Published online Apr 28, 2016. doi: 10.3748/wjg.v22.i16.4226
Peer-review started: November 10, 2015
First decision: December 21, 2015
Revised: January 13, 2016
Accepted: January 30, 2016
Article in press: January 30, 2016
Published online: April 28, 2016
Processing time: 160 Days and 23.5 Hours
AIM: To assess the impact of eukaryotic elongation factor 1 alpha 2 (eEF1A2) on hepatocellular carcinoma (HCC) cell proliferation, apoptosis, migration and invasion, and determine the underlying mechanisms.
METHODS: eEF1A2 levels were detected in 62 HCC tissue samples and paired pericarcinomatous specimens, and the human HCC cell lines SK-HEP-1, HepG2 and BEF-7402, by real-time PCR and immunohistochemistry. Experimental groups included eEF1A2 silencing in BEL-7402 cells with lentivirus eEF1A2-shRNA (KD group) and eEF1A2 overexpression in SK-HEP-1 cells with eEF1A2 plasmid (OE group). Non-transfected cells (control group) and lentivirus-based empty vector transfected cells (NC group) were considered control groups. Cell proliferation (MTT and colony formation assays), apoptosis (Annexin V-APC assay), cell cycle (DNA ploidy assay), and migration and invasion (Transwell assays) were assessed. Protein levels of PI3K/Akt/NF-κB signaling effectors were evaluated by Western blot.
RESULTS: eEF1A2 mRNA and protein levels were significantly higher in HCC cancer tissue samples than in paired pericarcinomatous and normal specimens. SK-HEP-1 cells showed lower eEF1A2 mRNA levels; HepG2 and BEL-7402 cells showed higher eEF1A2 mRNA levels, with BEL-7402 cells displaying the highest amount. Efficient eEF1A2 silencing resulted in reduced cell proliferation, migration and invasion, increased apoptosis, and induced cell cycle arrest. The PI3K/Akt/NF-κB signaling pathway was notably inhibited. Inversely, eEF1A2 overexpression resulted in promoted cell proliferation, migration and invasion.
CONCLUSION: eEF1A2, highly expressed in HCC, is a potential oncogene. Its silencing significantly decreases HCC tumorigenesis, likely by inhibiting PI3K/Akt/NF-κB signaling.
Core tip: Whether eukaryotic elongation factor 1 alpha 2 (eEF1A2) affects hepatocellular carcinoma (HCC) biology is largely unknown. In this study, eEF1A2 mRNA and protein levels were significantly higher in HCC cancer tissue samples than in paired control specimens. Efficient eEF1A2 silencing resulted in reduced cell proliferation, migration and invasion, increased apoptosis, and induced cell cycle arrest; the PI3K/Akt/NF-κB signaling pathway was notably inhibited. Inversely, eEF1A2 overexpression resulted in promoted cell proliferation, migration and invasion. Our findings indicate that eEF1A2 is a potential oncogene, whose silencing significantly decreases HCC tumorigenesis, likely by inhibiting PI3K/Akt/NF-κB signaling.
- Citation: Qiu FN, Huang Y, Chen DY, Li F, Wu YA, Wu WB, Huang XL. Eukaryotic elongation factor-1α 2 knockdown inhibits hepatocarcinogenesis by suppressing PI3K/Akt/NF-κB signaling. World J Gastroenterol 2016; 22(16): 4226-4237
- URL: https://www.wjgnet.com/1007-9327/full/v22/i16/4226.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i16.4226
Human eukaryotic elongation factor 1 alpha 2 (eEF1A2) gene is located on chromosome 20q13.3 and participates in peptide chain elongation during protein translation, therefore playing a critical role in protein synthesis[1]. Traditionally, eEF1A2 was considered a housekeeping protein, with expression limited to the heart, brain and skeletal muscle[1,2]. However, recent studies have attributed more biological functions to eEF1A2 besides its role as an elongation factor. eEF1A2 has carcinogenic potential due to these diverse functions. Recent studies have demonstrated that eEF1A2 is highly expressed in tumors from multiple tissues, indicating a tight relation between eEF1A2 and tumor genesis, development and biological behaviors[3-7]. eEF1A2 is known to transmit signals from the cytoplasm to the nucleus through interaction with zinc finger protein 1 and subsequent interactions with a set of receptors with tyrosine kinase ability, thus promoting efficient cell proliferation[8]. Besides, eEF1A2 can also regulate cell apoptosis; high levels of rat eEF1A2 protect muscle cells from caspase-3-mediated apoptosis[9]. In addition, the interaction between eEF1A2 and peroxidase I protects cells from oxidative stress-induced apoptosis, involving the suppression of caspase-3 and caspase-8 pyrolysis[10]. Taken together, these findings indicate that eEF1A2 regulation of tumorigenesis may be associated with its apoptosis inhibitory ability[11]. In addition to cell proliferation and apoptosis regulation, eEF1A2 is also involved in cytoskeleton rearrangement, interacting with F-actin and shortening microtubules, thus playing an important role in tumor invasion and migration[11,12]. High eEF1A2 levels were reported to induce filopodia generation in breast cancer cells, enhancing their migration and invasion abilities[11]. Conversely, targeted silencing of eEF1A2 reduces the migration and invasion abilities of some tumor cells. Highly expressed in human breast cancer BT-549 cells and mouse plasmacytoma ABPC4 cells, eEF1A2 efficiently binds Akt and induces its phosphorylation, promoting tumor cell invasion and migration, and thereby increasing malignancy[13].
Primary hepatocellular carcinoma (HCC) is a common malignancy in China, whose annual cases exceed 50% of the global incidence[14]. The mechanism and potential therapeutic targets of HCC are hotspots in global liver cancer research. Recently, the microarray-discrepant genome hybridization technology was applied to analyze gene expression profiles and functions of candidate genes in HCC cancer tissues and cell lines. The results revealed exceptionally high expression of eEF1A2 in HCC, which is quite intriguing[15-17]. Akt is a major regulator in the PI3K/Akt/NF-κB signaling pathway, and can be activated upon phosphorylation to subsequently phosphorylate a series of downstream targets[18]. As an important target downstream of Akt, NF-κB participates in multiple biological processes such as cell proliferation, apoptosis, invasion and migration, by regulating the transcription of various genes[19]. EEF1A2 was identified as an upstream inducer of PI3K[17], which promotes cell migration, invasion and metastasis in pancreatic cancer[12]. However, there is no report about the effects of eEF1A2 silencing on HCC progression, and the mechanism underlying eEF1A2 involved in HCC is largely unknown.
This study aimed to assess the effect of eEF1A2 on HCC cell proliferation, apoptosis, invasion, and migration, and determine its effect on PI3K/Akt/NF-κB signaling in HCC. We found that eEF1A2 promotes HCC tumorigenicity, likely by regulating the PI3K/Akt/NF-κB signaling pathway. These findings indicate that eEF1A2 is a potential novel therapeutic target for HCC.
A total of 62 HCC patients were enrolled, who had surgical excision and pathology confirmation from October 2012 to December 2013 in Fujian Provincial Hospital. They included 52 males and 10 females of 54.3 ± 12.2 (18.0-73.0) years old. None of them had radiotherapy or chemotherapy before surgery. Liver cancer and paired pericarcinomatous tissue specimens in each patient were excised. A part of each tissue sample was fixed in 10% neutral buffered formalin for immunohistochemistry, while the remaining portion was kept in RNA preserving fluid (Beijing ComWin Biotech Co., Ltd.) overnight, before storage at -80 °C for real-time polymerase chain reaction (PCR).
Normal liver tissue specimens were obtained from 20 patients with liver hemangioma who had surgical excision from October 2012 to December 2013 in Fujian Provincial Hospital. They included 12 men and 8 women of 40.8 ± 10.4 (25.0-66.0) years old. These control patients were hepatitis and liver cirrhosis free. The excised peripheral liver hemangioma tissue specimens were treated as described above for liver cancer and paired pericarcinomatous tissue samples.
This study was approved by the Ethics Committee of Fujian Provincial Hospital; all patients provided signed informed consent.
The human HCC cell lines SK-HEP-1, HepG2 and BEL-7402 were purchased from Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (China). Cells were cultured in DMEM (Gibco, United States) supplemented with 10% fetal bovine serum (FBS, Gibco) and incubated at 37 °C in a humid environment containing 5% CO2.
According to eEF1A2 sequence in GenBank database (NM_001958), an interfering shRNA sequence targeting site 418 was designed as ACTACATCACCATCATCGA, with the control scrambled siRNA sequence TTCTCCGAACGTGTCACGT. Both shRNA fragments were cloned into the GV115 vector (Shanghai Genechem Biotech Co, Ltd, China) with Age I/EcoR I double restriction enzyme digestion sites. Reconstructed lentivirus vectors expressing GV115-eEF1A2-shRNA and the negative control were transformed into competent E. coli DH5α, and positive colonies were selected for PCR and sequencing. Reconstructed lentivirus expressing vectors, packing plasmids pHelper 1.0 and pHelper 2.0 were co-transfected into 293T cells with Lipofectamine 2000 (Invitrogen). Forty-eight hours after transfection, the lentivirus containing culture medium was collected and concentrated. After titration, lentiviruses were stored at -80 °C.
To assess the role of eEF1A2 in tumor cell proliferation, apoptosis and migration, a lentivirus expressing eEF1A2-shRNA was used to infect log-phase BEL-7402 cells. The virus containing culture medium was replaced with fresh DMEM supplemented with 10% FBS at 12 h after infection. Five days post lentivirus infection, three BEL-7402 cell groups were set up: KD (knockdown, cells infected with eEF1A2-shRNA lentivirus), NC (negative control, cells infected with negative control-shRNA lentivirus) and CON (cells without lentivirus infection). Cells were used for subsequent experiments, when lentiviral transfection efficiency was above 80%.
According to eEF1A2 sequence in GenBank database (NM_001958), the following primers were designed: forward 5’-GAGGATCCCCGGGTACCGGTCGCCACCATGGGCAAGGAGAAGACCCAC-3, and reverse 5’-TCCTTGTAGTCCATACCCTTGCCCGCCTTCTGCGCCTT CTGCGCCGACTTG-3’ for eEF1A2 cloning. Exogenous eEF1A2 was expressed in SK-HEP-1 cells via transduction of the lentivirus plasmid pGLV5-eEF1A2 (pGLV5 was from GenePharma Co, Ltd, China), to assess the role of eEF1A2 in tumor cell proliferation, apoptosis and migration. Three groups of cells were set up: OE (overexpression, cells infected with eEF1A2 lentivirus), NC (negative control, cells infected with negative control lentivirus) and CON (cells without lentivirus infection). The SK-HEP-1 cells were collected 5 d after infection, and used for RT-PCR or Western blot.
Total RNA was extracted from homogenized samples using TRIzol Reagent (Invitrogen, United States) and treated with DNase. A total of 62 HCC liver cancer and paired pericarcinomatous tissue specimens, 20 normal liver tissue samples, and log-phase HCC SK-HEP-1 and HepG2 cells were assessed. In addition, the three BEL-7402 cell groups (KD, NC, and CON) were analyzed after 5 d of culture post-lentiviral infection, as described above. For each sample, 2 μg of RNA were used for cDNA synthesis with a specific kit (Promega, United States). Real-time PCR was performed with SYBR Green I (Applied Biosystems, United States) on ABI 7300 (Applied Biosystems). The primers used are described in Table 1.
| Gene | Primers (5’-3’) | Length (bp) |
| eEF1A2 | GTCAAGGAAGTCAGCGCCTAC | 124 |
| TGAACCACGGCATGTTGGG | ||
| GAPDH | TGACTTCAACAGCGACACCCA | 121 |
| CACCCTGTTGCTGTAGCCAAA |
Liver tissues were fixed in 10% neutral formalin, and paraffin embedded. After sectioning, an SP immunohistochemistry kit (Fuzhou Maixin Biotech Co., Ltd, China) was used for staining. Rabbit anti-human eEF1A2 polyclonal antibody (1:50, Novus Biologicals, United States) was used for eEF1A2 detection according to the manufacturer’s instructions. Positive cells were identified by claybank particles in the cytoplasm. Five hundred cells were counted under a high power lens, and positive and negative staining considered with ≥ 10% and < 10% dyed cells, respectively.
BEL-7402 cells were cultured for 5 d post lentivirus infection, and lysed with cell lysis buffer (Hyclone-Pierce, United States) for total protein extraction. After quantification, 50 μg protein were separated by SDS-PAGE and electro-transferred onto PVDF membranes. After blocking, the membranes were incubated with primary antibodies, including rabbit anti-GAPDH, Akt, p-Akt, IκB, p-IκB (Ser32), p-NF-κB p65 (ser468), Bcl-2, MMP-9 and MMP-2 (Cell Signaling Technology, United States), rabbit anti-eEF1A2 (Novus Biologicals), rabbit anti-c-Myc (Santa Cruz, United States), and rabbit anti- NF-κB p65 (eBioscience, United States). Horseradish peroxidase (HRP)-conjugated goat-anti-rabbit IgG (KPL, United States) was used as a secondary antibody. After exposure and film development, protein bands were scanned with a Gel Doc gel image analyzer (Bio-Rad, United States). Relative protein levels were determined with GAPDH as a reference.
HCC cells infected with lentivirus for 3 d were seeded in 96-well plates at a density of 2 × 103 cells/well in 100 μL culture medium. Three replicates were set up for each group. After lentiviral infection of BEL-7402 cells followed by 1-5 d of culture, 10 μL of 5 mg/mL MTT (Sigma) were added into wells, and further incubated for 4 h at 37 °C. Then, culture medium was carefully removed followed by addition of 100 μL DMSO to dissolve the purple crystals. Finally, absorbance was read at 490 nm on an American stat Fax-2100microplate reader (Awareness Technology, United States). Cell proliferation curve was obtained with absorbance values and time on the vertical and horizontal axes, respectively.
HCC cells infected with lentivirus for 3 d were seeded in 6-well plates at a density of 4 × 102 cells /well. Three replicate wells were set up for each group, and cultured continuously for 14 d. Then, cells were washed twice with PBS, fixed with paraformaldehyde for 30 min, and submitted to Giemsa staining for 20 min. Cell colonies were counted under a microscope. Colony formation rate was derived as (colony numbers/inoculated cell numbers) × 100%.
Five days after lentivirus infection, cells were washed with PBS, and fixed with 70% ethanol (pre-cooled at 4 °C) for 1 h. After ethanol removal, cells were loaded with propidium iodide (PI) for 15 min in the dark, according to the DNA ploidy detection kit (Sigma, United States). Cell cycle distribution was analyzed by flow cytometry on FACS Calibur (BD Biosciences, United States) with > 10000 cells assessed for each sample.
After 5 d of lentivirus infection, HCC cells were washed with PBS, and assessed for apoptosis using the Annexin V-APC apoptosis detection kit (eBioscience) according to the manufacturer’s instructions. Flow cytometry was carried out on FACS Calibur (BD Biosciences).
Transwell chambers for cell migration assessment were purchased from Corning (United States). Those used for cell invasion assays were from BD Biosciences, with Matrigel on the filter membrane. The chambers were placed in 24-well plates, and 1 × 106 cells added to the upper chambers in 200 μL of serum free DMEM; meanwhile, 500 μL of complete medium were added to the bottom chambers. Incubation was carried out for 24 h at 37 °C with 5% CO2. Then, the culture medium in the upper chamber as well as cells on the filter membrane (invasion assay) were removed. The cells that had passed through the filter membrane were submitted to Giemsa staining for 30 min and dissolved in 180 μL of 10% acetic acid. 100 μL of the resulting solution were used for absorbance measurements at 570 nm on an American stat Fax-2100 microplate reader. Finally, cell migration and invasion rates were calculated, respectively.
Data are mean ± SD of at least three replicates. Student’s t-test was used to compare continuous measurement data with a normal distribution between two groups. One way analysis of variance (ANOVA) was employed while comparing three groups. Mann-Whitney U test was used to evaluate continuous data with a non-normal distribution; χ2 test was used to compare positive rates. Statistical analyses were performed with the SPSS13.0 software (SPSS, United States). P < 0.05 was considered statistically significant.
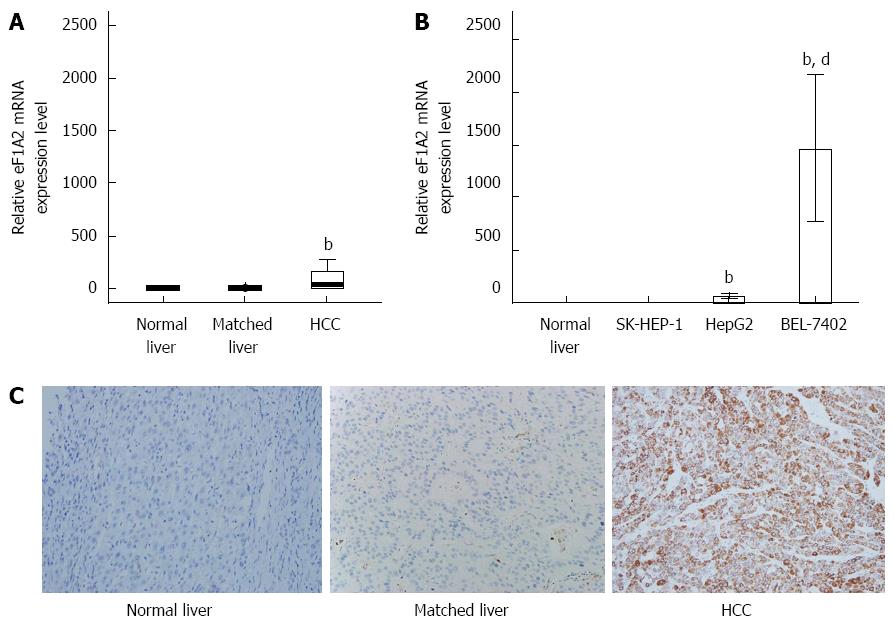
To determine the relationship between eEF1A2 and HCC, we assessed eEF1A2 expression levels in 62 HCC and paired pericarcinomatous tissue samples, 20 normal liver tissue specimens, and HCC cell lines. We found that median eEF1A2 mRNA levels in HCC tissue samples [35.27 (2.45-166.15)] were significantly higher compared with paired pericarcinomatous specimens [1.34 (0.78-1.68)] and normal liver tissue samples [1.22 (0.72-1.63)] (P < 0.01; Figure 1A). Besides, the HCC cell lines HepG2 and BEL-7402, but not SK-HEP-1, showed higher eEF1A2 mRNA levels compared with normal liver tissues (Figure 1B). Furthermore, immunohistochemistry data revealed that eEF1A2 was mainly expressed in the cytoplasm. Importantly, 47 HCC tissues showed positive eEF1A2 expression (a positive rate of 75.8%), while only 8.1% of paired pericarcinomatous tissue samples stained for eEF1A2 (47/62) (Table 2). No eEF1A2 positive sample was found in normal liver tissues (Table 2). Accordingly, eEF1A2 protein levels in HCC were significantly higher than values obtained for paired pericarcinomatous and normal liver tissues (P < 0.01) (Figure 1C).
| eEF1A2 positive tissue/total tissue | Positive rate | |
| HCC | 47/62 | 75.8% |
| Paired pericarcinomatous | 5/62 | 8.1% |
| Normal liver | 0/20 | 0% |
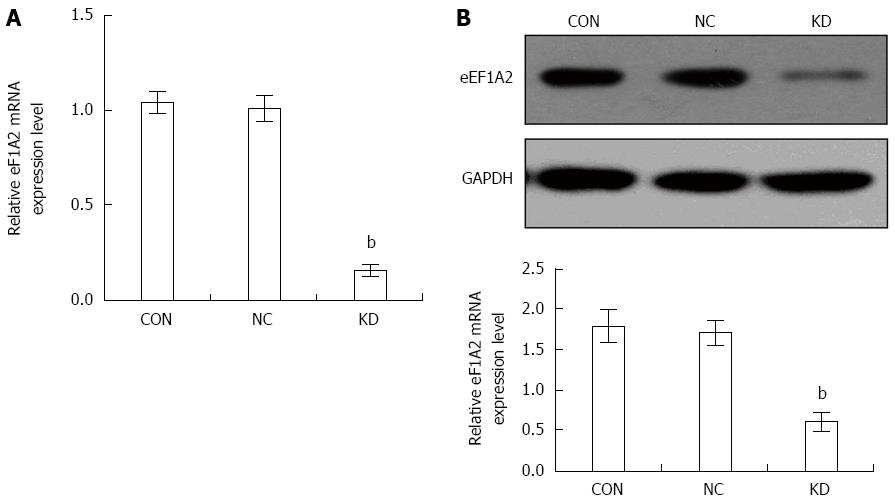
BEL-7402 cells were selected for subsequent studies for its higher eEF1A2 expression compared with the other cell lines (Figure 1B). BEL-7402 cells were infected with lentivirus expressing eEF1A2-shRNA for 5 d. Then, eEF1A2 mRNA levels were decreased by 84.10 ± 3.32% in the KD group compared with the NC group (P < 0.01) as shown in Figure 2A. In agreement, eEF1A2 protein amounts in the KD group were reduced by 64.47% ± 7.07% compared with the values obtained for the NC group (P < 0.01) (Figure 2B). There were no differences between the NC and CON groups in eEF1A2 mRNA or protein levels (Figure 2A and B).
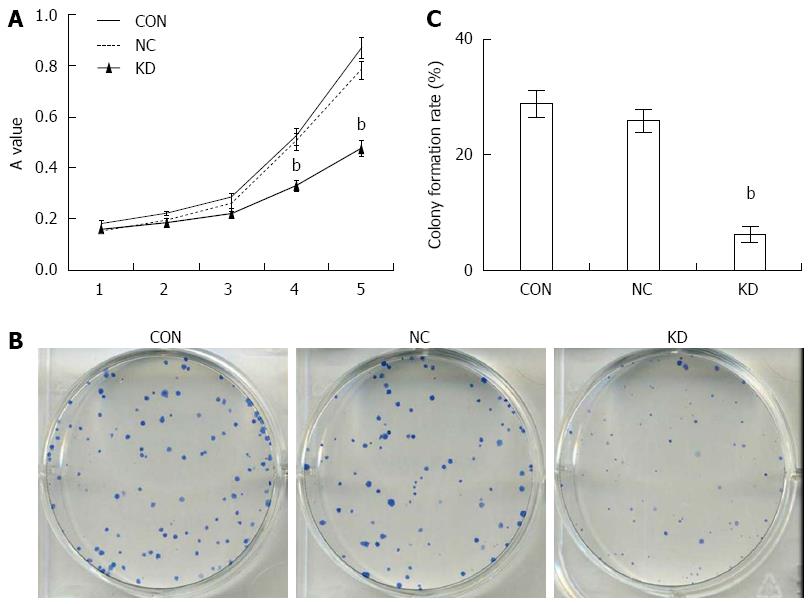
The accelerated proliferation of tumor cells is a crucial mechanism in tumor deterioration. MTT results showed that BEL-7402 cells of the NC and CON groups began to proliferate at 2 d of culture, entering into log-phase at 3 d, with a normal growth curve. In contrast, cell proliferation in the KD group was remarkably suppressed, with significantly lower proliferation obtained at 4 and 5 d compared with the NC group (P < 0.01): inhibition rates of 34.36% ± 4.65% and 39.44% ± 3.94% were obtained, respectively (Figure 3A). These results were further confirmed by the colony formation assay; compared with the NC group, the KD group showed an overtly declined colony formation rate of 6.70% ± 6.08% (P < 0.01) (Figure 3B and C).
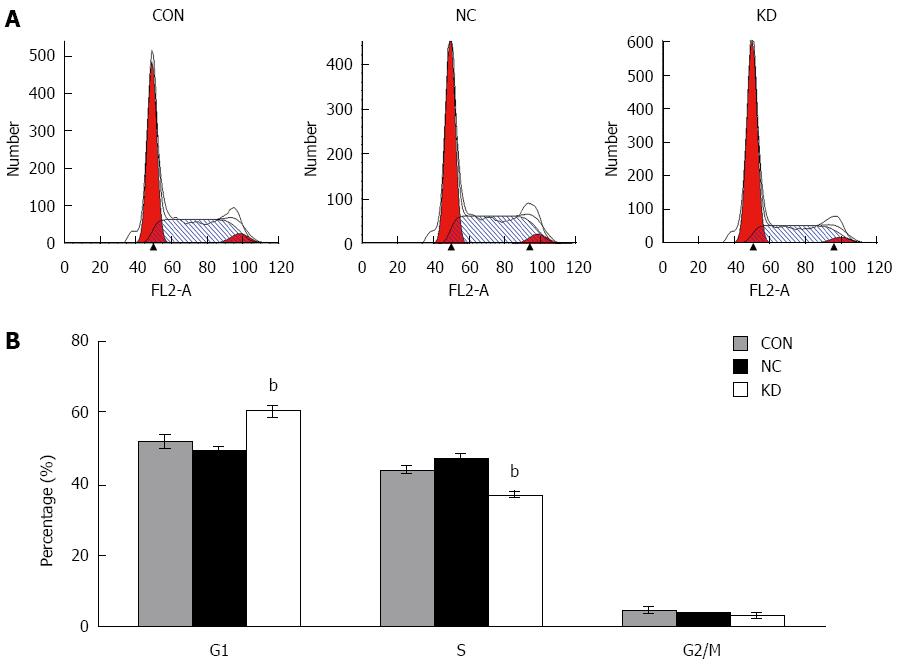
To determine the cause of decreased cell proliferation in eEF1A2 deprived BEL-7402 cells, the effect of eEF1A2 knockdown on cell cycle progression was analyzed by flow cytometry. DNA ploidy detection indicated that after lentivirus infection, BEL-7402 cells showed 60.13% ± 2.16% of G0/G1 cells, a much higher rate than that of the NC group (49.28% ± 1.29%, P < 0.05); consequently, S phase rates were significantly decreased in the KD group compared with the NC group (P < 0.01) (Figure 4A and B).
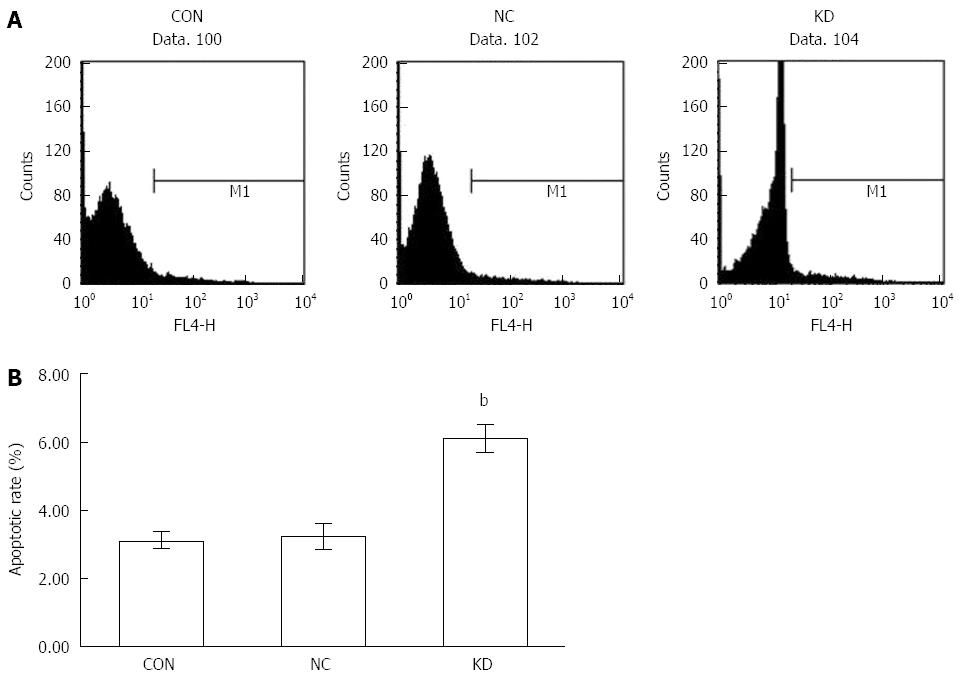
Another effective way to inhibit tumor progression is to promote apoptosis. In an Annexin V-APC assay, we showed that after lentivirus infection, BEL-7402 cells in the KD group showed a high apoptosis rate (6.09% ± 0.40%), significantly increased compared with those of the NC (3.24% ± 0.39%) and CON (3.12% ± 0.25%) groups (P < 0.01) as shown in Figure 5.
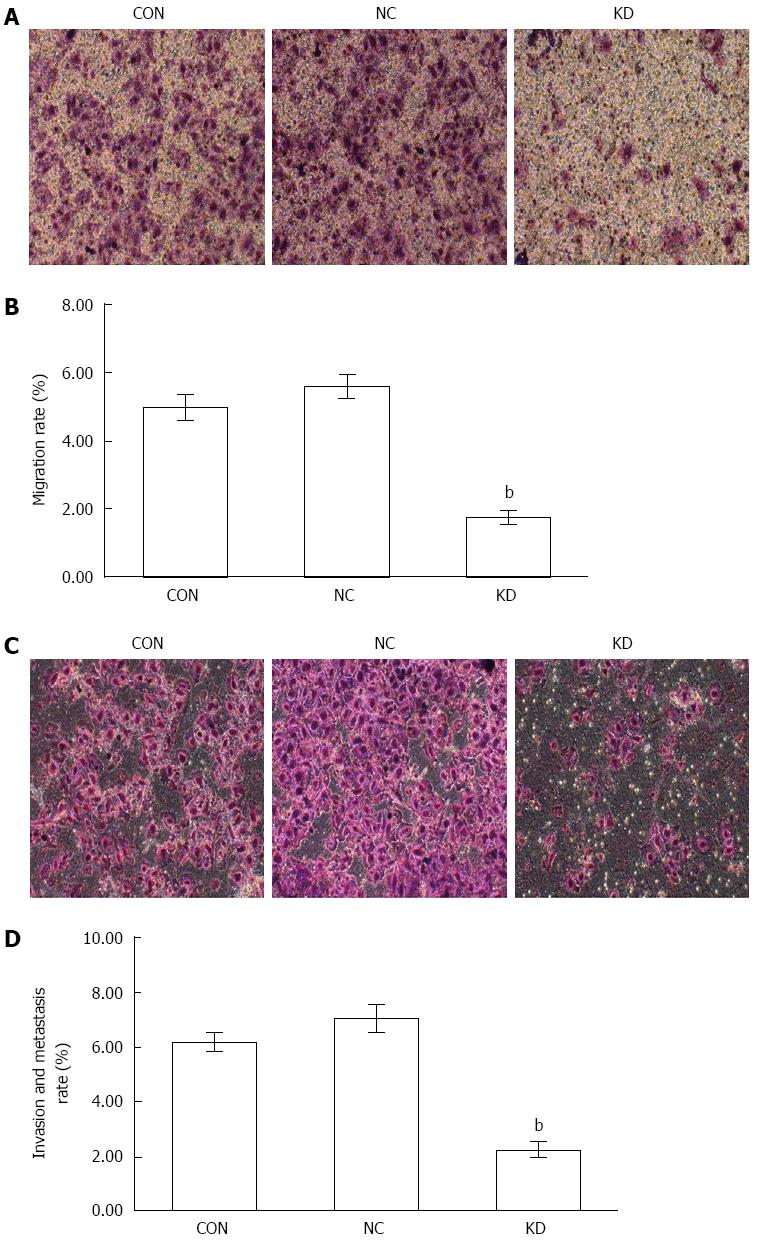
Migration and invasion are important strategies in tumor worsening, and their inhibition effectively prevents tumor progression. Transwell assays demonstrated that the migration rate of BEL-7402 cells in the KD group (1.76 ± 0.22) was much lower compared with the NC group (5.62 ± 0.31) (Figure 6A and B). Similarly, BEL-7402 cells in the KD group showed a significantly decreased invasion rate (2.26 ± 0.27) compared with the NC group (7.06 ± 0.50, P < 0.01) (Figure 6C and D).
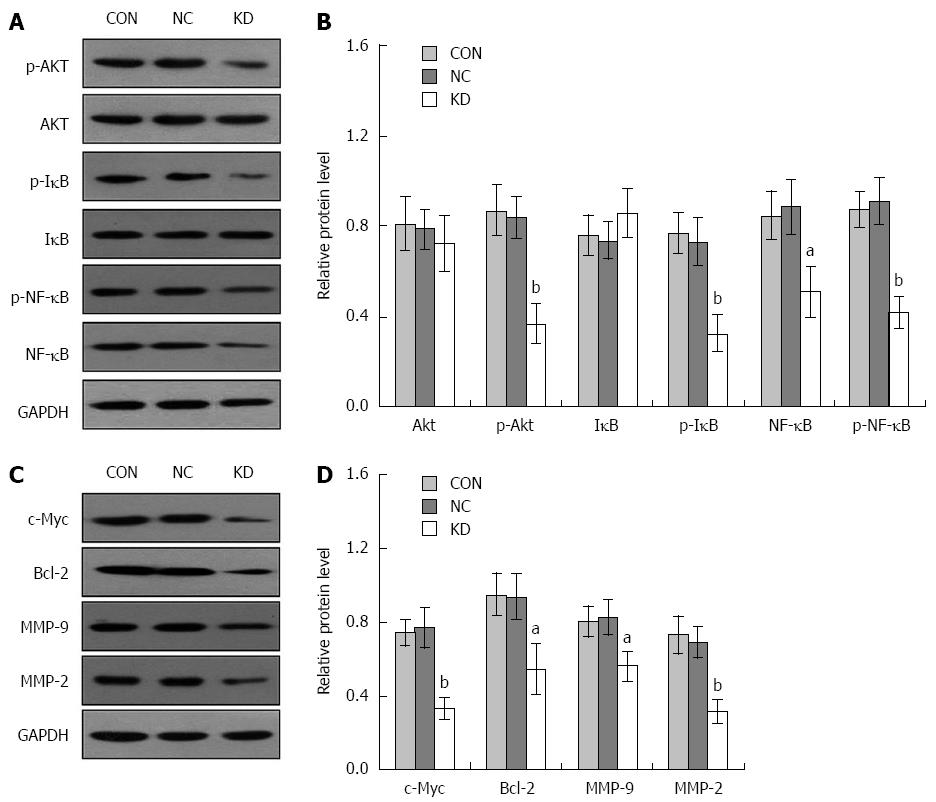
To further explore the molecular mechanism underlying the eEF1A2 effect in BEL-7402 cells, activation of the PI3K/AKT/NF-κB signaling pathway was assessed. As shown in Figure 7A and B, eEF1A2 knockdown significantly suppressed p-Akt, p-IκB (Ser32), p-NF-κB (P < 0.01) and NF-κB (P < 0.05) protein levels in BEL-7402 cells. In addition, c-Myc, MMP-2 (P < 0.01), Bcl-2 and MMP-9 (P < 0.05) as downstream target genes regulated by NF-κB, were downregulated in the KD group compared with the NC group (Figure 7C and D).
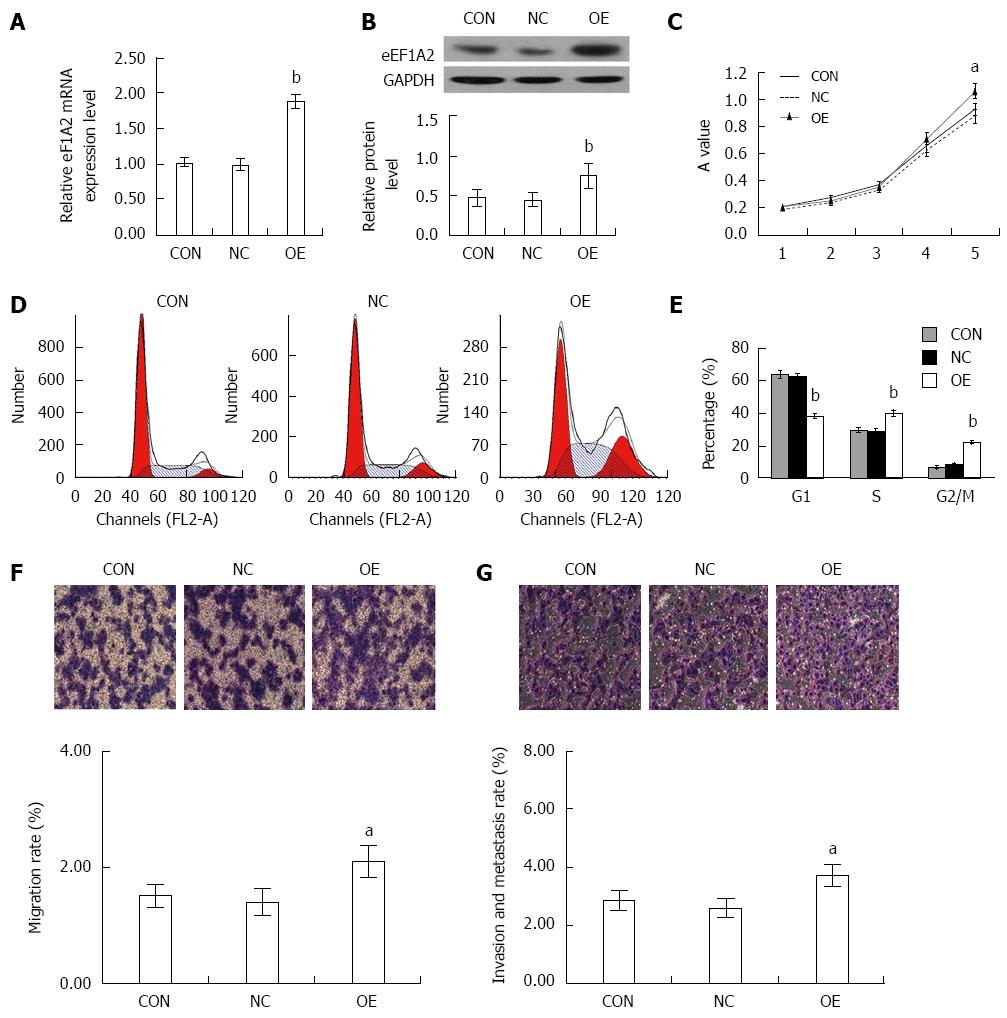
To evaluate how eEF1A2 affects the proliferation and invasion of HCC cells, we established SK-HEP-1 cell lines that overexpressed eEF1A2 using lentivirus-based overexpression eEF1A2 system. The levels of ectopic eEF1A2 mRNA and protein expression were considerably higher than those of endogenous eEF1A2 in the negative lentiviurs control (NC) and blank control cells (CON) as determined by qRT–PCR and Western blot (Figure 8A and B). Increased proliferation properties of the eEF1A2-expressing cells were observed by MTT assay (Figure 8C). Cell cycle progression was analyzed by flow cytometry. DNA ploidy detection indicated that overexpression of eEF1A2 induced lower G0/G1 and higher S and G2/M rates (Figure 8D and E). Transwell assays demonstrated that overexpression of eEF1A2 significantly increased the migration and invasion rates of SK-HEP cells (Figure 8F and G).
eEF1A2 has an important role in carcinogenesis and tumour metastasis; however, the mechanism underlying its activity remains unclear. In the present work, we indicated that eEF1A2, highly expressed in HCC, is a potential oncogene. Its silencing significantly suppresses cell proliferation, promotes apoptosis, and decreases migration and invasion rates in HCC cells, likely by inhibiting the PI3K/Akt/NF-κB signaling pathway. These findings indicate that eEF1A2 is a potential novel therapeutic target for HCC.
eEF1A2 is a multifunctional protein and potential oncogene. Anand et al[3] first proposed a possible role for eEF1A2 in the development of ovarian cancer. Besides ovarian cancer, the abnormally high expression of eEF1A2 has been described in breast, prostate, lung and gastrointestinal cancers[4-7]. The high eEF1A2 expression in malignant liver tumors has aroused increasing attention recently[15-17]. In the present work, we also found exceptionally high eEF1A2 expression in HCC cancer, which showed a positive rate of 75.8% as determined by immunohistochemistry. Taken together, these findings indicate a relationship between high eEF1A2 expression and HCC occurrence.
Due to the high eEF1A2 levels in the BEL-7402 cell line, the latter was selected for lentivirus-mediated knockdown of eEF1A2. As shown above, eEF1A2 silencing in BEL-7402 cells significantly inhibited cell proliferation, induced apoptosis, and caused cell cycle arrest in the G0/G1 phase. In addition, eEF1A2 suppression efficiently inhibited BEL-7402 migration and invasion. Inversely, overexpression of eEF1A2 in SK-HEP-1 cells increased cell proliferation, and promoted migration and invasion. These results was consistent with the role of eEF1A2 in pancreatic cancer, which indicated that overexpression of eEF1A2 promoted cell growth, survival, and invasion in pancreatic cancer[20]. These findings indicate a carcinogenic role for eEF1A2 in HCC, and its abnormally high expression is tightly related to proliferation, apoptosis, invasion and migration in HCC cells.
eEF1A2 promotes phosphorylation of tyrosine residues[8], and PI3K/Akt is an important pathway activated downstream of tyrosine phosphorylation. Constitutive activation of PI3K/Akt leads to decreased apoptosis and/or accelerated cell cycle, which result in cell malignancy[21]. Indeed, genesis and development of multiple malignant tumors are closely related to abnormal activation of the PI3K/Akt signaling pathway. Akt, a major regulator of PI3K/Akt signaling, is activated upon phosphorylation. Recent findings have revealed an Akt-specific binding site in eEF1A2[22], which is involved in the regulation of Akt phosphorylation and activity[11,13]. As shown above, eEF1A2 knockdown significantly inhibited Akt activity, indicating the ability of eEF1A2 to efficiently regulate Akt phosphorylation in HCC cells. As an important downstream target of Akt, NF-κB exerts its role by transmitting and amplifying Akt signal, which makes the PI3K/Akt/NF-κB signaling pathway a critical target for anti-malignant tumor treatment[19]. As a transcription factor, NF-κB usually forms the IκB/NF-κB complex under normal conditions, where its transcriptional activity is suppressed. Activated Akt separates and activates NF-κB from the IκB/NF-κB complex by phosphorylating IκB. On the other hand, by directly phosphorylating Ser529 and Ser536 sites on NF-κB already interacting with the related binding sites, Akt promotes its transcriptional activity. Upon activation, NF-κB regulates the expression of multiple genes involved in cell proliferation, apoptosis, invasion and migration, thus augmenting cell malignancy[19]. We demonstrated that eEF1A2 silencing inhibits IκB and NF-κB phosphorylation. These findings indicate the inhibitory impact of eEF1A2 knockdown on NF-κB transcriptional activity, which likely translates into altered NF-κB-mediated effects on proliferation, apoptosis, invasion and migration in tumor cells. Moreover, with NF-κB-binding sites in their gene sequences, the oncoprotein c-Myc[23], the anti-apoptosis factor Bcl-2[24], MMP-9[25] and MMP-2[26] are considered important targets downstream of NF-κB. As shown above, c-Myc, Bcl-2, MMP-9 and MMP-2 expression levels were significantly reduced in eEF1A2-deficient cells. These findings indicate that eEF1A2 affects HCC proliferation, migration, invasion and apoptosis likely by regulating the PI3K/Akt/NF-κB signaling pathway. Further studies are needed to unveil the mechanisms underlying the regulatory activity of eEF1A2 on PI3K/Akt/NF-κB signaling.
In conclusion, we propose a potential carcinogenic role of eEF1A2 in HCC cells. Indeed, eEF1A2 is involved in cell proliferation, apoptosis, migration and invasion during HCC carcinogenesis and development via regulation of PI3K/Akt/NF-κB signaling. Further studies are warranted to determine whether other signaling pathways play a role in eEF1A2-mediated carcinogenesis. Overall, our findings indicate eEF1A2 as a novel diagnostic marker and therapeutic target in HCC.
Human eukaryotic elongation factor 1 alpha 2 (eEF1A2) participates in peptide chain elongation during protein translation, therefore playing a critical role in protein synthesis and other biological pathways. Consequently, eEF1A2 has carcinogenic potential due to its diverse functions. However, the role of eEF1A2 in hepatocellular carcinoma (HCC) remains largely unknown.
High eEF1A2 levels induce filopodia generation in breast cancer cells, enhancing their malignancy, at least in part by modulating the Akt signaling pathway.
eEF1A2 overexpression resulted in promoted hepatocellular carcinoma cell proliferation, migration and invasion. Conversely, its silencing resulted in opposite effects, with decreased activity of the PI3K/Akt/NF-κB signaling pathway.
These findings suggest a potential carcinogenic role for eEF1A2 in HCC cells. Therefore, eEF1A2 should be considered a novel diagnostic marker and therapeutic target in HCC.
The human eukaryotic elongation factor 1 alpha 2 (eEF1A2) gene is located on chromosome 20q13.3.
In this study, the authors found significantly higher eEF1A2 mRNA and protein levels in HCC cancer tissue samples compared with paired control specimens. In accordance, they demonstrated that eEF1A2 knockdown resulted in decreased HCC malignancy, with PI3K/Akt/NF-κB signaling markedly repressed. Meanwhile, eEF1A2 overexpression resulted in increased malignancy potential of HCC cells. These data reveal eEF1A2 as a potential oncogene, which likely acts through PI3K/Akt/NF-κB signaling.
P- Reviewer: Iqbal M S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Knudsen SM, Frydenberg J, Clark BF, Leffers H. Tissue-dependent variation in the expression of elongation factor-1 alpha isoforms: isolation and characterisation of a cDNA encoding a novel variant of human elongation-factor 1 alpha. Eur J Biochem. 1993;215:549-554. [PubMed] |
| 2. | Kahns S, Lund A, Kristensen P, Knudsen CR, Clark BF, Cavallius J, Merrick WC. The elongation factor 1 A-2 isoform from rabbit: cloning of the cDNA and characterization of the protein. Nucleic Acids Res. 1998;26:1884-1890. [PubMed] |
| 3. | Anand N, Murthy S, Amann G, Wernick M, Porter LA, Cukier IH, Collins C, Gray JW, Diebold J, Demetrick DJ. Protein elongation factor EEF1A2 is a putative oncogene in ovarian cancer. Nat Genet. 2002;31:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 199] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Kulkarni G, Turbin DA, Amiri A, Jeganathan S, Andrade-Navarro MA, Wu TD, Huntsman DG, Lee JM. Expression of protein elongation factor eEF1A2 predicts favorable outcome in breast cancer. Breast Cancer Res Treat. 2007;102:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Zhu H, Lam DC, Han KC, Tin VP, Suen WS, Wang E, Lam WK, Cai WW, Chung LP, Wong MP. High resolution analysis of genomic aberrations by metaphase and array comparative genomic hybridization identifies candidate tumour genes in lung cancer cell lines. Cancer Lett. 2007;245:303-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Scaggiante B, Dapas B, Bonin S, Grassi M, Zennaro C, Farra R, Cristiano L, Siracusano S, Zanconati F, Giansante C. Dissecting the expression of EEF1A1/2 genes in human prostate cancer cells: the potential of EEF1A2 as a hallmark for prostate transformation and progression. Br J Cancer. 2012;106:166-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Duanmin H, Chao X, Qi Z. eEF1A2 protein expression correlates with lymph node metastasis and decreased survival in pancreatic ductal adenocarcinoma. Hepatogastroenterology. 2013;60:870-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (1)] |
| 8. | Panasyuk G, Nemazanyy I, Filonenko V, Negrutskii B, El’skaya AV. A2 isoform of mammalian translation factor eEF1A displays increased tyrosine phosphorylation and ability to interact with different signalling molecules. Int J Biochem Cell Biol. 2008;40:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Ruest LB, Marcotte R, Wang E. Peptide elongation factor eEF1A-2/S1 expression in cultured differentiated myotubes and its protective effect against caspase-3-mediated apoptosis. J Biol Chem. 2002;277:5418-5425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Chang R, Wang E. Mouse translation elongation factor eEF1A-2 interacts with Prdx-I to protect cells against apoptotic death induced by oxidative stress. J Cell Biochem. 2007;100:267-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Amiri A, Noei F, Jeganathan S, Kulkarni G, Pinke DE, Lee JM. eEF1A2 activates Akt and stimulates Akt-dependent actin remodeling, invasion and migration. Oncogene. 2007;26:3027-3040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Xu C, Hu DM, Zhu Q. eEF1A2 promotes cell migration, invasion and metastasis in pancreatic cancer by upregulating MMP-9 expression through Akt activation. Clin Exp Metastasis. 2013;30:933-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 13. | Li Z, Qi CF, Shin DM, Zingone A, Newbery HJ, Kovalchuk AL, Abbott CM, Morse HC. Eef1a2 promotes cell growth, inhibits apoptosis and activates JAK/STAT and AKT signaling in mouse plasmacytomas. PLoS One. 2010;5:e10755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 15. | Grassi G, Scaggiante B, Farra R, Dapas B, Agostini F, Baiz D, Rosso N, Tiribelli C. The expression levels of the translational factors eEF1A 1/2 correlate with cell growth but not apoptosis in hepatocellular carcinoma cell lines with different differentiation grade. Biochimie. 2007;89:1544-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Schlaeger C, Longerich T, Schiller C, Bewerunge P, Mehrabi A, Toedt G, Kleeff J, Ehemann V, Eils R, Lichter P. Etiology-dependent molecular mechanisms in human hepatocarcinogenesis. Hepatology. 2008;47:511-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Pellegrino R, Calvisi DF, Neumann O, Kolluru V, Wesely J, Chen X, Wang C, Wuestefeld T, Ladu S, Elgohary N. EEF1A2 inactivates p53 by way of PI3K/AKT/mTOR-dependent stabilization of MDM4 in hepatocellular carcinoma. Hepatology. 2014;59:1886-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 18. | Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta. 2004;1697:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 552] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 19. | Ma Y, Wang J, Liu L, Zhu H, Chen X, Pan S, Sun X, Jiang H. Genistein potentiates the effect of arsenic trioxide against human hepatocellular carcinoma: role of Akt and nuclear factor-κB. Cancer Lett. 2011;301:75-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Cao H, Zhu Q, Huang J, Li B, Zhang S, Yao W, Zhang Y. Regulation and functional role of eEF1A2 in pancreatic carcinoma. Biochem Biophys Res Commun. 2009;380:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E. PI3K/AKT signaling pathway and cancer: an updated review. Ann Med. 2014;46:372-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 879] [Article Influence: 79.9] [Reference Citation Analysis (0)] |
| 22. | Lau J, Castelli LA, Lin EC, Macaulay SL. Identification of elongation factor 1alpha as a potential associated binding partner for Akt2. Mol Cell Biochem. 2006;286:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | La Rosa FA, Pierce JW, Sonenshein GE. Differential regulation of the c-myc oncogene promoter by the NF-kappa B rel family of transcription factors. Mol Cell Biol. 1994;14:1039-1044. [PubMed] |
| 24. | Viatour P, Bentires-Alj M, Chariot A, Deregowski V, de Leval L, Merville MP, Bours V. NF- kappa B2/p100 induces Bcl-2 expression. Leukemia. 2003;17:1349-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Andela VB, Gordon AH, Zotalis G, Rosier RN, Goater JJ, Lewis GD, Schwarz EM, Puzas JE, O’Keefe RJ. NFkappaB: a pivotal transcription factor in prostate cancer metastasis to bone. Clin Orthop Relat Res. 2003;S75-S85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Felx M, Guyot MC, Isler M, Turcotte RE, Doyon J, Khatib AM, Leclerc S, Moreau A, Moldovan F. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NF-kappaB in human osteosarcoma. Clin Sci (Lond). 2006;110:645-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |