Published online Apr 28, 2016. doi: 10.3748/wjg.v22.i16.4120
Peer-review started: July 6, 2015
First decision: August 26, 2015
Revised: September 24, 2015
Accepted: December 12, 2015
Article in press: December 14, 2015
Published online: April 28, 2016
Processing time: 290 Days and 10.9 Hours
AIM: To study the therapeutic effects of mesenchymal stem cells (MSCs) and an interleukin-1 receptor antagonist (IL-1Ra) in acute liver failure.
METHODS: Chinese experimental miniature swine (15 ± 3 kg, 5-8 mo) were obtained from the Laboratory Animal Centre of the Affiliated Drum Tower Hospital of Nanjing University Medical School. Acute liver failure was induced via 85% hepatectomy, and animals were treated by MSC transplantation combined with IL-1Ra injection. Blood samples were collected for hepatic function analysis, and the living conditions and survival time were recorded. Liver injury was histologically analyzed. Hepatic cell regeneration and apoptosis were studied by Ki67 immunohistochemistry and terminal deoxynucleotidyl transferase dUTP nick end labeling, respectively. The levels of protein kinase B and nuclear factor-κB expression were analyzed by Western blotting.
RESULTS: MSCs were infected with a lentivirus for expression of green fluorescent protein (GFP) for subsequent identification; 97.3% of the MSCs were positive for GFP as assessed by flow cytometry. Additional flow cytometric analysis of cell surface marker expression demonstrated that > 90% of GFP-expressing MSCs were also positive for CD29, CD44, and CD90, indicating that most of these cells expressed typical markers of MSCs, and the population of MSCs was almost pure. Transplantation of MSCs in combination with 2 mg/kg IL-1Ra therapy significantly improved survival time compared to the acute liver failure model group (35.3 ± 6.7 d vs 17.3 ± 5.5 d, P < 0.05). Combined therapy also promoted improvement in serum inflammatory cytokines and biochemical conditions. The observed hepatic histopathologic score was significantly lower in the group with combined therapy than in the model group (3.50 ± 0.87 vs 8.17 ± 1.26, P < 0.01). In addition, liver cell apoptosis in the combined therapy group was significantly inhibited (18.1 ± 2.1% vs 70.8 ± 3.7%, P < 0.01), and hepatic cell regeneration increased. A significant increase in protein kinase B expression and decrease in nuclear factor-κB expression were observed (P < 0.01), which supports their important roles in liver regeneration.
CONCLUSION: MSCs and IL-1Ra had a synergistic effect in liver regeneration via regulation of inflammation and apoptotic signaling.
Core tip: Combination therapy is superior to any method of mesenchymal stem cell (MSC) transplantation used alone. This study shows that combination therapy improved liver function, inhibited apoptosis, and prolonged the survival of swine with acute liver failure (ALF). Transplanted MSCs may participate in liver regeneration by promoting proliferation and inhibiting apoptosis during the initial stage of ALF following antagonism of interleukin-1 inflammatory signaling. Combination therapy with MSC transplantation and an interleukin-1 receptor antagonist, which enables restoration from ALF and liver reconstruction in swine, is a promising treatment option for ALF patients in the future.
- Citation: Sang JF, Shi XL, Han B, Huang X, Huang T, Ren HZ, Ding YT. Combined mesenchymal stem cell transplantation and interleukin-1 receptor antagonism after partial hepatectomy. World J Gastroenterol 2016; 22(16): 4120-4135
- URL: https://www.wjgnet.com/1007-9327/full/v22/i16/4120.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i16.4120
Acute liver failure (ALF) is characterized by severe liver cell damage due to virus infection, drugs, toxins, alcohol, or as a result of hepatectomy. ALF leads to hepatic encephalopathy, hepatorenal syndrome, severe infection, multiple organ failure, and even death[1]. Combating ALF requires either reduction of liver cell necrosis or stimulation of liver cell regeneration. This can be achieved with drug therapy, artificial liver therapy, stem cell transplantation, and liver transplantation[2]. Even though artificial liver therapy has the potential to reduce mortality, its efficacy is limited[3]. So far, liver transplantation is the most effective treatment for ALF. However, it has its own limitations due to many difficulties, including severe donor shortage, numerous complications, immune rejection, use of immunosuppressive agents, and high medical costs[4].
In recent years, stem cell therapy has become a new area of investigation for ALF treatment. This is mainly because stem cells are abundant, show low immunogenicity, and have the potential to differentiate into hepatocyte-like cells[5]. A large body of evidence suggests that mesenchymal stem cells (MSCs) could differentiate in vitro and in vivo into liver-like cells with partial hepatic functions under appropriate environmental conditions[6,7]. Given that autologous cell transplantation helps to prevent immunologic rejection, which is always a major obstacle for orthotopic liver transplantation, MSCs could be regarded as seeding cells for transplantation in relation to the treatment of liver diseases[8].
Severe inflammation as a result of ALF leads to necrosis of a large number of liver cells and is caused by acetaminophen, idiosyncratic drug reactions, hepatitis B, or seronegative hepatitis. The occurrence of ALF also involves various inflammatory factors and cytokines, and its pathogenesis is closely related to liver cell apoptosis[9-11]. In recent years, experimental studies have demonstrated that microcirculatory dysfunction and an inflammatory environment are determinants of ALF, and proinflammatory mediators such as interleukin (IL)-1, IL-2, and tumor necrosis factor (TNF)-α are the key players[12]. One study showed that the levels of these cytokines in patients with ALF were significantly higher than in healthy individuals and patients with chronic hepatitis[13]. IL-1 may be a main driver of late inflammation, which leads to further injury. IL-1 is considered to be a primary proinflammatory cytokine because of its ability to stimulate expression of many inflammation-associated genes through the IL-1 signaling cascade[14].
The IL-1 receptor antagonist (IL-1Ra) is a natural IL-1 antagonist that can block the inflammatory process by competitively binding to the IL-1 receptor with equal avidity to IL-1. IL-1Ra inhibits the stimulation of downstream signaling, thereby reducing inflammation[15]. Imbalance between IL-1 and IL-1Ra has been observed in a variety of inflammatory diseases including ALF[16]. IL-1Ra, which is significantly associated with the level of liver inflammation, is an independent marker unaffected by obesity, alcohol consumption, or insulin resistance[17]. IL-1Ra can inhibit hepatocellular apoptosis in mice with ALF induced by acetaminophen and significantly improve their survival rate[18]. Therefore, we hypothesized that reducing inflammation in acutely injured liver would benefit the efficacy of MSC transplantation in patients with ALF. In this study, IL-1Ra was injected through the portal vein along with MSCs to reduce liver inflammation in a swine model of ALF. Liver function before and after MSC transplantation with or without IL-Ra was compared by measuring the changes in serum levels of alanine aminotransferase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), and γ-glutamyl transpeptidase (γ-GT). In addition, pathologic injury and hepatic cell apoptosis were also examined. The outcome of this study appears promising and may improve the clinical application of MSCs.
Chinese experimental miniature swine (15 ± 3 kg, 5-8 mo) were obtained from the Laboratory Animal Centre of the Affiliated Drum Tower Hospital of Nanjing University Medical School. Animals were maintained under standard conditions. All animal procedures were approved by the Animal Care Ethics Committee of Nanjing Drum Tower Hospital. Every effort was made to minimize any suffering of the animals used in this study.
Porcine MSCs were isolated as described previously[19]. Bone marrow aspirates were collected from the iliac crests. MSCs were separated by density gradient centrifugation over a Ficoll histopaque layer (20 min, 400 ×g, density: 1.077 g/mL) (TBD, Tianjin, China) and cultured in low-glucose Dulbecco’s modified Eagle’s medium (Gibco of Thermo Fisher Scientific, Waltham, MA, United States) supplemented with 10% fetal bovine serum (Gibco), 100 IU/mL penicillin, and 100 μg/mL streptomycin (Gibco). The non-adherent cells were removed after the first 24 h incubation, and medium was changed every 3-4 d. When the cells reached 80% confluence, they were trypsinized using 2.5 g/L trypsin-EDTA (Gibco) and replated at a density of 1 × 104/cm2 for further expansion. The cells were infected with a lentivirus encoding the gene for green fluorescent protein (GFP), and the multiplicity of infection was determined by fluorescence inverted phase-contrast microscopy and flow cytometry. MSCs were characterized by flow cytometry (FACScan; Becton Dickinson, Franklin Lakes, NJ, United States) with phycoerythrin-conjugated antibodies against CD29 (VMRD, Pullman, WA, United States), CD44, and CD90 (Becton Dickinson); isotypic antibodies were used as controls.
The swine model of ALF was established as described previously[20]. The swine were starved for 8 h before the operation. Initial sedation was achieved with a deep intramuscular injection of ketamine (15-20 mg/kg) and chlorpromazine (6-8 mg/kg), administered 15 min after atropine (0.01 mg/kg). Oxygen saturation and heart rate were monitored throughout the operation, and anesthesia was maintained using 1.5% halothane in oxygen titrated to provide anesthesia. Normal saline (1 L) and 5% dextrose (500 mL) were administered intravenously during the surgical procedure. For 85% hepatectomy, left trilobectomy was performed, together with partial right-posterior-lobe resection but without hepatic pedicle occlusion. Parts of the right posterior and caudate lobes were retained to leave a residual hepatic volume of 15% of the normal volume. Computed tomography (CT) and CT reconstruction were performed to visualize the residual liver volume after hepatectomy, and B-scan ultrasound examination was performed to detect ascites.
All animals received the following subcutaneous injections: 5 mL 10% glucose, 0.1 mL analgesics (ketorolac; Whanin Pharm, Seoul, South Korea), and 0.1 mL ceftriaxone (Rocephin; Roche Holding AG, Basel, Switzerland). Animals had ad libitum access to 20% glucose solution for drinking and standard laboratory chow.
Twenty-four swine with injured livers were randomly divided into four groups (n = 6 each): a model control group, a group receiving IL-1Ra via peripheral vein injection, a group receiving MSC transplantation via portal vein injection, and a combined therapy group. The model control group received 40 mL normal saline via the portal vein 1 d after surgery. The IL-1Ra group received an injection of 2 mg/kg IL-1Ra (Institute of Process Engineering, Chinese Academy of Sciences, China) via the ear vein at 18 h, 2 d, and 4 d after surgery. The MSC transplant group received 1 × 108 GFP-MSCs suspended in 40 mL normal saline via the portal vein 24 h after surgery. The combined therapy group received 2 mg/kg IL-1Ra via the ear vein 18 h after surgery, followed by MSC transplantation 6 h after IL-1Ra injection, and finally 2 mg/kg IL-1Ra was again injected via the ear vein at 2 d and 4 d after surgery.
Blood samples were collected before and after MSC transplantation. Venous blood samples were drawn preoperatively and at 1 d, 3 d, 5 d, 7d, and 14 d after MSC transplantation (day 0) for biochemical analysis. Serum levels of ALT, AST, ALP, and γ-GT were monitored, and the degree of liver function estimated. Inflammatory cytokines IL-1β, IL-6, and TNF-α were also measured by ELISA (Corbett Life Science, Sydney, Australia) preoperatively, intraoperatively, and at 1 d, 3 d, 5 d, 7 d, and 14 d after MSC transplantation.
Seven days after cell transplantation, animal liver tissues were surgically collected under general anesthesia. To trace transplanted MSCs, GFP expression in frozen tissue sections was analyzed by fluorescent microscopy. For histologic analysis, liver tissue was fixed in 10% neutral-buffered formalin and embedded in paraffin. Sections of 5-μm thickness were affixed to slides, deparaffinized, and stained with hematoxylin and eosin to determine morphologic changes. Liver injury was histologically interpreted and scored in all samples. Histopathology was independently evaluated by three pathologists blinded to the treatment and scored in terms of steatosis, necrosis, and inflammation as follows: 0, normal; 1, mild changes; 2, mild to moderate severity; 3, moderate severity; and 4, maximum severity. The scores were added for each animal to create a composite score. Sections were photographed with a Leitz Aristoplan microscope (Wetzlar, Germany). The slices were subjected for further anti-Ki67 (EnoGene Biotech Co., Ltd., New York, NY, United States) immunohistochemical staining. To determine liver cell proliferation, six high-powered fields from each section were analyzed to obtain an average number of Ki67+ cells (Ki67 index).
Hepatic apoptosis was detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (BioBox, Nanjing, China)[21]. Paraffin sections for histologic assessment were deparaffinized, rehydrated, and then rinsed in PBS. After endogenous peroxidase activity was blocked by methanol, a permeability solution (1 g/L Triton X-100 in 0.1% sodium citrate), TUNEL reaction solution, and Converter-POD were added. Each slice was stained by 3,3-diaminobenzidine, and apoptosis was observed by microscopy. The brown staining in the nucleus represented apoptotic cells. Three fields were randomly selected in each slice under high-power field (400 ×) and the percentage of TUNEL+ cells was used to estimate the apoptosis rate.
Western blotting was performed as previously described[22]. Seven days after cell transplantation, surgically collected animal liver tissues were frozen in liquid nitrogen and lysed with buffer containing 20 mmol/L Tris (pH 7.4), 250 mmol/L NaCl, 2 mmol/L EDTA (pH 8.0), 0.1% Triton X-100, 0.01 mg/mL aprotinin, 0.005 mg/mL leupeptin, 0.4 mmol/L PMSF, and 4 mmol/L NaVO4. The protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad Laboratories, Hercules, CA, United States) and an equal amount of protein was separated by SDS-PAGE. After separation, the proteins were electrotransferred onto nitrocellulose membranes and blotted with primary antibodies against protein kinase B (Akt), nuclear factor (NF)-κB p65, and β-actin (EnoGene) at 1:1000 dilutions. The membranes were washed and exposed to horseradish-peroxidase-conjugated secondary antibody for 1 h, and signals were finally detected by ECL reagent (GE Healthcare, Little Chalfont, United Kingdom). The signal band intensities were analyzed using Image J software and the results are expressed as fold change relative to the internal control (β-actin).
All assays were repeated three times to ensure reproducibility. Results are expressed as mean ± SD. Differences between the control and treated groups were analyzed by Student’s unpaired t test and one-way analysis of variance. The comparison of survival time was achieved using the Kruskal-Wallis test. For the survival time, two-tailed P values ≤ 0.05 were considered as statistically significant. Analyses were performed with SPSS version 17.0 statistical software (SPSS Inc., Chicago, IL, United States).
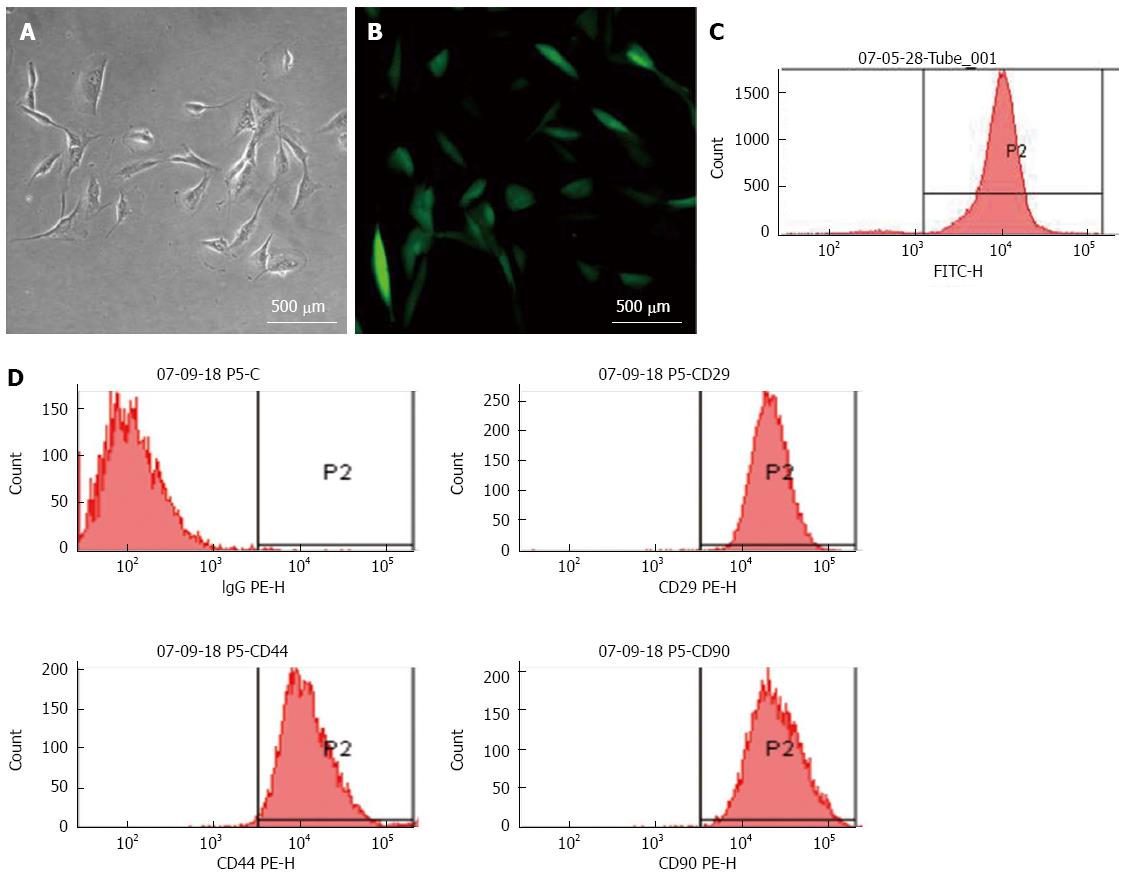
MSCs formed colonies after 24 h of plating when observed under microscope. They grew rapidly like fibroblasts, with a single nucleus. After passage 1, they looked like spindles or asters with a slim body. However, after passage 4, most of the miscellaneous cells were eliminated, leaving the uniformly fibroblast-like cells, which were MSCs. Infecting these cells with lentivirus expressing GFP protein resulted in nearly complete transfection as observed by fluorescence microscopy (Figure 1A and B). Flow cytometry revealed that approximately 97.3% of MSCs were GFP positive (Figure 1C). In addition, flow cytometry analysis of cell surface-marker expression demonstrated that > 90% of GFP-MSCs were positive for CD29, CD44, and CD90 expression (Figure 1D).
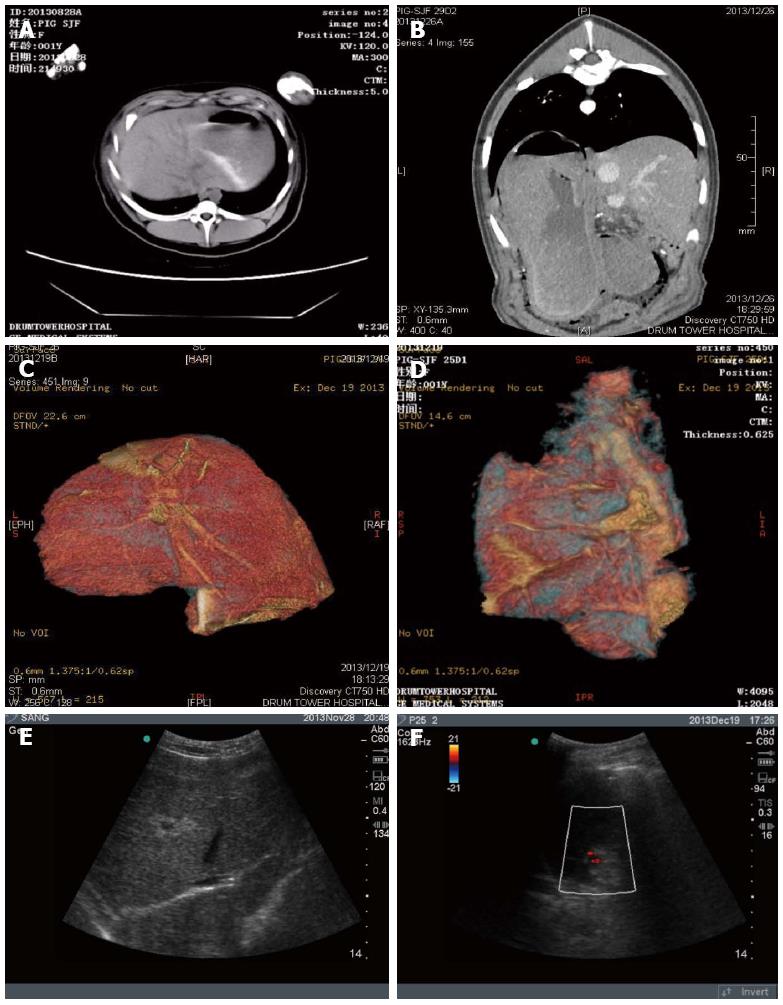
CT examination and reconstruction analysis of the residual liver volume in the hepatectomized animals indicated that approximately 85% of the liver was resected compared with normal swine liver (Figure 2A-D). After hepatectomy, animals displayed typical liver failure characteristics, such as a prolonged recovery time, hypobulia, drowsiness, reduced movement, weak response to stimulation, and emerging ocular hemorrhage. B-scan ultrasound revealed massive ascites (Figure 2E).
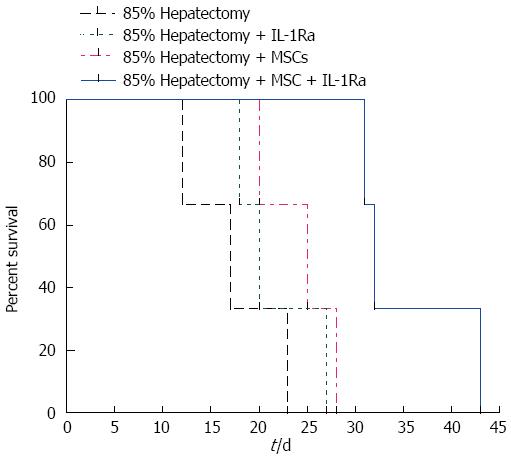
All swine in the hepatectomy group (no treatment) died within 25 d after surgery; the average survival time was 17.3 ± 5.5 d. No abdominal bleeding or vascular embolism was detected during autopsy examinations, indicating that animals died from liver failure and not surgical complications. However, the 25-d survival rates of the treatment group were 33.3%, 66.7%, and 100% in the IL-1Ra group, MSC transplantation group, and combined therapy group, respectively, with average survival times of 21.7 ± 4.7 d, 24.3 ± 4.0 d, and 35.3 ± 6.7 d, respectively. The difference in survival time between the hepatectomy and combined therapy groups was significant (P < 0.05) (Figure 3).
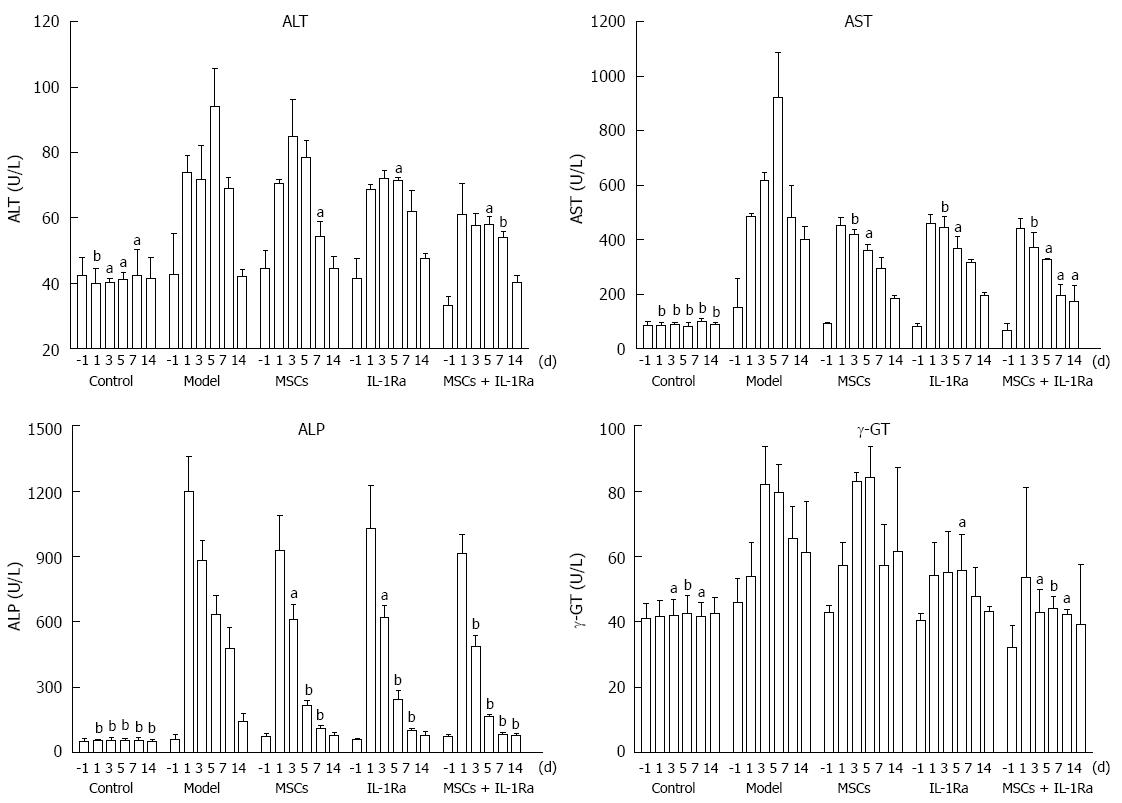
Preoperative and serial postoperative measurements of serum ALT, AST, ALP, and γ-GT are shown in Figure 4. Serum levels of ALT and AST were significantly elevated within 1-7 d after hepatectomy, whereas ALP levels were significantly elevated within 1-14 d after hepatectomy (all P < 0.05). γ-GT level was significantly elevated within 3-7 d (P < 0.05 or < 0.01) after hepatectomy. These data demonstrated that acute liver injury was successfully achieved after hepatectomy. Postoperative measurements revealed that the greatest improvements were found in the combined therapy group. The serum levels of ALT in the model group were significantly lower than in the combined therapy group on days 5 (P < 0.05) and 7 (P < 0.01), in the IL-1Ra group on day 5 (P < 0.05), and the MSC transplantation group on day 7 (P < 0.05). Similarly, the serum levels of AST were significantly lower in the combined therapy group than in the model group on days 3, 5, 7, and 14 (P < 0.01), and significantly lower than in the IL-1Ra and MSC transplantation groups on days 3 (P < 0.05), 5, and 7 (P < 0.01). The serum levels of ALP were significantly lower in the combined therapy group than in the model group on days 3 (P < 0.01), 5, 7, and 14 (P < 0.05), and significantly lower than in the IL-1Ra and MSC transplantation groups on days 3 (P < 0.01) and 5 (P < 0.05). The serum levels of γ-GT in the model group were significantly lower than in the combined therapy group on days 3 (P < 0.05), 5 (P < 0.01), and 7 (P < 0.05), and lower than the IL-1Ra group on day 5 (P < 0.05). There was no significant difference from the MSC transplantation group.
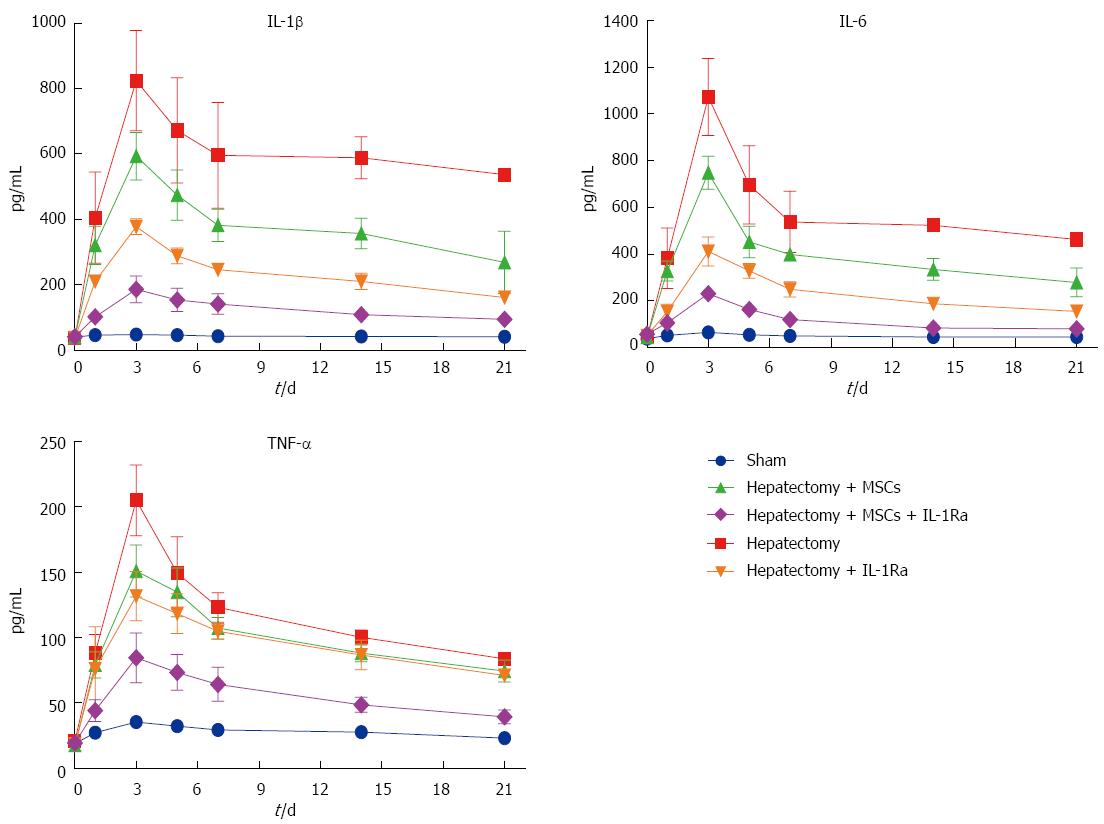
Following hepatectomy, the levels of inflammatory cytokines IL-1β, IL-6, and TNF-α increased significantly in all groups, reaching a peak within 3 d and lasting > 2 wk before gradually declining (Figure 5). The combined therapy group had the lowest levels of inflammatory cytokines, with IL-1β, IL-6 and TNF-α levels significantly lower than in the model group on days 1-14 (all P < 0.05). The IL-1Ra group also had significantly decreased IL-1β levels on days 3-14 (all P < 0.05), decreased IL-6 levels on days 1-14 (all P < 0.05), and decreased TNF-α levels on day 3 (P < 0.05). MSC transplantation alone had a weaker influence on inflammatory cytokines, as IL-1β levels were only significantly decreased on day 14 (P < 0.05), IL-6 levels decreased only on days 3 and 14 (P < 0.05), and TNF-α levels decreased only on day 3 (P < 0.05).
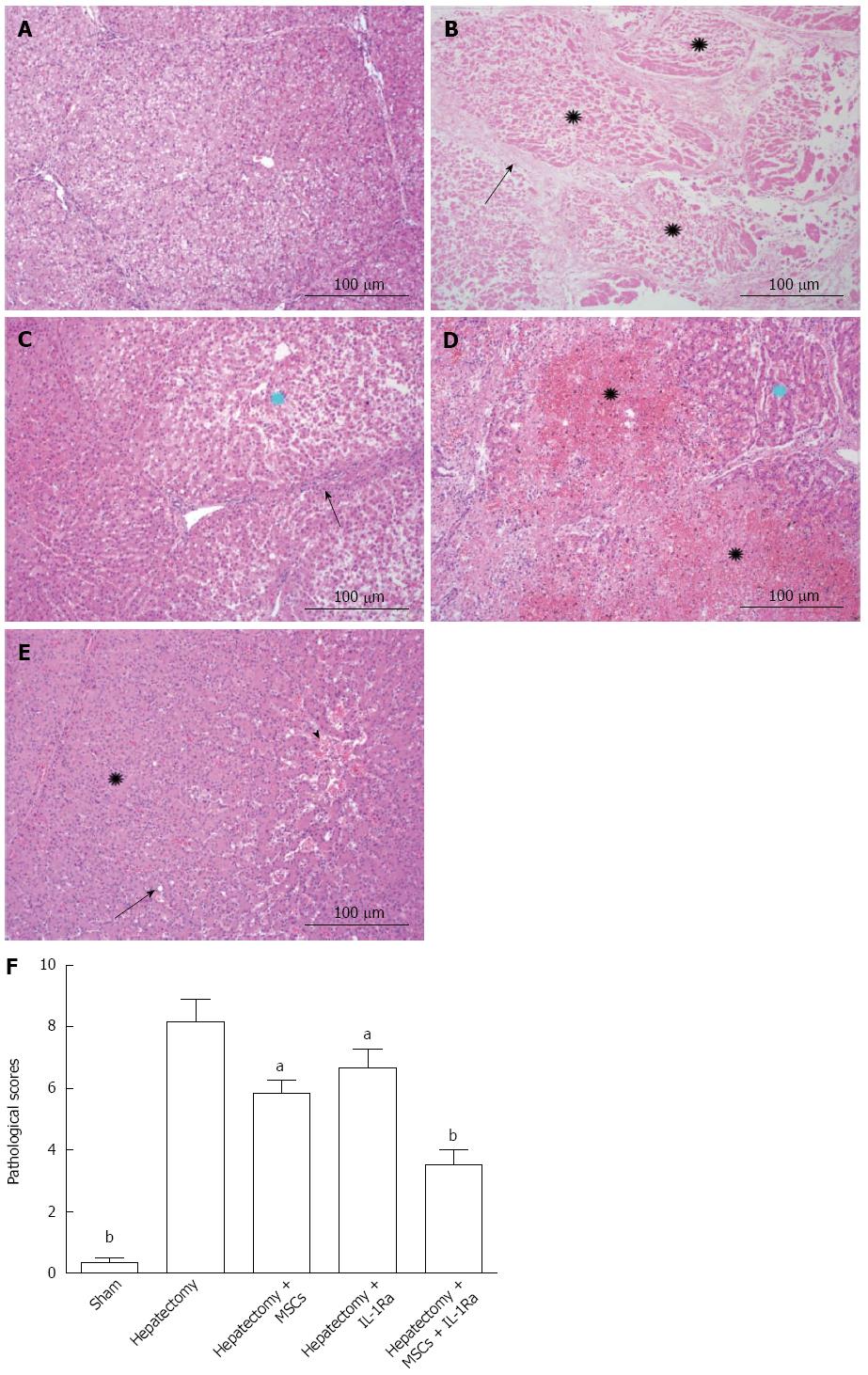
Histopathologic studies of swine liver tissue from normal sham-operated animals showed that the liver lobular structure was clear, arranged radially around the central vein in liver cells, and liver lobules were separated by slender connective tissue. In biliary epithelium, no cell degeneration, necrosis, or non-proliferation was observed (Figure 6A). However, liver tissue samples from the injured model group after hepatectomy demonstrated diffuse necrosis with extensive bridging and proliferation of inflammatory cells around the periportal area. Severe hepatic necrosis was seen in most of the lobules, along with hepatic sinusoidal dilatation, congestion, and hemorrhage. Liver cell necrosis appeared in the hemorrhagic area. Only a small number of liver cells remained on the edges of the hepatic lobules (Figure 6B). This group showed the most severe liver damage, while in contrast, the therapy groups had less inflammatory cell infiltration and relatively complete lobular architectures. The combined therapy group displayed the most apparent improvements among all the groups. The lobular architecture could be recognized and liver cells were arranged radially around the central vein. Liver lobules were surrounded by interlobular connective tissue, and few inflammatory cells were observed. Combined therapy substantially protected against liver injury, relieved liver sinusoidal dilatation and congestion, and reduced the level of liver cell degeneration and necrosis (Figure 6E).
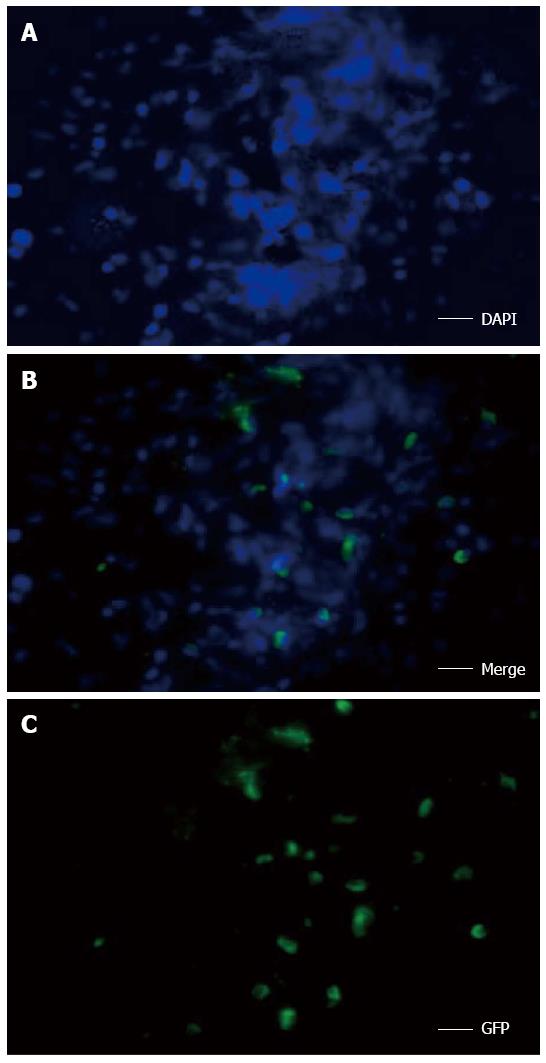
After hepatic resection, the pathologic score was 8.17 ± 1.26, which was significantly higher than that of the sham-operated group (0.33 ± 0.29; P < 0.01) (Figure 6F). The histopathologic score in the combined therapy group (3.50 ± 0.87) was significantly decreased compared with hepatectomized model group (P < 0.01). However, there was no significant difference in the IL-1Ra (6.67 ± 1.04) and MSC transplantation (5.83 ± 0.76) groups (Figure 6F). In addition, frozen sections of liver tissue were examined using fluorescence microscopy. The green fluorescent signal for GFP-MSCs and the blue staining (DAPI) signal for nuclei were distributed around the hepatic lobules in the combined therapy group, representing even distribution of MSCs (Figure 7).
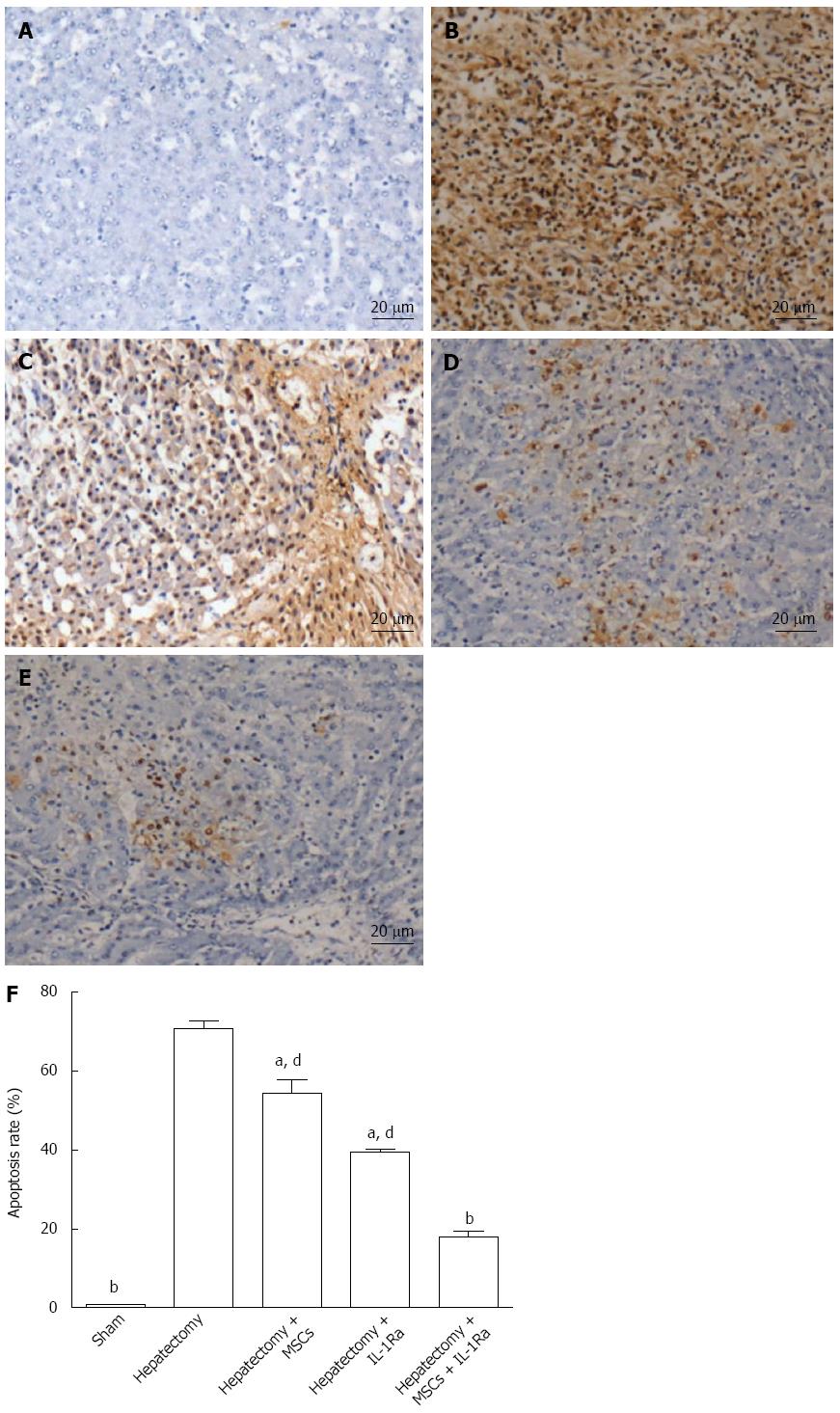
To determine the effect of combination therapy on ALF-related apoptosis, a TUNEL assay was performed (Figure 8). The cells in the model control group had many positively stained apoptotic cells that were round with brown nuclei (Figure 8B). The percentage of TUNEL-positive cells relative to the total cell count was used to estimate the apoptosis rate. The number of apoptotic cells in the sham group was low (0.8% ± 0.2%), while their number significantly increased following hepatectomy (70.8% ± 3.7%; P < 0.01) (Figure 8F). Apoptotic cells were also detected in the combined therapy group (Figure 8E); however, the apoptosis rate (18.1% ± 2.1%) was significantly lower compared to the IL-1Ra and MSC transplantation groups (39.5% ± 1.2% and 54.5% ± 6.0%, respectively) (P < 0.01) (Figure 8F).
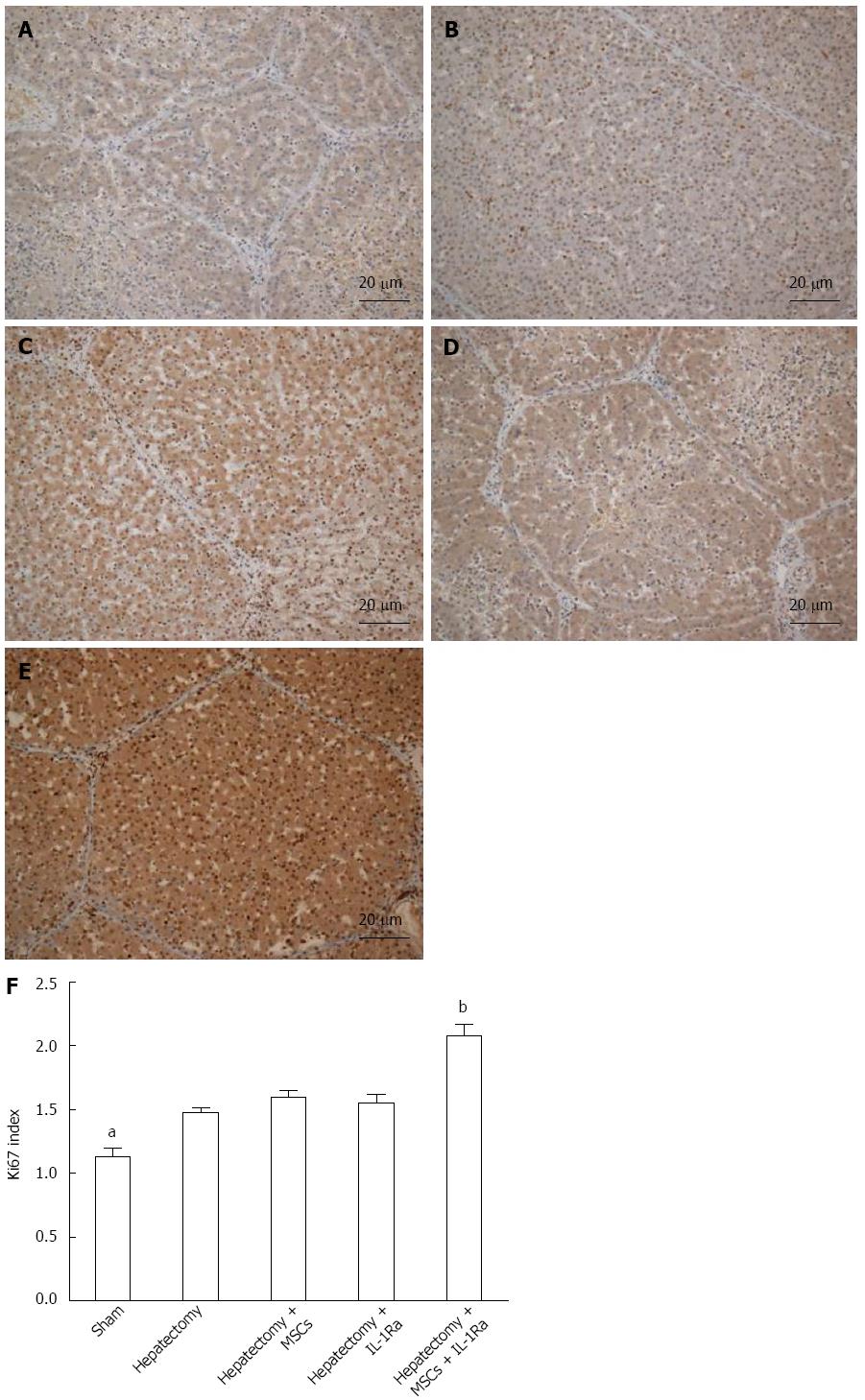
To quantify hepatic regeneration, the expression of Ki67 was analyzed by immunohistochemistry (Figure 9). The expression of Ki67 was significantly increased in the hepatectomy group compared with the sham group (P < 0.05). The Ki67 index was also upregulated in the MSC transplantation and IL-1Ra groups, but the differences were not significant. By contrast, Ki67 expression was significantly increased in the combined therapy group (P < 0.01).
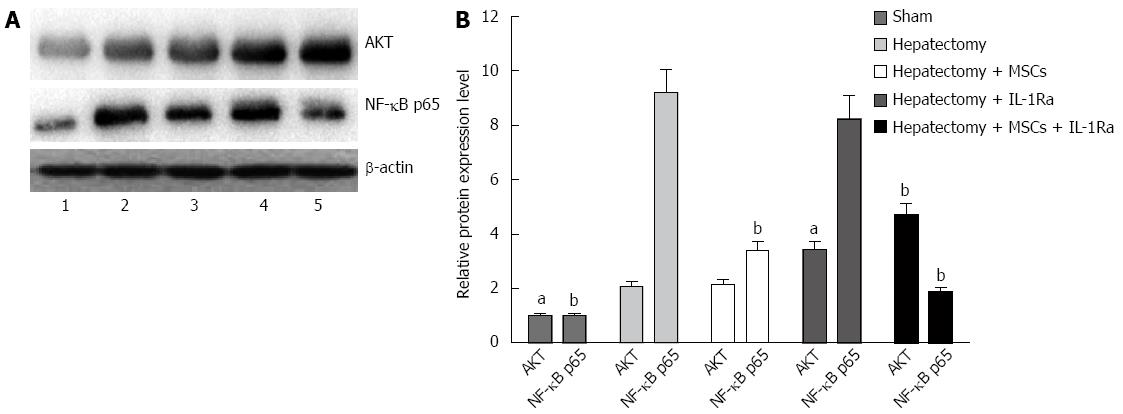
To investigate whether Akt and NF-κB signaling pathways mediate hepatocyte injury, the expression levels of these two proteins were assessed by Western blotting. The expression levels of Akt and NF-κB were significantly increased in the model group compared with the sham group (P < 0.05) (Figure 10). Compared to the model group, the MSC transplantation group had significantly decreased expression of NF-κB (P < 0.01), but similar levels of Akt expression. Conversely, the IL-1Ra group showed significantly increased Akt expression (P < 0.05), with no change in NF-κB compared to the model group. In the combined therapy group, expression levels of both Akt and NF-κB were significantly different compared to the model group (P < 0.01).
ALF is a severe disease that aggravates liver cell necrosis. Partial hepatectomy leads to compensatory hypertrophy and hyperplasia in the remaining lobes of the liver to replace the lost functional mass. However, large-scale hepatectomy may cause ALF, the biggest problem in clinical settings. Bone-marrow-derived MSCs may thus be a potential therapeutic choice, because their mesodermal origin represents multipotent adult stem cells with the potential for self-renewal[23]. Petersen et al[24] and Schwartz et al[25] showed that MSCs possess the ability to differentiate into hepatocytes, both in vitro and in vivo. However, despite the ability of MSCs to differentiate, the limited MSC transplantation efficiency is a major obstacle due to microcirculatory dysfunction and inflammatory environment. Proinflammatory mediators, such as IL-1, IL-2, and TNF-α, create an inflammatory environment in ALF, leading to reduced survival and differentiation of the transplanted MSCs, thereby limiting the therapeutic role of the transplantation[26]. Increased levels of proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, are seen in all hepatectomized animals[27].
Exogenous IL-1Ra reduces some proinflammatory mediators, thus providing a better inflammatory environment[28]. This results in increased liver cell proliferation and enhanced MSC transplantation efficiency. This effect may last at least three weeks with combined therapy. Indeed, in the combined therapy group, a continuous reduction in proinflammatory mediators was observed, which could be attributed to the disrupted inflammatory cycle caused by IL-1Ra in the early phase. This reduction was slightly ameliorated when MSCs were transplanted alone, but better results were achieved in the combined therapy group.
In our study, combined therapy resulted in: (1) reduction of liver injury biomarkers; (2) improvement in hepatic functional parameters; and (3) increased survival, as compared with IL-1Ra or MSC transplantation alone. Combined therapy improved liver serology of ALT, AST, ALP, and γ-GT, and provided a long-term survival benefit, observed as a significantly higher survival time compared to untreated animals. Furthermore, the histopathologic scores in the combined therapy group were significantly decreased compared with those in the model group. Combined therapy inhibited hepatocellular apoptosis and promoted liver cell proliferation in swine with ALF, thereby significantly improving their survival rate. Higher MSC transplantation efficiency may directly lead to a higher hepatocyte differentiation rate, proliferation level, and better liver function. In addition, proliferation in the combined therapy group was significantly higher than in the IL-1Ra or MSC transplantation groups alone. Therefore, we conclude that the combined therapy group had the best liver function. These results also suggest that the animals with lower levels of inflammatory cytokines have a higher MSC implantation rate. It is therefore important to improve the efficacy of MSC transplantation and to reduce the inflammation levels.
We further studied the potential mechanism of combined therapy in hepatic protection. Although much is known about the cytokines (hepatocyte priming) and growth factors (stimulating cell cycle progression) involved, a link between these two signaling pathways has yet to be illustrated. Akt has been shown to play an important role in the compensatory recovery of liver mass following resection by regulating hepatocyte hypertrophy[29]. In addition to its role in leukocyte trafficking, signaling and phagocytosis, Akt also plays an important role in cell survival and proliferation. Similarly, elevation of Akt expression in the combined therapy group in our study supports higher liver cell proliferation and inhibition of apoptosis.
Hepatectomy-induced liver injury results in increased hepatocellular apoptosis as well as inflammation. This strongly inhibits the therapeutic effect of MSC transplantation. Effective MSC transplantation may induce secretion of growth factors that participate in the priming phase of liver regeneration, which makes hepatocytes responsive to growth factors such as hepatocyte growth factor, epidermal growth factor, and transforming growth factor-α, and promotes hepatocyte replication and liver growth in vivo[30]. Pathways such as Akt signaling are central to protecting hepatocytes from apoptosis and to enhanced hepatic repair after liver injury. Inactivation of the Akt pathway results in delayed liver regeneration in mice[31]. The antiapoptotic effect of the Akt signaling pathway is via activation of Bcl-2, which in turn inhibits the apoptotic mediator, caspase-3[32].
The production of cytokines is a consequence of extensive liver necrosis as well as infection/sepsis, which frequently complicates ALF. Hepatectomy leads to increased levels of endotoxin in the portal circulation, thereby activating Kupffer cells to produce toxic mediators that cause liver injury[33]. Transcription factor NF-κB, a key regulator of genes involved in inflammation, is activated by endotoxin or oxidative stress, which results in its translocation to the nucleus and subsequent transcription of target genes[34]. NF-κB has long been considered as a prototypical proinflammatory signaling molecule, largely due to the activation of NF-κB by proinflammatory cytokines such as IL-1 and TNF-α. In the present study, hepatectomy caused a compensatory increase in Akt and NF-κB expression. However, combined therapy decreased NF-κB levels, which likely then results in reduced expression of genes involved in inflammation, an effect that is important for MSC transplantation and liver regeneration after hepatectomy. It is generally considered that inhibition of apoptosis and promotion of cell proliferation are possible major outcomes of intraportal MSC transplantation with IL-1Ra administration.
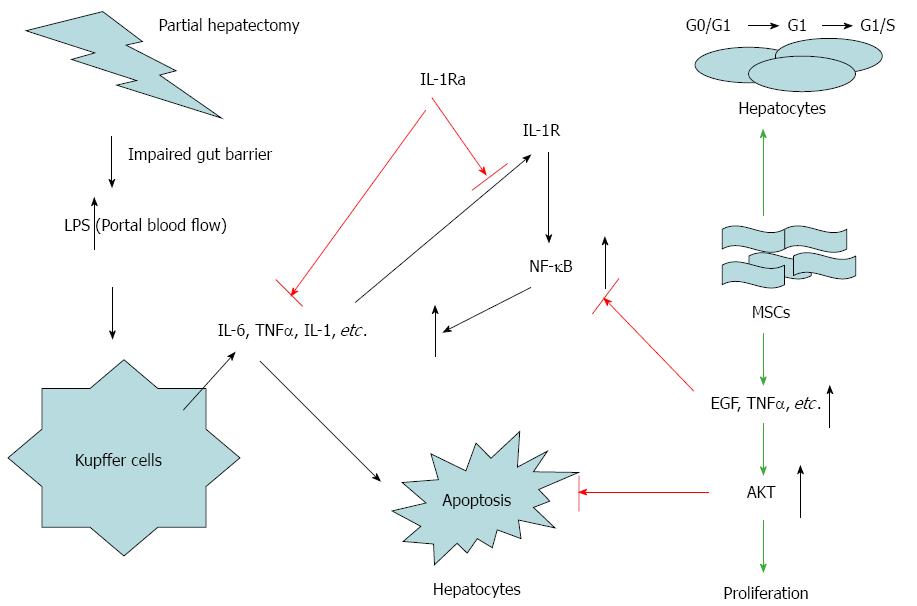
As Akt and NF-κB are important regulators of hepatic regeneration following partial hepatectomy, we propose a dual-action mechanism for combination therapy involving these two signaling molecules: (1) inhibition of NF-κB leads to decreased IL-1, IL-6, and TNF-α expression, which results in better MSC transplantation with IL-Ra administration, thus promoting regeneration of liver cells; (2) upregulation of Akt expression due to growth factors (hepatocyte growth factor, epidermal growth factor, transforming growth factor-α) induces hepatocyte survival, DNA synthesis and cell division (Figure 11).
In summary, combination therapy appears to be superior to MSC transplantation alone. Our data revealed that combination therapy could improve liver function, inhibit apoptosis, and prolong the survival time of swine with ALF. The transplanted MSCs may quickly participate in liver regeneration by promoting proliferation and inhibiting apoptosis during the initial stage of ALF, after the IL-1 inflammation signaling pathway is blocked by IL-Ra. Combination therapy with IL-1Ra and MSC transplantation, which enables restoration from acute liver injury and reconstruction in swine, is a promising treatment option for patients with ALF. This increased understanding of the liver regeneration cascade in MSC transplantation combined with IL-1Ra administration could lead to improved clinical outcomes for treatment of acute or chronic liver failure.
Acute liver failure (ALF) is characterized by severe liver cell damage caused by virus infection, drugs, toxins, alcohol, or hepatectomy. ALF leads to hepatic encephalopathy, hepatorenal syndrome, severe infection, multiple organ failure, and even death. Combating ALF requires either reduction of liver cell necrosis or stimulation of liver cell regeneration. So far, liver transplantation is the most effective treatment for ALF. However, it has its own limitations, including severe donor shortage, numerous complications, immune rejection, use of immunosuppressive agents, and high medical costs.
In recent years, therapy with stem cells has become a new area of investigation for ALF treatment. This is mainly attributable to their abundant availability, low immunogenicity, and potential of differentiating into hepatocyte-like cells. Experimental studies have demonstrated that microcirculatory dysfunction and an inflammatory environment are determinants of ALF, and proinflammatory mediators such as interleukin (IL)-1, IL-2, and tumor necrosis factor-α are the key players in ALF.
In this study, the authors hypothesized that reducing inflammation in the acutely injured liver can benefit the efficacy of mesenchymal stem cell (MSC) transplantation in ALF patients. IL-1 receptor antagonist (IL-1Ra) was injected to reduce liver inflammation, in addition to MSC transplantation through the portal vein in a swine model of ALF. The liver functions before and after MSC transplantation with or without IL-Ra were compared by measuring the changes in serum levels of alanine aminotransferase, aspartate transaminase, alkaline phosphatase, and γ-glutamyl transpeptidase. In addition, pathologic injury and hepatic cell apoptosis were also examined.
This study revealed that combination therapy improves liver function, inhibits apoptosis, and prolongs the survival of swine with ALF. The transplanted MSCs may quickly participate in liver regeneration by promoting proliferation and inhibiting apoptosis during the initial stage of ALF, after the IL-1 inflammation signaling pathway is blocked by IL-Ra. Combination therapy with IL-1Ra and MSC transplantation, which enables restoration from acute liver injury and reconstruction in swine, is a promising treatment option for patients with ALF in the future. This enhanced understanding of the liver regeneration cascade in MSC transplantation combined with IL-1Ra administration could lead to improved clinical outcomes for the treatment of acute or chronic liver failure.
IL-1Ra is a natural IL-1 antagonist that can block the inflammatory process by competitively binding to the IL-1 receptor with equal avidity to IL-1. It inhibits the stimulation of downstream signaling and thereby reduces inflammation.
This study provided a new way to restore or treat ALF. The result of the study showed that combination therapy with MSCs and IL-1Ra can increase liver regeneration after hepatectomy in a swine model of ALF. The study appears promising and demonstrates the preclinical application of MSCs.
P- Reviewer: Gao B, Mendez I, Wang GY S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang DN
| 1. | Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 847] [Article Influence: 70.6] [Reference Citation Analysis (2)] |
| 2. | Hoyer DP, Munteanu M, Canbay A, Hartmann M, Gallinat A, Paul A, Saner FH. Liver transplantation for acute liver failure: are there thresholds not to be crossed? Transpl Int. 2014;27:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Pan XN, Zheng LQ, Lai XH. Bone marrow-derived mesenchymal stem cell therapy for decompensated liver cirrhosis: a meta-analysis. World J Gastroenterol. 2014;20:14051-14057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | O’Grady J. Timing and benefit of liver transplantation in acute liver failure. J Hepatol. 2014;60:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Buzhor E, Leshansky L, Blumenthal J, Barash H, Warshawsky D, Mazor Y, Shtrichman R. Cell-based therapy approaches: the hope for incurable diseases. Regen Med. 2014;9:649-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Ma HC, Shi XL, Ren HZ, Yuan XW, Ding YT. Targeted migration of mesenchymal stem cells modified with CXCR4 to acute failing liver improves liver regeneration. World J Gastroenterol. 2014;20:14884-14894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Deng C, Qin A, Zhao W, Feng T, Shi C, Liu T. Up-regulation of CXCR4 in rat umbilical mesenchymal stem cells induced by serum from rat with acute liver failure promotes stem cells migration to injured liver tissue. Mol Cell Biochem. 2014;396:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Volarevic V, Nurkovic J, Arsenijevic N, Stojkovic M. Concise review: Therapeutic potential of mesenchymal stem cells for the treatment of acute liver failure and cirrhosis. Stem Cells. 2014;32:2818-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Raicevic G, Najar M, Najimi M, El Taghdouini A, van Grunsven LA, Sokal E, Toungouz M. Influence of inflammation on the immunological profile of adult-derived human liver mesenchymal stromal cells and stellate cells. Cytotherapy. 2015;17:174-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Qingqing M, Xin Z, Meizhong S. Bone marrow mesenchymal stem cells altered the immunoregulatory activities of hepatic natural killer cells. Clin Res Hepatol Gastroenterol. 2014;38:689-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Xiao JQ, Shi XL, Ma HC, Tan JJ, Lin-zhang Q, Ding YT. Administration of IL-1Ra chitosan nanoparticles enhances the therapeutic efficacy of mesenchymal stem cell transplantation in acute liver failure. Arch Med Res. 2013;44:370-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Nguyen NT, Vierling JM. Acute liver failure. Curr Opin Organ Transplant. 2011;16:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Nakae H, Yonekawa T, Narita K, Endo S. Are proinflammatory cytokine concentrations reduced by plasma exchange in patients with severe acute hepatic failure? Res Commun Mol Pathol Pharmacol. 2001;109:65-72. [PubMed] |
| 14. | Jensen LE, Muzio M, Mantovani A, Whitehead AS. IL-1 signaling cascade in liver cells and the involvement of a soluble form of the IL-1 receptor accessory protein. J Immunol. 2000;164:5277-5286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Volarevic V, Al-Qahtani A, Arsenijevic N, Pajovic S, Lukic ML. Interleukin-1 receptor antagonist (IL-1Ra) and IL-1Ra producing mesenchymal stem cells as modulators of diabetogenesis. Autoimmunity. 2010;43:255-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 140] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Sekiyama KD, Yoshiba M, Thomson AW. Circulating proinflammatory cytokines (IL-1 beta, TNF-alpha, and IL-6) and IL-1 receptor antagonist (IL-1Ra) in fulminant hepatic failure and acute hepatitis. Clin Exp Immunol. 1994;98:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 133] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Pihlajamäki J, Kuulasmaa T, Kaminska D, Simonen M, Kärjä V, Grönlund S, Käkelä P, Pääkkönen M, Kainulainen S, Punnonen K. Serum interleukin 1 receptor antagonist as an independent marker of non-alcoholic steatohepatitis in humans. J Hepatol. 2012;56:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Hu J, Yan D, Gao J, Xu C, Yuan Y, Zhu R, Xiang D, Weng S, Han W, Zang G. rhIL-1Ra reduces hepatocellular apoptosis in mice with acetaminophen-induced acute liver failure. Lab Invest. 2010;90:1737-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Brückner S, Tautenhahn HM, Winkler S, Stock P, Jonas S, Dollinger M, Christ B. Isolation and hepatocyte differentiation of mesenchymal stem cells from porcine bone marrow--”surgical waste” as a novel MSC source. Transplant Proc. 2013;45:2056-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Eshkenazy R, Dreznik Y, Lahat E, Zakai BB, Zendel A, Ariche A. Small for size liver remnant following resection: prevention and management. Hepatobiliary Surg Nutr. 2014;3:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 21. | Yuan H, Li L, Zheng W, Wan J, Ge P, Li H, Zhang L. Antidiabetic drug metformin alleviates endotoxin-induced fulminant liver injury in mice. Int Immunopharmacol. 2012;12:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Zhang ZF, Lu J, Zheng YL, Hu B, Fan SH, Wu DM, Zheng ZH, Shan Q, Liu CM. Purple sweet potato color protects mouse liver against d-galactose-induced apoptosis via inhibiting caspase-3 activation and enhancing PI3K/Akt pathway. Food Chem Toxicol. 2010;48:2500-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Zhang S, Chen L, Liu T, Zhang B, Xiang D, Wang Z, Wang Y. Human umbilical cord matrix stem cells efficiently rescue acute liver failure through paracrine effects rather than hepatic differentiation. Tissue Eng Part A. 2012;18:1352-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1795] [Cited by in RCA: 1669] [Article Influence: 64.2] [Reference Citation Analysis (0)] |
| 25. | Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 255] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 26. | Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 373] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 27. | Xu CS, Jiang Y, Zhang LX, Chang CF, Wang GP, Shi RJ, Yang YJ. The role of Kupffer cells in rat liver regeneration revealed by cell-specific microarray analysis. J Cell Biochem. 2012;113:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, Min SY, Kurt-Jones EA, Szabo G. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476-3489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 587] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 29. | Jackson LN, Larson SD, Silva SR, Rychahou PG, Chen LA, Qiu S, Rajaraman S, Evers BM. PI3K/Akt activation is critical for early hepatic regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1401-G1410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Vandermeulen M, Grégoire C, Briquet A, Lechanteur C, Beguin Y, Detry O. Rationale for the potential use of mesenchymal stromal cells in liver transplantation. World J Gastroenterol. 2014;20:16418-16432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Pan N, Lv X, Liang R, Wang L, Liu Q. Suppression of graft regeneration, not ischemia/reperfusion injury, is the primary cause of small-for-size syndrome after partial liver transplantation in mice. PLoS One. 2014;9:e93636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Wang W, Du Z, Yan J, Ma D, Shi M, Zhang M, Peng C, Li H. Mesenchymal stem cells promote liver regeneration and prolong survival in small-for-size liver grafts: involvement of C-Jun N-terminal kinase, cyclin D1, and NF-κB. PLoS One. 2014;9:e112532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Nanji AA, Jokelainen K, Tipoe GL, Rahemtulla A, Thomas P, Dannenberg AJ. Curcumin prevents alcohol-induced liver disease in rats by inhibiting the expression of NF-kappa B-dependent genes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G321-G327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 193] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 34. | Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2497] [Cited by in RCA: 3631] [Article Influence: 226.9] [Reference Citation Analysis (0)] |