Published online May 14, 2016. doi: 10.3748/wjg.v22.i18.4438
Peer-review started: February 18, 2016
First decision: March 21, 2016
Revised: March 25, 2016
Accepted: April 7, 2016
Article in press: April 7, 2016
Published online: May 14, 2016
Processing time: 77 Days and 4.9 Hours
Great progress has been made in the field of liver transplantation over the past two decades. This progress, however, also brings up the next set of challenges: First, organ shortage remains a major limitation, and accounts for a large proportion of wait list mortality. While living donation has successfully increased the total number of liver transplants done in Asian countries, the total number of such transplants has been stagnant in the western hemisphere. As such, there has been a significant effort over the past decade to increase the existing deceased donor pool. This effort has resulted in a greater use of liver allografts following donation after cardiac death (DCD) along with marginal and extended criteria donors. Improved understanding of the pathophysiology of liver allografts procured after circulatory arrest has not only resulted in better selection and management of DCD donors, but has also helped in the development of mechanical perfusion strategies. Early outcomes demonstrating the clinical applicability of both hypothermic and normothermic perfusion and its potential to impact patient survival and allograft function have generated much interest. Second, long-term outcomes of liver transplant recipients have not improved significantly, as recipients continue to succumb to complications of long-term immunosuppression, such as infection, malignancy and renal failure. Furthermore, recent evidence suggests that chronic immune-mediated injury to the liver may also impact graft function.
Core tip: Organ shortage remains a major limitation in liver transplantation, and there has been a significant effort over the past decade to increase the existing deceased donor pool. Recent advances have included better selection and management of donors after circulatory arrest, application of hypothermic and normothermic perfusion, minimization of standard immunosuppression and use of new immunosuppressive medications. Additionally, there has been renewed emphasis and understanding of liver immunology and the impact of antibody-mediated rejection. Together, these advances have allowed for expansion of the donor pool with concurrent improved patient outcomes.
- Citation: Jadlowiec CC, Taner T. Liver transplantation: Current status and challenges. World J Gastroenterol 2016; 22(18): 4438-4445
- URL: https://www.wjgnet.com/1007-9327/full/v22/i18/4438.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i18.4438
Over the last several decades, the field of transplantation has witnessed much change yet, among all solid organ transplants, arguably the greatest advances have been in liver transplantation. Today, liver transplantation is universally accepted as the only treatment option for end-stage liver disease, acute fulminant hepatic failure, hepatocellular carcinoma, hilar cholangiocarcinoma and several metabolic disorders. Advancements in surgical technique, perioperative management and immunosuppressive therapy have yielded excellent short-term graft and patient survival outcomes which have become the expected norm. With these advances comes the next set of challenges: organ shortage and improving long-term outcomes of the liver transplant recipients.
The disparity between the number of available liver allografts and transplant candidates continue to grow worldwide. In Asia, this problem has been successfully addressed by ever-increasing numbers of living-donor liver transplantation (LDLT). In the western countries, however, the number of LDLT has not seen a significant change for over a decade, and the demand for deceased donor liver allografts continue to increase. Thus, substantial effort has been put in to expand the existing deceased donor pool.
In the early 1990s, the use of livers procured from donation after cardiac death (DCD) donors was an early attempt to narrow the disparity between organs and recipients in need[1]. The early experience with DCD livers was not favorable, as prolonged donor warm ischemia time and ischemia-reperfusion injury likely played a large role in the high rate of primary non-function, hepatic artery thrombosis, ischemic cholangiopathy, and allograft failure[2,3]. With increasing experience, however, factors associated with improved outcomes have been identified and more centers have begun to use these donors[4-6].
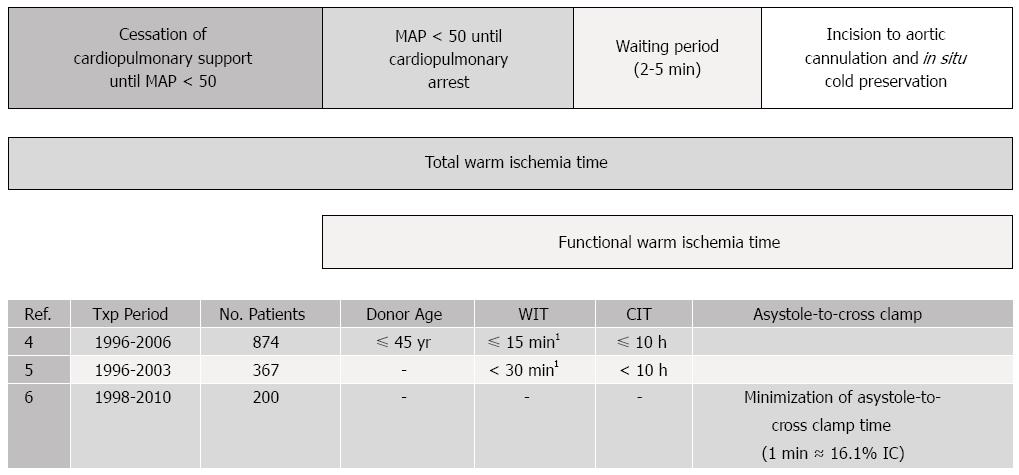
To date, there have been several large retrospective analyses which have identified DCD donor-related variables associated with recipient outcomes (Figure 1). Young age (45 ± 10 years old), short donor warm ischemia time (less than thirty minutes), and limited cold ischemia time (less than ten hours) have been shown to improve allograft outcomes[4-6]. In the Mayo Clinic experience, every minute added to the warm ischemia period between asystole and cross-clamp was found to be associated with a 16.1 percent increase in the odds of ischemic cholangiopathy[6]. Recipient factors associated with improved DCD outcomes generally concentrate on avoidance of sicker patients: excluding patients requiring retransplantation, patients with renal failure (creatinine > 2.0 mg/dL) and patients on life support[5]. The correlation between these factors and outcomes is likely related to minimization of both cold ischemia time and further allograft injury through recipient instability. As a result, by applying these donor and recipient criteria, DCD outcomes have improved considerably with recipient survival and allograft function similar to that of donation after brain death (DBD) donors[4-6].
Despite these improved outcomes, many DCD livers continue to go unused as a result of unacceptable donor parameters and concern for poor allograft function. The concept of mechanical perfusion for solid organ transplantation was originally introduced in the late 1960s by Belzer et al[7] and renewed interest returned following a report by Moers et al[8] in 2000 which, through a prospective randomized control trial, demonstrated a decreased incidence of delayed graft function and improved graft survival in in kidney transplant recipients. Since then, hypothermic machine perfusion in kidney transplantation has gained a widespread use. Although suboptimal, static hypothermic cold storage remains the primary method for liver preservation, largely because of its cost effectiveness, simplicity, and logistics. At present, there is a large and apparent need to optimize preservation particularly for DCD, marginal, and extended-criteria donor organs. These allografts are subject to a greater risk of ischemia-reperfusion injury which occurs as a result of donor warm ischemia time, aortic cross-clamping and initiation of cold ischemia, rewarming during graft implantation, and finally full reperfusion (Figure 1). It is here that the utility of mechanical perfusion has emerged as potential solution to this problem.
Hypothermia slows cellular metabolism and prolongs the amount of time an organ can be deprived of oxygen without loss of viability (Table 1). In liver transplantation, hypothermic perfusion has shown similar benefits. Authors Guarrera et al[9] have demonstrated that hypothermic perfusion decreases the extent of graft injury and subsequent clinical studies by the same group have shown improved allograft function, lower serum transaminases and decreased hospital stay as compared to matched historic cold storage liver allografts from DBD donors[10,11]. Equally beneficial results have been reported in DCD liver allografts[12,13]. In a trial using human DCD livers, Hypothermic Oxygenated PErfusion (HOPE) was applied for 1 to 2 h prior to implantation[12]. Functional warm ischemia time (MAP < 50 mmHg to cold flush) in this group ranged from 22 to 41 min and postoperative allograft function was normal in the entire cohort. In a follow-up period of 8.5 mo, no evidence of intrahepatic biliary complications was noted. As a continuation to this study, authors Dutkowski et al[13] recently published their results evaluating DCD livers treated with HOPE along with matched static cold storage DCD livers. As anticipated, results for DCD livers subjected to HOPE were superior with decreased graft injury, decreased intrahepatic cholangiopathy and biliary complications and improved 1-year graft survival[13]. Similarly, hypothermic machine perfusion has also been applied to extended criteria DBD donor liver allografts with encouraging outcomes, such as decreased allograft dysfunction (19% vs 30%), improved patient survival at one year (84% vs 80%) and a reduction in biliary complications (13% vs 43%)[11].
| Hypothermic mechanical perfusion | Normothermic mechanical perfusion | |
| Temperature | 4 °C | 37 °C |
| Pathophysiology | Reduced cellular metabolism | Continued cellular metabolism |
| Non-functioning liver during preservation | Functioning liver during preservation | |
| Perfusate | No specific requirements, oxygen can be used | Continuous delivery of nutrients and oxygen needs to be maintained |
| Logistics | Similar to that of hypothermic mechanical perfusion currently used for renal allografts | More complex |
| Benefits | Logistically easier | Allows for the assessment of metabolic and synthetic function during preservation |
| May provide benefit in marginal livers | May provide benefit in marginal livers | |
| Potential use in steatotic livers | ||
| Felt to reduce ischemia reperfusion injury |
Normothermic perfusion is technically more demanding, as it requires a continuous delivery of nutrients and oxygen in order to maintain ongoing cellular metabolism[14]. In theory, this setup reduces the ischemia-reperfusion injury and may thereby allow for the safe transplantation of marginal liver allografts. Normothermic mechanical perfusion can also allow for the assessment of liver function during the pre-implantation period (Table 1). Although these advantages are notable, historically, there have been difficulties in maintaining stable perfusion[15]. In a preclinical work by op den Dries et al[16], discarded DCD human donor livers were subjected to normothermic mechanical perfusion following cold ischemia times of 6.9 ± 1.9 h (WIT 15.5 ± 2.4 min). After six hours of normothermic perfusion (37 °C), bile production was observed and histological examination showed well-preserved liver morphology without signs of hepatocellular ischemia, biliary injury, or sinusoidal damage[16]. Currently, there are several human trials underway comparing normothermic liver preservation with static cold storage. The first Phase I, non-randomized, prospective trial using the normothermic mechanical perfusion unit enrolled twenty transplanted livers in United Kingdom and was completed in 2014. Results from this trial demonstrated the safety and feasibility of normothermic machine preservation in human liver allografts (personal communication). In the interim, a multicenter, randomized controlled trial with enrollment plan for 260 liver allografts (130 using OrganOx©; 130 using static cold storage) is under way.
The application of mechanical perfusion to marginal donors is foreseeable as hepatic steatosis has become increasingly common in the United States and the incidence of deceased donors with steatotic livers has concurrently increased. Allografts with severe steatosis, defined as greater than sixty percent of the parenchyma, are known to carry a high risk of primary non-function[17,18]. On the contrary, liver allografts with mild steatosis (< 30%) yield results similar to those of non-steatotic liver allografts[17,18]. The outcomes of liver allografts with moderate steatosis (30% to 60%) remain variable, and often depend upon additional factors such as recipient stability, ischemia time, and mechanism of donation (i.e., DBD vs DCD). Impaired microcirculation secondary to increased hepatocyte volume is theorized to be responsible for the relative susceptibility of steatotic livers to ischemia[19]. Cellular edema accompanying ischemia-reperfusion likely results in further obstruction of the sinusoids, thereby exacerbating this injury. Accordingly, steatotic DCD liver allografts are generally discarded as these scenarios combine several risk factors. As such, the application of mechanical perfusion may help salvage steatotic livers. At present, preclinical and clinical studies are lacking however Bessems et al[20] evaluated mechanical perfusion using a steatotic rat model in which they compared static cold storage and hypothermic oxygenated mechanical perfusion. Results from this animal model found that preservation of steatotic livers stored in standard cold storage resulted in more cellular injury. By comparison, the steatotic livers subjected to hypothermic oxygenated mechanical perfusion showed improved bile production and higher ATP levels[20].
Although the short-term outcomes after liver transplantation have been excellent worldwide, long-term outcomes remain suboptimal. The top causes of late mortality after liver transplantation are allograft failure, cardiovascular events, infection, malignancy and renal failure[21]. There is a clear and direct link between these and the long-term use of immunosuppressive medications. As a result, in the past decade, a significant effort has been put forth to minimize immunosuppression in liver transplantation.
Renal insufficiency is the strongest predictor of late mortality following liver transplantation[21]. Because of this, the use of renal sparing immunosuppression protocols both during the induction and maintenance periods following liver transplantation has been introduced into the clinical practice over the past decade.
Mammalian target of rapamycin inhibitors: A number of large trials evaluated the role of mammalian target of rapamycin inhibitors (mTOR) in liver transplantation. The Preservation of Renal Function in Liver Transplant Recipients with Certican Therapy (PROTECT) study[22], a randomized controlled trial sought to evaluate the effect of conversion from calcineurin inhibitors (CNI) to everolimus starting four weeks following liver transplantation. Unfortunately, at one year, the study results proved to be inconclusive. No difference in glomerular filtration rater (GFR) was noted using the Cockcroft-Gault formula. Moreover, despite lack of clear benefit, the study did, however, observe a higher rate of infections, anemia, leukopenia and hyperlipidemia in the Everolimus treatment group[22].
By comparison, a second trial by Masetti et al[23] in 2010 showed some benefit in using mTOR inhibitors in liver transplantation. In this randomized control trial, cyclosporine (CsA) was used for ten days following liver transplantation. Patients were then randomized to Everolimus or cyclosporine plus mycophenolate mofetil (MMF). At twelve months, GFR was better in the Everolimus group and no differences were noted in patient survival, acute rejection or hepatic artery thrombosis[23]. Among the patients with a GFR less than 60 mL/min per 1.73 m2, the Everolimus treatment group showed a benefit. Accordingly, conclusions of the study were that early withdrawal of CsA followed by Everolimus monotherapy in de novo liver transplant patients was associated with an improvement in renal function, although, a criticism of the study has been the baseline difference between the two groups with regard to the starting GFR.
In Spare-the-Nephron Trial[24], patients on CNI/MMF for the first four weeks post-transplant were randomized to either continue the same regimen or convert to Sirolimus/MMF. A mean increase in patient GFR was noted in the Sirolimus group, however the composite endpoint demonstrated non-inferiority of Sirolimus to CNIs when used with MMF[24]. Review of other smaller studies evaluating the conversion from CNI to mTOR inhibitors in liver transplant patients with renal insufficiency reveals similar variable results[25-28]. A meta-analysis of the use of Sirolimus in liver transplant recipients with CNI-induced renal insufficiency found no significant improvement in GFR, risk of death or graft failure[29]. Findings were, however, significant for an increased risk of infection, mouth ulcers and treatment discontinuation[29]. Given this variability, the role of mTOR inhibitors continues to evolve with much indecision and hesitancy surrounding their use for renal insufficiency. Newer roles, specifically with regard to benefit in hepatocellular carcinoma, likewise remain to be fully realized.
Induction therapy and delayed introduction of calcineurin inhibitors: Delayed introduction of CNI following liver transplantation may, theoretically, help decrease the negative impact of CNI on renal function[30-33]. Among de novo liver transplant recipients who had pre-existing renal insufficiency, Thymoglobulin induction and delayed introduction of CNI resulted in lower serum creatinine, a higher estimated GFR, and less dependence on dialysis at twelve months[30]. Similar strategies have been employed using newer generation anti-interleukin-2-receptor antibodies (Basiliximab, Daclizumab) for induction[32,33]. The ReSpECT trial, comparing standard Tacrolimus vs reduced-dose Tacrolimus and Daclizumab induction and delayed reduced-dose Tacrolimus[34], demonstrated that the greatest decline in eGFR was seen in the standard Tacrolimus group with the least decline noted in the Daclizumab induction and delayed reduced-dose Tacrolimus group. Currently, at Mayo Clinic, Basiliximab induction is used in patients with renal insufficiency so as to delay initiation of CNI post-transplant.
Immunosuppression minimization/withdrawal: Of all the solid organs that are transplanted routinely, liver allograft appears to be unique, as approximately 19 percent of the recipients can achieve “operational” tolerance (off immunosuppression incidentally or obligatorily)[35,36]. Given these findings, several recent trials investigated elective withdrawal of immunosuppression (IS) in liver transplant recipients. In one such trial, 12 out of 20 (60%) pediatric liver transplant recipients were successfully weaned off IS, with no or minimal portal inflammation[37]. In a large European multi-center trial of 102 adult deceased donor liver transplant recipients, 41 (40%) achieved operational tolerance[38]. At present, more information is needed so as to better guide clinical decision making and similar trials with long-term follow-up will help identify parameters with predictive and diagnostic value regarding who could be successfully weaned off IS. Longitudinal follow-up of operationally tolerant patients will also further demonstrate whether long-term outcomes improve with limiting the recipients’ cumulative exposure to IS, particularly CNI.
Historically, liver allografts were thought to be spared from the HLA antibody-mediated injury, however recent findings have challenged this concept. The Baylor group initially reported their observation in liver recipients with chronic rejection, who had circulating donor-specific HLA-antibody (DSA) more often than in patients with no rejection[39]. Subsequently, they found that multiple IgG subclasses were found in the sera of chronic rejection patients, and the IgG3 subclass was associated with increased risk of graft loss[40]. A definitive cause-and-effect relationship remains to be demonstrated however, as these studies used post-transplant DSA for analyses and did not include protocol biopsies. In our own observation at Mayo Clinic, we did not find any DSA-mediated graft injury in the first year after transplantation[41], and over the long term, de novo DSA formation was preceded by liver allograft dysfunction (e.g., recurrent HCV cirrhosis)[42].
Based on these new observations, it appears that DSA may indeed cause chronic liver allograft injury, although less frequently than in kidney or hear transplantation. In order to better understand the impact of DSA on both early and late outcomes in liver transplantation, longitudinal prospective studies, similar to those done in kidney transplantation, correlating DSA (both pre- and posttransplant at routine intervals) with clinical outcomes and graft histology through protocol biopsies will need to be done. These studies will ideally be designed to analyze DSA in detail, target antigen expression in the allograft, and take into account immunosuppression and compliance issues in the recipients. In addition, histologic and genetic evidence of endothelial cell injury and microvascular inflammation (hallmark of DSA-mediated injury in kidney allografts) will need to be investigated in liver transplant recipients with circulating DSA (Table 2)[43-58].
| Kidney transplantation | Liver transplantation | |
| DSA specificity and levels | ↑ risk hyperacute rejection[44]; ↑ DSA in new onset late kidney allograft dysfunction[45]; acute AMR is associated with high posttransplant DSA levels[45] | ↑ DSA in recipients with CR, presence of IgG3 subclass associated with ↑ risk of graft loss[40] |
| C4d deposition in microvasculature | Graft failure significantly worse in the presence of C4d+ staining[44]; C4d+ is a marker of antibody-mediated injury[44,46] | C4d+ staining nonspecific; In the presence of DSA, linear portal capillary and sinusoidal staining observed in ACR; DSA negative C4d+ staining found in biliary strictures and recurrent liver disease[47] |
| DSA subtypes, C1q binding | ↓ graft survival and ↑ risk for AMR with C1q-binding DSA[48,49] | Limited data[50] |
| Microvascular inflammation | Peritubular capillaritis is a possible predictor of chronic AMR[51]; subclinical AMR may contribute to development of CAN[52] | No current data |
| EC activation by light microscopy | EC and BM ultrastructural abnormalities in glomerular and peritubular capillaries are early markers of TXG[53] | No current data |
| Gene expression profile of chronic AMR | Defined genetic profile in AMR[54,55] | No current data |
| Therapeutic-trials to prevent DSA-associated injury | Bortezomib (plasma cell-targeted therapy) as a possible antihumoral therapy[56]; plasma exchange for AMR[57,58] | No current data |
Along with the advances made in liver transplantation over the past thirty years have come a new set of challenges. As the demand for liver transplantation continues to grow worldwide, more collaborative studies are needed to narrow the disparity between the number of available deceased donor liver allografts and wait-listed patients, and to improve the long-term outcomes of the liver recipients.
P- Reviewer: Bramhall S, Liu QD, Makisalo H S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Casavilla A, Ramirez C, Shapiro R, Nghiem D, Miracle K, Bronsther O, Randhawa P, Broznick B, Fung JJ, Starzl T. Experience with liver and kidney allografts from non-heart-beating donors. Transplantation. 1995;59:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 273] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Merion RM, Pelletier SJ, Goodrich N, Englesbe MJ, Delmonico FL. Donation after cardiac death as a strategy to increase deceased donor liver availability. Ann Surg. 2006;244:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Lee DD, Singh A, Burns JM, Perry DK, Nguyen JH, Taner CB. Early allograft dysfunction in liver transplantation with donation after cardiac death donors results in inferior survival. Liver Transpl. 2014;20:1447-1453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Lee KW, Simpkins CE, Montgomery RA, Locke JE, Segev DL, Maley WR. Factors affecting graft survival after liver transplantation from donation after cardiac death donors. Transplantation. 2006;82:1683-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Mateo R, Cho Y, Singh G, Stapfer M, Donovan J, Kahn J, Fong TL, Sher L, Jabbour N, Aswad S. Risk factors for graft survival after liver transplantation from donation after cardiac death donors: an analysis of OPTN/UNOS data. Am J Transplant. 2006;6:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 6. | Taner CB, Bulatao IG, Willingham DL, Perry DK, Sibulesky L, Pungpapong S, Aranda-Michel J, Keaveny AP, Kramer DJ, Nguyen JH. Events in procurement as risk factors for ischemic cholangiopathy in liver transplantation using donation after cardiac death donors. Liver Transpl. 2012;18:100-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Belzer FO, Ashby BS, Gulyassy PF, Powell M. Successful seventeen-hour preservation and transplantation of human-cadaver kidney. N Engl J Med. 1968;278:608-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 131] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Moers C, Pirenne J, Paul A, Ploeg RJ; Machine Preservation Trial Study Group. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2012;366:770-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 9. | Guarrera JV, Estevez J, Boykin J, Boyce R, Rashid J, Sun S, Arrington B. Hypothermic machine perfusion of liver grafts for transplantation: technical development in human discard and miniature swine models. Transplant Proc. 2005;37:323-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 87] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Henry SD, Nachber E, Tulipan J, Stone J, Bae C, Reznik L, Kato T, Samstein B, Emond JC, Guarrera JV. Hypothermic machine preservation reduces molecular markers of ischemia/reperfusion injury in human liver transplantation. Am J Transplant. 2012;12:2477-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 11. | Guarrera JV, Henry SD, Samstein B, Reznik E, Musat C, Lukose TI, Ratner LE, Brown RS, Kato T, Emond JC. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am J Transplant. 2015;15:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 12. | Dutkowski P, Schlegel A, de Oliveira M, Müllhaupt B, Neff F, Clavien PA. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol. 2014;60:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 267] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 13. | Dutkowski P, Polak WG, Muiesan P, Schlegel A, Verhoeven CJ, Scalera I, DeOliveira ML, Kron P, Clavien PA. First Comparison of Hypothermic Oxygenated PErfusion Versus Static Cold Storage of Human Donation After Cardiac Death Liver Transplants: An International-matched Case Analysis. Ann Surg. 2015;262:764-770; discussion 770-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 288] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 14. | Brockmann J, Reddy S, Coussios C, Pigott D, Guirriero D, Hughes D, Morovat A, Roy D, Winter L, Friend PJ. Normothermic perfusion: a new paradigm for organ preservation. Ann Surg. 2009;250:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Neuhaus P, Blumhardt G. Extracorporeal liver perfusion: applications of an improved model for experimental studies of the liver. Int J Artif Organs. 1993;16:729-739. [PubMed] |
| 16. | op den Dries S, Karimian N, Sutton ME, Westerkamp AC, Nijsten MW, Gouw AS, Wiersema-Buist J, Lisman T, Leuvenink HG, Porte RJ. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant. 2013;13:1327-1335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 226] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 17. | Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 333] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 18. | D’Alessandro AM, Kalayoglu M, Sollinger HW, Hoffmann RM, Reed A, Knechtle SJ, Pirsch JD, Hafez GR, Lorentzen D, Belzer FO. The predictive value of donor liver biopsies for the development of primary nonfunction after orthotopic liver transplantation. Transplantation. 1991;51:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 281] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Teramoto K, Bowers JL, Kruskal JB, Clouse ME. Hepatic microcirculatory changes after reperfusion in fatty and normal liver transplantation in the rat. Transplantation. 1993;56:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 146] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Bessems M, Doorschodt BM, Kolkert JL, Vetelainen RL, van Vliet AK, Vreeling H, van Marle J, van Gulik TM. Preservation of steatotic livers: a comparison between cold storage and machine perfusion preservation. Liver Transpl. 2007;13:497-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420-1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 585] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 22. | Fischer L, Klempnauer J, Beckebaum S, Metselaar HJ, Neuhaus P, Schemmer P, Settmacher U, Heyne N, Clavien PA, Muehlbacher F. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation--PROTECT. Am J Transplant. 2012;12:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 154] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 23. | Masetti M, Montalti R, Rompianesi G, Codeluppi M, Gerring R, Romano A, Begliomini B, Di Benedetto F, Gerunda GE. Early withdrawal of calcineurin inhibitors and everolimus monotherapy in de novo liver transplant recipients preserves renal function. Am J Transplant. 2010;10:2252-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Teperman L, Moonka D, Sebastian A, Sher L, Marotta P, Marsh C, Koneru B, Goss J, Preston D, Roberts JP; Spare-the-Nephron Trial Liver Transplantation Study Group. Calcineurin inhibitor-free mycophenolate mofetil/sirolimus maintenance in liver transplantation: the randomized spare-the-nephron trial. Liver Transpl. 2013;19:675-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Eisenberger U, Sollinger D, Stickel F, Burckhardt B, Frey FJ. Relationship between renal resistance index and renal function in liver transplant recipients after cessation of calcineurin inhibitor. Clin Transplant. 2009;23:499-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Shenoy S, Hardinger KL, Crippin J, Desai N, Korenblat K, Lisker-Melman M, Lowell JA, Chapman W. Sirolimus conversion in liver transplant recipients with renal dysfunction: a prospective, randomized, single-center trial. Transplantation. 2007;83:1389-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Watson CJ, Gimson AE, Alexander GJ, Allison ME, Gibbs P, Smith JC, Palmer CR, Bradley JA. A randomized controlled trial of late conversion from calcineurin inhibitor (CNI)-based to sirolimus-based immunosuppression in liver transplant recipients with impaired renal function. Liver Transpl. 2007;13:1694-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | DuBay D, Smith RJ, Qiu KG, Levy GA, Lilly L, Therapondos G. Sirolimus in liver transplant recipients with renal dysfunction offers no advantage over low-dose calcineurin inhibitor regimens. Liver Transpl. 2008;14:651-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Asrani SK, Leise MD, West CP, Murad MH, Pedersen RA, Erwin PJ, Tian J, Wiesner RH, Kim WR. Use of sirolimus in liver transplant recipients with renal insufficiency: a systematic review and meta-analysis. Hepatology. 2010;52:1360-1370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Bajjoka I, Hsaiky L, Brown K, Abouljoud M. Preserving renal function in liver transplant recipients with rabbit anti-thymocyte globulin and delayed initiation of calcineurin inhibitors. Liver Transpl. 2008;14:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Soliman T, Hetz H, Burghuber C, Györi G, Silberhumer G, Steininger R, Mühlbacher F, Berlakovich GA. Short-term versus long-term induction therapy with antithymocyte globulin in orthotopic liver transplantation. Transpl Int. 2007;20:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Asrani SK, Kim WR, Pedersen RA, Charlton MR, Kremers WK, Therneau TM, Rosen CB, Dean PG. Daclizumab induction therapy in liver transplant recipients with renal insufficiency. Aliment Pharmacol Ther. 2010;32:776-786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 33. | Lin CC, Chuang FR, Lee CH, Wang CC, Chen YS, Liu YW, Jawan B, Chen CL. The renal-sparing efficacy of basiliximab in adult living donor liver transplantation. Liver Transpl. 2005;11:1258-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Neuberger JM, Mamelok RD, Neuhaus P, Pirenne J, Samuel D, Isoniemi H, Rostaing L, Rimola A, Marshall S, Mayer AD. Delayed introduction of reduced-dose tacrolimus, and renal function in liver transplantation: the ‘ReSpECT’ study. Am J Transplant. 2009;9:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 209] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 35. | Mazariegos GV, Reyes J, Marino IR, Demetris AJ, Flynn B, Irish W, McMichael J, Fung JJ, Starzl TE. Weaning of immunosuppression in liver transplant recipients. Transplantation. 1997;63:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 290] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 36. | Assy N, Adams PC, Myers P, Simon V, Minuk GY, Wall W, Ghent CN. Randomized controlled trial of total immunosuppression withdrawal in liver transplant recipients: role of ursodeoxycholic acid. Transplantation. 2007;83:1571-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, Alonso EM, Philogene MC, Ikle D, Poole KM. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012;307:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 38. | Benítez C, Londoño MC, Miquel R, Manzia TM, Abraldes JG, Lozano JJ, Martínez-Llordella M, López M, Angelico R, Bohne F. Prospective multicenter clinical trial of immunosuppressive drug withdrawal in stable adult liver transplant recipients. Hepatology. 2013;58:1824-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 39. | O’Leary JG, Kaneku H, Susskind BM, Jennings LW, Neri MA, Davis GL, Klintmalm GB, Terasaki PI. High mean fluorescence intensity donor-specific anti-HLA antibodies associated with chronic rejection Postliver transplant. Am J Transplant. 2011;11:1868-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 40. | Kaneku H, O’Leary JG, Taniguchi M, Susskind BM, Terasaki PI, Klintmalm GB. Donor-specific human leukocyte antigen antibodies of the immunoglobulin G3 subclass are associated with chronic rejection and graft loss after liver transplantation. Liver Transpl. 2012;18:984-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 41. | Taner T, Gandhi MJ, Sanderson SO, Poterucha CR, De Goey SR, Stegall MD, Heimbach JK. Prevalence, course and impact of HLA donor-specific antibodies in liver transplantation in the first year. Am J Transplant. 2012;12:1504-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 42. | Taner T, Heimbach JK, Rosen CB, Nyberg SL, Park WD, Stegall MD. Decreased chronic cellular and antibody-mediated injury in the kidney following simultaneous liver-kidney transplantation. Kidney Int. 2016;89:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 43. | Taner T, Stegall MD, Heimbach JK. Antibody-mediated rejection in liver transplantation: current controversies and future directions. Liver Transpl. 2014;20:514-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 44. | Patel R, Terasaki PI. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med. 1969;280:735-739. [PubMed] |
| 45. | Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, Gourishankar S, Grande J, Halloran P, Hunsicker L. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 389] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 46. | Burns JM, Cornell LD, Perry DK, Pollinger HS, Gloor JM, Kremers WK, Gandhi MJ, Dean PG, Stegall MD. Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant. 2008;8:2684-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 47. | Ali S, Ormsby A, Shah V, Segovia MC, Kantz KL, Skorupski S, Eisenbrey AB, Mahan M, Huang MA. Significance of complement split product C4d in ABO-compatible liver allograft: diagnosing utility in acute antibody mediated rejection. Transpl Immunol. 2012;26:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, Suberbielle C, Frémeaux-Bacchi V, Méjean A, Desgrandchamps F. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369:1215-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 685] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 49. | Yabu JM, Higgins JP, Chen G, Sequeira F, Busque S, Tyan DB. C1q-fixing human leukocyte antigen antibodies are specific for predicting transplant glomerulopathy and late graft failure after kidney transplantation. Transplantation. 2011;91:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 50. | Del Bello A, Congy-Jolivet N, Danjoux M, Muscari F, Lavayssière L, Esposito L, Cardeau-Desangles I, Guitard J, Dörr G, Milongo D, Suc B, Duffas JP, Alric L, Bureau C, Guilbeau-Frugier C, Rostaing L, Kamar N. De novo donor-specific anti-HLA antibodies mediated rejection in liver-transplant patients. Transpl Int. 2015;28:1371-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 51. | Lerut E, Naesens M, Kuypers DR, Vanrenterghem Y, Van Damme B. Subclinical peritubular capillaritis at 3 months is associated with chronic rejection at 1 year. Transplantation. 2007;83:1416-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Haas M, Montgomery RA, Segev DL, Rahman MH, Racusen LC, Bagnasco SM, Simpkins CE, Warren DS, Lepley D, Zachary AA. Subclinical acute antibody-mediated rejection in positive crossmatch renal allografts. Am J Transplant. 2007;7:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 53. | Wavamunno MD, O’Connell PJ, Vitalone M, Fung CL, Allen RD, Chapman JR, Nankivell BJ. Transplant glomerulopathy: ultrastructural abnormalities occur early in longitudinal analysis of protocol biopsies. Am J Transplant. 2007;7:2757-2768. [PubMed] |
| 54. | Dean PG, Park WD, Cornell LD, Gloor JM, Stegall MD. Intragraft gene expression in positive crossmatch kidney allografts: ongoing inflammation mediates chronic antibody-mediated injury. Am J Transplant. 2012;12:1551-1563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 55. | Sis B, Jhangri GS, Riopel J, Chang J, de Freitas DG, Hidalgo L, Mengel M, Matas A, Halloran PF. A new diagnostic algorithm for antibody-mediated microcirculation inflammation in kidney transplants. Am J Transplant. 2012;12:1168-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 56. | Everly MJ, Everly JJ, Susskind B, Brailey P, Arend LJ, Alloway RR, Roy-Chaudhury P, Govil A, Mogilishetty G, Rike AH. Bortezomib provides effective therapy for antibody- and cell-mediated acute rejection. Transplantation. 2008;86:1754-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 310] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 57. | Bentall A, Tyan DB, Sequeira F, Everly MJ, Gandhi MJ, Cornell LD, Li H, Henderson NA, Raghavaiah S, Winters JL. Antibody-mediated rejection despite inhibition of terminal complement. Transpl Int. 2014;27:1235-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 58. | Stegall MD, Gloor J, Winters JL, Moore SB, Degoey S. A comparison of plasmapheresis versus high-dose IVIG desensitization in renal allograft recipients with high levels of donor specific alloantibody. Am J Transplant. 2006;6:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 322] [Article Influence: 16.9] [Reference Citation Analysis (0)] |