylori oral infection is nonetheless present, and the oral cavity represents a secondary site for H. pylori colonization.
Core tip: Recent studies designed to test the role of the oral cavity as a significant reservoir for Helicobacter pylori (H. pylori) and that used more appropriate methodologies have produced contrasting facts with respect to the existence of oral H. pylori. In this article, the author presents evidence supporting the oral cavity as a second colonized site for H. pylori, besides primarily residing in the stomach, which plays a significant role in H. pylori diagnosis, transmission, and treatment. Additionally, this article introduces new technology for the diagnosis, cell culture, and treatment of oral H. pylori.
- Citation: Yee JK. Helicobacter pylori colonization of the oral cavity: A milestone discovery. World J Gastroenterol 2016; 22(2): 641-648
- URL: https://www.wjgnet.com/1007-9327/full/v22/i2/641.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i2.641
Over the past twenty years, there has been disagreement as to whether oral infection by Helicobacter pylori (H. pylori) exists or not - with no definite conclusion. It was proposed that no living H. pylori exists in the oral cavity, and that the positive results detected by PCR from oral samples indicate the presence of H. pylori fragments, rather than living bacteria, or are due to reflux from the stomach. H. pylori could not be cultivated from PCR-positive samples. The H. pylori coming from stomach reflux was thought only to survive in the oral cavity for a few hours because of the high oxygen concentration.
If the above proposed idea is correct, then the fragment or dead H. pylori should not have any negative effect on the drug eradication of H. pylori infections of the stomach[1,2]. However, H. pylori has been found in the oral cavity in urea breath test (UBT)-negative patients who had no reflux of H. pylori from the stomach. Also, in a clinical trial, there was a close relationship between oral and stomach H. pylori infections. Obviously, if living H. pylori exists in the oral cavity, and is present either before or after the stomach drug treatment, it raises significant issues regarding the treatment protocols[3].
The aim of this review article was to list all evidence that contradicts the proposed idea, and thus indicates living H. pylori does exist in the oral cavity. Why do we have so many disparate views on the facts regarding whether oral H. pylori exists? Because we lack a technology to easily detect oral H. pylori. Therefore, the author introduces a new technology for the diagnosis and treatment of oral H. pylori infection.
There are a number of studies using PCR as the indicated research tool; PCR is a sensitive and reliable test for detecting oral H. pylori. Wang et al[3] report a clinical trial that included a total of 159 symptomatic individuals with stomach pain and 118 asymptomatic individuals with no stomach complaints; patients were recruited and tested using the saliva H. pylori antigen test (HPS), the H. pylori flagella test (HPF), the UBT, and the PCR test, which were also confirmed by saliva culture. It was found that the H. pylori antigen exists in the oral cavity in UBT-negative individuals. In the absence of stomach infection, patients may still have the H. pylori antigen in the mouth.
The study on clinical efficacy of H. pylori detection using PCR treatment outcomes of the clarithromycin-based genotypic resistance test that show real-time PCR is efficacious for H. pylori detection[4]. Also, a nested PCR assay is at least as sensitive as histology, and may be useful for H. pylori detection in the oral cavity of patients compared with endoscopic examination[5]. Real-time PCR in the sub-gingival plaque of chronic periodontitis patients indicated H. pylori may be present[6]. Furthermore, PCRs have shown their usefulness in examining the potential virulence of coccoid forms of H. pylori[6].
All of the above studies show that PCR is a sensitive and reliable test that can detect living H. pylori.
The oral cavity has two parts: hypoxic and non-hypoxic. Gingivitis is often caused by six bacteria (Prevotella intermedia, Porphyromonas gingivalis, Fusobacterium nucleatum, Actinobacillus actinomycetemcomitans, Tannerella forsythia, and Treponema denticola) that are hypoxic and can grow in the mouth. Why, then, would H. pylori not be able to grow? The subgingival plaque of the oral cavity has microaerophilic environments favorable for the growth of this bacterium, and H. pylori was detected in the supragingival plaque of individuals with H. pylori gastric diseases by a rapid urease test and real-time PCR analysis[7]. There, the same strain of H. pylori in plaque and gastric mucosa was observed. There is a highly significant association between periodontal disease and colonization of H. pylori in dental plaque. Periodontal disease and H. pylori infection were prevalent in more than 50% of the population. There was also a positive correlation between periodontal disease and H. pylori. based on seropositivity and rapid urease test-positivity in a community of Indians[8]. The study showed a positive association between H. pylori and oral lesions, such as ulcerative/inflammatory lesions, squamous cell carcinoma, and primary lymphoma[9]. Román-Román et al[10] simultaneously detected H. pylori in saliva and in gastric biopsies and found the same vacA genotypes in both sample types from the same patient. They suggested that saliva could be the transmitting and reinfecting vector for stomach H. pylori infection. H. pylori has recently been detected in the oral cavity and oropharynx[11]. In this study, authors focused on real-time PCR analysis of cagA and vacA genes of H. pylori strains in tonsils and tonsillar squamous cell cancer and compared them with H. pylori strains obtained from the gastric mucosa of the same patients. Their findings of oral presence of H. pylori without concurrent stomach infection was confirmed using UBT. The results showed that more than one H. pylori strain can be present in the oropharynx and stomach in the same patient. Although H. pylori DNA was verifiable by PCR in several plaque and root canal samples, bacterial colonies could only be grown from root canals, but not from plaque. These colonies were unequivocally identified as H. pylori by microscopic, genetic, and biochemical approaches. The root canals of endodontic-infected teeth may be a reservoir for live H. pylori that could serve as a potential source for transmission[12,13]. When H. pylori infection was studied in children, a positive association between the presence of H. pylori and oral hygiene was found together with the periodontal status[14,15]. H. pylori was detected in subgingival dental plaques. A review article with twenty-three studies and including 1861 patients showed that the prevalence of gastric and dental plaque H. pylori coinfection was 49.7% (95%CI: 16.0%-83.4%), and the percent of agreement between the dental plaque, H. pylori status and the gastric H. pylori was estimated at 82%[16]. However, there is not enough evidence for the efficacy of dental treatment on prevention of recurrent gastric H. pylori infection.
H. pylori has been reported to be present in 0-40% of the cases with head and neck cancer. A higher percentage has been identified in laryngeal and pharangeal cancer. The results of another study suggest a possible association between H. pylori and increased risk of oral cancer[17]. H. pylori may be present in the subgingival plaque samples of patients with chronic periodontitis, showing positive coinfection with chronic periodontitis[18,19].
There are a number of studies that show when patients received drug treatment for stomach H. pylori, the drug did not eliminate oral H. pylori. Also, a study shows that mouth-rinse treatment alone or combined with periodontal treatment can, to some extent, reduce the prevalence of oral H. pylori and improve the eradication rate of gastric H. pylori[3,20]. The patients who had received therapy were again H. pylori-positive while they were still carrying H. pylori in dental plaques, which showed that successful eradication of gastric H. pylori does not guarantee prevention of reinfection. A new strategy that indicates concomitant eradication in oral and gastric colonization can result in clearance of H. pylori infection[21].
A review article indicated that recent studies have not only shown that the microorganism can be detected fairly consistently from the oral cavity, but also that the chances of recurrence of H. pylori infection are higher among patients who harbor the organism in the oral cavity. Furthermore, initial results from clinical trials have shown that H. pylori-positive dyspeptic patients may benefit from periodontal therapy[22].
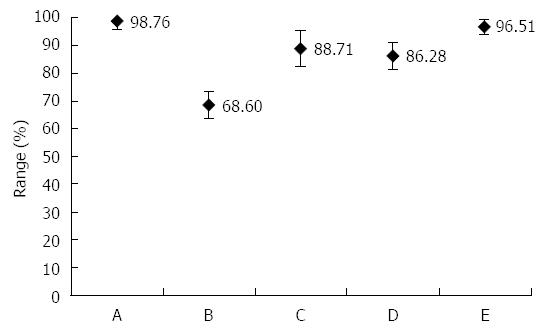
In the PCR-positive saliva sample, can H. pylori be confirmed by culture? The answer is yes! One study showed that H. pylori from a saliva sample can be cultured in individuals with all positive test results (HPS and HPF) of the oral cavity[3]. Based on the culture, they calculated the sensitivity, specificity, accuracy, and positive- and negative-predictive values of HPS. For the results of the comparison study, see Figure 1.
One of the views against oral H. pylori exists because oral and stomach H. pylori have different genotypes. One study showed that more than one H. pylori strain can be present in the oropharynx and stomach in the same patient[11]. The oropharyngeal infection seems to be independent of the gastric infection. However, there are a number of studies showing that oral and stomach H. pylori have the same genotype. Regarding a high similarity in genotype of H. pylori isolates from saliva, stomach, and stool, one study supports the idea that fecal - oral is the main route of H. pylori transmission, and the oral cavity may serve as a reservoir for H. pylori; however, remarkable genotype diversity among stomach, saliva, and stool samples showed that more than one H. pylori genotype may exist in the same patient[23]. The vacA genotypes have been detected in oral cavities from patients without dyspepsia[24]. The presence of H. pylori in the oral cavity was more frequent in seropositive subjects without dyspepsia symptoms, and could represent the source of gastric infection and bacterial transmission. The data suggest that more than one H. pylori strain may exist in the mouth of asymptomatic persons.
Occurrence of the same strain of H. pylori simultaneously in plaque and gastric mucosa has also been observed[7]. A positive correlation was obtained between the collected indices and quantity of H. pylori colonization.
The author had communicated with the Nobel Laureate, Dr. Robin Warren, regarding oral H. pylori. He indicated in this letter, “We have never managed to culture H. pylori from food, water, or the mouth, at least not while I was involved with the work. I think you should be very careful talking about ‘live antigens’ and “oral H. pylori” in the absence of a definite culture. If you have antigens in saliva or plaque, you should state exactly that and state the method used to demonstrate the antigens. With today’s highly sensitive immune and PCR tests, I could well believe your suggestion of oral antigens from gastric reflux, but with a negative and correctly done breath test, I would usually expect the stomach to be clear of H. pylori, in which case reflux would not put those antigens in the mouth. How long antigens could stay in the mouth after treatment of H. pylori infection would be a matter for another study. I would suggest you use a series of patients for endoscopy, as we did, and fully examine the mucosa for H. pylori and the saliva and plaque for H pylori, however you do it. Then repeat the process after treatment. However, talking about gastric reflux in the absence of proven gastric H. pylori is dangerous. We did find rare cases, usually with very low numbers of bacteria in the stomach, that gave false-negative breath tests, but you seem to have quite a few possible false positives. I think they would need to be fully investigated with endoscopy, CLOtest, culture, and histology before making too many comments on them in a printed paper. You need to be very careful to get the full facts correct and avoid incorrect suggestive statements.”
If a patient suffers from H. pylori infection, there is good reason to suspect that there are antigens in the mouth due to stomach reflux. But, with a UBT-negative patient, we still detected antigens in the oral cavity, and not only in a few patients; instead, we observed it in a large number of patients. Further, the prevalence of H. pylori infection of the oral cavity in gastric H. pylori-positive patients compared with gastric H. pylori-negative patients is significantly different (80% vs 23%).
Now, why are there antigens in the mouth? Someone may say it comes from food contamination. However, we found live antigens inside dental plaques. The dental plaque can be defined as a complex microbial community, with greater than 1010 bacteria per milligram. It has been estimated that as many as 400 distinct bacterial species may be found in a plaque. Inorganic components are also found in dental plaques, which are so-called as biofilm. Thus, food contamination may not be a good explanation for the presence of oral H. pylori antigens.
Regardless of whether the source of antigens in the oral cavity is from stomach or food, as long as the bacteria live in the mouth, it will be a key issue. Does colonization of H. pylori exist in the oral cavity? If we assume it does, then we have several issues to follow upon: (1) H. pylori recurrence rate is high in Asia due to oral H. pylori; (2) drug treatments are not effective on oral H. pylori due to dental plaque structure; and (3) the eradication rate is getting lower with each treatment.
To date, the exact mode and route of transmission of the microorganism is still unknown. The successful detection of H. pylori DNA from dental plaque and saliva in our lab draws attention to the possible importance of oral - oral transmission. Our study showed that systemic therapy failed to clear H. pylori from dental plaque despite its clearance from the stomach[3]. This evidence suggests that the oral cavity may be another niche for H. pylori and may be the source infection/reinfection.
Why would the traditional treatment of gastric infection be ineffective against oral infection? It is reasonable to hypothesize that H. pylori survives in moderate-to-advanced dental biofilms because the architecture and the microcosm of these periodontal conditions promote a viable habitat for microaerophilic and anaerobic microorganisms. Because dental biofilms can provide urea, urease-producing bacteria such as H. pylori may have improved viability in this periodontal environment, as antibiotics have difficulty penetrating the bacterial biofilm structure. The microbial ecology of the oral cavity is highly complex, richly diverse, and not yet well understood. Poor periodontal health may be associated with H. pylori infections in the oral cavity and with poverty status. This is why the efficacy of drug treatment on gastric H. pylori is lower than has been reported when good patient compliance is achieved to (60% vs 80%-90%).
The progressive loss of efficacy of standard eradication therapies has made the treatment of H. pylori more challenging than ever. Endoscopic-guided antibiotic susceptibility testing had previously been suggested to guide treatment after failure of second-line therapies. However, its role has expanded over the years, in accordance with the current Maastricht Guidelines. Several authors have dealt with this topic, developing both efficacy trials and cost-effectiveness trials against resistant H. pylori infections as well as infections in naïve patients. However, results are not homogeneous enough to provide definite advice, because antibiotic resistance is not the only reason for treatment failure. Moreover, the culture-guided approach is fraught with many practical issues, such as the availability of both endoscopy units and microbiology laboratories, and the need for a standard of quality that cannot be satisfied everywhere. Finally, pre-treatment susceptibility testing should be part - and not the only weapon - of a targeted, personalized strategy to overcome H. pylori infection[25].
A meta-analysis published in 2011 indicated that the prevalence of H. pylori infection in the oral cavities of gastric H. pylori-positive patients was significantly higher than in gastric H. pylori-negative patients (45.0% vs 23.9%)[26]. The pooled OR was 3.61 and the 95%CI: was 1.91-6.82 (P < 0.0001). Different diagnostic methods produced different pooled ORs with PCR the highest (OR = 5.11, 95%CI: 2.08-12.54, P = 0.0004) and the rapid urease test the lowest (OR = 2.00, 95%CI: 0.80-5.00, P = 0.14). The 44.8% (91/203) prevalence of H. pylori infection in the oral cavity in patients with clinical and/or histologic gastroesophageal diseases was significantly higher than the 13.2% (21/159) in patients with non-ulcerous dyspepsia or healthy controls (OR = 5.15, 95%CI: 2.97-8.92, P < 0.00001). The eradication efficiency in the stomach was 85.8% (187/218), while in the oral cavity it was only 5.7% (9/158) (OR = 55.59, P < 0.00001). H. pylori was more difficult to eradicate in the oral cavity than in the stomach, and may be a source of reinfection.
Another recent meta-analysis in 2014 included 48 articles reporting on the association between saliva and plaque and H. pylori-infection, twelve clinical trials, and a meta-analysis[27]. They found a close relation between H. pylori infection in the oral cavity and the stomach. The mouth is the first extra-gastric reservoir.
There is a meta-analysis regarding the relationship between the existence of H. pylori in dental plaque and in the stomach of patients that included twenty-three studies with 1861 patients[16]. Their results show that the prevalence of co-infection of gastric and dental plaque H. pylori was 49.7% (95%CI: 16.0%-83.4%) and the percent of agreement between the dental plaque H. pylori status and the gastric H. pylori was estimated as 82%.
Another recent meta-analysis in 2014 indicated that dental plaque can act as a reservoir, and proper oral hygiene maintenance is essential to prevent reinfection[28].
Global pediatric clinical studies have reported a decreasing tendency in the overall rate of H. pylori eradication. Antibiotic drug resistance to H. pylori, which has been reported to vary widely between geographic regions, is mainly associated with treatment failure in these patients[29-39]. Due to the rising prevalence of antimicrobial resistance, mainly to clarithromycin, efficacy of standard, triple therapies has declined to unacceptably low levels in most parts of the world. Molecular testing methods are currently available for the characterization of H. pylori therapeutic susceptibility, including genotypic detection of macrolide resistance and evaluation of the cytochrome P450 2C19 status known to affect the metabolism of proton pump inhibitors; these data show increasing antibiotic resistance[30].
The global problem of H. pylori infection and its increasing antibiotic resistance has been analyzed[31]. New data concerns the role of the bacterium in various clinical conditions; the indications of H. pylori testing, diagnostic procedures, and eradication-treatment regimens have been reported. The molecular tests can be used to detect H. pylori and clarithromycin and/or fluoroquinolone resistance in gastric biopsies without necessitating culture[32].
H. pylori therapy in clinical practice is becoming progressively more difficult. A review article indicated the rate of eradication failure has dramatically risen in many countries due to resistance to antibiotics[37]. This review summarized important studies regarding H. pylori therapy published from April 2013 to April 2014 that indicated that the emerging problem of quinolone resistance remains a worry. Individualized therapy, based on factors such as antimicrobial information, resistance data, and CYP2C19 metabolism, may well be the most notable future trend to emerge in 2016.
Several strategies have been proposed to increase the H. pylori eradication rate, including the prolongation of the treatment duration to 14 d, the use of a four-drug regimen (quadruple, sequential, and concomitant treatments), and the use of novel antibiotics, such as levofloxacin. However, triple therapy remains the most widely accepted first-line treatment regimen in Brazil, the United States, and throughout Europe. Because this therapy is limited by resistance to clarithromycin, other therapeutic regimens have been investigated worldwide[39]. A study indicated that eradication has no effect on infection in the esophagus, which may become a reason for increasing drug resistance[40].
All of the above data indicate that drug resistance has significantly increased in past years. One of the reasons for this is because we all focus on the drug resistance issue and ignore the oral cavity infected by H. pylori that increases reinfection of the stomach; as a result, more antibiotics are used in repeated treatments. It is now time that we look a different way; if we clean the oral H. pylori, then the clinician can use reduced amounts of drugs to complete the treatment for H. pylori infection.
There are three important technologies developing in order make a strong foundation for a colonized site in the oral cavity. PCR is a highly sensitivity test for oral H. pylori, but it is not convenient in clinical settings. So, first, a high-sensitivity and -specificity test in saliva should be established. Then we will have a much easier time of running clinical trials on a large number of patients to obtain a greater number of data in order to find the positive correlation between oral and stomach H. pylori infection. Second, and most important, is developing a cell culture technology suitable for detecting low-concentration H. pylori in the oral cavity. Once a new cell culture method is established, then we could determine if HPS technology can be confirmed by cell culture data. As a final step, we need to develop a technology, rather than an antibiotic drug, to eliminate H. pylori from the oral cavity.
The most common bacteria causing infection across the world is H. pylori, which colonizes the human stomach. These bacteria have also been detected in some extra-gastric ecologic niches, such as the oral cavity and water. However, the results of H. pylori detection in extra-gastric ecologic niches are controversial. The UBT does not detect H. pylori in the oral cavity. Improvement of the sensitivity and the specificity of the detection methods appears to be one of the main bottleneck issues in providing compelling evidence[41,42].
HPS for oral urease: Oral urease was specifically detected in saliva using a lateral flow immunochromatographic test device. The device for H. pylori antigen detection in saliva was identical to the device used for oral urease detection. The HPS test for saliva employed a monoclonal antibody that was developed against oral urease. Test procedure: No food or drink was allowed one hour prior to the test. A swab was put under the tongue for at least one minute. The swab was swirled vigorously for fifteen seconds in a buffer solution, then we expunged as much liquid as possible from the swab by pressing and rotating the fiber portion against the wall of the tube. Two to three drops of saliva/buffer mixture were added into the sample well. As the test kit begins to work, one will see a purple color move across the result window in the center of the test disk. The presence of two color bands (“T” band and “C” band) within the result window indicates a positive result. The presence of only one purple-color band indicates a negative result. Specificity: An in-house study was conducted with three separate lots of the HPS test to determine its specificity. The following common oral bacteria were applied: Actinomyces naeslundii, Actinomyces odontolyticus, Bifidobacterium dentium, Corynebacterium matruchotii, Gemella haemolysans, Granulicatella adiacens, Streptococcus gordonii, S. salivarius, S. sanguinis, and Veillonella parvula. All of the above were analyzed and did not show interference or cross-reactivity with the test. Sensitivity: The test’s sensitivity was 10 ng/mL HPS antigen[43].
Krajden et al[44] in 1989 first reported on the culture of H. pylori gastritis in seventy-one patients with plaque; one plaque culture result was positive, and of all seventy-one saliva cultures, none of the patients presented a positive. Since then, many scientists perform oral H. pylori cultures, but are rarely successful. Indeed, culture-positive rates are very low among published studies from various countries. The key reasons for the difficulty of cultivating oral H. pylori result from oral specimen collection, preservation, small colonies of H. pylori culture, and competition with other oral bacteria and H. pylori colonies. It seems the use of conventional stomach bacteria culturing techniques for the culture of oral H. pylori has reached its limit. Alterations to this method are required to obtain a high positive rate of oral H. pylori culture. Some authors simply make premature conclusions that “oral H. pylori cannot be cultured” and “the oral cavity is not a colonized site”, which has become the main theoretical basis of some scholars opposing oral H. pylori colonization.
A study using an “artificial ammonia cloud” greatly improved the positive rate of oral H. pylori culture[3]. It made the medium more suitable for nutrients for H. pylori growth and reproduction. The application of the artificial ammonia cloud technology is for the special treatment of saliva, as it protected H. pylori in the saliva sample, whereas a medium with low H. pylori simulated a stomach environment, with a strong acid to kill other bacteria. Thus, H. pylori can grow better in an oral H. pylori-culture medium.
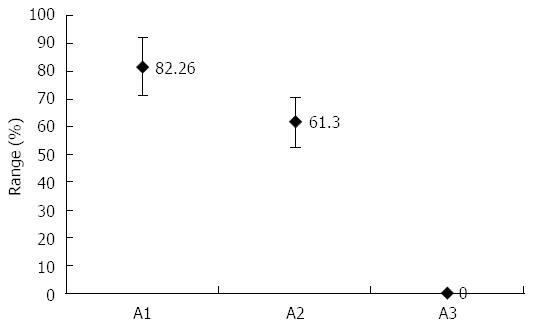
There are reports that indicate drug eradication on stomach H. pylori with no effect on oral H. pylori[45]. In the food industry, polylysine (L) and the glycerol monolaurate (GM) are used in preserving meat products. The L is typically produced as a homo-polypeptide of approximately twenty-five to thirty lysine residues. In contrast to a normal peptide bond that is linked by an alphacarbon group, the lysine amino acids are molecularly linked by the epsilon amino group and the carboxyl group. L belongs to the group of cationic polymers[46]. In water, L contains a positively charged hydrophilic amino group. It is adsorbed electrostatically to the cell surface of the bacteria, followed by a stripping of the outer membrane. This eventually leads to abnormal distribution of the cytoplasm, causing damage to the H. pylori cell. GM is the monoester formed from glycerol and lauric acid. H. pylori is extremely sensitive to GM. However, there are no reports of L or GM killing H. pylori in vivo. As both have had a safe record in the food industry, they have been tested to see whether they can eliminate H. pylori in the oral cavity. Patients who received treatment of LGM mouthwash and drug eradication showed 82.26% (within a 95%CI) effective results within one month of treatment[3] (Figure 2).
More important is the classic H. pylori eradication programs in which there are no clear measures of oral H. pylori; as H. pylori traditional treatment occurs, frequent relapses become more critical.
In the oral cavity there exists a live H. pylori that has negative influences on the eradication of stomach infection. As long as we agree with the idea of a second colonized site within the oral cavity, the rate for successful eradication of H. pylori will increase.
P- Reviewer: Biernat MM, Iwanicki A S- Editor: Yu J L- Editor: Filopodia E- Editor: Ma S
| 1. | Marshall B. Helicobacter pylori infection of the Seventh National Forum of China. 2012 Aug 26-27; Beijing, China. . |
| 2. | Al-Ahmad A, Kürschner A, Weckesser S, Wittmer A, Rauberger H, Jakob T, Hellwig E, Kist M, Waidner B. Is Helicobacter pylori resident or transient in the human oral cavity? J Med Microbiol. 2012;61:1146-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Wang XM, Yee KC, Hazeki-Taylor N, Li J, Fu HY, Huang ML, Zhang GY. Oral Helicobacter pylori, its relationship to successful eradication of gastric H. pylori and saliva culture confirmation. J Physiol Pharmacol. 2014;65:559-566. [PubMed] |
| 4. | Liu Q, Qi D, Kang J, Jin Y, Liu W, Gao W, Hou P, Lu J. Efficacy of real-time PCR-based detection of Helicobacter pylori infection and genotypic resistance-guided quadruple therapy as the first-line treatment for functional dyspepsia with Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2015;27:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Ismail H, Morgan C, Griffiths P, Williams J, Jenkins G. A Newly Developed Nested PCR Assay for the Detection of Helicobacter pylori in the Oral Cavity. J Clin Gastroenterol. 2015;Epub ahead of print. [PubMed] |
| 6. | Duś I, Dobosz T, Manzin A, Loi G, Serra C, Radwan-Oczko M. Role of PCR in Helicobacter pylori diagnostics and research--new approaches for study of coccoid and spiral forms of the bacteria. Postepy Hig Med Dosw (Online). 2013;67:261-268. [PubMed] |
| 7. | Bharath TS, Reddy MS, Dhanapal R, Raj Kumar NG, Neeladri Raju P, Saraswathi T. Molecular detection and corelation of Helicobacter pylori in dental plaque and gastric biopsies of dyspeptic patients. J Oral Maxillofac Pathol. 2014;18:19-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Nisha KJ, Nandakumar K, Shenoy KT, Janam P. Periodontal disease and Helicobacter pylori infection: a community-based study using serology and rapid urease test. J Investig Clin Dent. 2014;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Irani S, Monsef Esfahani A, Bidari Zerehpoush F. Detection of Helicobacter pylori in Oral Lesions. J Dent Res Dent Clin Dent Prospects. 2013;7:230-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 10. | Román-Román A, Giono-Cerezo S, Camorlinga-Ponce M, Martínez-Carrillo DN, Loaiza-Loeza S, Fernández-Tilapa G. vacA genotypes of Helicobacter pylori in the oral cavity and stomach of patients with chronic gastritis and gastric ulcer. Enferm Infecc Microbiol Clin. 2013;31:130-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Lukeš P, Pavlík E, Potužníková B, Plzák J, Nártová E, Doseděl J, Katra R, Sterzl I, Betka J, Astl J. Comparison of Helicobacter pylori genotypes obtained from the oropharynx and stomach of the same individuals - a pilot study. Prague Med Rep. 2012;113:231-239. [PubMed] |
| 12. | Hirsch C, Tegtmeyer N, Rohde M, Rowland M, Oyarzabal OA, Backert S. Live Helicobacter pylori in the root canal of endodontic-infected deciduous teeth. J Gastroenterol. 2012;47:936-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Ogaya Y, Nomura R, Watanabe Y, Nakano K. Detection of Helicobacter pylori DNA in inflamed dental pulp specimens from Japanese children and adolescents. J Med Microbiol. 2015;64:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Tsami A, Petropoulou P, Kafritsa Y, Mentis YA, Roma-Giannikou E. The presence of Helicobacter pylori in dental plaque of children and their parents: is it related to their periodontal status and oral hygiene? Eur J Paediatr Dent. 2011;12:225-230. [PubMed] |
| 15. | Boyanova L, Panov V, Yordanov D, Gergova G, Mitov I. Characterization of oral Helicobacter pylori strain by 4 methods. Diagn Microbiol Infect Dis. 2013;77:287-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Navabi N, Aramon M, Mirzazadeh A. Does the presence of the Helicobacter pylori in the dental plaque associate with its gastric infection? A meta-analysis and systematic review. Dent Res J (Isfahan). 2011;8:178-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Dayama A, Srivastava V, Shukla M, Singh R, Pandey M. Helicobacter pylori and oral cancer: possible association in a preliminary case control study. Asian Pac J Cancer Prev. 2011;12:1333-1336. [PubMed] |
| 18. | Diouf A, Seck-Diallo AM, Faye M, Benoist HM, Sembene M, Diallo PD, Martinez-Gomis J, Sixou M. [Prevalence of Helicobacter pylori detected by real-time PCR in the subgingival plaque of patients with chronic periodontitis]. Odontostomatol Trop. 2011;34:5-12. [PubMed] |
| 19. | Silva DG, Stevens RH, Macedo JM, Albano RM, Falabella ME, Fischer RG, Veerman EC, Tinoco EM. Presence of Helicobacter pylori in supragingival dental plaque of individuals with periodontal disease and upper gastric diseases. Arch Oral Biol. 2010;55:896-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Song HY, Li Y. Can eradication rate of gastric Helicobacter pylori be improved by killing oral Helicobacter pylori? World J Gastroenterol. 2013;19:6645-6650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Abadi AT, Mobarez AM, Teymournejad O, Karbalaei M. Concomitant Colonization of Helicobacter pylori in Dental Plaque and Gastric Biopsy. J Pathog. 2014;2014:871601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Anand PS, Kamath KP, Anil S. Role of dental plaque, saliva and periodontal disease in Helicobacter pylori infection. World J Gastroenterol. 2014;20:5639-5653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 92] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (2)] |
| 23. | Momtaz H, Souod N, Dabiri H, Sarshar M. Study of Helicobacter pylori genotype status in saliva, dental plaques, stool and gastric biopsy samples. World J Gastroenterol. 2012;18:2105-2111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 64] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (2)] |
| 24. | Fernández-Tilapa G, Axinecuilteco-Hilera J, Giono-Cerezo S, Martínez-Carrillo DN, Illades-Aguiar B, Román-Román A. vacA genotypes in oral cavity and Helicobacter pylori seropositivity among adults without dyspepsia. Med Oral Patol Oral Cir Bucal. 2011;16:e175-e180. [PubMed] |
| 25. | Cammarota G, Ianiro G, Bibbò S, Di Rienzo TA, Masucci L, Sanguinetti M, Gasbarrini A. Culture-guided treatment approach for Helicobacter pylori infection: review of the literature. World J Gastroenterol. 2014;20:5205-5211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Zou QH, Li RQ. Helicobacter pylori in the oral cavity and gastric mucosa: a meta-analysis. J Oral Pathol Med. 2011;40:317-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 27. | Adler I, Muiño A, Aguas S, Harada L, Diaz M, Lence A, Labbrozzi M, Muiño JM, Elsner B, Avagnina A. Helicobacter pylori and oral pathology: relationship with the gastric infection. World J Gastroenterol. 2014;20:9922-9935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 28. | Al Sayed A, Anand PS, Kamath KP, Patil S, Preethanath RS, Anil S. Oral Cavity as an Extragastric Reservoir of Helicobacter pylori. ISRN Gastroenterol. 2014;2014:261369. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Seo JH, Woo HO, Youn HS, Rhee KH. Antibiotics resistance of Helicobacter pylori and treatment modalities in children with H. pylori infection. Korean J Pediatr. 2014;57:67-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of Helicobacter pylori infection: Past, present and future. World J Gastrointest Pathophysiol. 2014;5:392-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 31. | Iwańczak F, Iwańczak B. Treatment of Helicobacter pylori infection in the aspect of increasing antibiotic resistance. Adv Clin Exp Med. 2012;21:671-680. [PubMed] |
| 32. | de Korwin JD. [New recommendations for the diagnosis and the treatment of Helicobacter pylori infection]. Presse Med. 2013;42:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Shiota S, Yamaoka Y. Strategy for the treatment of Helicobacter pylori infection. Curr Pharm Des. 2014;20:4489-4500. [PubMed] |
| 34. | Iwańczak F, Iwańczak B. [H. pylori infections in children: clinical, diagnostic and treatment implications]. Pol Merkur Lekarski. 2013;35:187-190. [PubMed] |
| 35. | Vakil N, Vaira D. Treatment for H. pylori infection: new challenges with antimicrobial resistance. J Clin Gastroenterol. 2013;47:383-388. [PubMed] |
| 36. | Gisbert JP. [Diseases linked to Helicobacter pylori infection]. Gastroenterol Hepatol. 2014;37 Suppl 3:40-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Cui R, Zhou L. Helicobacter pylori infection: an overview in 2013, focus on therapy. Chin Med J (Engl). 2014;127:568-573. [PubMed] |
| 38. | O’Connor A, Vaira D, Gisbert JP, O’Morain C. Treatment of Helicobacter pylori infection 2014. Helicobacter. 2014;19 Suppl 1:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Dos Santos AA, Carvalho AA. Pharmacological therapy used in the elimination of Helicobacter pylori infection: a review. World J Gastroenterol. 2015;21:139-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Cellini L, Grande R, Artese L, Marzio L. Detection of Helicobacter pylori in saliva and esophagus. New Microbiol. 2010;33:351-357. [PubMed] |
| 41. | Amiri N, Abiri R, Eyvazi M, Zolfaghari MR, Alvandi A. The frequency of Helicobacter pylori in dental plaque is possibly underestimated. Arch Oral Biol. 2015;60:782-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Mesquita B, Gonçalves MJ, Pacheco P, Lopes J, Salazar F, Relvas M, Coelho C, Pacheco JJ, Velazco C. Helicobacter pylori identification: a diagnostic/confirmatory method for evaluation. Curr Microbiol. 2014;69:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 43. | Yee KC, Wei MH, Yee HC, Everett KD, Yee HP, Hazeki-Talor N. A screening trial of Helicobacter pylori-specific antigen tests in saliva to identify an oral infection. Digestion. 2013;87:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Krajden S, Fuksa M, Anderson J, Kempston J, Boccia A, Petrea C, Babida C, Karmali M, Penner JL. Examination of human stomach biopsies, saliva, and dental plaque for Campylobacter pylori. J Clin Microbiol. 1989;27:1397-1398. [PubMed] |
| 45. | Miyabayashi H, Furihata K, Shimizu T, Ueno I, Akamatsu T. Influence of oral Helicobacter pylori on the success of eradication therapy against gastric Helicobacter pylori. Helicobacter. 2000;5:30-37. [PubMed] |
| 46. | Shima S, Matsuoka H, Iwamoto T, Sakai H. Antimicrobial action of epsilon-poly-L-lysine. J Antibiot (Tokyo). 1984;37:1449-1455. [PubMed] |